A Review on the Application of Deep Eutectic Solvents in Polymer-Based Membrane Preparation for Environmental Separation Technologies
Abstract
1. Context
2. Properties of Deep Eutectic Solvents
2.1. Classification of Deep Eutectic Solvents
2.2. Physicochemical Properties of Deep Eutectic Solvents
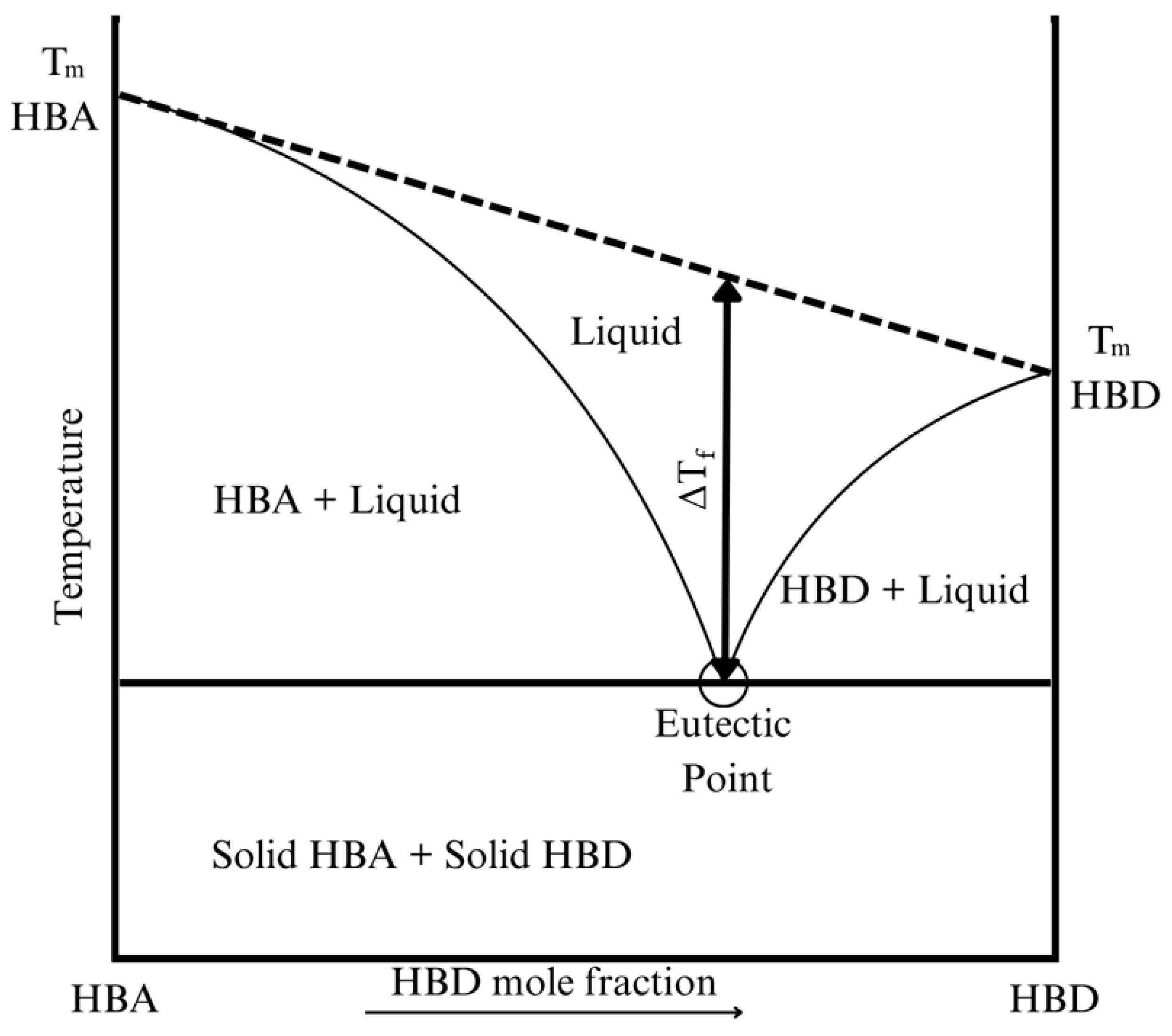
2.2.1. Melting Point of Deep Eutectic Solvents
2.2.2. Surface Tension of Deep Eutectic Solvents
2.2.3. Viscosity of Deep Eutectic Solvents
2.2.4. Density of Deep Eutectic Solvents
3. Use of Deep Eutectic Solvents in Polymer Membrane Fabrication
| Work | Base/Support Material (Solvent) | DES Code (Molar Ratio) | HBA | HBD | Nature of DES | CDES, % | M.P.T. (Non-Solvent) | rp, nm | ε, % | WCA, ° | Membrane Morphology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Use of DESs as additive and/or modifier | |||||||||||
| [99] | PES (DMAc) | ChCl/EG (1:2) | Choline chloride | Ethylene glycol | Hydrophilic | 0.5–4.0 | NIPS (H2O) | 3.4–12.1 | 48.4–64.0 | 42.3–48.2 | Dense top layer and a finger-like structure in the bulk |
| [98] | PES (DMAc) | L-M/CSA (5:1) | L-Menthol | 10-camphorsulfonic acid | Neutral | 0.2–1.0 | NIPS (H2O) | 0.73–0.85 | 70.5–82.7 | ~66 | Top dense layer, an intermediate finger-like layer and a sponge-like bottom |
| [115] | PVDF (AC) | [BMIM][Br]/DEG (2:1, 1:1, 1:2) | 1-butyl-3-methyl-imidazolium bromide | Diethylene glycol | Hydrophilic | 1.25–5.00 | CE | Dense | |||
| [116] | PA (H2O/Hx)/PSf (DMF) | ChCl/Urea (1:2) | Choline chloride | Urea | Hydrophilic | 1–10 | IP | 27.7–36.6 | Dense top PA layer and sponge-like PSf support | ||
| [117] | PA (H2O/Hx)/PSf | Th/AA (1:1, 1:2, 1:3, 1:4) | Thymol | Acetic acid | Hydrophilic | 0.0025–0.02 | CAIP | Dense top PA layer and porous PSf support | |||
| [118] | PA (H2O/Hx)/PES | CA/ChCl/GLY (x:1:2, ternary DES with x ranging from 0.24 to 0.96 mol%) | Choline chloride | Glycerol | Hydrophilic | 10 | IP | 47–64 | Cellular/Nodular structure | ||
| Citric acid | Citric acid | ||||||||||
| [119] | PAN (DMF) | SAT/Urea (1:2) | Sodium acetate trihydrate | Urea | Hydrophilic | ~3–~15 | ES | 50–70 | Porous | ||
| [120] | PI (NMP + DMAc) | ChCl/EG (1:2) | Choline chloride | Ethylene glycol | Hydrophilic | NIPS (H2O + NMP) | 135–152 | 42–67 | 58–74 | Finger-like microporous | |
| [121] | PAI (NMP) | ZnCl2/AA (1:3) | Zinc chloride | Acetamide | Hydrophilic | 5–50 | CE | 65.3–84.1 | Dense. Macrovoids appeared at DES concentrations of >20% | ||
| [122] | PEBAX (H2O + EtOH) | [EMIM][Cl]/EG (1:1) | 1-ethyl-3-methylimidazolium chloride | Ethylene glycol | Hydrophilic | ~5–~15 | CE | Dense | |||
| [EMIM][Cl]/Lev (1:2 1:1) | 1-ethyl-3-methylimidazolium chloride | Levulinic acid | Hydrophilic | ||||||||
| [123] | CS-CMC (H2O + AcA) | ChCl/Urea (1:2) | Choline chloride | Urea | Hydrophilic | CE | Dense | ||||
| [124] | CS (H2O + AcA) | PPRO/GLU (5:1) | Protonated L-proline | Glucose | Hydrophilic | 5 | CE | 50 | Dense | ||
| [125] | CS (H2O + AcA) | PCA/SULF (1:3) | Protonated 2-Pyrrolidone-5-carboxylic acid | Sulfolane | Hydrophilic | 5 | CE | 100 | Dense | ||
| [126] | CS (H2O + AcA) | PRO/SULF (1:2) | L-proline | Sulfolane | Hydrophilic | 5 | CE | 34 | Dense | ||
| [127] | GO (H2O)/PES | ChCl/EG (1:2) | Choline chloride | Ethylene glycol | Hydrophilic | CE | 56.5–62.9 | Porous | |||
| Use of DESs as pore former | |||||||||||
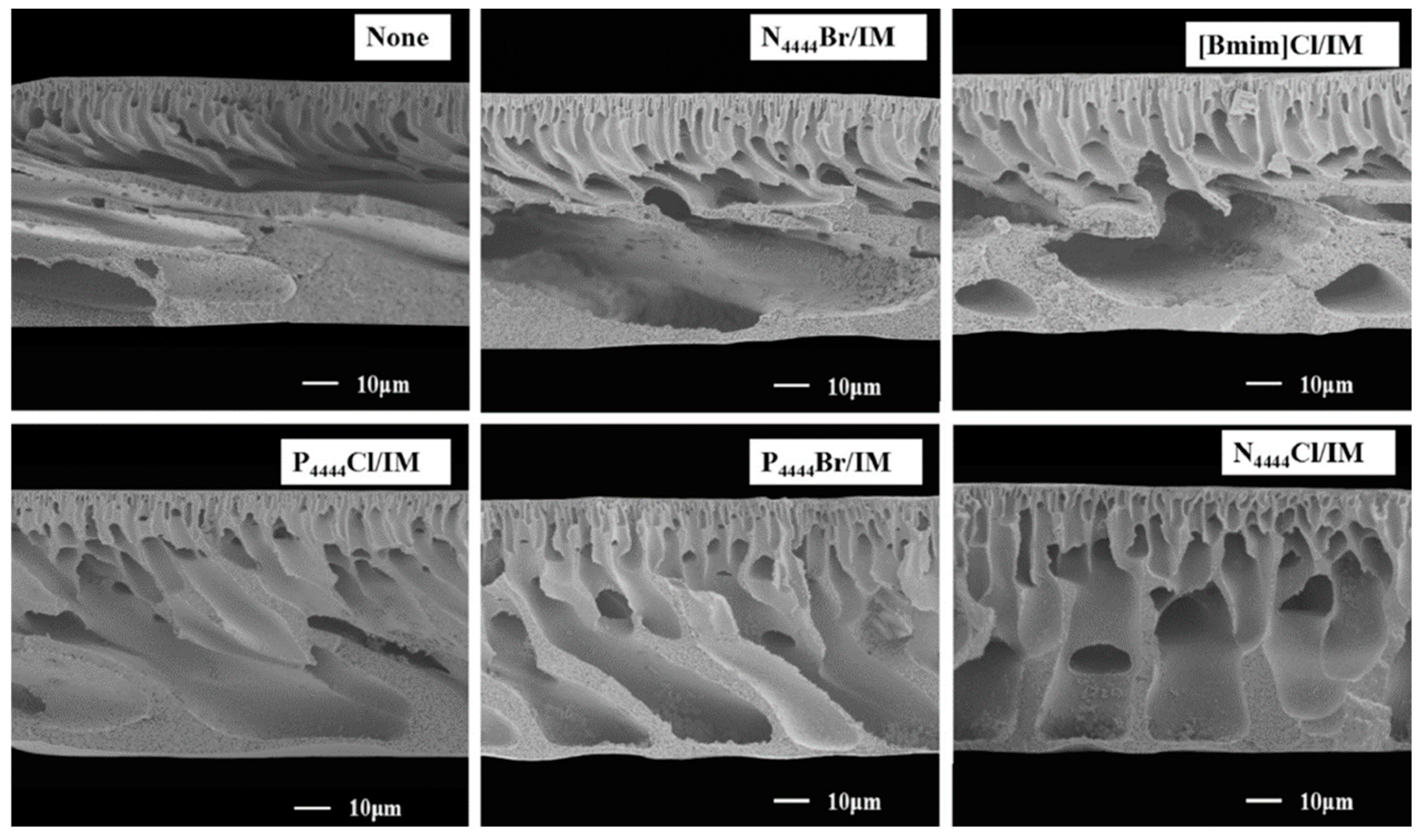
| [128] | PES (NMP) | [N4444][Cl]/IM (3:7) | Tetrabutylammonium chloride | Imidazole | Hydrophilic | 2 | NIPS (H2O) | 40.5 ± 1.2 | 86.5 ± 2.5 | All: Dense top layer and a porous sublayer with a finger-like and macrovoid structure | |
| [N4444][Br]/IM (3:7) | Tetrabutylammonium bromide | Imidazole | Hydrophilic | 2 | NIPS (H2O) | 32.2 ± 0.8 | 63.3 ± 1.8 | ||||
| [P4444][Cl]/IM (3:7) | Tetrabutylphosphonium chloride | Imidazole | Hydrophilic | 2 | NIPS (H2O) | 37.1 ± 0.8 | 78.4 ± 1.9 | ||||
| [P4444][Br]/IM (3:7) | Tetrabutylphosphonium bromide | Imidazole | Hydrophilic | 1–4 | NIPS (H2O) | 38.3 ± 1.0 (at 2%) | 83.6 ± 2.0 (at 2%) | ||||
| [BMIM][Cl]/IM (3:7) | 1-butyl-3-methylimidazolium chloride | Imidazole | Hydrophilic | 2 | NIPS (H2O) | 34.9 ± 0.6 | 68.1 ± 1.8 | ||||
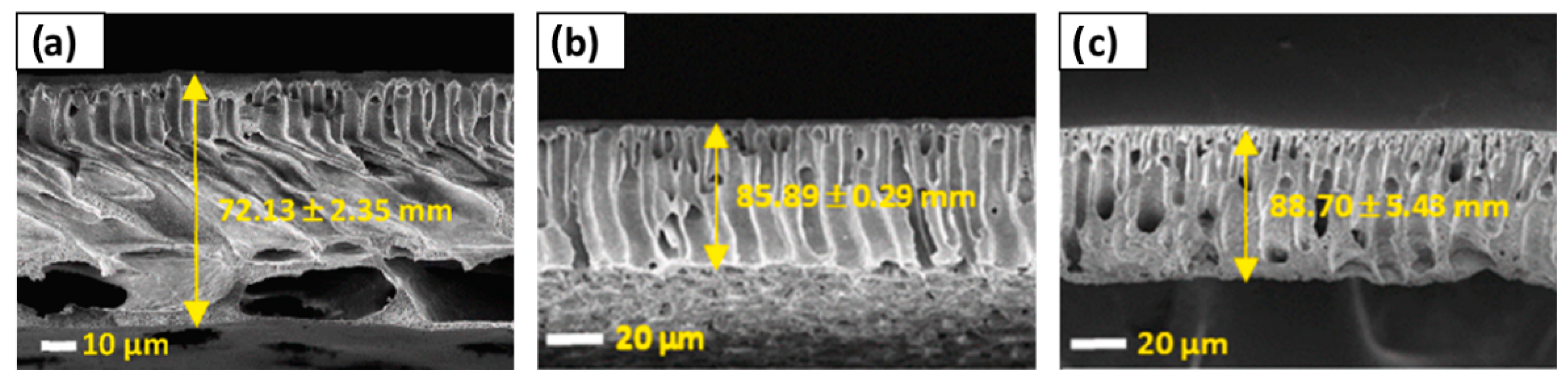
| [95] | PES (NMP) | DecA/[N4444][Cl] (2:1) | Tetrabutylammonium chloride | Decanoic acid | Hydrophobic | 1–4 | NIPS (H2O) | 12.90–16.94 | 62.46–74.10 | Dense top layer, intermediate finger-like sublayer, and macrovoids and sponge-like structure at the bottom. | |
| [129] | PES (DMSO) | ChCl/Urea (1:2, 1:3, 1:4, 1:5) | Choline chloride | Urea | Hydrophilic | 1 | NIPS (H2O) | 14.24–29.46 | 70–93 | Porous | |
| [130] | PES (NMP) | ChCl/IA (1:1) | Choline chloride | Itaconic acid | Hydrophilic | 0.5–0.8 | NIPS (H2O) | 1.96–2.93 | 71.0–73.2 | 60.9–66.2 | Dense top layer and a finger-like intermediate layer with or without macrovoids |
| [131] | PSE (DMF) | ZnCl2/EG (1:4) | Zinc chloride | Ethylene glycol | Hydrophilic | 1–10 | NIPS (H2O) | 24.4–54.3 | 77–84 | Dense top layer and a finger-like and/or macrovoids with a sponge-like structure in the bulk | |
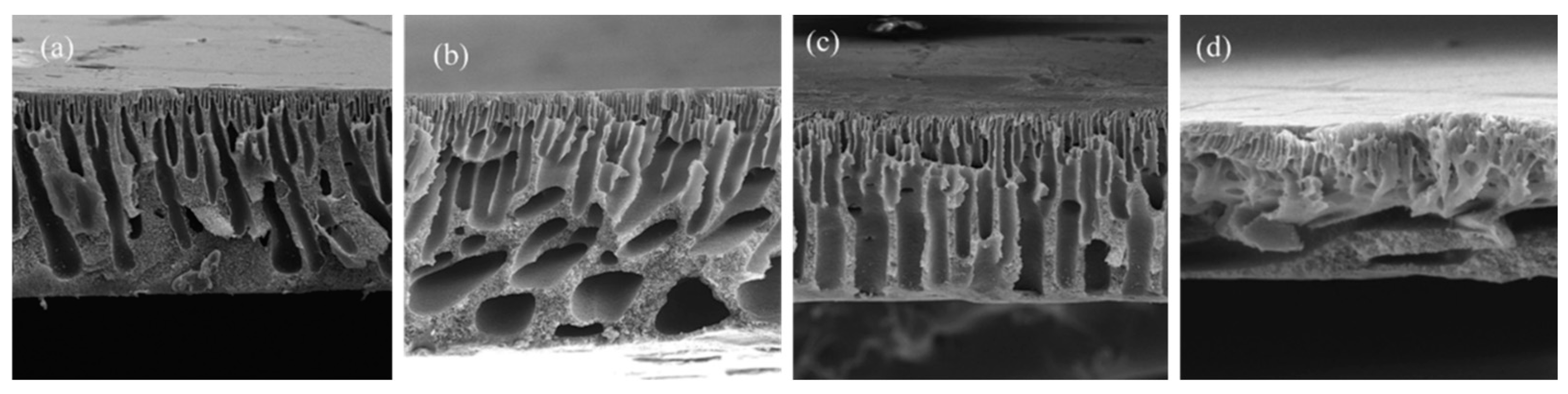
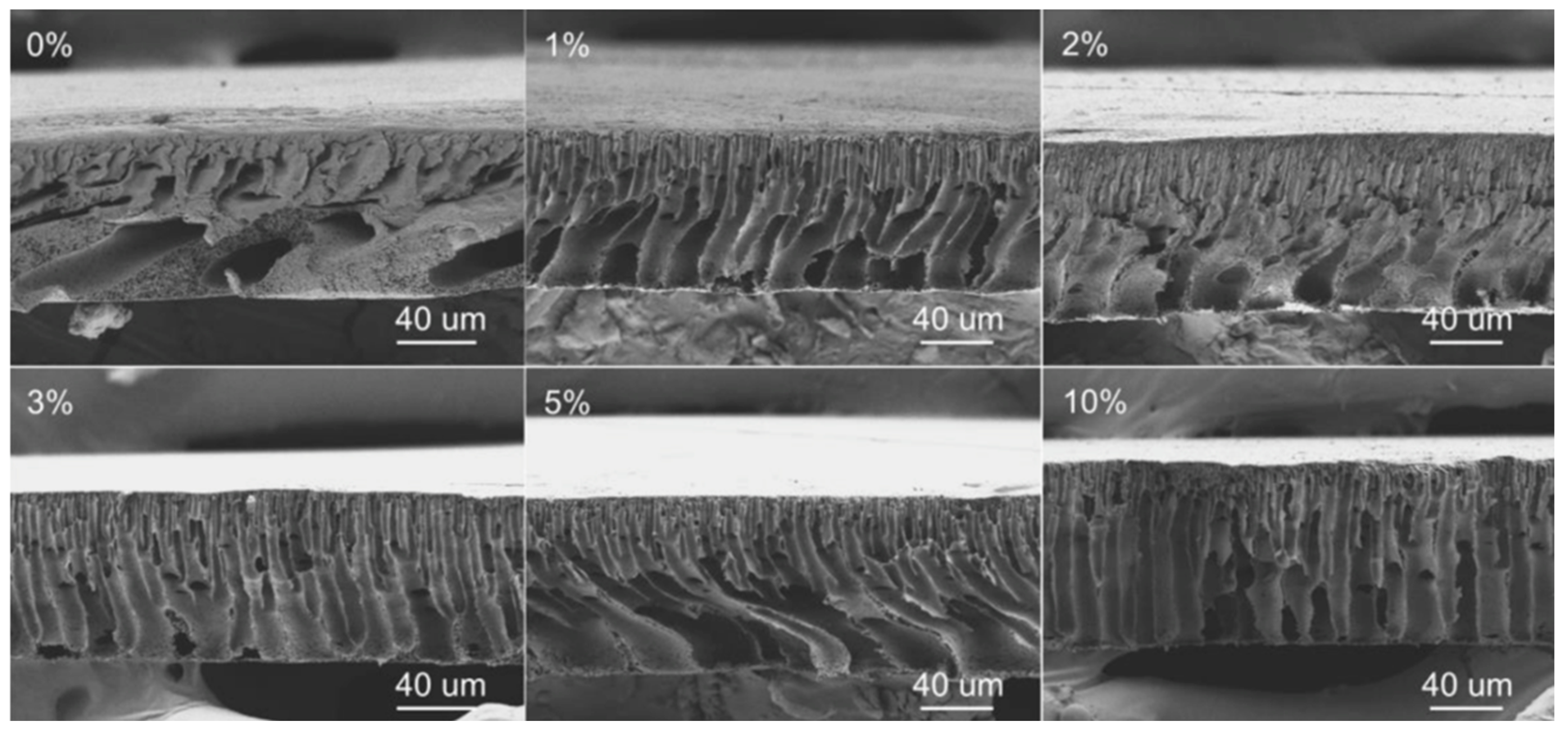
| [85] | PVDF (DMF) | ChCl/Urea (1:2) | Choline chloride | Urea | Hydrophilic | 2 | NIPS * NIPS * NIPS * NIPS * NIPS * * (H2O) | 532 ± 152 | ~71 | All: Thin porous layer and a finger-like with macrovoids bulk. Macrovoids diminished as DES% increased | |
| ChCl/GLY (1:2) | Choline chloride | Glycerol | Hydrophilic | 2 | 326 ± 88 | ~67 | |||||
| ChCl/ZnCl2 (1:2) | Choline chloride | Zinc chloride | Hydrophilic | 2 | 350 ± 67 | ~60 | |||||
| ChCl/LA (1:2) | Choline chloride | Lactic acid | Hydrophilic | 2 | 296 ± 138 | ~62 | |||||
| ChCl/GLU (1:2) | Choline chloride | Glucose | Hydrophilic | 2 | 282 ± 45 | ~75 | |||||
| [132] | PSf (NMP) | ChCl/FR (1:1) | Choline chloride | D-(-)-Fructose | Hydrophilic/Hydrophobic | 1–4 | NIPS (H2O) | 4.4–17.0 | 55–88 | 36–75 | Dense top layer and a sponge, finger-like, and macrovoid sublayer |
| Use of DESs as solvent or cosolvent | |||||||||||
| [96] | PVDF (DES) | [NMA]/AA (1.9:1) | N-methylacetamide | Acetamide | Hydrophilic | 81–85 | NTIPS * NTIPS * NTIPS * * (H2O) | 0.53–4.64 | 80–90 | 55–60 | All: dense top layer, intermediate finger-like sublayer and macrovoided sublayer |
| [NMA]/NMU (4.1:1) | N-methylacetamide | N-methylurea | Hydrophilic | 83 | 5.37 | ~90 | ~50 | ||||
| [NMA]/NN′-DMU (2.8:1) | N-methylacetamide | N,N′ -dimethylurea | Hydrophilic | 83 | 3.20 | ~80 | ~70 | ||||
| [97] | PVDF (DES + PolarClean/TEP) | PTSA/TBnA MsO (1:1) | Benzyl-trimethylammonium mesylate | p-toluensulphonic acid | Hydrophilic | 60 | NIPS (H2O/IPA) | 80–150 | 82–85 | 104–110 | Sponge- and finger-like with macrovoids |
| PhAA/TMG (2:1) | Trimethyl glycine | Phenyl acetic acid | Hydrophobic | 60 | NIPS (H2O/IPA) | 150–280 | 80–85 | 104–114 | Spherulitic | ||
| PAN (DES + DMSO) | GLYA/TMG (2:1) (+)CSA/SB3-MIM (1.5:1) | Trimethyl glycine (3-(1-methyl-1H-imidazole-3-ium-3 -yl) propane -1-sulfonate) | Glycolic acid (1S)-(+)-10- camphorsulfonic acid | Hydrophilic Hydrophilic | 60 | NIPS (H2O) | 230 ± 10 | 86 ± 2 | ~43 | Finger-like with macrovoids | |
| (+)CSA/SB3-4 (2:1) | 3-(N,N-dimethybutylammonio) propane-1-sulfonate | (1S)-(+)-10- camphorsulfonic acid | Hydrophilic | 60 | NIPS (H2O) | 60 ± 10 | 82 ± 2 | ~42 | Dense top layer and finger-like bulk | ||
| [133] | PA (H2O +DES/Hx)/PES | ChCl/EG (1:2) | Choline chloride | Ethylene glycol | Hydrophilic | 10–90 | IP | 49–67 | Porous | ||
| [134] | Lignin (DES) | PA/Urea (2:1) | Urea | Propionic acid | Hydrophilic | 78 | NIPS (H2O) | 66–76 | Finger-like | ||
| Use of DESs as non-solvent or a component in the coagulation bath in NIPS | |||||||||||
| [100] | PVDF (DMAc) | BET/LA (1:2) | Betaine | Lactic acid | Hydrophilic | 1–20 | NIPS (H2O) | 60–80 | Finger-like macrovoids | ||
| Use of DESP as additive and/or modifier | |||||||||||
| [101] | PA (H2O/Hx)/PI (NMP) | β-CD/MA (1:5, 1:10) | β-Cyclodextrin | L-malic acid | Hydrophilic | 100 (DESP used for coating) | IP | 47–79 | 6–17 | Dense top layer and a porous bulk | |
| [135] | CS (H2O) | β-CD/LA (1:3–1:8) | β-Cyclodextrin | Lactic acid | Hydrophilic | CE | Change from dense at highly porous as DESP concentration increased | ||||
| Work | Base/ Support Material | DES Code | Rq, nm | Application | Performance | Concluding Remarks |
|---|---|---|---|---|---|---|
| Use of DESs as additive and/or modifier | ||||||
| [99] | PES | ChCl/EG | 7.5–12.9 | NF for dye separation | PWF up to 241.3 L m2 h−1 at 3 bar with a BSA rejection of 98.9% and RG19 dye removal of 99.2% | The use of DESs always increased the PWF, and the antifouling properties were improved due to a smoother surface. The maximum PWF was obtained when using a membrane containing a 2% of DES. |
| [98] | PES | L-M/CSA | 1.7–9.54 * | NF for pharmaceutical separation | PWF up to 111.5 L m2 h−1 with a ceftriaxone and amoxicillin rejection of 99.6 and 99.2%, respectively | The optimum performance membrane characteristics were obtained when using a membrane containing 0.2% of DES. Antifouling properties were also improved due to a smoother surface and higher negative surface charge. |
| [115] | PVDF | [BMIM][Br]/DEG | GS for removal of SO2 | SO2 permeability reached up to 17,480 Barrer (0.2 bar, 40 °C) and SO2/N2 and a ultrahigh SO2/CO2 selectivity of 3690 and 211, respectively. | The increase of DES content and [Bmim]-to-DEG ratio improved the SO2 permeability and SO2/N2 selectivity. The maximum performance was achieved with the membrane containing 50% of DES (molar ratio of 2:1) and the membrane performance was stable for at least 100h. | |
| [116] | PA/PSf | ChCl/Urea | 12.6–30.1 | RO for water desalination | PWF up to 56.7 L m2 h−1 with a NaCl rejection of 96.4% | The membrane modified with 1% of DES showed the best performance. Antifouling properties specially increased at DES concentration < 5% due to a smoother surface and the negative charge induced by the DESs. |
| [117] | PA/PSf | Th/AA | 49.54–70.00 | RO for water treatment | PWF up to 80.39 L m2 h−1 with a NaCl rejection of 98% at the optimal DES dosage. | Optimal DES dosage and molar ratio of 0.0025% and 1:3. This membrane also presented the best long-term performance stability with a flux decline of 9% after 24 h. In addition, the membrane presented the highest fouling resistance, showing a 1.5% flux decline after 180 min in operation with a NaCl/humic acid-containing solution, which was attributed to the roughness, water contact angle and Zeta potential reduction. Moreover, the use of DESs increased the membrane resistance to chlorine agents, improving the chemical washing efficiency of these membranes. |
| [118] | PA/PES | CA/ChCl/GLY | 29.7–44.2 * | NF for ground and drinking water treatment | PWF up to 39.5 L m2 h−1 bar−1 with a Na2SO4 rejection of 98.8% | Best membrane performance obtained for the DESs containing 0.72 mol% of citric acid. Antifouling properties also enhanced due to a higher hydrophilicity and electronegativity (lower zeta potential). |
| [119] | PAN | SAT/Urea | Not evaluated | |||
| [120] | PI | ChCl/EG | 33.8–118.9 | UF for aqueous phenol removal | PWF up to 300 L m2 h−1 with a phenol removal efficiency of 96% | The use of DES-coated nanosilica as nanofillers enhanced the membrane pore structure (increased pore size) and chemistry (increased hydrophilicity) for phenol removal, being the 2% nanoparticles load the optimal value for maximizing membrane performance |
| [121] | PAI | ZnCl2/AA | PV for water/IPA separation | Total flux between 30 and ~110 g m−2 h−1 with a separation factor between 200 and 800 at optimal DES content for a water in feed from 5 to 20%. | Total flux and separation factor inversely proportional and highly dependent of DES content. Total flux increased but separation factor drastically decreased as DES content rose. Optimal DES content established at 10%. | |
| GS for O2/He/N2 separation | He, O2 and N2 permeabilities of 5.32, 0.27 and 0.017 Barrer, respectively, and O2/N2, He/O2, and He/N2 selectivity of 15.9, 19.7 and 0.33, respectively. | Permeability coefficient for all gases increased with increasing DES content in membranes. However, the He/N2 and He/O2 selectivity decreased whilst O2/N2 selectivity increased. | ||||
| [122] | PEBAX | [EMIM][Cl]/EG | GS for natural gas desulfuration | H2S permeability up to 1928 Barrer, and H2S/CO2, and H2S/CH4 selectivity up to 14.35 and 242.0, respectively. | The inclusion of DESs in membrane involved DES-H2S interactions, which improved the H2S separation efficiency, making the [Emim]Cl/Lev DES the most efficient. | |
| [EMIM][Cl]/Lev | ||||||
| [123] | CS-CMC | ChCl/Urea | Proton exchange membrane for fuel cells | Proton conductivity up to 1.57·10−2 S/cm | DESs acted as a plasticizer which increased the thermal degradation stability of the membrane and promoted the proton conductivity. Also, DESs led to a smoother and more homogeneous morphology. | |
| [124] | CS | PPRO/GLU | 3.0 ± 0.5 | PV for ethanol dehydration | Total permeate flux of 0.242 and 0.389 kg m−2 h−1 and separation factor of 1425 and 831.7 at temperature of 20 and 50 °C, respectively | Incorporation of DESs in membrane preparation improved the mass transfer of water molecules respect to ethanol, thus enhancing pervaporation yield and permeation. |
| [125] | CS | PCA/SULF | 23 ± 0.5 | PV for ethanol dehydration | Total permeate flux of 0.3 and 0.449 kg m−2 h−1 and separation factor of 518 and374 at temperature of 20 and 50 °C, respectively | Incorporation of DESs reduced the membrane mass transfer resistance, contributing to the increase in the permeate flux. However, membrane selectivity decreased respect to the CS bare membrane. |
| [126] | CS | PRO/SULF | PV for MeOH-MTBE azeotropic mixture separation | At 45 °C: total flux of 73 kg m−2 h−1 and separation factor of 1 At 25 °C: total flux of 8 kg m−2 h−1 and separation factor of 35 | Higher separation factors were obtained with the crosslinked CS membranes even though the total flux drastically decreased respect to the non-crosslinked membrane. DESs incorporation in the membrane led to a decreased in specific mechanical properties such as Young’s modulus and tensile strength due to plasticization. | |
| [127] | GO/PES | ChCl/EG | 60.2–60.7 | NF for dye desalination | PWF up to 124.8 ± L m2 h−1 bar−1 with a high dye rejection:
| The use of DESs as additive considerably increased the PWF due to the lower wettability, the enlargement of nanochannels after the DES functionalization, and the reduced yet high negative surface charge, among other factors. Moreover, Na2SO4 salt rejection was low in around 5% while keeping high dye rejection. In addition, GO/DES membranes presented an enhanced antiadsorption of dye properties and a flux recovery of 74–100% after four filtration cycles. |
| Use of DESs as pore former | ||||||
| [128] | PES | [N4444][Cl]/IM | 9.5 ± 0.2 | UF for water treatment | PWF up to 781 L m2 h−1 with a BSA rejection of 97.7% at 2 bars | DESs as pore former increased the PWF due to the formation of nanovoids and enlargement of membrane pores. The maximum PWF was obtained for the N4444Cl/IM DES. However, DES-based membranes presented lower elongation at break and tensile strength than the PES bare membrane. |
| [N4444][Br]/IM | 6.4 ± 0.4 | |||||
| [P4444][Cl]/IM | 9.1 ± 0.4 | |||||
| [P4444][Br]/IM | 9.2 ± 0.2 | |||||
| [BMIM][Cl]/IM | 7.3 ± 0.2 | |||||
| [125] | PES | DecA/[N4444][Cl] | UF for water treatment | PWF up to 142.84 L m2 h−1 with a pepsin, egg albumin and BSA rejection of 91.5, 97.3 and 99.0%, respectively, at 2 bar | DES addition as porogen improved the water flux and protein rejection ratio of the membrane. The maximum membrane performance was obtained when using a 2% DES concentration. | |
| [129] | PES | ChCl/Urea | UF for dairy wastewater treatment | PWF up to 233.9 L m2 h−1 with a rejection rates of TSS, TDS, BOD, and COD were stated at about 90, 88, 93, and 97%, respectively. | DES-based membranes significantly increased the permeate flux due to the larger pores, being the maximum PWF for the ChCl:Urea at molar ratio of 1:4. However, DES-based membrane are prone to be fouled in a short-time period with a permeate flux reduction of ~35% in 6 h of operation. | |
| [130] | PES | ChCl/IA | 28.43–30.48 | NF for anionic and cationic dye separation | PWF up to 257.14 L m2 h−1 bar−1 with a dye rejection:
| The PWF was improved due to the induced hydrophily and roughness by DESs. In addition, the use of DESs drastically decreased the Mg, Na and Ca salt rejection, which improved the membrane selectivity. DES-based membranes slightly enhanced the antifouling properties. |
| [131] | PSE | ZnCl2/EG | 3.6–9.5 * | UF for water treatment | PWF of 212.3 L m2 h−1 with a BSA, HA and SA rejection of 96.4, 82.7 and 97.4%, respectively, at 1 bar for the optimal DES doping content of 3% | Increase in DES doping content increased pore size, leading to a higher PWF even though the rejection of BSA, HA and SA drastically declined. Thus, the optimal DES content was stablished at 3%. In addition, membranes tended to suffer from a more severe fouling at DES content > 3% due to a higher water flux and surface roughness. |
| [129] | PES | ChCl/Urea | 28.393 | MF for water treatment | PWF = 62 L m2 h−1; BSA rejection = 45% | DESs application improved membrane thermal stability, even though overall tensile strength of the membranes were decreased from 4.24 to 2.92 MPa. Among the five tested DESs, glycerol-based DESs yielded the best membrane parameters and performances, showing an increase in permeate flux and maintaining an acceptable BSA rejection. Almost all membranes presented higher antifouling properties than the pristine PVDF due to a higher hydrophilicity. |
| ChCl/GLY | 24.338 | PWF = 52 L m2 h−1; BSA rejection = 65% | ||||
| ChCl/ZnCl | 21.832 | PWF = 38 L m2 h−1; BSA rejection = 64% | ||||
| ChCl/LA | 28.958 | PWF = 6 L m2 h−1; BSA rejection = 100% | ||||
| ChCl/GLU | 36.970 | PWF = 19 L m2 h−1; BSA rejection = 100% | ||||
| [132] | PSf | ChCl/FR | 15.748–25.955 | UF for stormwater treatment | PWF up to 125 L m2 h−1 bar−1 at the optimal DES concentration of 3%. | The use of FR-based DESs allowed tailoring membrane surface properties and pore size by adjusting DES concentration. DES-based membranes presented improved mechanical properties, and remarkable antifouling properties. |
| Use of DESs as solvent or cosolvent | ||||||
| [130] | PVDF | [NMA]/AA | NF for water treatment | PWF = 97 L m2 h−1; BSA rejection = 96% | [NMA]/AA is considered as the optimal DES, leading to a high PWF and BSA rejection due to the favorable combination of membrane pore size, porosity, hydrophilicity, morphology and low mass transfer resistance. The use of DESs as solvent could drastically reduce the membrane fabrication cost due to the simplicity and low cost of these DESs compared to the toxic conventional ones and even other green solvents. | |
| [NMA]/NMU | PWF = 112 L m2 h−1; BSA rejection = 85% | |||||
| [NMA]/NN′-DMU | PWF = 60 L m2 h−1; BSA rejection = 95% | |||||
| [97] | PVDF | PTSA/TBnA MsO | 16–26 * | UF for water treatment | PWF up to ~2300 L m2 h−1 bar−1 and MB+ rejection ~75% | Differences in membrane pore size governed the water permeance and rejection of methylene blue cation dye (MB+) of the DES-based membranes. Thus, large pore sizes led to a higher PWF and membranes with similar pore sizes presented similar PWF. |
| PhAA/TMG | 19–34 * | PWF up to 3243 L m2 h−1 bar−1 | ||||
| PAN | GLYA/TMG (+)CSA/SB3-MIM | 33 * | PWF = 2479 L m2 h−1 bar−1; MB+ rejection = 69% | |||
| (+)CSA/SB3-4 | 16 * | PWF = 874 L m2 h−1 bar−1 | ||||
| [133] | PA | ChCl/EG | 23.6–40.9 * | NF for water treatment | PWF up to 43.3 L m2 h−1 bar−1 with a Na2SO4 rejection of 99.3% at the optimal DES concentration. | Optimal DES concentration was 60% in the solvent. PWF increased a 143% respect to the bare membrane without using DESs. In addition, PWF and salt rejection kept constant during 6 days, indicating that DES-based membrane was stable. Sulfated salts (Na2SO4, MgSO4) presented a very high rejection whilst chlorine salts (MgCl2, NaCl) rejection was < 50%. Zeta potential of membranes decreased with DES concentration, which could improve antifouling properties against organic matter. |
| [134] | Lignin | PA/Urea | UF for molecular separation in the pharmaceutical and chemical industries | At 22% lignin in dope solution:
| The best outcomes were observed for membranes prepared at 22% lignin dissolved in a DESs with a molar ratio of 2:1. These membranes were suitable for treating aqueous and organic solvents. Regarding the membrane stability, an initial methanol flux decline of ~50% was observed in the first 20 h, even though the flux kept stable up to nearly 150 h. | |
| Use of DESs as non-solvent or a component in the coagulation bath in NIPS | ||||||
| [136] | PVDF | BET/LA | 52.6–119 | Static adsorption for rare earth ions separation | Adsorption capacity up to 39.3, 40.2, and 45.9 mg g−1 for Nd, Sm and Dy, respectively. | DES concentration of 5% led to the highest adsorption capacity which was higher than the bare membrane. DES addition in coagulation bath promoted the migration of hydrophilic functional groups in the membrane to lower epidermal layer, improving the adsorption capacity. |
| MF/UF for rare earth ions separation | PWF up to 400 L m2 h−1 at 1% DES. Initial membrane flux up to 4.5 µmol m−2 s−1 at the optimal DES concentration. | PWF decreased as DES concentration increased due to a lower wettability behavior. Optimal DES concentration of 5%. Membrane kept the 70% of its initial flux after 7 operation cycles using EDTA as stripping solution and water for membrane flushing at the end of each cycle. | ||||
| Use of DESP as additive and/or modifier | ||||||
| [101] | PA/PI | β-CD/MA | 42–109 * | FO for recovery of organic solvents | EtOH flux up to ~10 L m2 h−1 with a monascorubrin rejection of ~98%. | Solvent flux and monascorubrin rejection were significantly enhanced after the incorporation of a DESs interlayer. These effects were attributed to the large amount of hydrogen bonding induced by the DESs, and the β-cyclodextrin forming the interlayer has a cavity structure to prevent monascorubrin from penetrating through the composite membrane. The stability of the membrane with DESs interlayer was enhanced, showing an EtOH flux of ~7.5 L m2 h−1 after 600 min of operation. Flux decline was attributed to pore blocking, fouling, and swelling which destroy the membrane structure. |
| [135] | CS | β-CD/LA | Adsorption of dyes | Maximum adsorbent capacity for methyl orange of 203.5 mg g−1 at the optimal conditions with a removal efficiency up to 93%. | The highest adsorption efficiency was obtained at a DESs molar ratio of 1:4, 15 mL of PEG as pore former, dye solution pH of 5, methyl orange concentration of 20 mg L−1, and an adsorbent dose of 2 mg. Adsorption of methyl orange in DES-based membranes was favored by a higher influence of electrostatic interactions. | |
3.1. Deep Eutectic Solvents as Components or Additives in Membrane Preparation
3.2. Deep Eutectic Solvents as Solvent or Co-Solvent in Membrane Preparation
3.3. Deep Eutectic Solvents as Pore Formers in Membrane Preparation
3.4. Other Uses of Deep Eutectic Solvents in Membrane Preparation
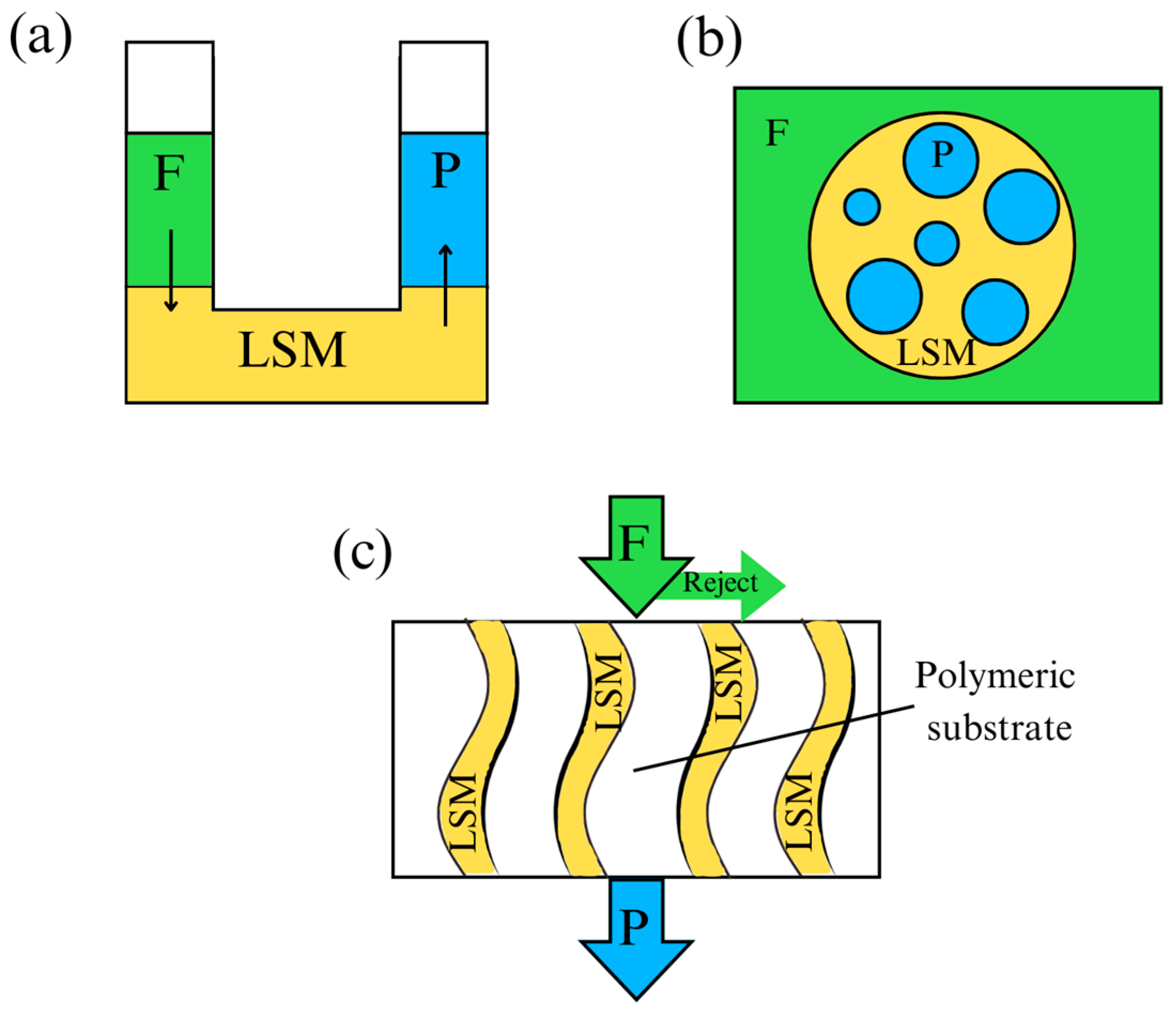
4. Use of Deep Eutectic Solvents in Liquid Membranes
5. Concluding Remarks and Challenges of Deep Eutectic Solvents in Membrane Design, Functionalization and Applications
- Regarding the use of DESs during membrane preparation, DESs could also be used as a substitute of the non-solvent component of the non-solvent induced phase separation (NIPS) process since the polarity of some DESs has been widely reported [98].
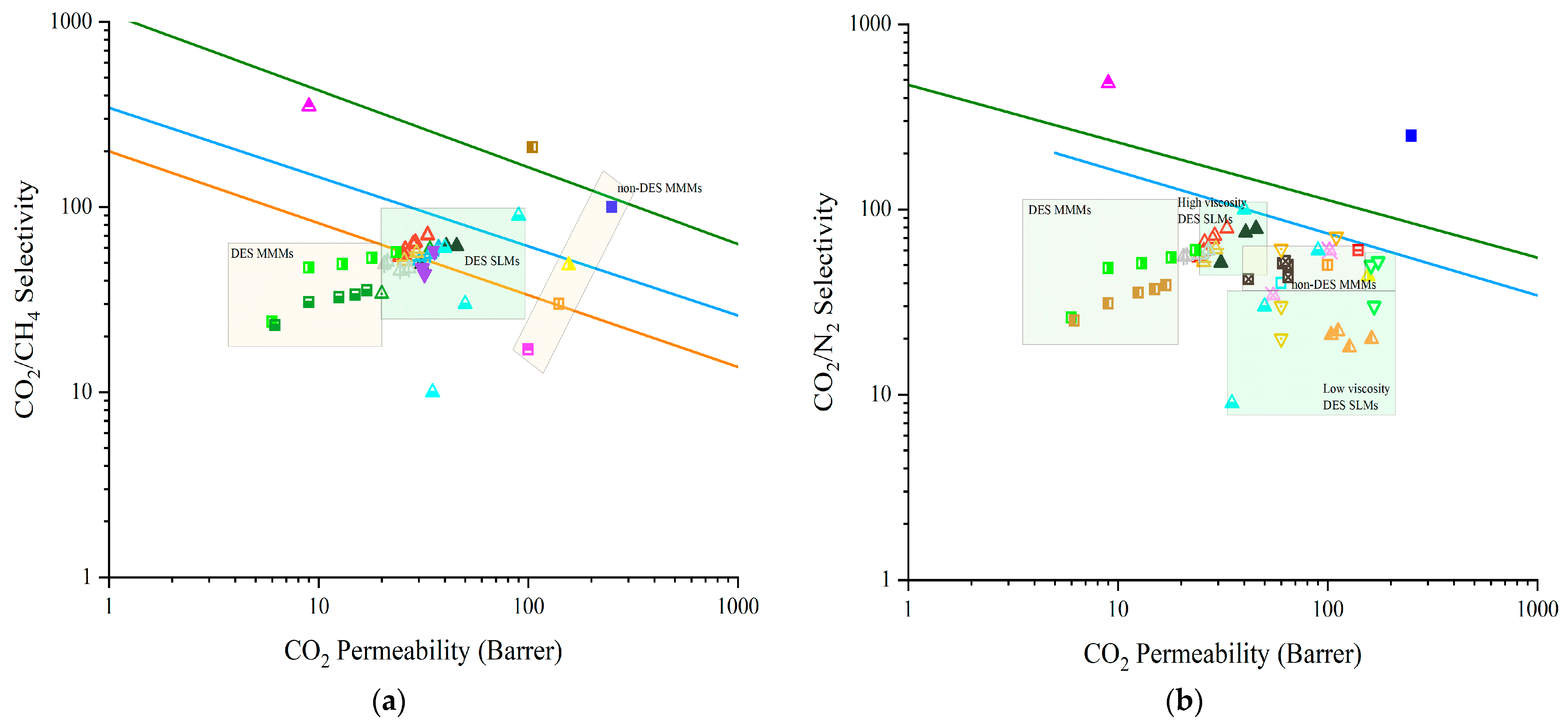
- Enhancing the retention of DESs inside supported liquid membranes (SLMs) is fundamental for the success of any operation. The most important step is to improve the low stability of the developed SLMs. The high DESs loss that most of the studied DES-based SLMs suffer needs to be palliated by moving onto new types of DESs. Ideally, attraction between DESs and CO2 should be stronger than van der Waals bonds, but still reversible as non-covalent bonds.
- Metal-based DESs (types I, II, and IV) offer a tailored design for the gas separation application without losing the characteristic advantages of supported liquid membranes (SLM) technology [190]. This tunability englobes forementioned physicochemical properties of the DESs such as viscosity or surface tension and also chemical affinity of the additive towards the gas to separate.
- On this line, innovative DESs might also be developed to be used for both pore-forming and SLM applications. DESs based on natural resources like sugar or amino acids [191] might be adequate for these purposes as renewable and solvents. Alternatively, membrane materials can be designed for DESs to modify them. Such is the case of graphene oxide (GO) matrixes, whose interlayer space can be tuned depending on the amount of DESs applied for the SLM fabrication, potentially varying its permeability [167].
- The use of DESs in the synthesis of membranes, either as pore formers or co-solvents, should explore new fields of environmental applications: The pore-forming ability of these additives may be controlled until tailoring nanopores for separations in gas phase or separations of gas from liquid effluents. The latter application could also be explored with DES-based SLMs. The removal of methane [192] or phosphorus [17] from liquid effluents is a field that needs to be further developed with the help of DES-based SLMs. Non-ionic (type V) DESs could be an answer to this aspect and a new niche to discover with this technology.
- Also, in this respect, further functionalization of membranes, before or after the formation of DES-based SLMs, may constitute a pathway of future investigation in this field. Apart from performing DESs immobilization within the membrane’s pores, additional functionalization might be implemented by grafting silicon-based compounds on the surface of the membrane, improving its hydrophobicity and opening new processes to perform decarbonization operations [193].
- Finally, on an environmental perspective, life cycle analysis of the developed DES-based SLMs should be performed to assess the impact of these products to the ecosystem in terms of biodegradability of the employed membranes and toxicity of the DESs that has been introduced, especially on a post-service point of view.
Author Contributions
Funding
Conflicts of Interest
List of Symbols and Abbreviations
| (1S)+B3:B28-(+)-10-camphorsulphonic acid | (+)CSA |
| 1-butyl-3-methyl-imidazolium bromide | [BMIM][Br] |
| 1-butyl-3-methyl-imidazolium chloride | [BMIM][Cl] |
| 1-ethyl-3-methyl-imidazolium chloride | [EMIM]Cl |
| Tetramethylammonium species | [N1111] + |
| Tetraethylammonium molecules | [N2222] + |
| Tetrabutylammonium molecules | [N4444] + |
| Tetrabutylammonium bromide | [N4444][Br] |
| Tetrabutylammonium chloride | [N4444][Cl] |
| Tetrabutylphosphonium bromide | [P4444][Br] |
| Tetrabutylphosphonium chloride | [P4444][Br] |
| Acetamide | AA |
| Acetone | AC |
| Acetic acid | AcA |
| Betaine | BET |
| Bulk liquid membranes | BLMs |
| Citric acid | CA |
| Co-solvent-assited interfacial polymerization | CAIP |
| Casting evaporation | CE |
| Methane | CH4 |
| Choline chloride | ChCl |
| Carbon dioxide | CO2 |
| Coumarine | Cou |
| Chitosan | CS |
| Diethanolamine | DEA |
| Decanoic acid | DecA |
| Deep Eutectic Solvent | DES |
| Polymeric deep eutectic solvents | DESP |
| Nanoparticles ethaline coated | DES-SiO2 |
| N,N-Dimethyl acetamide | DMAc |
| Dimethyl formamide | DMF |
| Dimethyl sulfoxide | DMSO |
| Ethylenediaminetetraacetic acid | EDTA |
| Ethylene glycol | EG |
| Emulsion liquid membranes | ELMs |
| Electrospinning | ES |
| Choline chloride: ethylene glycol (1:2) deep eutectic solvent | Ethaline |
| Ethanol | EtOH |
| Fructose | FR |
| Greenhouse gases | GHGs |
| Glucose | GLU |
| Glycerol | GLY |
| Glycolic acid | GLYA |
| Graphene oxide | GO |
| Gas separation | GS |
| Hydrogen bond acceptor | HBA |
| Hydrogen bond donor | HBD |
| Itaconic acid | IA |
| Ionic Liquids | ILs |
| Imidazole | IM |
| Interfacial polymerization | IP |
| Isopropanol | IPA |
| DL-lactic acid | LA |
| Levulinic acid | Lev |
| L-malic acid | MA |
| Malic acid | MalA |
| Monoethanolamine | MEA |
| Microfiltration | MF |
| Mixed-matric membranes | MMMs |
| Membrane preparation technique | MPT |
| Nytrogen | N2 |
| Natural Deep Eutectic Solvents | NADESs |
| Nanofiltration | NF |
| 4-formyl-morpholine | NFM |
| Non-solvent induced phase separation | NIPS |
| N-methyl acetamide | NMA |
| N-methyl-pyrrilidone | NMP |
| N-methyl urea | NMU |
| N,N-dimethyl urea | NNDMU |
| Non-solvent induced phase separation with temperature gradient | NTIPS |
| Oxalic acid | OxA |
| Polyamide | PA |
| Polyacrylic acid | PAA |
| Propionic acid | PAc |
| Polyamide-imide | PAI |
| Polyacrylamide | PAM |
| Polyacrylonitrile | PAN |
| Protonated 2-pyrrolidone-5-carboxylic acid | PCA |
| Permeability of methane | PCH4, Barrer |
| Permeability of carbon dioxide | PCO2, Barrer |
| Poly (ethylene glycol) | PEG |
| Polyethersulfone | PES |
| Phenyl acetic acid | PhAA |
| Permeability of nitrogen | PN2, Barrer |
| Polypropylene | PP |
| Protonated L-proline | PPRO |
| L-proline | PRO |
| Polysulfate | PSE |
| Polysulfone | PSF |
| Polytetrafluoroethylene | PTFE |
| p-toluensulphonic acid | PTSA |
| Pervaporation | PV |
| Polyvinylidene fluoride | PVDF |
| Poly (vinyl pyrrolidone) | PVP |
| Pure water flux | PWF, L m−2 h−1 |
| Radius of the average size-void | r, m |
| Mean roughness | Ra, nm |
| Reverse osmosis | RO |
| Pore size | rp, nm |
| Root mean square roughness | Rq, nm |
| 3-(N,N-dimethybutyammonio) propane-1-sulfonate | SB3-4 |
| (3-(1-methyl-1H-imidazole-3-ium-3-yl) propane-1-sulfonate) | SB3-MIM |
| Scanning Electron Microscopy | SEM |
| Supported liquid membranes | SLMs |
| Tartaric acid | TarA |
| Benzyl-trimethylammonium mesylate | TBnA MsO |
| Triethyl phosphate | TEP |
| Thin film composite | TFC |
| Thymol | Th |
| Trimethyl glycine | TMG |
| Ultrafiltration | UF |
| Static water contact angle | WCA, º |
| Selectivity of carbon dioxide with respect methane | α CO2/CH4 |
| Selectivity of carbon dioxide with respect nitrogen | α CO2/N2 |
| β-cyclodextrin | β-CD |
| Surface tension | γ |
| Overall porosity | ε, % |
References
- United States Environmental Protection Agency. Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases#:~:text=Total%20U.S.%20Emissions%20in%202022,of%20these%20greenhouse%20gas%20emissions (accessed on 8 September 2024).
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, Challenges, and Perspectives of Biogas Cleaning, Upgrading, and Utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Gleick, P.H.; Cooley, H. Annual Review of Environment and Resources Freshwater Scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Commission, E.; for Climate Action, D.-G. Going Climate-Neutral by 2050—A Strategic Long-term Vision for a Prosperous, Modern, Competitive and Climate-Neutral EU Economy; Publications Office: Luxembourg, 2019. [Google Scholar]
- The White House. FACT SHEET: President Biden to Catalyze Global Climate Action through the Major Economies Forum on Energy and Climate. Available online: https://www.whitehouse.gov/briefing-room/statements-releases/2023/04/20/fact-sheet-president-biden-to-catalyze-global-climate-action-through-the-major-economies-forum-on-energy-and-climate/ (accessed on 8 September 2024).
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A Review of Emerging Trends in Membrane Science and Technology for Sustainable Water Treatment. J. Clean. Prod. 2020, 266, 121867. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Yuan, Z.; Lin, Z.; Zhang, M.; Li, Y.; Tang, J.; Liang, Z.; Li, Y.; Chen, L.; et al. Boosting Membranes for CO2 Capture toward Industrial Decarbonization. Carbon Capture Sci. Technol. 2023, 7, 100117. [Google Scholar] [CrossRef]
- Joint Research Centre. Energy in a Low-Carbon World: Trade in Fossil Fuels Plummets, Global Energy Self-Sufficiency Increases. Available online: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/low-carbon-world-trade-fossil-fuels-plummets-2023-01-26_en (accessed on 8 September 2024).
- Sweetapple, C.; Fu, G.; Butler, D. Identifying Sensitive Sources and Key Control Handles for the Reduction of Greenhouse Gas Emissions from Wastewater Treatment. Water Res. 2014, 62, 249–259. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Bouyahyi, M.; Duchateau, R. Potential of Functionalized Polyolefins in a Sustainable Polymer Economy: Synthetic Strategies and Applications. Acc. Chem. Res. 2022, 55, 1985–1996. [Google Scholar] [CrossRef]
- Ramírez-Martínez, M.; Aristizábal, S.L.; Szekely, G.; Nunes, S.P. Bio-Based Solvents for Polyolefin Dissolution and Membrane Fabrication: From Plastic Waste to Value-Added Materials. Green Chem. 2022, 25, 966–977. [Google Scholar] [CrossRef]
- Jayan, J.S.; Vindhyasurumi, A.; Lekshmi, A.G.; Appukuttan, S. Fluoropolymer Nanocomposite Membranes for Gas Separation Applications. In Advanced Fluoropolymer Nanocomposites: Fabrication, Processing, Characterization and Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 485–528. ISBN 9780323953351. [Google Scholar]
- Scholes, C.A. Blended Perfluoropolymer Membranes for Carbon Dioxide Separation by Miscible and Immiscible Morphologies. J. Membr. Sci. 2021, 618, 118675. [Google Scholar] [CrossRef]
- Sun, W.S.; Yin, M.J.; Zhang, W.H.; Li, S.; Wang, N.; An, Q.F. Tailor-Made Microstructures Lead to High-Performance Robust PEO Membrane for CO2 Capture via Green Fabrication Technique. Green Energy Environ. 2023, 8, 1389–1397. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Khalaf, A.; Ibrahim, A.A.; Halaweh, G. Cellulose Acetate Membranes: Fouling Types and Antifouling Strategies—A Brief Review. Processes 2023, 11, 489. [Google Scholar] [CrossRef]
- Bryan Trujillo Ruales, H.; Spadafora, A.; Fiore, P.; Veres, J.; Caravella, A.; Iulianelli, A. Reformer + Membrane Separator Plant for Decarbonized Hydrogen Production from Biogas/Biomethane: An Experimental Study Combined to Energy Efficiency and Exergy Analyses. Energy Convers. Manag. 2024, 315, 118748. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Martínez-Soria, V.; Izquierdo, M.; Chen, L.I.; Le Corre Pidou, K.; McAdam, E.J. Membrane-Assisted Reactive Crystallisation for the Recovery of Dissolved Phosphorus in Vivianite Form from Liquid Effluents. Sep. Purif. Technol. 2023, 326, 124712. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, R.; Barzagli, F.; Sanku, M.G.; Li, C.; Xiao, M. CO2 Absorption in Blended Amine Solvent: Speciation, Equilibrium Solubility and Excessive Property. Chem. Eng. J. 2023, 466, 143279. [Google Scholar] [CrossRef]
- Singh, V.K.; Anil Kumar, E. Measurement and Analysis of Adsorption Isotherms of CO2 on Activated Carbon. Appl. Therm. Eng. 2016, 97, 77–86. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New Approach for Biogas Purification Using Cryogenic Separation and Distillation Process for CO2 Capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Efficient CO2 Absorption and Low Temperature Desorption with Non-Aqueous Solvents Based on 2-Amino-2-Methyl-1-Propanol (AMP). Int. J. Greenh. Gas Control. 2013, 16, 217–223. [Google Scholar] [CrossRef]
- Cormos, A.M.; Burca, M.; Ilea, F.; Cristea, M.V. Process Control Strategy of Amine-Based Post-Combustion CO2 Capture Systems. Chem. Eng. Trans. 2019, 76, 757–762. [Google Scholar] [CrossRef]
- Romeo, L.M.; Minguell, D.; Shirmohammadi, R.; Andrés, J.M. Comparative Analysis of the Efficiency Penalty in Power Plants of Different Amine-Based Solvents for CO2Capture. Ind. Eng. Chem. Res. 2020, 59, 10082–10092. [Google Scholar] [CrossRef]
- Garcia, J.A.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Technical Analysis of CO2 Capture Pathways and Technologies. J. Env. Chem. Eng. 2022, 10, 108470. [Google Scholar] [CrossRef]
- Jiménez Robles, R. Membrane-Based Technologies for Recovery of Dissolved Methane and Phosphorous from Liquid Effluents: Membrane Surface Modification and Performance Enhancement. Ph.D. Thesis, University of Valencia, Valencia, Spain, 2023. [Google Scholar]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Memb. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, W.; Wang, Q.; Wu, Z.; Wang, Z. Designed Strategies of Nanofiltration Technology for Mg2+/Li+ Separation from Salt-Lake Brine: A Comprehensive Review. Desalination 2023, 546, 116205. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent Advances in Polymer Blend Membranes for Gas Separation and Pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Craveiro, R.; Neves, L.A.; Duarte, A.R.C.; Paiva, A. Supported Liquid Membranes Based on Deep Eutectic Solvents for Gas Separation Processes. Sep. Purif. Technol. 2021, 254, 117593. [Google Scholar] [CrossRef]
- Mubashir, M.; D’Angelo, F.N.; Gallucci, F. Recent Advances and Challenges of Deep Eutectic Solvent Based Supported Liquid Membranes. Sep. Purif. Rev. 2022, 51, 226–244. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep Eutectic Solvents and Their Applications as Green Solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Xin, K.; Zhou, P.; van Sint Annaland, M. Experimental Study on CO2 Separation Using Hydrophobic Deep Eutectic Solvent Based Supported Liquid Membranes. Sep. Purif. Technol. 2023, 310, 123129. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Zaidouni, S.; Basaid, K.; Mir, Y. Deep Eutectic Solvents as Sustainable Extraction Media for Plants and Food Samples: A Review. Sustain. Chem. Pharm. 2023, 31, 100937. [Google Scholar]
- Li, X.; Hou, M.; Han, B.; Wang, X.; Zou, L. Solubility of CO2 in a Choline Chloride + Urea Eutectic Mixture. J. Chem. Eng. Data 2008, 53, 548–550. [Google Scholar] [CrossRef]
- Pelaquim, F.P.; Barbosa Neto, A.M.; Dalmolin, I.A.L.; da Costa, M.C. Gas Solubility Using Deep Eutectic Solvents: Review and Analysis. Ind. Eng. Chem. Res. 2021, 60, 8607–8620. [Google Scholar] [CrossRef]
- Saeed, U.; Laeeq Khan, A.; Gilani, M.A.; Aslam, M.; Khan, A.U. CO2 Separation by Supported Liquid Membranes Synthesized with Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. Int. 2021, 28, 33994–34008. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Berton, P.; Shamshina, J. Deep Eutectic Solvents: Alternative Solvents for Biomass-Based Waste Valorization. Molecules 2022, 27, 6606. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep Eutectic Solvents vs Ionic Liquids: Similarities and Differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Azzouz, A.; Hayyan, M. Are Deep Eutectic Solvents Biodegradable? Process Saf. Environ. Prot. 2023, 176, 1021–1025. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Figoli, A.; Boczkaj, G. Deep Eutectic Solvents—A New Platform in Membrane Fabrication and Membrane-Assisted Technologies. J. Environ. Chem. Eng. 2022, 10, 106414. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic Hydrogen Bond Donors in the Formation of Non-Ionic Deep Eutectic Solvents: The Quest for Type v Des. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How Polar Are Choline Chloride-Based Deep Eutectic Solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Ghareh Bagh, F.S.; Shahbaz, K.; Mjalli, F.S.; Hashim, M.A.; Alnashef, I.M. Zinc (II) Chloride-Based Deep Eutectic Solvents for Application as Electrolytes: Preparation and Characterization. J. Mol. Liq. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Mares, M.L.; Ciocirlan, O.; Cojocaru, A.; Anicai, L. Physico-Chemical and Electrochemical Studies in Choline Chloride Based Ionic Liquid Analogues Containing Trivalent Chromium Chloride. Rev. Chim. 2013, 64, 815–824. [Google Scholar]
- Mu, M.; Zhang, X.; Yu, G.; Xu, R.; Liu, N.; Wang, N.; Chen, B.; Dai, C. Effective Absorption of Dichloromethane Using Deep Eutectic Solvents. J. Hazard. Mater. 2022, 439, 129666. [Google Scholar] [CrossRef]
- Lemaoui, T.; Boublia, A.; Darwish, A.S.; Alam, M.; Park, S.; Jeon, B.H.; Banat, F.; Benguerba, Y.; Alnashef, I.M. Predicting the Surface Tension of Deep Eutectic Solvents Using Artificial Neural Networks. ACS Omega 2022, 7, 32194–32207. [Google Scholar] [CrossRef]
- Zubeir, L.F.; Lacroix, M.H.M.; Kroon, M.C. Low Transition Temperature Mixtures as Innovative and Sustainable CO2 Capture Solvents. J. Phys. Chem. B 2014, 118, 14429–14441. [Google Scholar] [CrossRef] [PubMed]
- Jibril, B.; Mjalli, F.; Naser, J.; Gano, Z. New Tetrapropylammonium Bromide-Based Deep Eutectic Solvents: Synthesis and Characterizations. J. Mol. Liq. 2014, 199, 462–469. [Google Scholar] [CrossRef]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor. ChemSusChem 2020, 13, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.; Elbourne, A.; Greaves, T.L.; Bryant, G.; Bryant, S.J. Physico-Chemical Characterisation of Glycerol- and Ethylene Glycol-Based Deep Eutectic Solvents. J. Mol. Liq. 2024, 394, 123777. [Google Scholar] [CrossRef]
- Aravena, P.; Cea-Klapp, E.; Gajardo-Parra, N.F.; Held, C.; Garrido, J.M.; Canales, R.I. Effect of Water and Hydrogen Bond Acceptor on the Density and Viscosity of Glycol-Based Eutectic Solvents. J. Mol. Liq. 2023, 389, 122856. [Google Scholar] [CrossRef]
- Mero, A.; Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A.; Guazzelli, L. Comparison of Physicochemical and Thermal Properties of Choline Chloride and Betaine-Based Deep Eutectic Solvents: The Influence of Hydrogen Bond Acceptor and Hydrogen Bond Donor Nature and Their Molar Ratios. J. Mol. Liq. 2023, 377, 121563. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Gilmore, M.; Swadzba-Kwasny, M.; Holbrey, J.D. Thermal Properties of Choline Chloride/Urea System Studied under Moisture-Free Atmosphere. J. Chem. Eng. Data 2019, 64, 5248–5255. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and Viscosities of (Choline Chloride + Urea) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Dou, W.; Yu, J.; Wang, X. Effect of Ethanol on the Density and Viscosity of Choline Chloride/Urea Eutectic System. J. Mol. Liq. 2023, 382, 121923. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Ibrahim, S.; Hayyan, A.; Hashim, M.A. Physical Properties of Ethylene Glycol-Based Deep Eutectic Solvents. J. Mol. Liq. 2019, 276, 794–800. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of Choline Chloride-Based Deep Eutectic Solvents: Experiments and Modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol Eutectics as Sustainable Solvent Systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Bardool, R.; Bakhtyari, A.; Duarte, A.R.C.; Raeissi, S. Simple and Global Correlation for the Densities of Deep Eutectic Solvents. J. Mol. Liq. 2019, 296, 111830. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, Y.M.; Al-Wahaibi, T.; Hashim, M.A. Glucose-Based Deep Eutectic Solvents: Physical Properties. J. Mol. Liq. 2013, 178, 137–141. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, M.M.; Branco, L.C.; Marrucho, I.M. Carbohydrates-Based Deep Eutectic Solvents: Thermophysical Properties and Rice Straw Dissolution. J. Mol. Liq. 2017, 247, 441–447. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit Sugar-Based Deep Eutectic Solvents and Their Physical Properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Crespo, E.A.; Silva, L.P.; Martins, M.A.R.; Bülow, M.; Ferreira, O.; Sadowski, G.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. The Role of Polyfunctionality in the Formation of [Ch]Cl-Carboxylic Acid-Based Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2018, 57, 11195–11209. [Google Scholar] [CrossRef]
- Ninayan, R.; Levshakova, A.S.; Khairullina, E.M.; Vezo, O.S.; Tumkin, I.I.; Ostendorf, A.; Logunov, L.S.; Manshina, A.A.; Shishov, A.Y. Water-Induced Changes in Choline Chloride-Carboxylic Acid Deep Eutectic Solvents Properties. Colloids Surf. A Physicochem. Eng. 2023, 679, 132543. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Fu, L.; Yang, Y.; Wang, Y.; Hu, X.; Wang, F.; Mu, T. Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Ind. Eng. Chem. Res. 2019, 58, 12741–12750. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Sert, M.; Arslanoğlu, A.; Ballice, L. Conversion of Sunflower Stalk Based Cellulose to the Valuable Products Using Choline Chloride Based Deep Eutectic Solvents. Renew. Energy 2018, 118, 993–1000. [Google Scholar] [CrossRef]
- Guo, W.; Hou, Y.; Ren, S.; Tian, S.; Wu, W. Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. J. Chem. Eng. Data 2013, 58, 866–872. [Google Scholar] [CrossRef]
- Deepika; Juneja, S.; Pandey, S.; Deepika; Juneja, S.; Pandey, S. Water Miscibility, Surface Tension, Density, and Dynamic Viscosity of Hydrophobic Deep Eutectic Solvents Composed of Capric Acid, Menthol, and Thymol. J. Chem. Eng. Data 2022, 67, 3400–3413. [Google Scholar] [CrossRef]
- Li, K.; Jin, Y.; Jung, D.; Park, K.; Kim, H.; Lee, J. In Situ Formation of Thymol-Based Hydrophobic Deep Eutectic Solvents: Application to Antibiotics Analysis in Surface Water Based on Liquid-Liquid Microextraction Followed by Liquid Chromatography. J. Chromatogr. A 2020, 1614, 460730. [Google Scholar] [CrossRef]
- Shi, R.; Zhou, F.; Chen, Y.; Liu, Z.; Liu, S.; Mu, T. Magnetic Deep Eutectic Solvents: Formation and Properties †. Phys. Chem. Chem. Phys. 2022, 24, 20073–20081. [Google Scholar] [CrossRef]
- Sarjuna, K.; Ilangeswaran, D. Preparation of Some Zinc Chloride Based Deep Eutectic Solvents and Their Characterization. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 33, pp. 2767–2770. [Google Scholar]
- Alhadid, A.; Mokrushina, L.; Minceva, M. Formation of Glassy Phases and Polymorphism in Deep Eutectic Solvents. J. Mol. Liq. 2020, 314, 113667. [Google Scholar] [CrossRef]
- Tiecco, M.; Grillo, A.; Mosconi, E.; Kaiser, W.; Del Giacco, T.; Germani, R. Advances in the Development of Novel Green Liquids: Thymol/Water, Thymol/Urea and Thymol/Phenylacetic Acid as Innovative Hydrophobic Natural Deep Eutectic Solvents. J. Mol. Liq. 2022, 364, 120043. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of Novel, Moisture-Stable, Lewis-Acidic Ionic Liquids Containing Quaternary Ammonium Salts with Functional Side Chains. Chem. Commun. 2001, 1, 2010–2011. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, Hydrogen Bonding and Rheology in Choline Chloride Deep Eutectic Solvents as a Function of the Hydrogen Bond Donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
- Velez, C.; Acevedo, O. Simulation of Deep Eutectic Solvents: Progress to Promises. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1598. [Google Scholar] [CrossRef]
- Schaeffer, N.; Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Carvalho, P.J.; Russina, O.; Triolo, A.; Paccou, L.; Guinet, Y.; Hedoux, A.; et al. Non-Ideality in Thymol + Menthol Type v Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 2203–2211. [Google Scholar] [CrossRef]
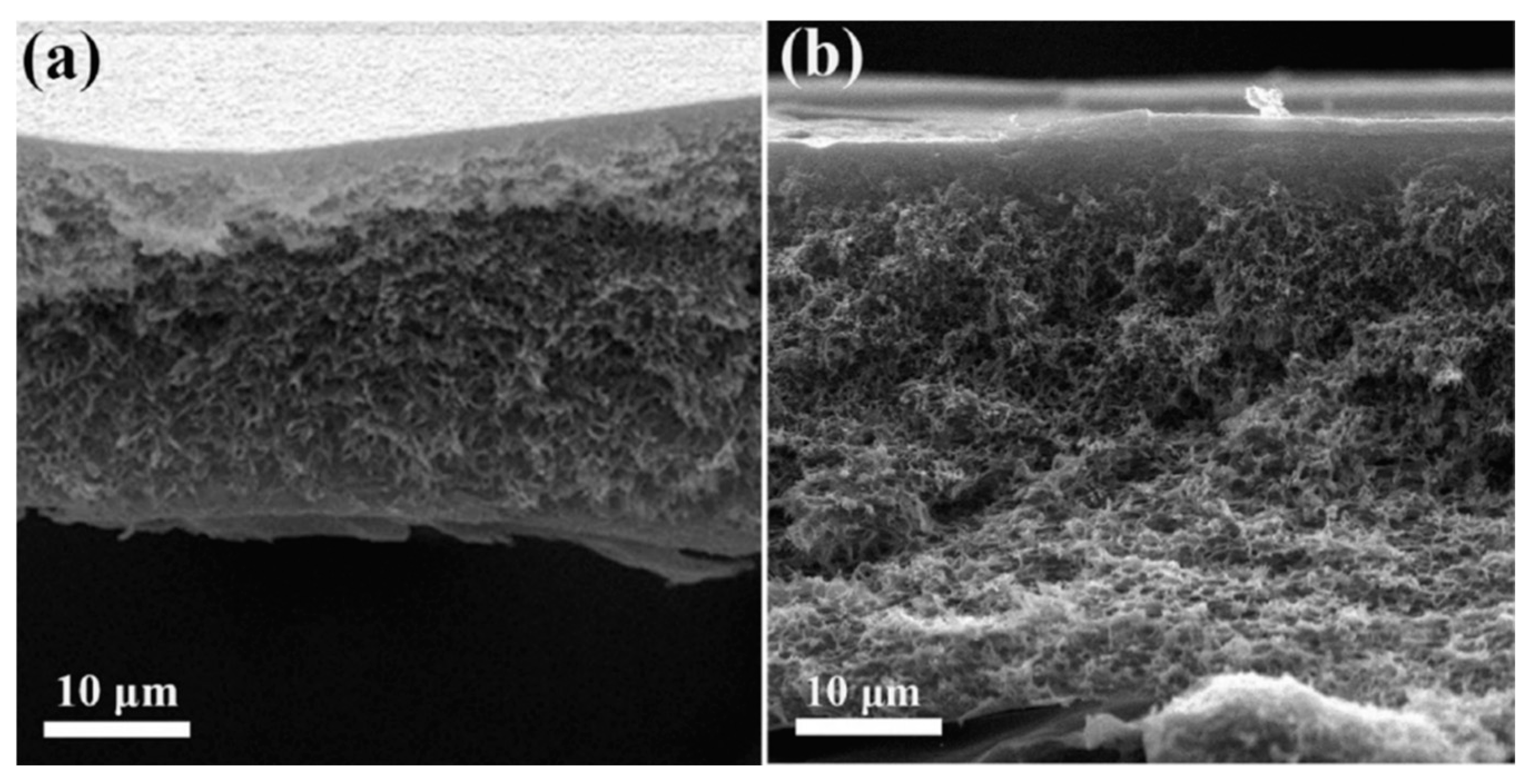
- Yeow, A.T.H.; Chan, M.K.; Ong, C.S.; Ho, K.C. Effects of Hydrogen Bond Donors on PVDF Membrane Modification Using Choline Chloride-Based Deep Eutectic Solvents. J. Ind. Eng. Chem. 2024, in press. [CrossRef]
- Mjalli, F.S.; Naser, J.; Jibril, B.; Alizadeh, V.; Gano, Z. Tetrabutylammonium Chloride Based Ionic Liquid Analogues and Their Physical Properties. J. Chem. Eng. Data 2014, 59, 2242–2251. [Google Scholar] [CrossRef]
- Haghbakhsh, R.; Parvaneh, K.; Raeissi, S.; Shariati, A. A General Viscosity Model for Deep Eutectic Solvents: The Free Volume Theory Coupled with Association Equations of State. Fluid Phase Equilib. 2018, 470, 193–202. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical Properties of Deep Eutectic Solvents: A Review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Haghbakhsh, R.; Duarte, A.R.C.; Raeissi, S. A Simple Model for the Viscosities of Deep Eutectic Solvents. Fluid Phase Equilib. 2020, 521, 112662. [Google Scholar] [CrossRef]
- Abbott, A.P. Application of Hole Theory to the Viscosity of Ionic and Molecular Liquids. ChemPhysChem 2004, 5, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Application of Hole Theory to Define Ionic Liquids by Their Transport Properties. J. Phys. Chem. B 2007, 111, 4910–4913. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, H.; Zhang, S.; Lu, X.; Ji, X. Effect of Water on the Density, Viscosity, and CO2 Solubility in Choline Chloride/Urea. J. Chem. Eng. Data 2014, 59, 3344–3352. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef] [PubMed]
- Morozov, O.S.; Shachneva, S.S.; Bulgakov, B.A.; Babkin, A.V.; Kepman, A.V. Effect of Different Pore-Forming Additives on the Formation of PVDF Microporous Membranes for Bucky-Gel Actuator. Eurasian Chem.-Technol. J. 2020, 22, 107–115. [Google Scholar] [CrossRef]
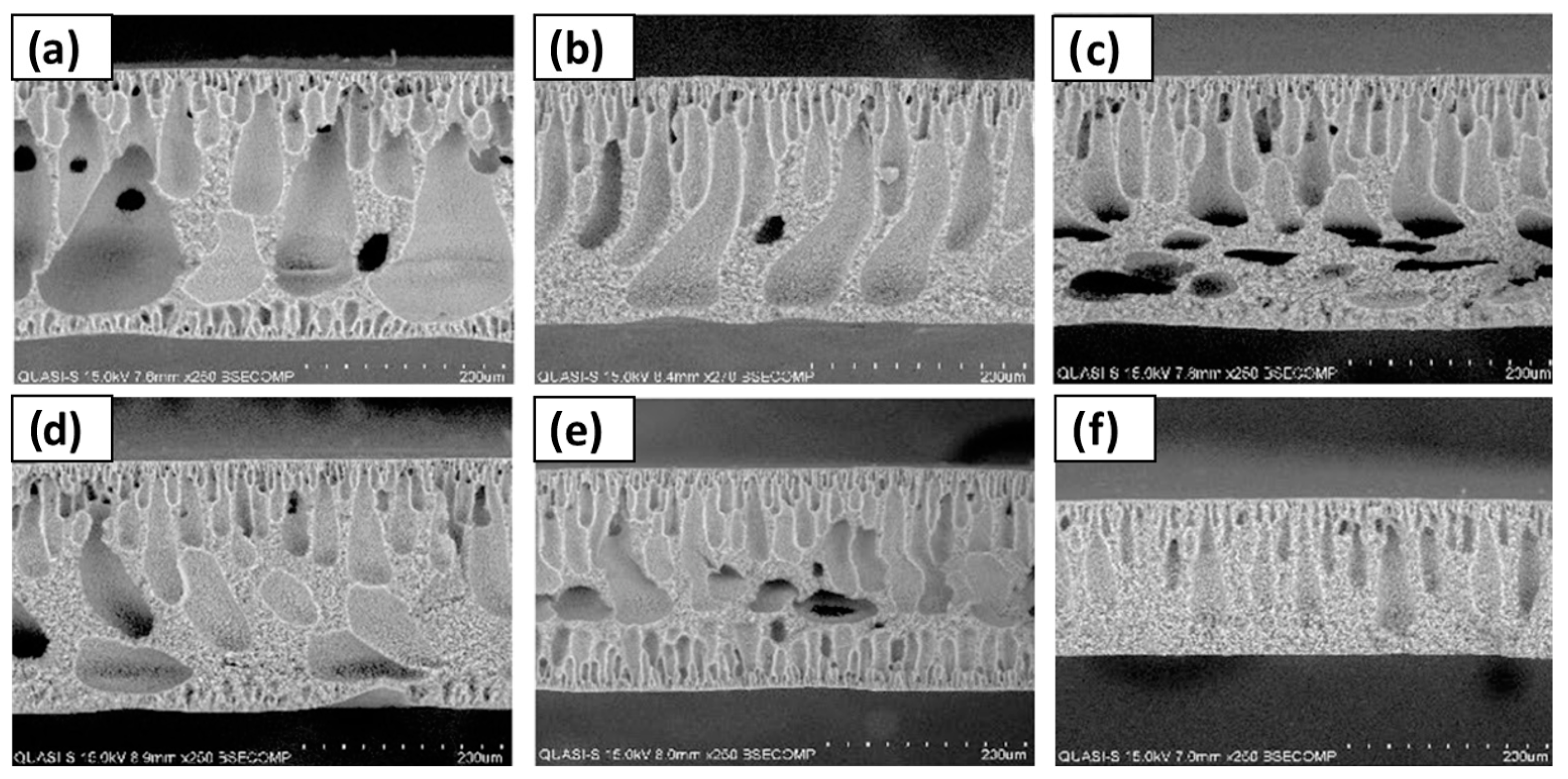
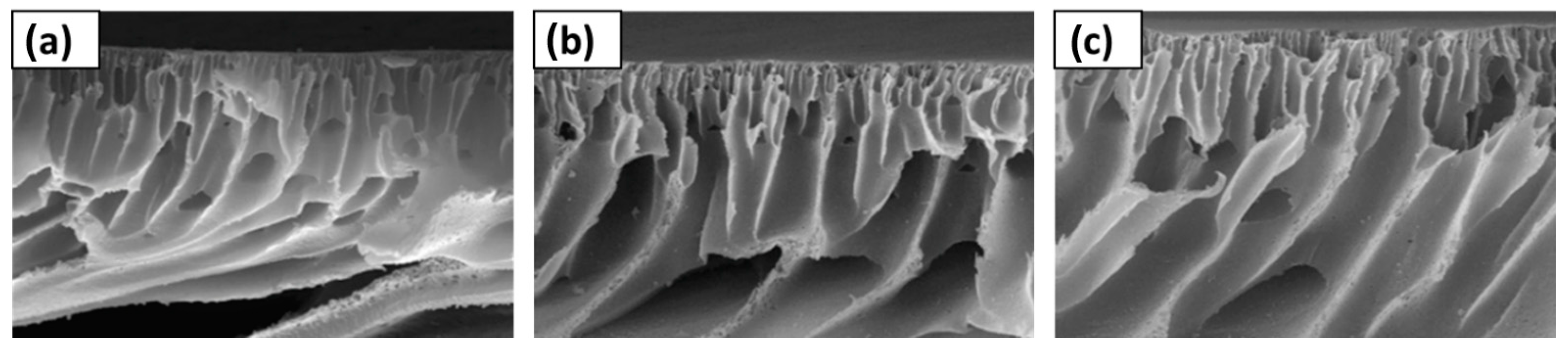
- Jiang, B.; Zhang, N.; Wang, B.; Yang, N.; Huang, Z.; Yang, H.; Shu, Z. Deep Eutectic Solvent as Novel Additive for PES Membrane with Improved Performance. Sep. Purif. Technol. 2018, 194, 239–248. [Google Scholar] [CrossRef]
- Ismail, N.; Pan, J.; Rahmati, M.; Wang, Q.; Bouyer, D.; Khayet, M.; Cui, Z.; Tavajohi, N. Non-Ionic Deep Eutectic Solvents for Membrane Formation. J. Memb. Sci. 2022, 646, 120238. [Google Scholar] [CrossRef]
- Russo, F.; Tiecco, M.; Galiano, F.; Mancuso, R.; Gabriele, B.; Figoli, A. Launching Deep Eutectic Solvents (DESs) and Natural Deep Eutectic Solvents (NADESs), in Combination with Different Harmless Co-Solvents, for the Preparation of More Sustainable Membranes. J. Memb. Sci. 2022, 649, 120387. [Google Scholar] [CrossRef]
- Moradi, G.; Rahimi, M.; Zinadini, S.; Shamsipur, M.; Babajani, N. Natural Deep Eutectic Solvent Modified Nanofiltration Membranes with Superior Antifouling Properties for Pharmaceutical Wastewater Treatment. Chem. Eng. J. 2022, 448, 137704. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Harifi-Mood, A.R. Ethaline Deep Eutectic Solvent as a Hydrophilic Additive in Modification of Polyethersulfone Membrane for Antifouling and Separation Improvement. J. Memb. Sci. 2020, 614, 118528. [Google Scholar] [CrossRef]
- Chen, L.; Cui, R.; Pan, W.; Dai, J.; Meng, M.; Dai, X.; Pan, J. Role of Natural Deep Eutectic Solvents (NADESs) in Coagulation Bath for PVDF-Based Membranes on Enhanced Permeation and Separation of Rare Earth Ions. J. Memb. Sci. 2023, 683, 121836. [Google Scholar] [CrossRef]
- Liang, J.; Huang, H.; Zhang, H.; Wu, Y.; Zhuang, Y. Preparation of Thin Film Composite (TFC) Membrane with DESPs Interlayer and Its Forward Osmosis (FO) Performance for Organic Solvent Recovery. Membranes 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Taghizadeh, A.; Vatanpour, V.; Ganjali, M.R.; Saeb, M.R. Deep Eutectic Solvents in Membrane Science and Technology: Fundamental, Preparation, Application, and Future Perspective. Sep. Purif. Technol. 2021, 258, 118015. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of Ionic Liquids with Membrane Technology: A New Approach for CO2 Separation. J. Memb. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Naziri Mehrabani, S.A.; Vatanpour, V.; Koyuncu, I. Green Solvents in Polymeric Membrane Fabrication: A Review. Sep. Purif. Technol. 2022, 298, 121691. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4248-9. [Google Scholar]
- Drioli, E.; Giorno, L. (Eds.) Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-44323-1. [Google Scholar]
- Hopp-Hirschler, M.; Safdari Shadloo, M.; Nieken, U. Viscous Fingering Phenomena in the Early Stage of Polymer Membrane Formation. J. Fluid Mech. 2019, 864, 97–140. [Google Scholar] [CrossRef]
- Wang, C.; Quan, X.; Liao, M.; Li, L.; Zhou, J. Computer Simulations on the Channel Membrane Formation by Nonsolvent Induced Phase Separation. Macromol. Theory Simul. 2017, 26, 1700027. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Xiang, M. Phase Separation in a PSf/DMF/Water System: A Proposed Mechanism for Macrovoid Formation. RSC Adv. 2014, 4, 42391–42402. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the Non-Solvent Induced Phase Separation (NIPS) Effect during the Fabrication of Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Cho, C.W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.S. Review of the Toxic Effects of Ionic Liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef]
- Wu, B.; Liu, W.W.; Zhang, Y.M.; Wang, H.P. Do We Understand the Recyclability of Ionic Liquids? Chem.—A Eur. J. 2009, 15, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tu, Z.; Yan, Z.; Zhang, X.; Hu, X.; Wu, Y. Deep Eutectic Solvent-Based Blended Membranes for Ultra-Super Selective Separation of SO2. J. Hazard. Mater. 2023, 460, 132515. [Google Scholar] [CrossRef]
- Seyyed Shahabi, S.; Azizi, N.; Vatanpour, V. Tuning Thin-Film Composite Reverse Osmosis Membranes Using Deep Eutectic Solvents and Ionic Liquids toward Enhanced Water Permeation. J. Memb. Sci. 2020, 610, 118267. [Google Scholar] [CrossRef]
- Dehqan, A.; Zinatizadeh, A.A.; Zinadini, S.; Harifi-Mood, A.; Seyyed Shahabi, S.; Vatanpour, V. Fabrication of High-Performance and High-Fouling Resistance Reverse Osmosis Membrane by a Natural Deep Eutectic Solvent (NDES) as a New Generation of Co-Solvents. J. Memb. Sci. 2024, 699, 122679. [Google Scholar] [CrossRef]
- Hao, Y.; Fang, Y.; Zhang, L.; Jiang, H.; Yang, N.; Jiang, B.; Sun, Y.; Zhang, L. Ternary Deep Eutectic Solvent Enhanced the Diffusion Control of Amine Monomer for Highly Permeable and Anti-Fouling Polyamide Nanofiltration Membranes. Sep. Purif. Technol. 2024, 330, 125371. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Chen, S.; Liu, W.; Chen, H. Fabrication of Multi-Functional Gelatin/Deep Eutectic Solvent/Polyacrylonitrile Nanofiber Membranes via Electrospinning. Soft Matter 2023, 19, 9315–9324. [Google Scholar] [CrossRef]
- Kuttiani Ali, J.; Maher Chabib, C.; Abi Jaoude, M.; Alhseinat, E.; Teotia, S.; Patole, S.; Hussain Anjum, D.; Qattan, I. Enhanced Removal of Aqueous Phenol with Polyimide Ultrafiltration Membranes Embedded with Deep Eutectic Solvent-Coated Nanosilica. Chem. Eng. J. 2021, 408, 128017. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Faykov, I.; Tataurov, M.; Dubovenko, R.; Shugurov, S. Development of Novel Polyamide-IMIDE/DES Composites and Their Application for Pervaporation and Gas Separation. Molecules 2021, 26, 990. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhang, P.; Zhang, X.; Hu, X.; Wu, Y. Engineering Highly Reversible Hydrogen Bonding Interaction in Pebax/Deep Eutectic Solvent Blended Membranes for Efficient Separation of H2S from CO2 and CH4. J. Memb. Sci. 2024, 699, 122618. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Walvekar, R.; Loh, K.S.; Khalid, M.; Lim, K.L. Effect of Deep Eutectic Solvent in Proton Conduction and Thermal Behaviour of Chitosan-Based Membrane. J. Mol. Liq. 2018, 269, 675–683. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Gontarek-Castro, E.; Karczewski, J.; Cabezas, R.; Merlet, G.; Araya-Lopez, C.; Boczkaj, G. Hybrid Cross-Linked Chitosan/Protonated-Proline:Glucose DES Membranes with Superior Pervaporation Performance for Ethanol Dehydration. J. Mol. Liq. 2022, 360, 119499. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Cichocki, Ł.; Plata-Gryl, M.; Boczkaj, G.; Galiano, F. Performance Tuning of Chitosan-Based Membranes by Protonated 2-Pyrrolidone-5-Carboxylic Acid-Sulfolane DES for Effective Water/Ethanol Separation by Pervaporation. Chem. Eng. Res. Des. 2023, 191, 401–413. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Msahel, A.; Galiano, F.; Serocki, M.; Ryl, J.; Hamouda, S.B.; Hafiane, A.; Boczkaj, G.; Figoli, A. Towards Azeotropic MeOH-MTBE Separation Using Pervaporation Chitosan-Based Deep Eutectic Solvent Membranes. Sep. Purif. Technol. 2022, 281, 119979. [Google Scholar] [CrossRef]
- Mehrabi, N.; Lin, H.; Aich, N. Deep Eutectic Solvent Functionalized Graphene Oxide Nanofiltration Membranes with Superior Water Permeance and Dye Desalination Performance. Chem. Eng. J. 2021, 412, 128577. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, N.; Zhang, L.; Sun, Y.; Huang, Z.; Wang, B.; Dou, H.; Guan, H. Enhanced Separation Performance of PES Ultrafiltration Membranes by Imidazole-Based Deep Eutectic Solvents as Novel Functional Additives. J. Memb. Sci. 2018, 564, 247–258. [Google Scholar] [CrossRef]
- Ashok Kumar, S.; Subathra, K.; Srinivasan, G.; Jayaraman, S.; Gnanasekaran, G.; Kanimozhi, G.; Govindaradjane, S. Impact of Tween-80 and Deep Eutectic Solvent-Based Micellar-Enhanced Ultrafiltration in Dairy Wastewater Treatment. Chem. Eng. Technol. 2021, 44, 913–922. [Google Scholar] [CrossRef]
- Saeb, Z.; Bide, Y.; Shokrollahzadeh, S. Structural and Separation Evaluation of Polysulfone-Based Loose NF Membrane Modified with Itaconic Acid-Choline Chloride Deep Eutectic Solvent as Additive. J. Env. Chem. Eng. 2024, 12, 112046. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Shen, Y.; Yin, M.J.; Wang, Z.P.; Wang, N.; Qin, Z.; An, Q.F. Polysulfate Membrane Prepared with a Novel Porogen for Enhanced Ultrafiltration Performance. Chem. Eng. J. Adv. 2022, 12, 100397. [Google Scholar] [CrossRef]
- Elhamarnah, Y.; Hey, T.; Lipnizki, F.; Qiblawey, H. Investigating the Impact of Stormwater Fouling on Polysulfone Ultrafiltration Membranes Modified with Deep Eutectic Solvents. J. Water Process Eng. 2023, 56, 104362. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, B.; Hao, Y.; Yang, N.; Zhang, L.; Zhang, C.; Sun, Y.; Xiao, X.; Zhang, L. Engineering Highly Permeable Thin-Film Composite Nanofiltration Membranes by Strengthening the Diffusion Control of Amine Monomer via Deep Eutectic Solvent. J. Memb. Sci. 2023, 678, 121689. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Aristizábal, S.L.; Silva, L.; Qasem, E.A.; Chisca, S.; Upadhyaya, L.; Althobaiti, D.; Coutinho, J.A.P.; Nunes, S.P. A Lignin-Based Membrane Fabricated with a Deep Eutectic Solvent. Green Chem. 2023, 25, 4769–4780. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Hai, X.; Yang, Z.; Li, X.; Chen, M.; Yuan, M.; Xiong, H.; Gao, Y.; Wang, L.; et al. Adsorption of Methyl Orange by Porous Membranes Prepared from Deep Eutectic Supramolecular Polymer-Modified Chitosan. Environ. Res. 2023, 236, 116778. [Google Scholar] [CrossRef]
- Young, T.-H.; Cheng, L.-P.; Lin, D.-J.; Fane, L.; Chuang, W.-Y. Mechanisms of PVDF Membrane Formation by Immersion-Precipitation in Soft (1-Octanol) and Harsh (Water) Nonsolvents. Polymer 1999, 40, 5315–5323. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and Synthesis of Polymeric Membranes Using Water-Soluble Pore Formers: An Overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Fernandes, C.S.; Md Nordin, N.A.H.; Bilad, M.R.; Matsuura, T.; Putra, Z.A.; Wirzal, M.D.H.; Jaafar, J. Explication of Hydrophobic Silica as Effective Pore Former for Membrane Fabrication. Appl. Surf. Sci. Adv. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Chung, T.S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of Polyethylene Glycol on Membrane Formation and Properties of Hydrophilic Sulfonated Polyphenylenesulfone (SPPSU) Membranes. J. Memb. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, J.; Zhang, Y.; Xing, D.; Yang, F.; Huang, J.; Huang, S.; Shao, L. Green Glycerol Tailored Composite Membranes with Boosted Nanofiltration Performance. J. Memb. Sci. 2022, 663, 121064. [Google Scholar] [CrossRef]
- Marino, T.; Figol, A. Arsenic Removal by Liquid Membranes. Membranes 2015, 5, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Krull, F.F.; Fritzmann, C.; Melin, T. Liquid Membranes for Gas/Vapor Separations. J. Memb. Sci. 2008, 325, 509–519. [Google Scholar] [CrossRef]
- Eljaddi, T.; Lebrun, L.; Hlaibi, M. Principle and Classification Criteria of Membranes Different Type of Liquid Membranes Mechanism of Facilitated Transport on Liquid Membranes Review on Mechanism of Facilitated Transport on Liquid Membranes. J. Membr. Sci. Res. 2017, 3, 199–208. [Google Scholar] [CrossRef]
- Yesil, H.; Tugtas, A.E. Removal of Heavy Metals from Leaching Effluents of Sewage Sludge via Supported Liquid Membranes. Sci. Total Environ. 2019, 693, 133608. [Google Scholar] [CrossRef]
- Li, S.; Lindsay, H.; Mannari, V.; Texter, J. Liquid Polymerized Ionic Liquids for Energy Storage Applications. J. Mol. Liq. 2023, 376, 121403. [Google Scholar] [CrossRef]
- Ramdin, M.; De Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Yang, Q.; Seelam, L. Recent Advances in Supported Liquid Membrane Technology. Sep. Purif. Technol. 2007, 53, 171–177. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A Review of Recent Trends and Emerging Perspectives of Ionic Liquid Membranes for CO2 Separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic Liquids Combined with Membrane Separation Processes: A Review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Sikander, A.B.; Anjum, T.; Khan, A.L.; Gilani, M.A.; Raja, A.A.; Yasin, M. Exploring the Potential of Highly Selective Deep Eutectic Solvents (DES) Based Membranes for Dehydration of Butanol via Pervaporation. Chemosphere 2022, 305, 135480. [Google Scholar] [CrossRef]
- Kim, S.; Shi, H.; Lee, J.Y. CO2 Absorption Mechanism in Amine Solvents and Enhancement of CO2 Capture Capability in Blended Amine Solvent. Int. J. Greenh. Gas. Control 2016, 45, 181–188. [Google Scholar] [CrossRef]
- Waseem, M.; Al-Marzouqi, M.; Ghasem, N. A Review of Catalytically Enhanced CO2-Rich Amine Solutions Regeneration. J. Environ. Chem. Eng. 2023, 11, 110188. [Google Scholar] [CrossRef]
- Jiang, B.; Dou, H.; Zhang, L.; Wang, B.; Sun, Y.; Yang, H.; Huang, Z.; Bi, H. Novel Supported Liquid Membranes Based on Deep Eutectic Solvents for Olefin-Paraffin Separation via Facilitated Transport. J. Memb. Sci. 2017, 536, 123–132. [Google Scholar] [CrossRef]
- Wen, S.; Wang, T.; Zhang, X.; Hu, X.; Wu, Y. Deep Eutectic Solvents Formed by Novel Metal-Based Amino Acid Salt and Dihydric Alcohol for Highly Efficient Capture of CO2. J. Environ. Chem. Eng. 2024, 12, 112533. [Google Scholar] [CrossRef]
- Cheng, N.N.; Li, Z.L.; Lan, H.C.; Xu, W.L.; Huang, K. Remarkable NH3 Absorption in Metal-Based Deep Eutectic Solvents by Multiple Coordination and Hydrogen-Bond Interaction. AIChE J. 2022, 68, e17660. [Google Scholar] [CrossRef]
- Xu, M.; Dou, H.; Peng, F.; Yang, N.; Xiao, X.; Tantai, X.; Sun, Y.; Jiang, B.; Zhang, L. Ultra-Stable Copper Decorated Deep Eutectic Solvent Based Supported Liquid Membranes for Olefin/Paraffin Separation: In-Depth Study of Carrier Stability. J. Memb. Sci. 2022, 659, 120775. [Google Scholar] [CrossRef]
- Deng, R.; Sun, Y.; Bi, H.; Dou, H.; Yang, H.; Wang, B.; Tao, W.; Jiang, B. Deep Eutectic Solvents As Tuning Media Dissolving Cu+ Used in Facilitated Transport Supported Liquid Membrane for Ethylene/Ethane Separation. Energy Fuels 2017, 31, 11146–11155. [Google Scholar] [CrossRef]
- Dietz, C.H.J.T.; Kroon, M.C.; Di Stefano, M.; Van Sint Annaland, M.; Gallucci, F. Selective Separation of Furfural and Hydroxymethylfurfural from an Aqueous Solution Using a Supported Hydrophobic Deep Eutectic Solvent Liquid Membrane. Faraday Discuss. 2018, 206, 77–92. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Type V Deep Eutectic Solvents: Design and Applications. Curr. Opin. Green Sustain. Chem. 2022, 35, 100612. [Google Scholar] [CrossRef]
- de Castro, A.M.; Prasavath, D.; Bevilaqua, J.V.; Portugal, C.A.M.; Neves, L.A.; Crespo, J.G. Role of Water on Deep Eutectic Solvents (DES) Properties and Gas Transport Performance in Biocatalytic Supported DES Membranes. Sep. Purif. Technol. 2021, 255, 117763. [Google Scholar] [CrossRef]
- Ishaq, M.; Gilani, M.A.; Bilad, M.R.; Faizan, A.; Raja, A.A.; Afzal, Z.M.; Khan, A.L. Exploring the Potential of Highly Selective Alkanolamine Containing Deep Eutectic Solvents Based Supported Liquid Membranes for CO2 Capture. J. Mol. Liq. 2021, 340, 117274. [Google Scholar] [CrossRef]
- Ishaq, M.; Gilani, M.A.; Ahmad, F.; Afzal, Z.M.; Arshad, I.; Bilad, M.R.; Ayub, K.; Khan, A.L. Theoretical and Experimental Investigation of CO2 Capture through Choline Chloride Based Supported Deep Eutectic Liquid Membranes. J. Mol. Liq. 2021, 335, 116234. [Google Scholar] [CrossRef]
- Saeed, U.; Khan, A.U.; Khan, A.L.; Gilani, M.A.; Bilad, M.R. Separation of Carbon Dioxide by Potassium Carbonate Based Supported Deep Eutectic Liquid Membranes: Influence of Hydrogen Bond Donor. J. Membr. Sci. Res. 2022, 8, 526587. [Google Scholar] [CrossRef]
- Saeed, U.; Laeeq Khan, A.; Amjad Gilani, M.; Roil Bilad, M.; Ullah Khan, A. Supported Liquid Membranes Comprising of Choline Chloride Based Deep Eutectic Solvents for CO2 Capture: Influence of Organic Acids as Hydrogen Bond Donor. J. Mol. Liq. 2021, 335, 116155. [Google Scholar] [CrossRef]
- Saeed, U.; Khan, A.L.; Gilani, M.A.; Bilad, M.R.; Khan, A.U. Supported Deep Eutectic Liquid Membranes with Highly Selective Interaction Sites for Efficient CO2 Separation. J. Mol. Liq. 2021, 342, 117509. [Google Scholar] [CrossRef]
- Ishaq, M.; Gilani, M.A.; Afzal, Z.M.; Bilad, M.R.; Nizami, A.S.; Rehan, M.; Tahir, E.; Khan, A.L. Novel Poly Deep Eutectic Solvents Based Supported Liquid Membranes for CO2 Capture. Front Energy Res. 2020, 8, 595041. [Google Scholar] [CrossRef]
- Lin, H.; Gong, K.; Ying, W.; Chen, D.; Zhang, J.; Yan, Y.; Peng, X. CO2-Philic Separation Membrane: Deep Eutectic Solvent Filled Graphene Oxide Nanoslits. Small 2019, 15, 1904145. [Google Scholar] [CrossRef]
- Lin, H.; Gong, K.; Hykys, P.; Chen, D.; Ying, W.; Sofer, Z.; Yan, Y.; Li, Z.; Peng, X. Nanoconfined Deep Eutectic Solvent in Laminated MXene for Efficient CO2 Separation. Chem. Eng. J. 2021, 405, 126961. [Google Scholar] [CrossRef]
- Nowosielski, B.; Warmińska, D.; Cichowska-Kopczyńska, I. CO2 Separation Using Supported Deep Eutectic Liquid Membranes Based on 1,2-Propanediol. ACS Sustain. Chem. Eng. 2023, 11, 4093–4105. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.F.; Wang, Z.; Yao, J. Nanocellulose Membrane with Double-Salt Deep Eutectic Solvent for Efficient Carbon Capture. Sep. Purif. Technol. 2024, 347, 127614. [Google Scholar] [CrossRef]
- Mehdi, M.S.; Fakhar-e-Alam, M.; Asif, M.; Rehman, J.; Alshgari, R.A.; Jamal, M.; Zaman, S.U.; Umar, M.; Rafiq, S.; Muhammad, N.; et al. Deep Eutectic Solvent Coated Cerium Oxide Nanoparticles Based Polysulfone Membrane to Mitigate Environmental Toxicology. Molecules 2023, 28, 7162. [Google Scholar] [CrossRef] [PubMed]
- Saif-ur-Rehman; Zaman, M.K.U.; Ahsan Waseem, M.; Zaman, S.U.; Shozab Mehdi, M. Deep Eutectic Solvent-Functionalized Mesoporous Silica SBA-15-Based Mixed Matrix Polymeric Membranes for Mitigation of CO2. Eng. Proc. 2021, 12, 61. [CrossRef]
- Yuan, Y.; Qiao, Z.; Xu, J.; Wang, J.; Zhao, S.; Cao, X.; Wang, Z.; Guiver, M.D. Mixed Matrix Membranes for CO2 Separations by Incorporating Microporous Polymer Framework Fillers with Amine-Rich Nanochannels. J. Memb. Sci. 2021, 620, 118923. [Google Scholar] [CrossRef]
- Ferrari, H.Z.; Bernard, F.; dos Santos, L.; Dias, G.; Le Roux, C.; Micoud, P.; Martin, F.; Einloft, S. Enhancing CO2/N2 and CO2/CH4 Separation in Mixed Matrix Membrane: A Comprehensive Study on Pebax®1657 with SSMMP/IL for Improved Efficiency. Polym. Eng. Sci. 2024, 64, 2875–2893. [Google Scholar] [CrossRef]
- Silva, L.P.; Qasem, E.; Upadhyaya, L.; Esposito, R.; Górecki, R.; Coutinho, J.A.P.; Carvalho, P.J.; Nunes, S.P. Encapsulated Amino Acid-Based Ionic Liquid for CO2 Separation Membranes. ACS Sustain. Chem. Eng. 2024, 12, 300–309. [Google Scholar] [CrossRef]
- Lian, S.; Zhao, Q.; Zhang, Z.; Li, R.; Song, C. Tailored Interfacial Microenvironment of Mixed Matrix Membranes Based on Deep Eutectic Solvents for Efficient CO2 Separation. Sep. Purif. Technol. 2023, 307, 122753. [Google Scholar] [CrossRef]
- Lian, S.; Li, R.; Zhang, Z.; Liu, Q.; Song, C.; Lu, S. Improved CO2 Separation Performance and Interfacial Affinity of Composite Membranes by Incorporating Amino Acid-Based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 272, 118953. [Google Scholar] [CrossRef]
- Elhamarnah, Y.A.; Nasser, M.; Qiblawey, H.; Benamor, A.; Atilhan, M.; Aparicio, S. A Comprehensive Review on the Rheological Behavior of Imidazolium Based Ionic Liquids and Natural Deep Eutectic Solvents. J. Mol. Liq. 2019, 277, 932–958. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S.W. Polymeric Membranes for CO2 Separation and Capture. J. Memb. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Rindfleisch, F.; Dinoia, T.P.; Mchugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Ashworth, C.R.; Matthews, R.P.; Welton, T.; Hunt, P.A. Doubly Ionic Hydrogen Bond Interactions within the Choline Chloride-Urea Deep Eutectic Solvent. Phys. Chem. Chem. Phys. 2016, 18, 18145–18160. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, W.K.; Chiang, K.Y. Amine-Functionalized Biogenic Silica Incorporation Effect on Poly (Ether-Block-Amide) Membrane CO2/N2 Separation Performance. J. Memb. Sci. 2023, 680, 121732. [Google Scholar] [CrossRef]
- Katare, A.; Sharma, S.; Horo, H.; Bhowmick, S.; Kundu, L.M.; Mandal, B. An Investigation on the Effects of Both Amine Grafting and Blending with Biodegradable Chitosan Membrane for CO2 Capture from CO2/N2 Gas Mixtures. Chem. Eng. J. 2023, 466, 143215. [Google Scholar] [CrossRef]
- Nemestóthy, N.; Bakonyi, P.; Németh, Z.; Bélafi-Bakó, K. Evaluation of Pectin-Reinforced Supported Liquid Membranes Containing Carbonic Anhydrase: The Role of Ionic Liquid on Enzyme Stability and CO2 Separation Performance. J. CO2 Util. 2018, 24, 59–63. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural Deep Eutectic Solvent Mediated Pretreatment of Rice Straw: Bioanalytical Characterization of Lignin Extract and Enzymatic Hydrolysis of Pretreated Biomass Residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using Natural Deep Eutectic Solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; You, T.; Zhang, X.; Xu, F.; Wu, Y. Short-Time Deep Eutectic Solvent Pretreatment for Enhanced Enzymatic Saccharification and Lignin Valorization. Green Chem. 2019, 21, 3099–3108. [Google Scholar] [CrossRef]
- Elizondo Sada, O.M.; Hiemstra, I.S.A.; Chorhirankul, N.; Eppink, M.; Wijffels, R.H.; Janssen, A.E.M.; Kazbar, A. Pressure-Driven Membrane Processes for the Recovery and Recycling of Deep Eutectic Solvents: A Seaweed Biorefinery Case Study. Biotechnol. Rep. 2024, 43, e00849. [Google Scholar] [CrossRef]
- Liang, X.; Fan, W.; Zhang, Y.; Guo, Y. Regional Recovery and Controllable Regeneration of Brønsted Acidic Deep Eutectic Solvent in Biomass Fractionation via Bipolar Membrane Electrodialysis. Sep. Purif. Technol. 2024, 348, 127796. [Google Scholar] [CrossRef]
- Mishra, D.K.; Gopakumar, G.; Pugazhenthi, G.; Siva Brahmmananda Rao, C.V.; Nagarajan, S.; Banerjee, T. Molecular and Spectroscopic Insights into a Metal Salt-Based Deep Eutectic Solvent: A Combined Quantum Theory of Atoms in Molecules, Noncovalent Interaction, and Density Functional Theory Study. J. Phys. Chem. A 2021, 125, 9680–9690. [Google Scholar] [CrossRef]
- Janicka, P.; Przyjazny, A.; Boczkaj, G. Novel “Acid Tuned” Deep Eutectic Solvents Based on Protonated L-Proline. J. Mol. Liq. 2021, 333, 115965. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Gabaldón, C.; Badia, J.D.; Izquierdo, M.; Martínez-Soria, V. Recovery of Dissolved Methane through a Flat Sheet Module with PDMS, PP, and PVDF Membranes. Sep. Purif. Technol. 2022, 282, 120057. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Izquierdo, M.; Martínez-Soria, V.; Martí, L.; Monleón, A.; Badia, J.D. Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation. Membranes 2023, 13, 314. [Google Scholar] [CrossRef] [PubMed]








| HBA | Chemical Structure | HBD | Chemical Structure |
|---|---|---|---|
| Choline Chloride (ChCl) | 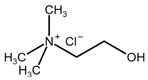 | Urea |  |
| Betaine |  | Glycerol | 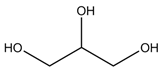 |
| ZnCl2 | 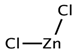 | Ethylene Glycol |  |
| Thymol | 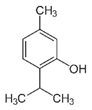 | Decanoic acid | 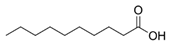 |
| Tetrabutylammonium Bromide ([N4444][Br]) |  | Oxalic Acid |  |
| Type | HBA | HBD |
|---|---|---|
| I | Ammonium, phosphonium, metallic salts, and other compounds that can be classified as [Cm+]n [An−]m | Metallic halides |
| II | Ammonium, phosphonium, metallic salts, and other compounds that can be classified as [Cm+]n [An−]m | Hydrated metal halides |
| III | Ammonium, phosphonium, metallic salts, and other compounds that can be classified as [Cm+]n [An−]m | Compounds containing amides (CONH2), carboxylic (COOH), or hydroxyl (OH) groups. |
| IV | Metallic halides | Compounds containing amides (CONH2), carboxylic (COOH), or hydroxyl (OH) groups. |
| V | Non-ionic compounds | |
| Type | HBA | HBD | Molar Ratio | Melting Point (K) | Surface Tension (mN·m−1) (298 K) | Viscosity (mPa·s) (298 K) | Density (g·cm−3) (298 K) | Refs. |
|---|---|---|---|---|---|---|---|---|
| I | ChCl | ZnCl2 | 1:2 | 325.3 | 415 | [44] | ||
| I | ChCl | ZnCl2 | 1:3 | 320.4 | 57.5 | [44] | ||
| I | [P4444][Br] | ZnCl2 | 1:2 | 341.0 | 2359 | [44] | ||
| I | [P4444][Br] | ZnCl2 | 1:3 | 343.0 | 6196 | [44] | ||
| II | ChCl | [CrCl3·6H2O] | 2:1 | 9074 | 1.38 | [45] | ||
| II | ChCl | [CrCl3·6H2O] | 1:2 | 4819 | 1.58 | [45] | ||
| III | [P4444][Cl] | Levulinic Acid | 1:2 | 167 | 1.02 | [46] | ||
| III | [MTP][Br] | Ethylene Glycol | 1:1 | 47.5 | [47] | |||
| III | [N1111][Cl] | Lactic Acid | 1:2 | 756 | 1.14 | [48] | ||
| III | [N2222][Cl] | Lactic Acid | 1:2 | 441 | 1.11 | [48] | ||
| III | [N3333][Br] | Glycerol | 1:2 | 51.6 | [49] | |||
| III | [N3333][Br] | Ethylene Glycol | 1:3 | 46.6 | [49] | |||
| III | [N4444][Cl] | Lactic Acid | 1:2 | 890 | 1.02 | [48] | ||
| III | [N4444][Br] | Acetic Acid | 1:1 | 34.5 | [47] | |||
| III | [N4444][Br] | Oxalic Acid | 1:1 | 42.7 | [47] | |||
| III | Betaine | Urea | 1:2 | 359 | [50] | |||
| III | Betaine | Ethylene Glycol | 1:3 | 56 | 36 | 1.13 | [51,52,53] | |
| III | Betaine | Glycerol | 1:2 | 1.22 | [53] | |||
| III | ChCl | Urea | 1:2 | 285 | 52 | 828 | 1.19 | [54,55,56,57] |
| III | ChCl | Ethylene Glycol | 1:2 | 237 | [58] | |||
| III | ChCl | Ethylene Glycol | 1:3 | 59 | 31 | 1.11 | [51,53,59] | |
| III | ChCl | Glycerol | 1:2 | 233 | 57.2 | 400 | 1.19 | [53,60,61] |
| III | ChCl | Glucose | 1:2 | 8000 | [62] | |||
| III | ChCl | Glucose | 1:1 | 73 | 9000 | 1.27 | [62,63] | |
| III | ChCl | Glucose | 2:1 | 71.6 | [62] | |||
| III | ChCl | D-Fructose | 1:1 | 293 | [64] | |||
| III | ChCl | D-Fructose | 2:1 | 283 | [64] | |||
| III | ChCl | Citric Acid | 1:2 | 344 | [65] | |||
| III | ChCl | Lactic Acid | 1:1 | 1160 | 1.17 | [66] | ||
| III | ChCl | Lactic Acid | 1:1 +20% water | 25 | 1.15 | [66] | ||
| III | ChCl | Lactic Acid | 1:2 | 47.4 | [67] | |||
| III | ChCl | Lactic Acid | 1:4 | 44.4 | [67] | |||
| III | ChCl | Levulinic Acid | 1:2 | 39.4 | [47] | |||
| III | ChCl | Malic Acid | 1:1.5 | 325 | [65] | |||
| III | ChCl | Malic Acid | 1:1 | 11,000 | 1.27 | [66] | ||
| III | ChCl | Malic Acid | 1:1 +20% water | 60 | 1.23 | [66] | ||
| III | ChCl | Malonic Acid | 1:1 | 283 | 65.7 | 1389 | 1.23 | [68,69] |
| III | ChCl | Oxalic Acid | 1:1 | 307 | 8953 | 1.26 | [66,68,69] | |
| III | ChCl | Tartaric Acid | 1:2 | 310 | [70] | |||
| III | ChCl | Tartaric Acid | 2:1 | 89,967 | 1.18 | [70] | ||
| III | ChCl | O-cresol | 1:3 | 1.07 | [71] | |||
| III | ChCl | Phenol | 1:3 | 1.09 | [71] | |||
| III | Menthol | Octanoic Acid | 1:1 | 26.7 | [47,72] | |||
| III | Menthol | Decanoic Acid | 1:1 | 16 | 0.89 | [72] | ||
| III | Thymol | Octanoic Acid | 1:1 | 29.1 | [47] | |||
| III | Thymol | Decanoic Acid | 1:1 | 285 | 12 | 0.93 | [72,73] | |
| III | Thymol | Decanoic Acid | 1:2 | 28.4 | [47] | |||
| IV | CoCl2· 6 H2O | Lactic Acid | 1:6 | 235.9 | 35 | 160 | 1.34 | [74] |
| IV | NiCl· 6 H2O | Urea | 1:2 | 235.3 | 89.9 | 500 | 1.58 | [74] |
| IV | ZnCl2 | Lactic Acid | 1:1 | 1.12 | [75] | |||
| V | Thymol | Menthol | 2:1 | 285 | 27 | 0.95 | [72,76] | |
| V | Thymol | Menthol | 1:1 | 257 | 37 | 0.93 | [72,76] | |
| V | Thymol | Menthol | 1:2 | 267 | 44 | 0.92 | [72,76] | |
| V | Thymol | Urea | 1:2 | 268 | [77] |
| Type of Configuration | Overview | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Bulk Liquid Membranes (BLM) | U-shaped tube with 3 phases: a feed and a receiving phase in contact with a membrane phase | Good for preliminary selectivity and kinetic transfer measurements High stability | Very low surface area Very low scalability | [142] |
| Emulsion Liquid Membranes (ELM) | Double emulsion: acceptor inside a membrane phase, which strips the feed | High recovery of metal species High mass transfer | High number of parameters to control Difficult stability control | [143] |
| Supported Liquid Membranes (SLM) | Polymeric membrane with pores filled with a liquid phase, which facilitates transport | Low cost Easy preparation High mechanical stability | Possible sweep of liquid phase Must operate at low pressures | [30] |
| Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Direct immersion | Easy to perform Low cost | Time-consuming Not valid for viscous DESs | [150] |
| N2 pressure | Ensures impregnation of all DESs Short immobilization time | Requires a more complex setup | [34,146,147] |
| Vacuum | High pore filling Simple assembly | Higher DESs consumption Requires vacuum pump | [32] |
| Membrane Support | Carrier | Molar Ratio | (Barrer) | (Barrer) | (Barrer) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Supported liquid membranes (SLMs) | ||||||||
| PTFE | ChCl:Urea | 1:2 | 90.0 | 1.0 | 1.7 | 90.0 | 60.0 | [27] |
| ChCl:Gly | 1:2 | 40.0 | 0.7 | 0.4 | 60.0 | 100.0 | ||
| ChCl:EG | 1:2 | 50.0 | 1.7 | 1.7 | 30.0 | 30.0 | ||
| ChCl:OxA | 1:2 | 35.0 | 3.5 | 3.9 | 10.0 | 9.0 | ||
| ChCl:LA + 4% water | 1:2 | 110.0 | 1.5 | 71 | [160] | |||
| ChCl:LA + 9% water | 1:2 | 60.0 | 1.0 | 61 | ||||
| ChCl:LA + 27% water | 1:2 | 60.0 | 2.0 | 30 | ||||
| ChCl:LA + 36% water | 1:2 | 60.0 | 3.0 | 20 | ||||
| PVDF | ChCl-MEA | 1:8 | 32.2 | 0.45 | 0.41 | 70.5 | 78.4 | [161] |
| ChCl-DEA | 1:8 | 27.7 | 0.44 | 0.40 | 63.0 | 69.3 | ||
| ChCl-Urea | 2:1 | 43.5 | 0.72 | 0.58 | 60.4 | 73.4 | [162] | |
| K2CO3-Gly | 1:6 | 34 | 0.58 | - | 59 | - | [163] | |
| ChCl-OxA | 1:1 | 35.5 | 0.61 | - | 58.6 | - | [164] | |
| ChCl-Urea | 1:1 | 38.0 | 0.65 | 0.52 | 58.5 | 73.9 | [162] | |
| Bet-MalA | 1:1 | 29.3 | 0.52 | 0.48 | 56.4 | 61.1 | [34] | |
| Bet-Urea | 1:3 | 33.4 | 0.6 | - | 55.7 | - | [165] | |
| ChCl-T E A | 1:8 | 25.1 | 0.46 | 0.42 | 54.6 | 59.8 | [161] | |
| ChCl-MalA | 1:1 | 33.3 | 0.61 | - | 54.5 | - | [164] | |
| Be-TartA | 1:1 | 25.6 | 0.5 | 0.49 | 51.1 | 52.1 | [34] | |
| ChCl-PAA | 20:1 | 21.3 | 0.42 | 0.38 | 50.7 | 56.1 | [166] | |
| ChCl-PAA | 15:1 | 20.6 | 0.42 | 0.37 | 49.0 | 55.6 | ||
| ChCl-TartA | 1:1 | 27.5 | 0.56 | - | 49.0 | - | [164] | |
| ChCl-Urea | 1:2 | 29.4 | 0.63 | 0.6 | 46.7 | 49.2 | [162] | |
| ChCl-PAM | 20:1 | 27.0 | 0.58 | 0.45 | 46.6 | 60.0 | [166] | |
| ChCl-PAM | 15:1 | 24.5 | 0.54 | 0.43 | 45.4 | 57.0 | ||
| Bet-Gly | 1:3 | 29.3 | 0.65 | - | 45.1 | - | [165] | |
| Bet-EG | 1:3 | 30.5 | 0.72 | - | 42.3 | - | ||
| K2CO3-EG | 1:6 | 20 | 0.59 | - | 34 | - | [163] | |
| Thy:Cou + 20% NFM | 1:1 | 161 | 3.2 | 49.9 | [32] | |||
| Thy:Cou + 40% NFM | 1:1 | 174.1 | 3.3 | 52.4 | ||||
| Thy-Cou | 1:1 | 166.6 | - | 5.55 | - | 30.0 | ||
| GO | ChCl-EG | 1:4 | 9 | 0.02 | 0.02 | ~350 | ~480 | [167] |
| Ti-Nanosheets | ChCl-EG | 1:4 | 105.0 | 0.5 | 0.42 | 210 | 250 | [168] |
| PP | ChCl-1,2-propanediol | 1:3 | 104 | - | 3 | - | 29 | [169] |
| 1:4 | 112 | - | 5 | - | 22 | |||
| Cellulose nanofibers | ChCl-ZnCl2 | 1:2 | 155.8 | 3.2 | 3.6 | 48.4 | 43.6 | [170] |
| Polymer membranes | ||||||||
| PSF | 6.2 | 0.3 | 0.2 | 23.0 | 25 | [171] | ||
| PSF/THF | 23.5 | 0.11 | 0.10 | 57 | 60 | [172] | ||
| Mixed-matrix membranes (MMM) | ||||||||
| PVA + amine + HMMP-1 nanoparticles MMM | ~250 | ~2.5 | ~1 | ~100 | ~250 | [173] | ||
| Pebax® + SSMMP% + [BMIM][NTf2] MMM | 140 | 4.7 | 2.3 | 30 | 60 | [174] | ||
| [N4444][l-prolinate] + pebax | 100 | 6 | 2 | 17 | 50 | [175] | ||
| Pebax® + Zeolite imidazole framework MMM | 60 | 1.5 | 40 | [176] | ||||
| [N16,1,1,1][Br][AcH] − Ceria 2% + PSF MMM | 9.0 | 0.3 | 0.3 | 30.5 | 31 | [171] | ||
| [N16,1,1,1][Br]:[AcH] − Ceria 4% + PSF MMM | 12.5 | 0.4 | 0.4 | 32.5 | 35.5 | |||
| [N16,1,1,1][Br]:[AcH] − Ceria 6% + PSF MMM | 15.0 | 0.4 | 0.4 | 33.5 | 37 | |||
| [N16,1,1,1][Br]:[AcH] − Ceria 8% + PSF MMM | 17.0 | 0.5 | 0.4 | 35.5 | 39 | |||
| PSF/THF + ChCl:DecA 1:1/SBA 5% | 18 | 0.17 | 0.16 | 53 | 55 | [172] | ||
| PSF/THF + ChCl:DecA 1:1/SBA 10% | 13 | 0.27 | 0.25 | 49 | 51 | |||
| PSF/THF + ChCl:DecA 1:1/SBA 15% | 9 | 0.38 | 0.38 | 47 | 48 | |||
| L-arginine EG 1:5 Pebax® 0% | 65.0 | 1.5 | 43 | [177] | ||||
| L-arginine EG 1:5 Pebax® 5% | 65 | 1.3 | 50 | |||||
| L-arginine:EG 1:5 Pebax® 10% | 61.0 | 1.2 | 51 | |||||
| L-arginine:EG 1:5 Pebax® 15% | 63.0 | 1.2 | 52.5 | |||||
| L-arginine:EG 1:5 Pebax® 20% | 42.0 | 1 | 42 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco-Velasco, G.; Gálvez-Subiela, A.; Jiménez-Robles, R.; Izquierdo, M.; Cháfer, A.; Badia, J.D. A Review on the Application of Deep Eutectic Solvents in Polymer-Based Membrane Preparation for Environmental Separation Technologies. Polymers 2024, 16, 2604. https://doi.org/10.3390/polym16182604
Marco-Velasco G, Gálvez-Subiela A, Jiménez-Robles R, Izquierdo M, Cháfer A, Badia JD. A Review on the Application of Deep Eutectic Solvents in Polymer-Based Membrane Preparation for Environmental Separation Technologies. Polymers. 2024; 16(18):2604. https://doi.org/10.3390/polym16182604
Chicago/Turabian StyleMarco-Velasco, Gorka, Alejandro Gálvez-Subiela, Ramón Jiménez-Robles, Marta Izquierdo, Amparo Cháfer, and José David Badia. 2024. "A Review on the Application of Deep Eutectic Solvents in Polymer-Based Membrane Preparation for Environmental Separation Technologies" Polymers 16, no. 18: 2604. https://doi.org/10.3390/polym16182604
APA StyleMarco-Velasco, G., Gálvez-Subiela, A., Jiménez-Robles, R., Izquierdo, M., Cháfer, A., & Badia, J. D. (2024). A Review on the Application of Deep Eutectic Solvents in Polymer-Based Membrane Preparation for Environmental Separation Technologies. Polymers, 16(18), 2604. https://doi.org/10.3390/polym16182604










