Exploring the Impact of Bio-Based Plasticizers on the Curing Behavior and Material Properties of a Simplified Tire-Tread Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding and Mixing
2.2.1. Rubber Formulation
2.2.2. Mixing Procedure
2.3. Analytical Testing
2.3.1. Vulcanization
2.3.2. Cured Payne Effect
2.3.3. Equilibrium Swelling
- Vr = the volume percentage/fraction of rubber in a swollen sample
- V0 = the molar volume of the solvent used (106.9 cm3/mol for toluene)
- f = the functionality of crosslinks (assuming that tetra-functional crosslinks are formed, f = 4)
- χ = the Flory–Huggins rubber–solvent interaction parameter (for SBR in toluene, χ = 0.378 [16])
- v = the crosslink density per unit volume (mol/cm3)
2.3.4. Stress–Strain Behavior
2.3.5. Dynamical Properties
3. Results and Discussion
3.1. Rubber Compounds
3.1.1. Vulcanization
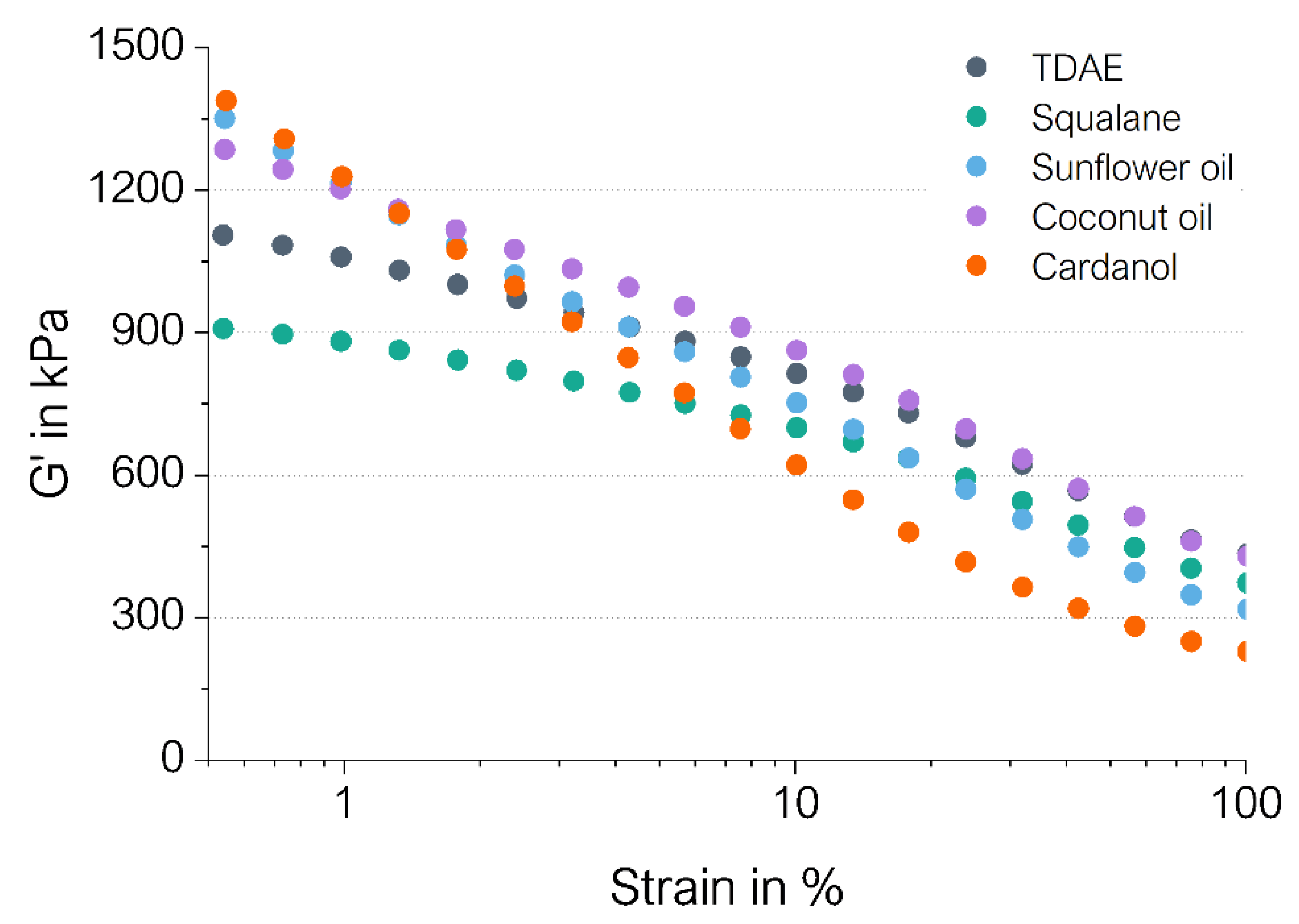
3.1.2. Cured Payne Effect
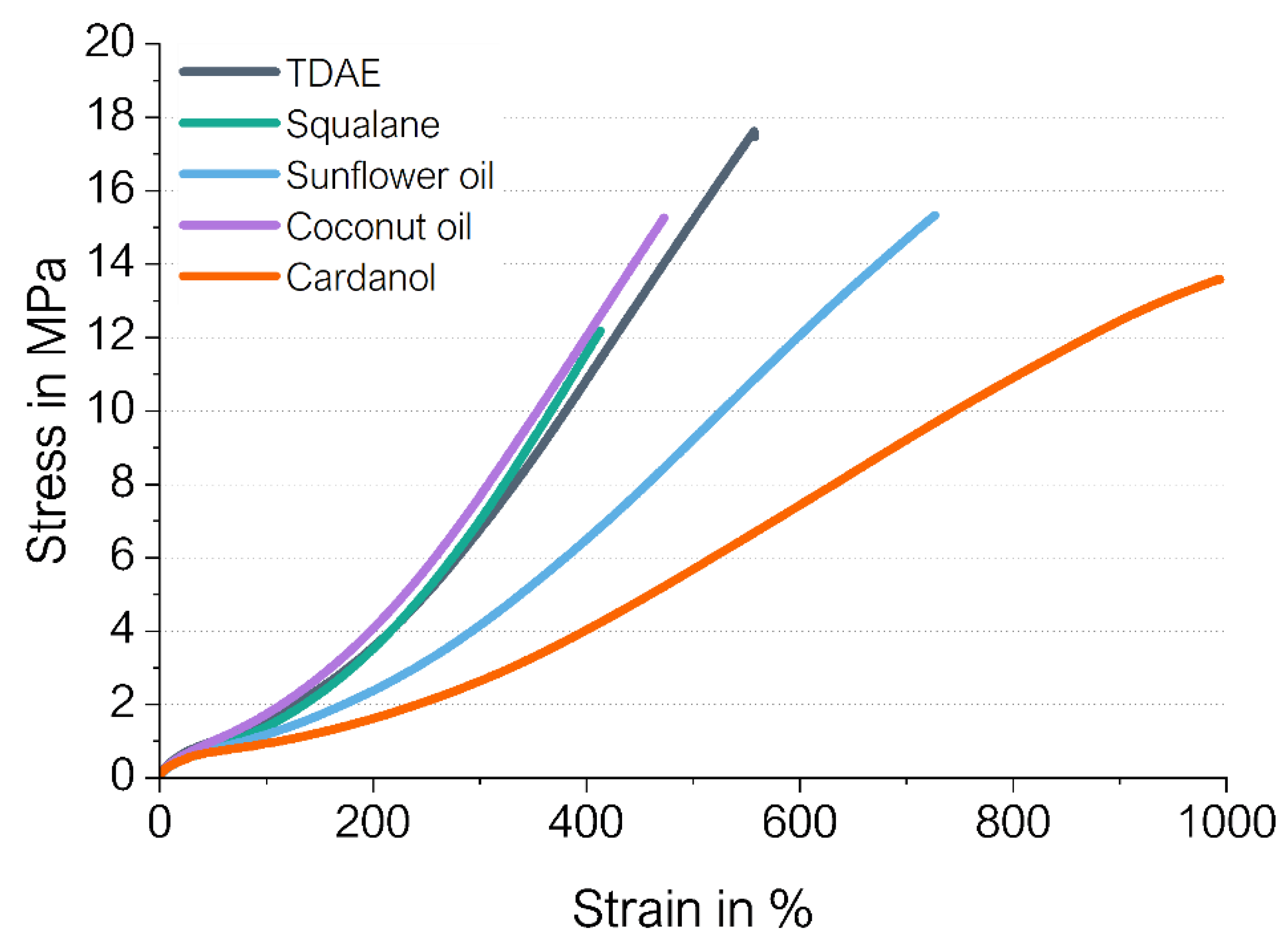
3.1.3. Stress–Strain Behavior
3.1.4. Dynamic Mechanical Analysis
3.2. Model Study Sulfur and Plasticizers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sustainability Driven: Accelerating Impact with the Tire Sector SDG Roadmap Tire Industry Project Sustainability Driven. Geneva, Switzerland, May 2021. Available online: https://sustainabilitydriven.info/ (accessed on 25 April 2024).
- Petchkaew, A.; Sahakaro, K.; Noordermeer, J.N.M. Petroleum-Based Safe Process Oils in NR, SBR and their Blends: Study on Unfilled Compounds. Part I. Oil Characteristics and Solubility Aspects. KGK Kautsch. Gummi Kunststoffe 2013, 66, 43–47. [Google Scholar]
- Rathi, A. Investigating Safe Mineral-Based and Bio-Based Process Oils for Tire Tread Application. Ph.D. Thesis, Department Mechanics of Solids, Surfaces, and Systems, University of Twente, Enschede, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Rathi, A.; Elsayed, M.; Krause-Rehberg, R.; Dierkes, W.K.; Noordermeer, J.W.; Bergmann, C.; Trimbach, J.; Blume, A. Effect of Aromatic Oil on the S-SBR/BR Blend Components Revealed using BDS and PALS. In Proceedings of the Deutsche Kautschuk Tagung, Nürnberg, Germany, 2–7 July 2018. [Google Scholar]
- Abbott, S.; Hansen, C.M.; Hiroshi, Y.; Hansen, C. Hansen Solubility Parameters in Practice, 5th ed.; 2008; Available online: https://www.hansen-solubility.com (accessed on 25 July 2023).
- Walters, P.; Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 1–27. [Google Scholar] [CrossRef]
- Mohamed, N.R.; Othman, N.; Shuib, R.K.; Hayeemasae, N. Perspective on Opportunities of Bio-Based Processing Oil to Rubber Industry: A Short Review. Iran. Polym. J. 2023, 32, 1455–1475. [Google Scholar] [CrossRef]
- Flanigan, C.; Beyer, L.; Klekamp, D.; Rohweder, D.; Haakenson, D. Using Bio-Based Plasticizers, Alternative Rubber. Rubber & Plastics News, 11 February 2013; 15–19. [Google Scholar]
- Alexander, M.; Thachil, E.T. The Effectiveness of Cardanol as Plasticiser, Activator, and Antioxidant for Natural Rubber Processing. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 107–125. [Google Scholar] [CrossRef]
- Othman, F.; Ismail, N. Coconut Oil Plasticizer as a Replacement of Petroleum Oil in Natural Rubber Compound: Physical and Mechanical Properties. In Charting the Sustainable Future of ASEAN in Science and Technology; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Ezzoddin, S.; Abbasian, A.; Zavieh, T.K.; Iji, H. Vegetable Oils, Sustainable Resources to be Replaced by High Aromatic Oil in Rubber Compounds. In Proceedings of the 11th International Seminar on Polymer Science and Technology, Tehran, Iran, 6–9 October 2014. [Google Scholar]
- Flanigan, C.; Beyer, L.; Klekamp, D.; Rohweder, D.; Terrill, E. Sustainable Processing Oils in Low RR Tread Compounds. Rubber & Plastics News. 2011. Available online: https://www.rubbernews.com/article/20100923/DATA01/309239947 (accessed on 10 August 2023).
- Siriwong, C.; Khansawai, P.; Boonchiangma, S.; Sirisinha, C.; Sae-Oui, P. The Influence of Modified Soybean Oil as Processing Aids in Tire Application. Polym. Bull. 2021, 78, 3589–3606. [Google Scholar] [CrossRef]
- Farshchi, N.; Abbasian, A. Inverse Gas Chromatography Study of Hansen Solubility Parameters of Rubber Process Oils (DAE, TDAE, MES, and NAP). Rubber Chem. Technol. 2020, 93, 297–318. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Blume, A.; Gruendken, M. Use of Liquid Polymers: Understanding the Structure-Property Relationship of Low-Molecular-Weight Liquid Polymers in Adjusted Blends of Sulfur-Cured S-SBR-Rich/Silica Formulations. Tire Technol. Int. 2020, 2020, 56–58. [Google Scholar]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- Duddey, J.E. Resins. In Rubber Compounding: Chemistry and Applications, 2nd ed.; Rogers, B., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 379–416. [Google Scholar]
- Boontawee, H.; Nakason, C.; Kaesaman, A.; Thitithammawong, A.; Chewchanwuttiwong, S. Benzyl Esters of Vegetable Oils as Processing Oil in Carbon Black–Filled SBR Compounding: Chemical Modification, Characterization, and Performance. Adv. Polym. Technol. 2017, 36, 320–330. [Google Scholar] [CrossRef]
- Hong, S.W. Antioxidants and Other Protectant System. In Rubber Compounding: Chemistry and Applications, 2nd ed.; Rodgers, B., Ed.; CRC Press: Bora Raton, FL, USA, 2016; pp. 420–459. [Google Scholar]
- Jin, J.; Noordermeer, J.W.M.; Dierkes, W.K.; Blume, A. The Effect of Silanization Temperature and Time on the Marching Modulus of Silica-Filled Tire Tread Compounds. Polymers 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The Effect of Filler-Filler and Filler-Elastomer Interaction on Rubber Reinforcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Nardelli, F.; Calucci, L.; Carignani, E.; Borsacchi, S.; Cettolin, M.; Arimondi, M.; Giannini, L.; Geppi, M.; Martini, F. Influence of Sulfur-Curing Conditions on the Dynamics and Crosslinking of Rubber Networks: A Time-Domain NMR Study. Polymers 2022, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Colvin, H. General-Purpose Elastomers. In Rubber Compounding: Chemistry and Applications, 2nd ed.; Rodgers, B., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 34–76. [Google Scholar]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of Glass Transition Temperatures: Binary Blends and Copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Edible Oils as Practical Phase Change Materials for Thermal Energy Storage. Appl. Sci. 2019, 9, 1627–1644. [Google Scholar] [CrossRef]
- Babazadeh, K.; Blume, A.; Zeeman, R.; Bandzierz, K. Optimized Laboratory Prediction of Wet Grip Performance of Tires. In Proceedings of the Tire Technology Expo, Hanover, Germany, 21–23 March 2023. [Google Scholar]



| Plasticizer | Mw in g/mol | ρ at 25 °C in g/cm3 | MP in °C | HSP in MPa1/2 | Chemical Structure | |
|---|---|---|---|---|---|---|
| TDAE | X | 0.95 | 27 * | δtot = 18.9 | X | |
| Squalane | 423 | 0.81 | −38 | δD = 15.9 δP = 0.1 δH = 0.1 δtot = 15.9 |  | |
| Sunflower oil | ~881 | 0.92 | −17 | δD = 16.5 δP = 1.7 δH = 3.6 δtot = 17.0 | 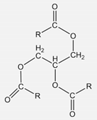 | where R = C16:0 (5%), C18:0 (6%), C18:1 (30%), C18:2 (59%) |
| Coconut oil | ~639 | 0.90 | 23–27 | δD = 16.3 δP = 2.3 δH = 2.6 δtot = 16.7 | 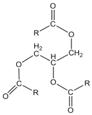 | where R = C8:0 (8%), C10:0 (7%), C12:0 (49%), C14:0 (8%), C16:0 (8%), C18:0 (2%), C18:1 (6%), C18:2 (2%) |
| Cardanol | ~302 | 0.93 | −30 | δD = 17.4 δP = 2.2 δH = 5.8 δtot = 18.5 | 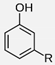 | where R = C15H27 |
| Ingredient | TDAE | Squalane | Sunflower Oil | Coconut Oil | Cardanol |
|---|---|---|---|---|---|
| S-SBR | 80 | 80 | 80 | 80 | 80 |
| BR | 20 | 20 | 20 | 20 | 20 |
| Silica | 80 | 80 | 80 | 80 | 80 |
| TESPD | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 |
| TDAE | 37.5 | X | X | X | X |
| Squalane | X | 37.5 | X | X | X |
| Sunflower oil | X | X | 37.5 | X | X |
| Coconut oil | X | X | X | 37.5 | X |
| Cardanol | X | X | X | X | 37.5 |
| Stearic acid | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Zinc oxide | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Sulfur | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| TBBS | 2 | 2 | 2 | 2 | 2 |
| DPG | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Time | Action |
|---|---|
| min:s | Stage 1: preheating 80 °C, 70 rpm, fill factor 72% |
| 0:00 | Addition of rubber |
| 1:00 | Addition of 2/3 silica, 2/3 silane |
| 2:30 | Addition of 1/3 silica, 1/3 silane, ZnO, stearic acid and plasticizer |
| 4:00 | 15 s sweep |
| 4:15 | Increase in torque (increase temperature to 130 °C) |
| 7:00 | Stop mixing (reaching 140 °C) |
| min:s | Stage 2: preheating 80 °C, 80 rpm, fill factor 69% |
| 0:00 | Addition of elastomer masterbatch |
| 0:50 | Addition of DPG |
| 1:00 | Increase in torque (increase temperature to 130 °C) |
| 5:00 | Stop mixing (reaching 140 °C) |
| min:s | Stage 3: preheating 50 °C, 50 rpm, fill factor 66% |
| 0:00 | Addition of elastomer masterbatch, curatives (sulfur and TBBS) |
| 3:00 | Stop mixing |
| Compound | t90 in min | t2 in min | MH in dNm | ML in dNm | CLD in mol/cm3 |
|---|---|---|---|---|---|
| TDAE | 37 | 14 | 12.6 | 1.3 | 184 ± 2 |
| Squalane | 37 | 14 | 10.8 | 1.1 | 174 ± 5 |
| Sunflower oil | 33 | 11 | 11.6 | 1.2 | 129 ± 3 |
| Coconut oil | 31 | 11 | 13.8 | 1.4 | 194 ± 3 |
| Cardanol | 36 | 6 | 10.3 | 0.8 | 92 ± 1 |
| Compound | G′0.56%–G′100% in kPa | G′100% in kPa |
|---|---|---|
| TDAE | 671 | 434 |
| Squalane | 536 | 373 |
| Sunflower oil | 1034 | 317 |
| Coconut oil | 857 | 429 |
| Cardanol | 1160 | 228 |
| Compound | Tensile Strength in MPa | Elongation at Break in % | Reinforcement Index M300/M100 |
|---|---|---|---|
| TDAE | 16.7 ± 1.7 | 510 ± 40 | 4.8 ± 0.3 |
| Squalane | 12.1 ± 1.7 | 420 ± 40 | 4.8 ± 0.3 |
| Sunflower oil | 15.6 ± 0.3 | 720 ± 7 | 3.5 ± 0.1 |
| Coconut oil | 14.4 ± 1.0 | 460 ± 20 | 4.4 ± 0.1 |
| Cardanol | 13.3 ± 0.6 | 990 ± 30 | 2.7 ± 0.1 |
| Compound | Tg in °C | Tan δ Peak Height | Tan δ at 0 °C | Tan δ at 60 °C |
|---|---|---|---|---|
| TDAE | −19 | 0.892 | 0.403 | 0.116 |
| Squalane | −39 | 0.783 | 0.208 | 0.106 |
| Sunflower oil | −37 | 0.678 | 0.191 | 0.131 |
| Coconut oil | −25 | 0.503 | 0.256 | 0.104 |
| Cardanol | −35 | 0.749 | 0.191 | 0.152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Elburg, F.; Grunert, F.; Aurisicchio, C.; di Consiglio, M.; di Ronza, R.; Talma, A.; Bernal-Ortega, P.; Blume, A. Exploring the Impact of Bio-Based Plasticizers on the Curing Behavior and Material Properties of a Simplified Tire-Tread Compound. Polymers 2024, 16, 1880. https://doi.org/10.3390/polym16131880
van Elburg F, Grunert F, Aurisicchio C, di Consiglio M, di Ronza R, Talma A, Bernal-Ortega P, Blume A. Exploring the Impact of Bio-Based Plasticizers on the Curing Behavior and Material Properties of a Simplified Tire-Tread Compound. Polymers. 2024; 16(13):1880. https://doi.org/10.3390/polym16131880
Chicago/Turabian Stylevan Elburg, Frances, Fabian Grunert, Claudia Aurisicchio, Micol di Consiglio, Raffaele di Ronza, Auke Talma, Pilar Bernal-Ortega, and Anke Blume. 2024. "Exploring the Impact of Bio-Based Plasticizers on the Curing Behavior and Material Properties of a Simplified Tire-Tread Compound" Polymers 16, no. 13: 1880. https://doi.org/10.3390/polym16131880
APA Stylevan Elburg, F., Grunert, F., Aurisicchio, C., di Consiglio, M., di Ronza, R., Talma, A., Bernal-Ortega, P., & Blume, A. (2024). Exploring the Impact of Bio-Based Plasticizers on the Curing Behavior and Material Properties of a Simplified Tire-Tread Compound. Polymers, 16(13), 1880. https://doi.org/10.3390/polym16131880









