Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse
Abstract
1. Introduction
2. Methods and Materials
2.1. Bacterial Cellulose Production
2.2. Batch Adsorption
2.3. The Desorption–Adsorption
- BC (0): Biomass cellulose without elution;
- BC (1): Biomass cellulose Elution 1;
- BC (2): Biomass cellulose Elution 2;
- BC (3): Biomass cellulose Elution 3.
2.4. Adsorption Models
2.5. FTIR
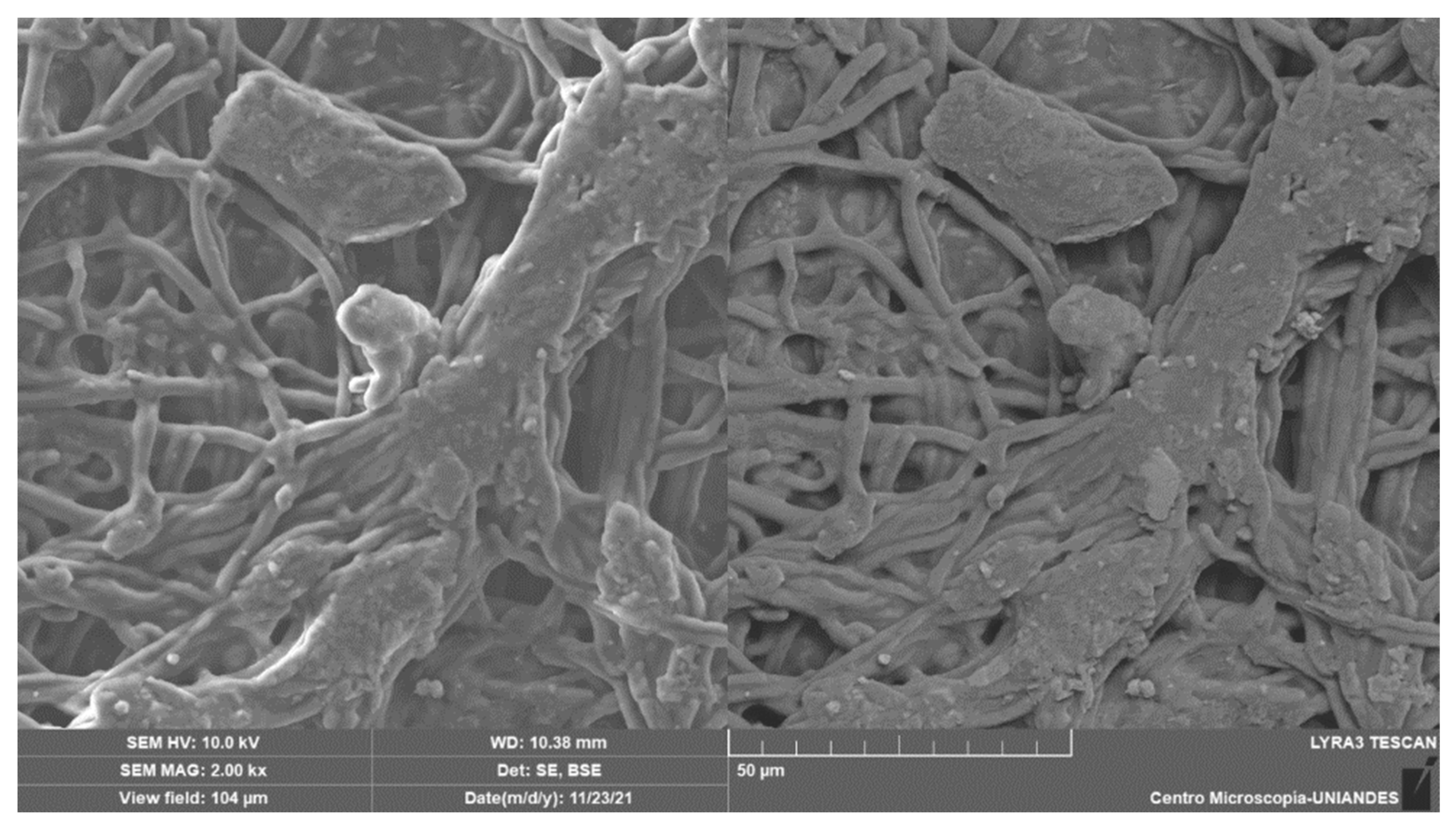
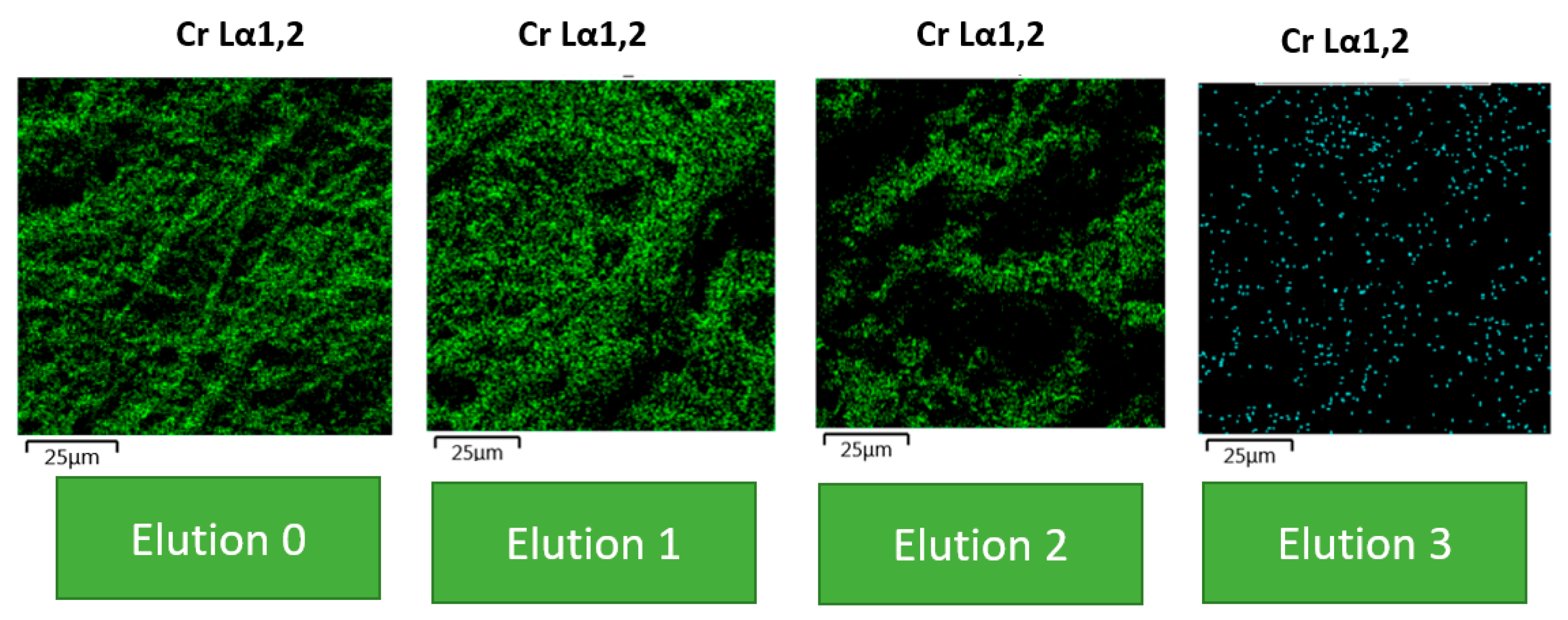
2.6. SEM and EDS Analysis
2.7. Measurement of the Pore Volume of Bacterial Cellulose
3. Result
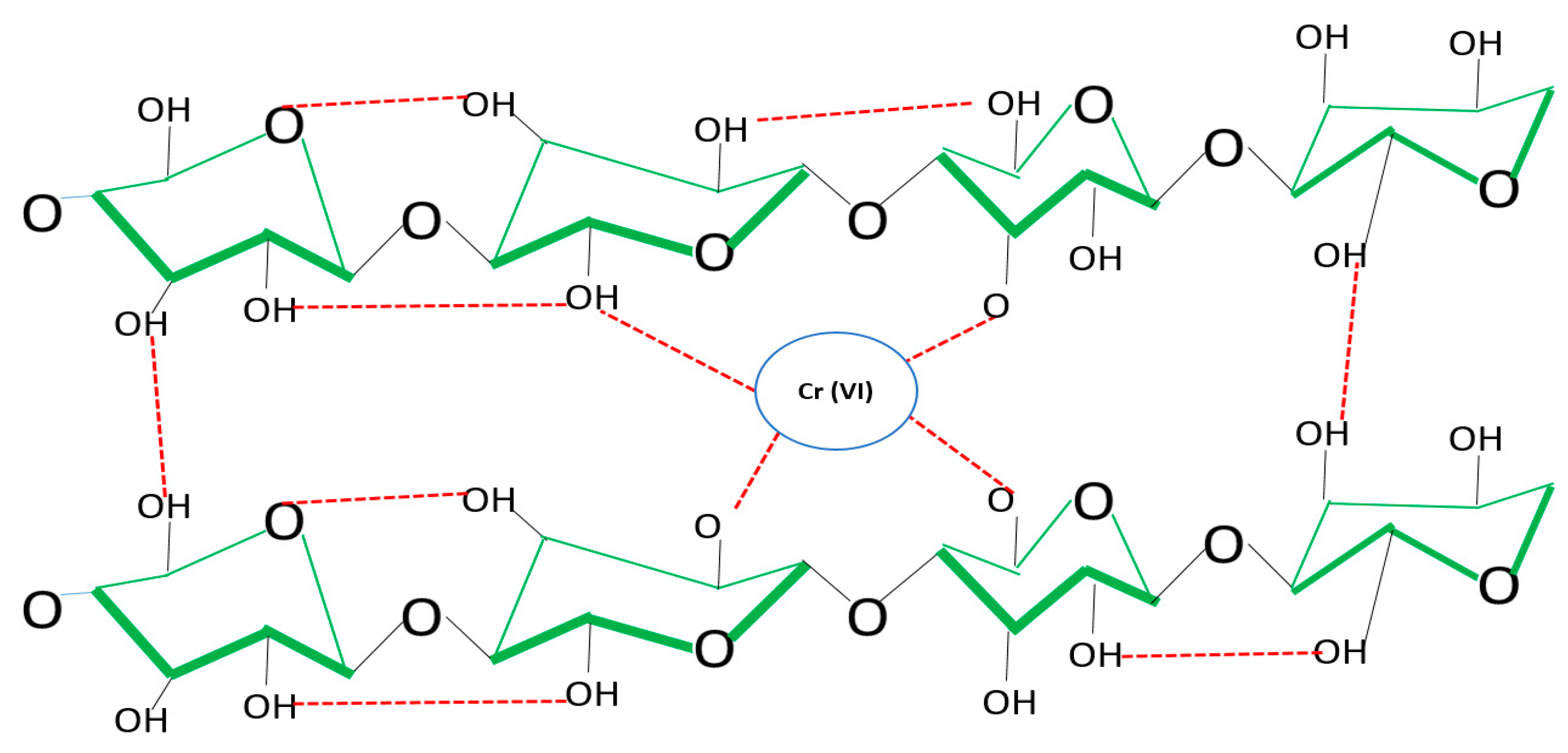
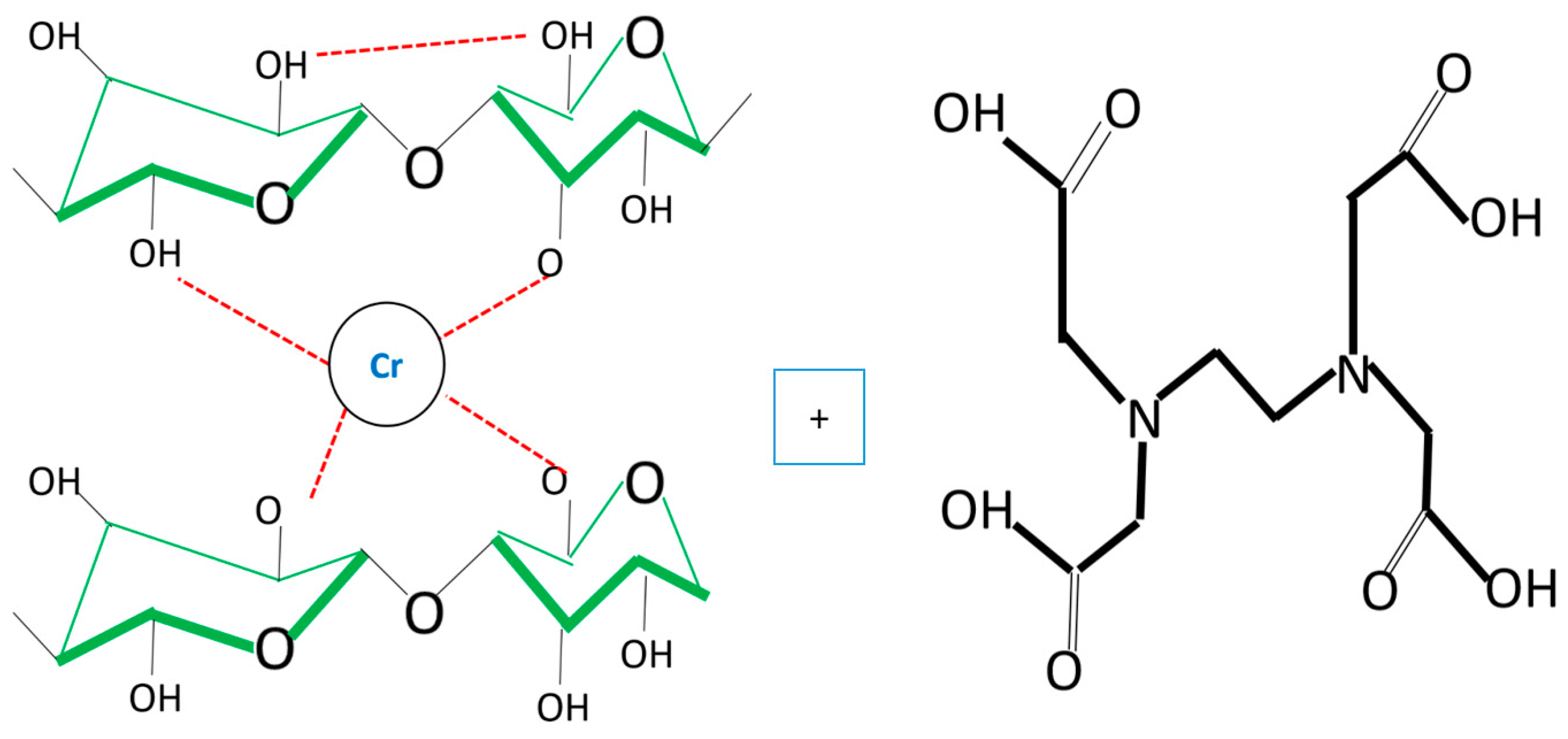
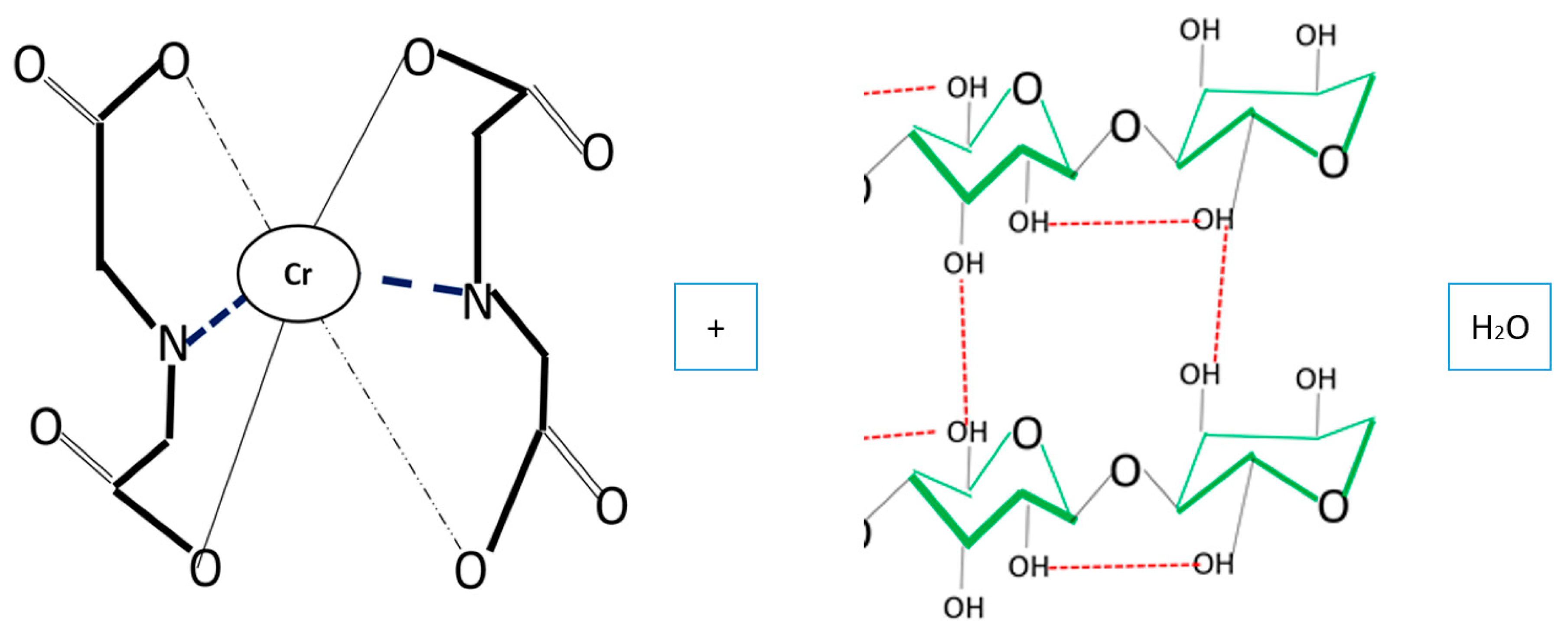
3.1. Adsorption Mechanism by Bacterial Cellulose Biomass
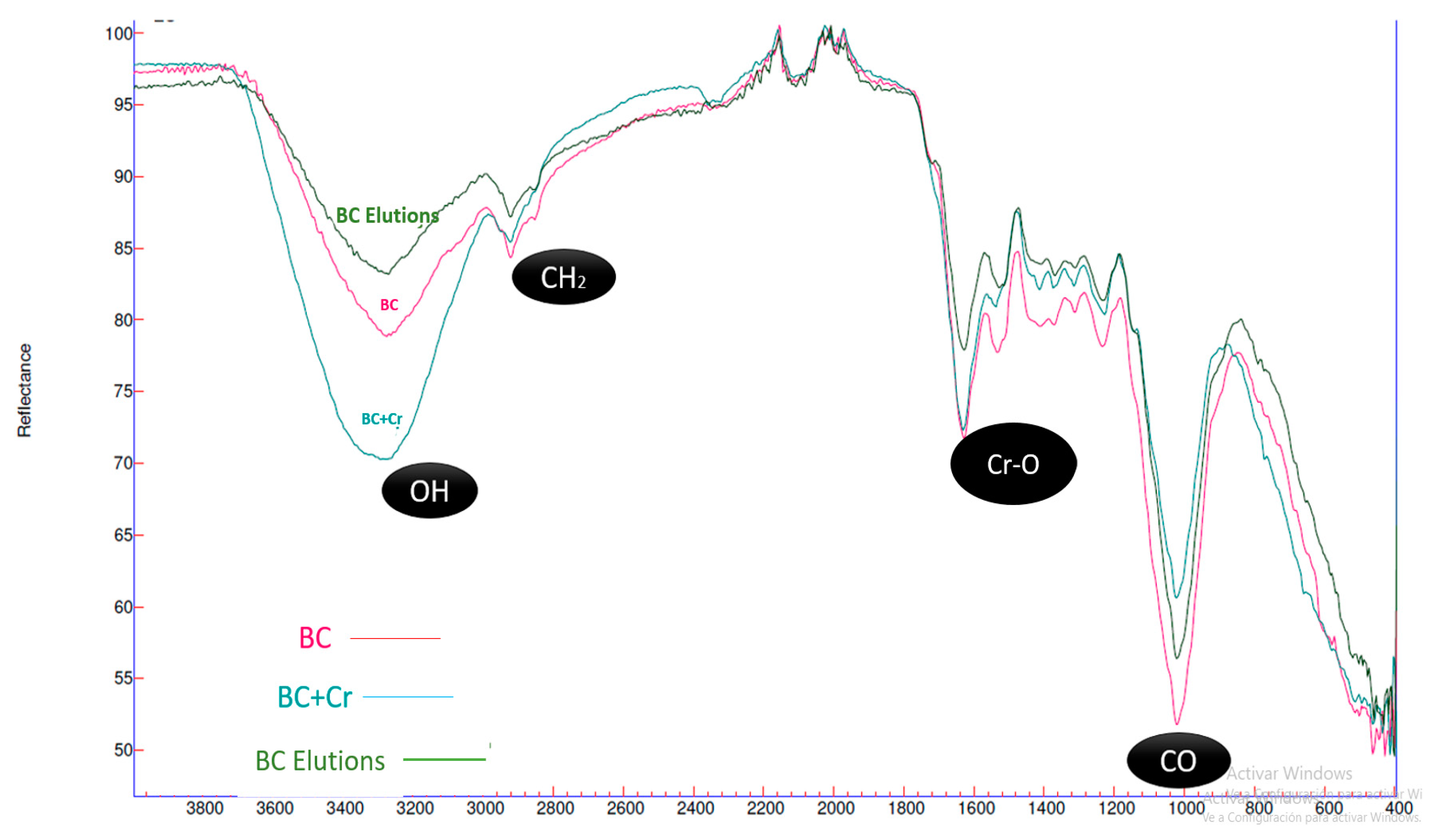
3.2. FTIR
3.3. Measurement of the Pore Volume of Bacterial Cellulose
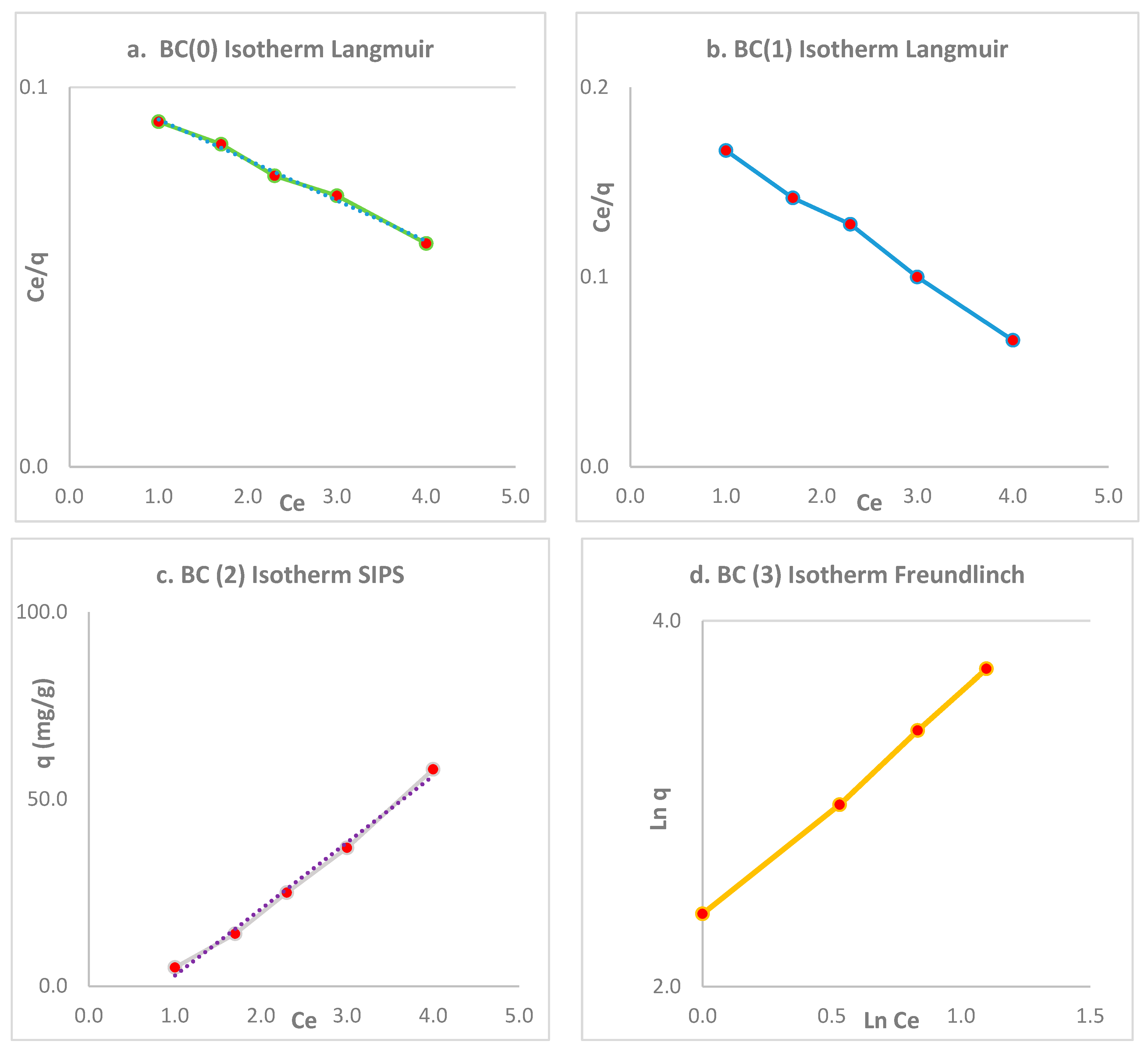
3.4. Isotherms
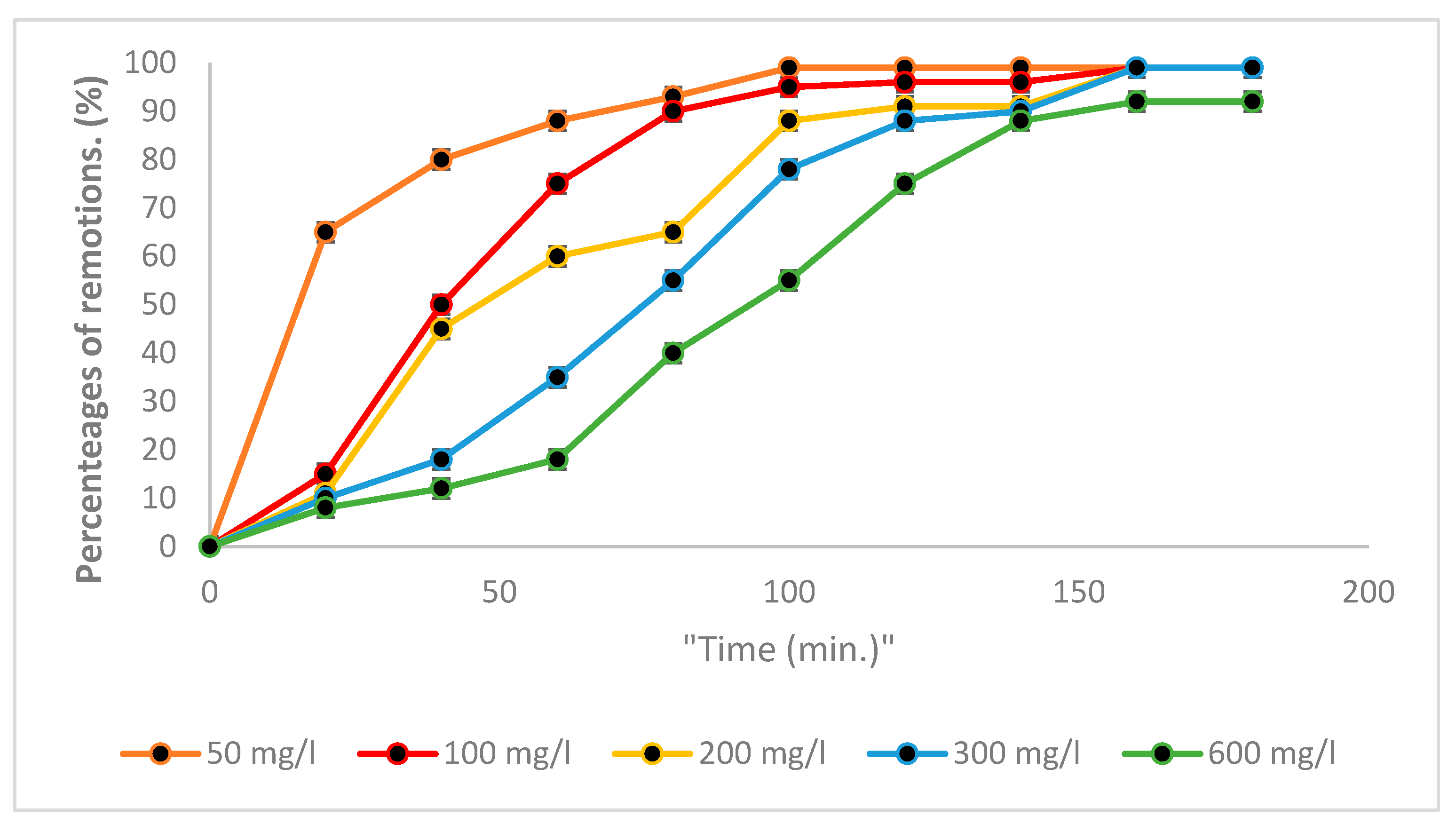
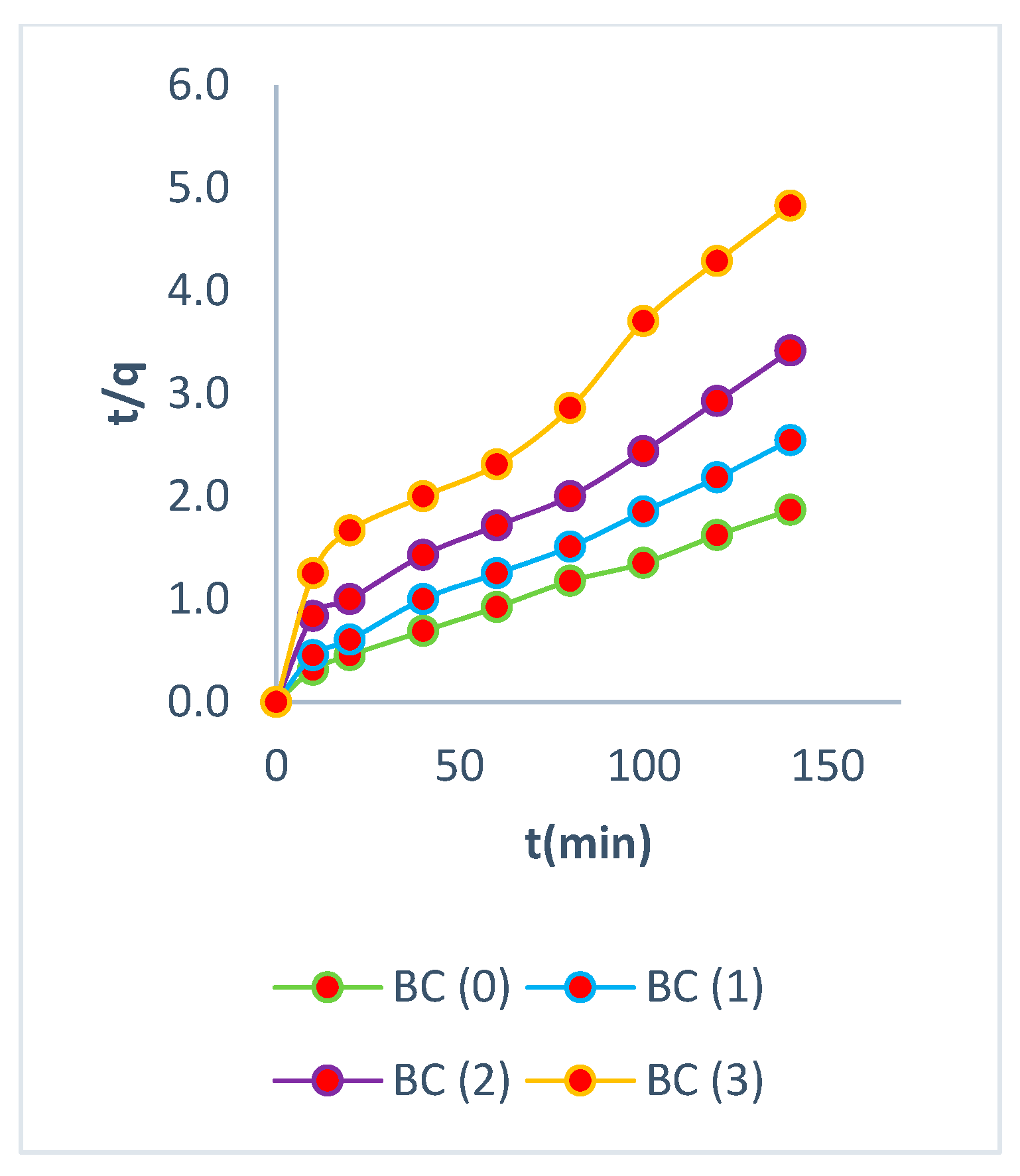
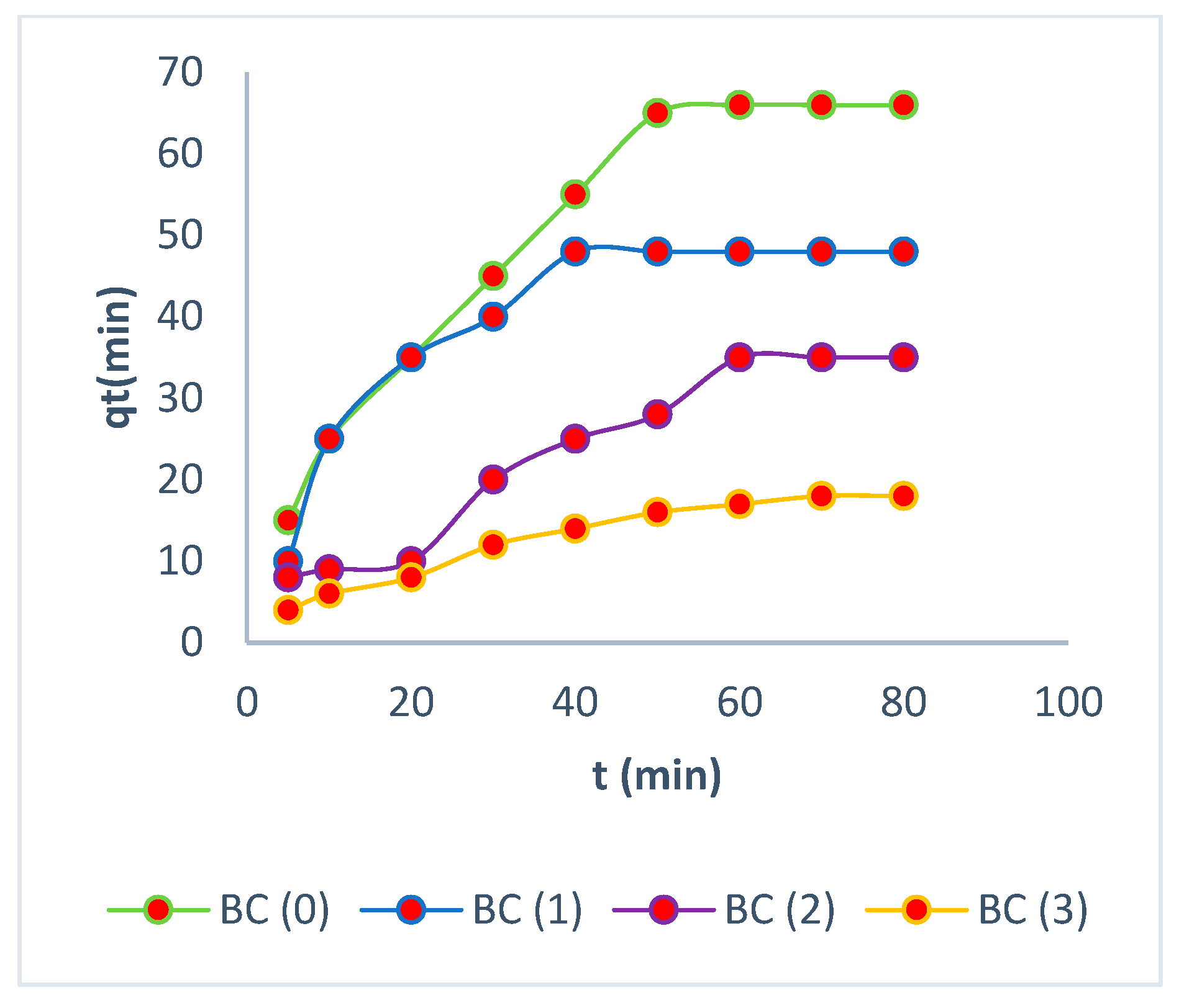
3.5. Kinetic Studies
3.6. Desorption Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sayago, U.F.C.; Gómez-Caicedo, M.I.; Mercado Suárez, Á.L. Design of a sustainable system for wastewater treatment and generation of biofuels based on the biomass of the aquatic plant Eichhornia Crassipes. Sci. Rep. 2024, 14, 11068. [Google Scholar]
- Sari, N.H.; Rangappa, S.S.M.; Siengchin, S. A review on cellulose fibers from Eichornia crassipes: Synthesis, modification, properties and their composites. J. Nat. Fibers 2023, 20, 2162179. [Google Scholar] [CrossRef]
- Sayago, U.F.C. The design of a sustainable industrial wastewater treatment system and the generation of biohydrogen from E. crassipes. Polymers 2024, 16, 893. [Google Scholar] [CrossRef] [PubMed]
- Carreño Sayago, U.F.; Castro, Y.P.; Rivera, L.R.C. Design of a fixed-bed column with vegetal biomass and its recycling for Cr (VI) treatment. Recycling 2022, 7, 71. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Castro, Y.P.; Rivera, L.R.C.; Mariaca, A.G. Estimation of equilibrium times and maximum capacity of adsorption of heavy metals by E. crassipes. Environ. Monit. Assess. 2020, 192, 1–16. [Google Scholar]
- Yang, H.R.; Li, S.S.; Shan, X.C.; Yang, C.; An, Q.D.; Zhai, S.R.; Xiao, Z.Y. Hollow polyethyleneimine/carboxymethyl cellulose beads with abundant and accessible sorption sites for ultra-efficient chromium (VI) and phosphate removal. Sep. Purif. Technol. 2021, 278, 119607. [Google Scholar] [CrossRef]
- Qing, Q.; Shi, X.Y.; Hu, S.Z.; Li, L.; Huang, T.; Zhang, N.; Wang, Y. Synchronously Enhanced Removal Ability and Stability of MXene through Biomimetic Modification. Langmuir 2023, 39, 9453–9467. [Google Scholar] [CrossRef]
- Li, L.; Shi, X.Y.; Huang, T.; Zhang, N.; Wang, Y. Synchronously enhanced storage stability and adsorption ability of MXene achieved by grafting polyethylenimine. J. Mater. Chem. A 2023, 11, 23438–23451. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Castro, Y.P. Development of a composite material between bacterial cellulose and E crassipes, for the treatment of water contaminated by chromium (VI). Int. J. Environ. Sci. Technol. 2022, 19, 6285–6298. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial cellulose and its applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Parte, F.G.B.; Santoso, S.P.; Chou, C.C.; Verma, V.; Wang, H.T.; Ismadji, S.; Cheng, K.C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.D.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C. Industrial-scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Sayago, U.F.C. Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI). Sci. Rep. 2021, 11, 9326. [Google Scholar] [CrossRef] [PubMed]
- Sayago, U.F.C. Design and Development of a Pilot-Scale Industrial Wastewater Treatment System with Plant Biomass and EDTA. Water 2023, 15, 3484. [Google Scholar] [CrossRef]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Yeo, J.C.C.; Zhu, Q.; Ye, E.; Loh, X.J.; Li, Z. Bacterial cellulose: Recent advances in biosynthesis, functionalization strategies and emerging applications. Eur. Polym. J. 2023, 199, 112446. [Google Scholar] [CrossRef]
- Peiravi-Rivash, O.; Mashreghi, M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Producing bacterial nano-cellulose and keratin from wastes to synthesize keratin/cellulose nanobiocomposite for removal of dyes and heavy metal ions from waters and wastewaters. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130355. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.Z.; Huang, T.; Zhang, N.; Wang, Y. Fabricating the ternary CeO2@ CNTs/CdSe composite with synchronously enhanced adsorption and photocatalytic activity toward water-soluble pollutants removal. Chem. Eng. J. 2023, 476, 146574. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Kiselev, E.G.; Nemtsev, I.V.; Vasiliev, A.D.; Kuzmin, A.P.; Shishatskaya, E.I. Bacterial cellulose (BC) and BC composites: Production and properties. Nanomaterials 2022, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchically porous poly (amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021, 600, 752–763. [Google Scholar] [CrossRef]
- Xiaorui, K.; Cong, Z.; Pin, X.; Zhanwen, D.; Zhijiang, C. Copper ion-imprinted bacterial cellulose for selectively removing heavy metal ions from aqueous solution. Cellulose 2022, 29, 4001–4019. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical review of bioadsorption on modified cellulose and removal of divalent heavy metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr (II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef]
- Guo, D.M.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Polyethylenimine-functionalized cellulose aerogel beads for efficient dynamic removal of chromium (VI) from aqueous solution. RSC Adv. 2017, 7, 54039–54052. [Google Scholar] [CrossRef]
- Yang, H.R.; Li, S.S.; Yang, C.; An, Q.D.; Zhai, S.R.; Xiao, Z.Y. Bi-layered hollow amphoteric composites: Rational construction and ultra-efficient sorption performance for anionic Cr (VI) and cationic Cu (II) ions. J. Colloid Interface Sci. 2022, 607, 556–567. [Google Scholar] [CrossRef]
- Luo, H.; Feng, F.; Yao, F.; Zhu, Y.; Yang, Z.; Wan, Y. Improved removal of toxic metal ions by incorporating graphene oxide into bacterial cellulose. J. Nanosci. Nanotechnol. 2020, 20, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Sayago, U.F.C.; Ballesteros, V.A.B. The Design of a Process for Adsorbing and Eluting Chromium (VI) Using Fixed-Bed Columns of E. crassipes with Sodium Tripolyphosphate (TPP). Water 2024, 16, 952. [Google Scholar]
- Ding, R.; Hu, S.; Xu, M.; Hu, Q.; Jiang, S.; Xu, K.; Tremblay, P.L.; Zhang, T. The facile and controllable synthesis of a bacterial cellulose/polyhydroxybutyrate composite by co-culturing Gluconacetobacter xylinus and Ralstonia eutropha. Carbohydr. Polym. 2021, 252, 117137. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.S.; Park, J.M. Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J. Hazard. Mater. 2006, 137, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.; Ibrahim, H.M.; Amr, A.; Asker, M.S.; El-Shafie, A. Preparation and characterization of ion exchanger based on bacterial cellulose for heavy metal cation removal. Egypt. J. Chem. 2019, 62 Pt 2, 457–465. [Google Scholar] [CrossRef]
- Tohamy, H.A.S. Fluorescence ‘Turn-on’Probe for Chromium Reduction, Adsorption and Detection Based on Cellulosic Nitrogen-Doped Carbon Quantum Dots Hydrogels. Gels 2024, 10, 296. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q.; Huang, J. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Hashem, A.; Taha, G.M.; Fletcher, A.J.; Mohamed, L.A.; Samaha, S.H. Highly efficient adsorption of Cd (II) onto carboxylated camelthorn biomass: Applicability of three-parameter isotherm models, kinetics, and mechanism. J. Polym. Environ. 2021, 29, 1630–1642. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B. Recent advances in the treatment of industrial wastewater from different celluloses in continuous systems. Polymers 2023, 15, 3996. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Mechanism of heavy metal ion biosorption by microalgal cells: A mathematic approach. J. Hazard. Mater. 2024, 463, 132875. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, R.N.; Liu, C.; Wang, Y.F.; Ouyang, X.K. Magnetic carboxylated cellulose nanocrystals as adsorbent for the removal of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2016, 93, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Long, Y.; Li, Q.; Chen, X.; Xu, X. Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int. J. Biol. Macromol. 2019, 126, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Phaenark, C.; Nasuansujit, S.; Somprasong, N.; Sawangproh, W. Moss biomass as effective biosorbents for heavy metals in contaminated water. Heliyon 2024, 10, e33097. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem 1906, 57, 1100–1107. [Google Scholar]
- Amar, M.B.; Mallek, M.; Valverde, A.; Monclús, H.; Myers, T.G.; Salvadó, V.; Cabrera-Codony, A. Competitive heavy metal adsorption on pinecone shells: Mathematical modelling of fixed-bed column and surface interaction insights. Sci. Total Environ. 2024, 917, 170398. [Google Scholar] [CrossRef]
- Chao, H.P.; Chang, C.C.; Nieva, A. Biosorption of heavy metals on Citrus maxima peel, passion fruit shell, and sugarcane bagasse in a fixed-bed column. J. Ind. Eng. Chem. 2014, 20, 3408–3414. [Google Scholar] [CrossRef]
- Safardastgerdi, M.; Ardejani, F.D.; Mahmoodi, N.M. Lignocellulosic biomass functionalized with EDTA dianhydride for removing Cu (II) and dye from wastewater: Batch and fixed-bed column adsorption. Miner. Eng. 2023, 204, 108423. [Google Scholar] [CrossRef]
- Solgi, M.; Mohamed, M.H.; Udoetok, I.A.; Steiger, B.G.; Wilson, L.D. Evaluation of a granular Cu-modified chitosan biocomposite for sustainable sulfate removal from aqueous media: A batch and fixed-bed column study. Int. J. Biol. Macromol. 2024, 260, 129275. [Google Scholar] [CrossRef]
- Lu, S.; Liu, W.; Wang, Y.; Zhang, Y.; Li, P.; Jiang, D.; Fang, C.; Li, Y. An adsorbent based on humic acid and carboxymethyl cellulose for efficient dye removal from aqueous solution. Int. J. Biol. Macromol. 2019, 135, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Ho, S.H.; Zhou, Y.; Ren, N.Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar]
- Liu, J.; Chen, T.-W.; Yang, Y.-L.; Bai, Z.-C.; Xia, L.-R.; Wang, M.; Lv, X.-L.; Li, L. Removal of heavy metal ions and anionic dyes from aqueous solutions using amide-functionalized cellulose-based adsorbents. Carbohydr. Polym. 2020, 230, 115619. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, G.; Pang, Y.; Chen, D.-Y.; Kong, L.-J.; Mehmood, S.; Subbaiah, M.V.; Rao, D.S.; Pavuluri, C.M.; Wen, J.-C.; Reddy, G.M. Modification of chitosan macromolecule and its mechanism for the removal of Pb (II) ions from aqueous environment. Int. J. Biol. Macromol. 2019, 136, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium (IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B. Development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Sci. Rep. 2023, 13, 1970. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Che, Y.; Xin, H. Novel complex gel beads composed of hydrolyzed polyacrylamide and chitosan: An effective adsorbent for the removal of heavy metal from aqueous solution. Bioresour. Technol. 2010, 101, 2558–2561. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal oxide-cellulose nanocomposites for the removal of toxic metals and dyes from wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef]
- Tohamy, H.A.S.; El-Sakhawy, M.; Kamel, S. Microwave-assisted synthesis of amphoteric fluorescence carbon quantum dots and their chromium adsorption from aqueous solution. Sci. Rep. 2023, 13, 11306. [Google Scholar] [CrossRef]
- Alipour, A.; Zarinabadi, S.; Azimi, A.; Mirzaei, M. Adsorptive removal of Pb (II) ions from aqueous solutions by thiourea-functionalized magnetic ZnO/nanocellulose composite: Optimization by response surface methodology (RSM). Int. J. Biol. Macromol. 2020, 151, 124–135. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Putro, J.N.; Santoso, S.P.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Nanocrystalline cellulose from waste paper: Adsorbent for azo dyes removal. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100260. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Mutesse, B.; Leswifi, T.Y.; Onyango, M.S. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 2019, 7, 103251. [Google Scholar] [CrossRef]
- Villalobos-Rodríguez, R.; Montero-Cabrera, M.E.; Esparza-Ponce, H.E.; Herrera-Peraza, E.F.; Ballinas-Casarrubias, M.L. Uranium removal from water using cellulose triacetate membranes added with activated carbon. Appl. Radiat. Isot. 2012, 70, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Yang, L.; Zhao, G.; Li, X.; Li, Y. Adsorption performance and mechanism of Cr (VI) using magnetic PS-EDTA resin from micro-polluted waters. Chem. Eng. J. 2012, 200, 480–490. [Google Scholar] [CrossRef]
- Novotnik, B.; Ščančar, J.; Milačič, R.; Filipič, M.; Žegura, B. Cytotoxic and genotoxic potential of Cr (VI), Cr (III)-nitrate and Cr (III)-EDTA complex in human hepatoma (HepG2) cells. Chemosphere 2016, 154, 124–131. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B.; Aguilar, A.M.L. Designing, Modeling and Developing Scale Models for the Treatment of Water Contaminated with Cr (VI) through Bacterial Cellulose Biomass. Water 2024, 16, 2524. [Google Scholar] [CrossRef]




| Model Isotherm | |||
|---|---|---|---|
| Freundlich equation | (1) | qe (mg/g) is the adsorption capacity at equilibrium; (mg/L) is the equilibrium concentration of adsorbents in solution; (mg/g) (L/mg) and n are constants for Freundlich [29]. | |
| Langmuir equation | (2) | = | (mg/g) is the adsorption capacity at equilibrium; (mg/g) is the maximum adsorption capacity; (mg/g) is a constant for Langmuir [25]. |
| Sheindorf– Rebuhn–Sheintuch equation (SIPS equation) | (3) | = | (mg/g) is the adsorption capacity at equilibrium; qm (mg/g) is the maximum adsorption capacity; Ms is a constant of SIPS [30]. |
| Model Kinetic | |||
| Pseudo-first order | (4) | qt and qe (mg/g) are the uptake amounts of pollution at equilibrium and time t (h); K1 (min−1) is the adsorption rate constant of the pseudo-first order [25,30]. | |
| Pseudo-second order | (5) | qt and qe (mg/g) are the uptake amounts of pollution at equilibrium and time t (h); K2 is a constant of the second-order model [25]. | |
| Intraparticle diffusion | (6) | qt (mg/g) is the uptake amount of pollution at equilibrium and time t (h); (mg/g)h0.5) is the intraparticle diffusion; C (mg/g) is the thickness of the boundary layer [25,30]. |
| Biomass | Mass (g) | Volume Mass (vBc) (mL) | Density Mas (pCb) g/mL | Mass of Particle (mg) | Volume Particle (mm) | Density of Particle (pp) | |
|---|---|---|---|---|---|---|---|
| BC (0) | 0.3 | 0.48 | 0.62 | 0.01 | 0.005 | 2 | 0.68 |
| BC (1) | 0.3 | 0.51 | 0.58 | 0.01 | 0.0066 | 1.5 | 0.61 |
| BC (2) | 0.3 | 0.54 | 0.55 | 0.01 | 0.007 | 1.29 | 0.57 |
| BC (3) | 0.3 | 0.57 | 0.52 | 0.01 | 0.008 | 1.17 | 0.55 |
| Isotherm | Constant | R2 | |
|---|---|---|---|
| BC | Langmuir | = 0.03; qm; 75 | 0.99 |
| Freundlich | = 0.16 | 0.91 | |
| SIPS | Ms = 0.99 | 0.97 | |
| Isotherm | Constant | R2 | |
| BC (1) | Langmuir | = 0.02; qm; 60 | 0.99 |
| Freundlich | = 0.11 | 0.92 | |
| SIPS | Ms = 0.99 | 0.95 | |
| Isotherm | Constant | R2 | |
| BC (2) | Langmuir | = 0.011; qm; 55 | 0.91 |
| Freundlich | = 0.10 | 0.92 | |
| SIPS | Ms = 0.55 | 0.95 | |
| Isotherm | Constant | R2 | |
| BC (3) | Langmuir | = 0.01; qm; 35 | 0.91 |
| Freundlich | = 0.09 | 0.98 | |
| SIPS | Ms = 0.01 | 0.91 |
| Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | qe (mg/g) | K1 (min) | R2 | qe (mg/g) | K2 × 10−3 (g/mg × min) | R2 | C (mg/g) | Kd (mg/g × 0.5 h) | R2 |
| BC(0) | 66 | 0.038 | 0.94 | 75 | 1.4 | 0.99 | 18.3 | 4.4 | 0.90 |
| BC(1) | 55 | 0.040 | 0.96 | 60 | 1.5 | 0.98 | 18.3 | 4.4 | 0.93 |
| BC(2) | 41 | 0.042 | 0.97 | 55 | 1.6 | 0.96 | 19.2 | 4.5 | 0.96 |
| BC(3) | 29 | 0.044 | 0.99 | 35 | 1.8 | 0.90 | 20.1 | 4.6 | 0.99 |
| Element | BC | BC (0)% | BC (1)% | BC (2)% | BC (3)% |
|---|---|---|---|---|---|
| Carbon | 46.8 | 44.67 | 46.67 | 47.67 | 48.67 |
| Oxygen | 48.2 | 46.94 | 45.94 | 47.3 | 48.3 |
| Cr (VI) | 0 | 13.3 | 8.5 | 6.3 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayago, U.F.C.; Ballesteros, V.B.; Aguilar, A.M.L. Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse. Polymers 2024, 16, 2605. https://doi.org/10.3390/polym16182605
Sayago UFC, Ballesteros VB, Aguilar AML. Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse. Polymers. 2024; 16(18):2605. https://doi.org/10.3390/polym16182605
Chicago/Turabian StyleSayago, Uriel Fernando Carreño, Vladimir Ballesteros Ballesteros, and Angelica María Lozano Aguilar. 2024. "Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse" Polymers 16, no. 18: 2605. https://doi.org/10.3390/polym16182605
APA StyleSayago, U. F. C., Ballesteros, V. B., & Aguilar, A. M. L. (2024). Bacterial Cellulose-Derived Sorbents for Cr (VI) Remediation: Adsorption, Elution, and Reuse. Polymers, 16(18), 2605. https://doi.org/10.3390/polym16182605








