Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems
Abstract
1. Introduction
2. Overview of Biopolymers and Biocomposites
2.1. Natural vs. Synthetic Biopolymers
2.2. Biocomposite Materials
2.3. Nanoparticles-Based Polymeric Biomaterials
3. Oculoplastic and Orbital Surgery Devices
3.1. The Applications of Biomaterials in the Repair of Orbital Floor Fractures
3.1.1. Overview of Orbital Floor Fractures and Ideal Properties of Biomaterials for Surgical Reconstruction
- Biocompatibility and Non-Toxicity: The ideal biomaterial should not induce allergic reactions or have carcinogenic potential. Furthermore, it is essential that it closely mimics the physical properties of the native orbital tissue, thereby ensuring seamless integration without undue stress or strain on adjacent structures.
- Long-term Acceptance: The material must be capable of being permanently accepted by the body. This implies that it should not elicit a chronic inflammatory response or be subject to rejection.
- Chemical Stability: The selected material must be chemically inert. This stability is crucial, as the material needs to withstand sterilization processes without degradation of its chemical properties, thus ensuring long-term functionality and safety within the complex orbital environment.
- Manipulability and Stability: During surgery, the ease of manipulation of the material is vital for precise placement and shaping. Once implanted, it should maintain its form and integrity, and resist any deformation that could compromise the reconstructed anatomy.
- Fixation Capability: Effective fixation to the host bone is fundamental for long-term implant success. Therefore, the material should be amenable to secure fixation using various methods, such as screws, wires, sutures, or adhesives, to provide stability and prevent displacement.
- Anti-Microbial and Bone Preservation: It is important that the material does not promote microbial growth, which could lead to infection, nor should it promote resorption of the underlying bone. Additionally, it must not distort or exert undue pressure on adjacent structures, thereby maintaining the integrity of the orbital contents.
- Radiopacity: For effective postsurgical assessment, the material should be radiopaque, allowing for clear visualization during radiological evaluations. This feature is essential for monitoring the position and condition of the implant over time.
- Cost-Effectiveness: While ensuring high standards of quality and functionality, the materials should also be cost-effective. Balancing quality and cost is key to making these essential medical devices available to a broader patient population, thereby enhancing their overall well-being and quality of life.
3.1.2. Current Biopolymers and Biocomposites for Orbital Floor Repair (Table 1)
| Material | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|
| Polylactic Acid (PLA) |
|
|
| [21] |
| Polyglactin 910 (Vicryl) |
|
|
| [22,23] |
| Polydioxanone (PDO) |
|
|
| [24,25,26,27,28] |
| Silicone |
|
|
| [29,30,31,32] |
| Porous Polyethylene (Medpor) |
|
|
| [33,34,35,36,37] |
| Medpor Titan |
|
|
| [38] |
| HAPEX™ |
|
|
| [39] |
3.1.3. Emerging Biopolymers, Biocomposites, and their Applications for Orbital Floor Reconstruction (Table 2)
| Material | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|
| Poly(trimethylene carbonate) (PTMC) | Combined with biphasic calcium phosphate particles and titanium mesh | Enhanced customizability, precision, neovascularization, bone growth | Requires further exploration for long-term efficacy | [40] |
| Bone marrow-derived mesenchymal stem cells (BMSCs) with β-TCP | Establishes osteoconductive environments for tissue regeneration | Improved tissue regeneration, accelerated healing | Challenge in translating findings to human clinical applications | [41] |
| HA nanoparticles in polyurethane scaffolds | Incorporation of nanoparticles | Enhanced mechanical strength, biocompatibility | Potential cytotoxicity, unclear stability, short half-life | [42] |
| Ce-doped ZnO nanoparticles in silk fibroin scaffolds | Nanoparticles with antibacterial properties | Strong antibacterial properties, favorable biocompatibility | Potential cytotoxicity, unclear stability | [43] |
| Dual-action coatings | Adaptable to physiological environment | Promising to address current challenges | Early stages of research | [44] |
3.2. The Applications of Biomaterials in Ocular Prosthesis
3.2.1. Overview of Ocular Prosthesis and Ideal Properties of Biomaterials for Restoring Functionality and Aesthetics
- Lightweight and Comfort: The prosthesis should be designed to be lightweight to promote maximum comfort for the patient. A heavy prosthesis can lead to discomfort and may cause strain on the surrounding orbital tissues. Therefore, the use of lightweight materials ensures that patients can perform their daily activities without feeling discomfort. It is important that this lightness in weight is achieved without compromising the durability or functionality of the prosthesis. An ideal lightweight prosthesis should be comfortable for prolonged wear while maintaining its structural integrity and functionality.
- Color Match to the Contralateral Eye: The prosthesis should be custom-tailored in color to match the characteristics of the contralateral eye as closely as possible. This approach ensures that the artificial eye is virtually indistinguishable from the natural eye color, thereby enhancing the overall aesthetic appearance.
- Texture and Integration with Facial Features: The prosthesis should mimic the natural eye not just in color but also in texture. This means that the surface of the prosthesis should feel similar to that of the natural eye when touched. This attention to detail in replicating the natural eye texture contributes significantly to a natural appearance and feel, enhancing the prosthesis’s integration with natural facial features.
- Hygiene and Maintenance: The design of the prosthesis should allow for easy and effective cleaning. Good hygiene is important to prevent infections and maintain the health of the surrounding orbital tissues. The surface and material of the prosthesis should not hold onto the bacteria and should be resistant to the build-up of deposits.
- Availability and Accessibility: The prosthesis should be readily available for re-placements or adjustments as needed. This means that the manufacturing processes should be sufficiently efficient to ensure that these prostheses are easily accessible to those in need. Availability is key to ensuring that patients can quickly obtain replacement or adjustment if their prosthesis becomes damaged or their physical needs change. This accessibility is essential for the continuous and comfortable use of prostheses, ensuring that patients do not face long periods of discomfort or lack of functionality.
3.2.2. Current Biopolymers and Biocomposites in Ocular Prosthetics
3.2.3. Emerging Biopolymers, Biocomposites, and Their Applications in Ocular Prosthetics
3.3. The Applications of Biomaterials in Posterior Lamellar Eyelid Reconstruction
3.3.1. Overview of Posterior Lamellar Eyelid Reconstruction and Ideal Properties for Tarsal Substitutes
- Structural Integrity and Durability: A tarsal substitute should be thin enough to not cause any discomfort or disruption in the eye’s anatomy, yet stable enough to maintain its shape and function over time. This durability is crucial to ensuring that the substitute can withstand the mechanical forces exerted during blinking and eye movements without deformation or deterioration.
- Biocompatibility: High biocompatibility is essential for minimizing adverse reactions from the body’s immune system. A biocompatible tarsal substitute would reduce the risk of rejection and other complications, such as irritation or infection, ensuring safer integration with the surrounding tissues.
- Tissue Integration: The ability to seamlessly merge into the peripheral tarsus is vital for a successful implant. This integration ensures that the substitute behaves as a natural component of the eye, thereby facilitating normal eyelid function. They should bond well with surrounding tissues without causing any structural weaknesses or abnormalities.
- Anti-Inflammatory: The ideal tarsal substitute should not provoke any inflammatory response. Therefore, it must be designed to avoid triggering the body’s immune response, which can lead to swelling, redness, and discomfort, thereby ensuring a more comfortable and effective healing process.
- Biomimetic Functionality: Mimicking the physical structures and biological functions of the native extracellular matrix is important as a substitute for effective function. This involves replicating the texture, elasticity, and strength of the natural tarsal plate, as well as its ability to interact with native cells and tissues to promote normal eyelid function.
- Cellular Support: The substitute should foster cell survival, proliferation, and growth. This provides a conducive environment for cells to adhere, grow, and function normally. This should encourage healthy tissue regeneration and integration, thereby contributing to the overall success and longevity of implants.
3.3.2. Current Types of Biomaterials and Approaches for Posterior Lamellar Eyelid Reconstruction
3.3.3. Emerging Biopolymers, Biocomposites, and Their Applications for Posterior Lamellar Eyelid Reconstruction
4. Applications of Hydrogels in Ophthalmology
4.1. Intraocular Lenses (IOLs)
4.2. Vitreous Substitutes
4.3. Ocular Wound Repair for Cornea Damage
5. Biopolymeric Drugs Delivery Systems
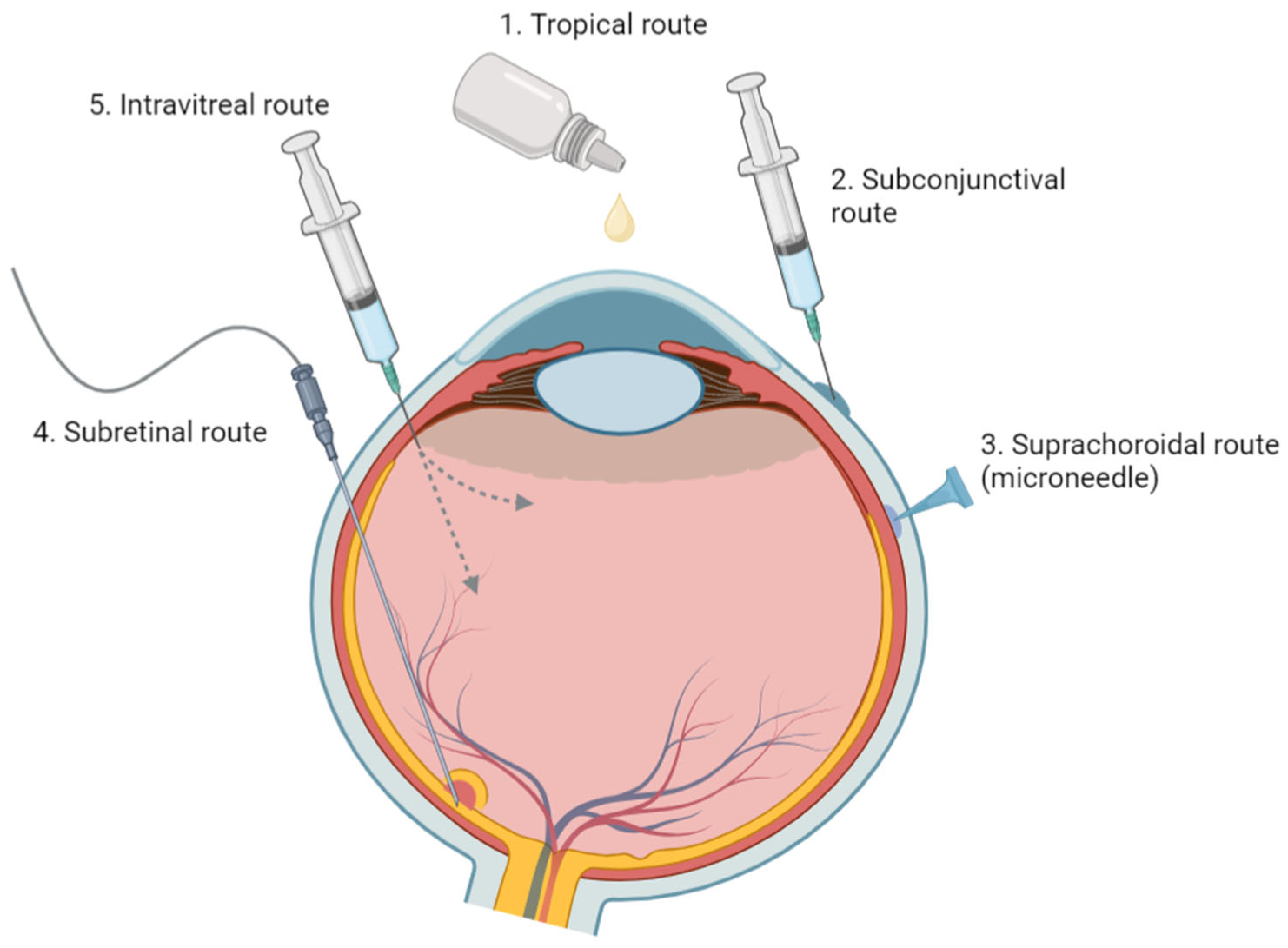
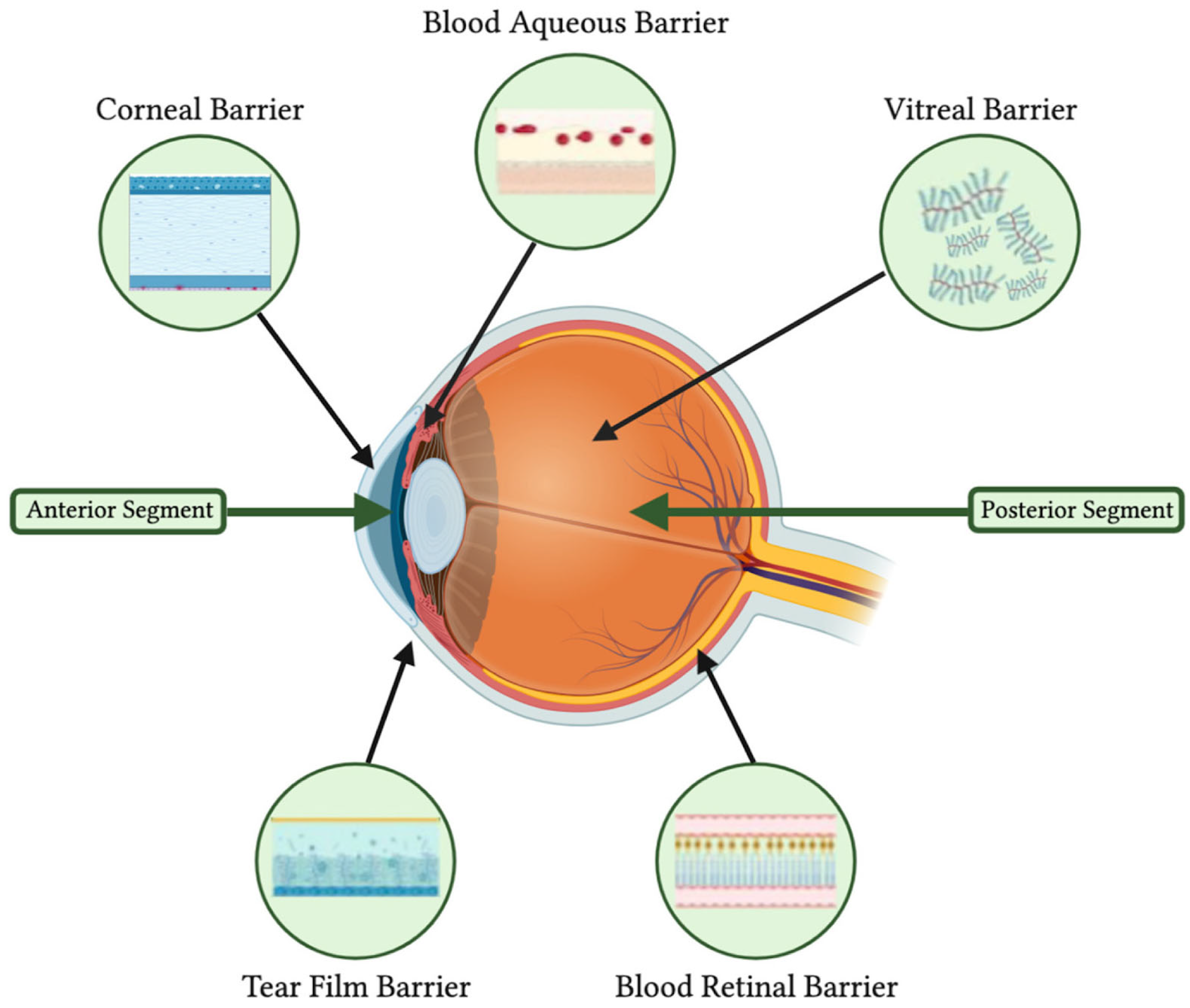
5.1. Anatomical and Physiological Barriers in Ocular Drug Delivery
5.2. Anterior Segment Diseases
5.2.1. Glaucoma
5.2.2. Dry Eye Disease
5.2.3. Cataracts
5.3. Posterior Segment Diseases
Age-Related Macular Degeneration (AMD) & Diabetic Retinopathy (DR)
5.4. Uveitis
5.4.1. Applications of Biopolymer-Based Hydrogels in Drug Delivery
5.4.2. Biodegradable Nano-Based Drug Delivery Systems
6. Current Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmus, M. Overview of Biomedical Materials. MRS Bull. 1991, 16, 33–38. [Google Scholar] [CrossRef]
- Carvalho, L.T.; Vieira, T.A.; Zhao, Y.; Celli, A.; Medeiros, S.F.; Lacerda, T.M. Recent Advances in the Production of Biomedical Systems Based on Polyhydroxyalkanoates and Exopolysaccharides. Int. J. Biol. Macromol. 2021, 183, 1514–1539. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.K.; Lee, D. Biopolymer Microparticles Prepared by Microfluidics for Biomedical Applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.A.; Roy, P.K.; Hossain, C.M.; Dutta, D.; Vichare, R.; Biswal, M.R. Starch-Based Nanomaterials in Drug Delivery Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–56. ISBN 978-0-12-820874-8. [Google Scholar]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable Liposome-Encapsulated Hydrogels for Biomedical Applications: A Marriage of Convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, D.M.; Contin, M.D.; Kolender, A.A.; D’Accorso, N. Polymeric Biocomposites from Renewable and Sustainable Natural Resources. In Polymeric and Natural Composites; Hasnain, M.S., Nayak, A.K., Alkahtani, S., Eds.; Advances in Material Research and Technology; Springer International Publishing: Cham, Germany, 2022; pp. 65–108. ISBN 978-3-030-70265-6. [Google Scholar]
- Maman, P.; Nagpal, M.; Aggarwal, G. Resorbable Polymer Fiber Reinforced Composites in Biomedical Application. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 121–166. ISBN 978-0-12-816909-4. [Google Scholar]
- Ramesh, M.; Deepa, C. Biocomposites for Biomedical Devices. In Green Biocomposites for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 287–300. ISBN 978-0-12-821553-1. [Google Scholar]
- Abidi, S.M.S.; Dar, A.I.; Acharya, A. Plant-Based Polymeric Nanomaterials for Biomedical Applications. In Nanomaterial-Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy; Acharya, A., Ed.; Springer Singapore: Singapore, 2020; pp. 129–158. ISBN 9789811542794. [Google Scholar]
- Nitta, S.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 Nanoparticle-Containing Polymers for Biomedical Applications: A Review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Burnstine, M.A. Clinical Recommendations for Repair of Isolated Orbital Floor Fractures. Ophthalmology 2002, 109, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Regan, W.F. Blow-Out Fracture of the Orbit*. Am. J. Ophthalmol. 1957, 44, 733–739. [Google Scholar] [CrossRef]
- Kulwin, D.R.; Leadbetter, M.G. Orbital Rim Trauma Causing a Blowout Fracture. Plast. Reconstr. Surg. 1984, 73, 969–970. [Google Scholar] [CrossRef]
- Waterhouse, N.; Lyne, J.; Urdang, M.; Garey, L. An Investigation into the Mechanism of Orbital Blowout Fractures. Br. J. Plast. Surg. 1999, 52, 607–612. [Google Scholar] [CrossRef]
- Warwar, R.E.; Bullock, J.D.; Ballal, D.R.; Ballal, R.D. Mechanisms of Orbital Floor Fractures: A Clinical, Experimental, and Theoretical Study. Ophthal. Plast. Reconstr. Surg. 2000, 16, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kersten, R.C.; Vagefi, M.R.; Bartley, G.B. Orbital “Blowout” Fractures: Time for a New Paradigm. Ophthalmology 2018, 125, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Poeschl, P.W.; Baumann, A.; Dorner, G.; Russmueller, G.; Seemann, R.; Fabian, F.; Ewers, R. Functional Outcome after Surgical Treatment of Orbital Floor Fractures. Clin. Oral Investig. 2012, 16, 1297–1303. [Google Scholar] [CrossRef]
- Waite, P.D.; Carr, D.D. The Transconjunctival Approach for Treating Orbital Trauma. J. Oral Maxillofac. Surg. 1991, 49, 499–503. [Google Scholar] [CrossRef]
- Baumann, A.; Ewers, R. Use of the Preseptal Transconjunctival Approach in Orbit Reconstruction Surgery. J. Oral Maxillofac. Surg. 2001, 59, 287–291. [Google Scholar] [CrossRef]
- Esmail, M.E.K.; Ibrahiem, M.F.K.; Abdallah, R.M.A.; Elshafei, A.M.K.; Gawdat, T.I. Resorbable Polylactic Acid Polymer Plates in Repair of Blow-Out Orbital Floor Fractures. Eur. J. Ophthalmol. 2021, 31, 1384–1390. [Google Scholar] [CrossRef]
- Eppley, B.L.; Sadove, A.M.; Havlik, R.J. Resorbable Plate Fixation in Pediatric Craniofacial Surgery. Plast. Amp Reconstr. Surg. 1997, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, J.A.; Wasserman, B.; Kraut, R. Use of Vicryl (Polyglactin-910) Mesh Implant for Repair of Orbital Floor Fracture Causing Diplopia: A Study of 28 Patients over 5 Years. Ophthal. Plast. Reconstr. Surg. 1993, 9, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, B.E.; Acar, C.; Kandzia, C.; Jochens, A.; Wiltfang, J.; Becker, S.T. Reconstruction of the Orbital Floor with Polydioxanone: A Long-Term Clinical Survey of up to 12 Years. Br. J. Oral Maxillofac. Surg. 2015, 53, 736–740. [Google Scholar] [CrossRef]
- Becker, S.T.; Terheyden, H.; Fabel, M.; Kandzia, C.; Möller, B.; Wiltfang, J. Comparison of Collagen Membranes and Polydioxanone for Reconstruction of the Orbital Floor After Fractures. J. Craniofac. Surg. 2010, 21, 1066–1068. [Google Scholar] [CrossRef]
- Gierloff, M.; Seeck, N.G.K.; Springer, I.; Becker, S.; Kandzia, C.; Wiltfang, J. Orbital Floor Reconstruction with Resorbable Polydioxanone Implants. J. Craniofac. Surg. 2012, 23, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Lizuka, T.; Mikkonen, P.; Paukku, P.; Lindqvist, C. Reconstruction of Orbital Floor with Polydioxanone Plate. Int. J. Oral Maxillofac. Surg. 1991, 20, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Burggasser, G.; Gauss, N.; Ewers, R. Orbital Floor Reconstruction with an Alloplastic Resorbable Polydioxanone Sheet. Int. J. Oral Maxillofac. Surg. 2002, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Prowse, S.J.B.; Hold, P.M.; Gilmour, R.F.; Pratap, U.; Mah, E.; Kimble, F.W. Orbital Floor Reconstruction: A Case for Silicone. A 12 Year Experience. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Klisovic, D.D.; Katz, S.E.; Lubow, M. The Wayward Implant: Orbital Silicone Plate Extrusion Associated with Squamous Epithelial Downgrowth and Infection. Orbit 2002, 21, 149–154. [Google Scholar] [CrossRef]
- Kohyama, K.; Arisawa, K.; Arisawa, Y.; Morishima, Y. Symptomatic Cystic Lesions as Late Post-Operative Complications of Silicone Implantation for Orbital Wall Fracture Reconstruction: A Long-Term Follow-Up Study. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 344–350. [Google Scholar] [CrossRef]
- Vichitvejpaisal, P.; Dalvin, L.A.; Lally, S.E.; Shields, C.L. Delayed Implant Infection with Cutibacterium acnes (Propionibacterium acnes ) 30 Years after Silicone Sheet Orbital Floor Implant. Orbit 2020, 39, 139–142. [Google Scholar] [CrossRef]
- Marella, V.G.; Rohit; Khetrapal, P.; Gangasani, A.; Bhanot, R.; Uppal, A. Titanium Mesh versus Medpor Implant in Orbital Floor Reconstructions: A Comparative Study. J. Pharm. Bioallied Sci. 2021, 13, S76–S79. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Saba, E.S.; Gupta, N.; Hendricks, T.M.; Singh, D.J. Alloplastic Reconstruction of Orbital Floor Fractures: A Systematic Review and Pooled Outcomes Analysis. Eur. J. Plast. Surg. 2020, 43, 109–116. [Google Scholar] [CrossRef]
- Suller, A.L.; Parikh, R.N.; Zhao, J.; Mahoney, N.R.; Campbell, A.A.; Siadati, S.; Eberhart, C.G.; Fu, R. Chocolate Cysts Associated With Porous Polyethylene Orbital Implants. Ophthal. Plast. Reconstr. Surg. 2021, 37, e75–e80. [Google Scholar] [CrossRef]
- Özkaya, N.K.; Erçöçen, A. Reconstruction of Orbital Floor Fractures Using a Porous Polyethylene Implant: Outcomes in the Early, Intermediate and Late Postoperative Periods. ENT Updat. 2020, 10, 321–325. [Google Scholar] [CrossRef]
- Pang, S.S.Y.; Fang, C.; Chan, J.Y.W. Application of Three-Dimensional Printing Technology in Orbital Floor Fracture Reconstruction. Trauma Case Rep. 2018, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.Y.; Merbs, S.L.; Grant, M.P.; Mahoney, N.R. Orbital Fracture Repair Outcomes with Preformed Titanium Mesh Implants and Comparison to Porous Polyethylene Coated Titanium Sheets. J. Cranio-Maxillofac. Surg. 2017, 45, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Downes, R.N.; Vardy, S.; Tanner, K.E.; Bonfield, W. Hydroxyapatite-Polyethylene Composite in Orbital Surgery. In Bioceramics; Elsevier: Amsterdam, The Netherlands, 1991; pp. 239–246. ISBN 978-0-7506-0269-3. [Google Scholar]
- Guillaume, O.; Geven, M.A.; Varjas, V.; Varga, P.; Gehweiler, D.; Stadelmann, V.A.; Smidt, T.; Zeiter, S.; Sprecher, C.; Bos, R.R.M.; et al. Orbital Floor Repair Using Patient Specific Osteoinductive Implant Made by Stereolithography. Biomaterials 2020, 233, 119721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bi, X.; Zhou, H.; Deng, Y.; Sun, J.; Xiao, C.; Gu, P.; Fan, X. Repair of Orbital Bone Defects in Canines Using Grafts of Enriched Autologous Bone Marrow Stromal Cells. J. Transl. Med. 2014, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- AL-Hamoudi, F.; Rehman, H.U.; Almoshawah, Y.A.; Talari, A.C.S.; Chaudhry, A.A.; Reilly, G.C.; Rehman, I.U. Bioactive Composite for Orbital Floor Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 10333. [Google Scholar] [CrossRef] [PubMed]
- Sarfaraz, S.; Khan, A.; Hameed, F.; Arshad, A.; Mutahir, Z.; Zeeshan, R.; Ijaz, K.; Chaudhry, A.A.; Khalid, H.; Rehman, I.; et al. Osteogenic and Antibacterial Scaffolds of Silk Fibroin/Ce-Doped ZnO for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1205–1216. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Moshfeghi, D.M.; Moshfeghi, A.A.; Finger, P.T. Enucleation. Surv. Ophthalmol. 2000, 44, 277–301. [Google Scholar] [CrossRef]
- Sokoya, M.; Cohn, J.; Kohlert, S.; Lee, T.; Kadakia, S.; Ducic, Y. Considerations in Orbital Exenteration. Semin. Plast. Surg. 2019, 33, 103–105. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Yang, X.; Fan, X.-L. The Evolution of Orbital Implants and Current Breakthroughs in Material Design, Selection, Characterization, and Clinical Use. Front. Bioeng. Biotechnol. 2022, 9, 800998. [Google Scholar] [CrossRef] [PubMed]
- Agahan, A.; Tan, A. Use of Hollow Polymethylmethacrylate as an Orbital Implant. Philipp. J. Ophthalmol. 2004, 29, 21–25. [Google Scholar]
- Groth, M.J.; Bhatnagar, A.; Clearihue, W.J.; Goldberg, R.A.; Douglas, R.S. Long-Term Efficacy of Biomodeled Polymethyl Methacrylate Implants for Orbitofacial Defects. Arch. Facial Plast. Surg. 2006, 8, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.; Aldoais, T.; Kaliki, S. Primary Orbital Polymethylmethacrylate Implant Following Primary Enucleation for Retinoblastoma: A Study of 321 Cases. Orbit 2021, 40, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Pine, K.R.; De Silva, K.; Zhang, F.; Yeoman, J.; Jacobs, R. Towards Improving the Biocompatibility of Prosthetic Eyes. Heliyon 2021, 7, e06234. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, L.; Boss, J.; Shah, C.T.; Droste, P.J.; Hassan, A.S. Alphasphere as a Successful Ocular Implant in Primary Enucleation and Secondary Orbital Implant Exchange. Orbit 2013, 32, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Jakobiec, F.A.; De Castro, D.K.; Mendoza, P.R.; Fay, A. Extruded, Partially Disintegrated, Poly-HEMA Orbital Implant (AlphaSphere). Ophthal. Plast. Reconstr. Surg. 2014, 30, e86–e91. [Google Scholar] [CrossRef] [PubMed]
- Neimkin, M.G.; Reggie, S.; Holds, J.B. Proptosis and Anterior Dislocation as a Late Noninflammatory Complication of Failure of Tissue Integration in the Alphasphere Implant. Ophthal. Plast. Reconstr. Surg. 2017, 33, S173–S175. [Google Scholar] [CrossRef]
- Yang, J.W.; Choi, J.; Lee, S.G.; Kim, D.S. Antibacterial Properties of Artificial Eyes Containing Nano-Sized Particle Silver. Orbit 2011, 30, 77–81. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, S.; Miola, M.; Perero, S.; Verné, E.; Coggiola, A.; Dolcino, D.; Ferraris, M. Novel Antibacterial Ocular Prostheses: Proof of Concept and Physico-Chemical Characterization. Mater. Sci. Eng. C 2016, 60, 467–474. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Wang, C.; Yao, K.; Gou, Z. Copper-Containing Mesoporous Bioactive Glass Coatings on Orbital Implants for Improving Drug Delivery Capacity and Antibacterial Activity. Biotechnol. Lett. 2014, 36, 961–968. [Google Scholar] [CrossRef] [PubMed]
- DeParis, S.W.; Zhu, A.Y.; Majumdar, S.; Tian, J.; Elisseeff, J.; Jun, A.S.; Mahoney, N.R. Effects of Collagen Crosslinking on Porcine and Human Tarsal Plate. BMC Ophthalmol. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; O’Connor, A.J.; Wood, J.; Casson, R.; Selva, D. Tissue Engineering in Ophthalmology: Implications for Eyelid Reconstruction. Ophthal. Plast. Reconstr. Surg. 2017, 33, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Pham, D.T.; O’Connor, A.J.; Wood, J.; Casson, R.; Selva, D.; Costi, J.J. The Biomechanics of Eyelid Tarsus Tissue. J. Biomech. 2015, 48, 3455–3459. [Google Scholar] [CrossRef] [PubMed]
- Ugradar, S.; Le, A.; Lesgart, M.; Goldberg, R.A.; Rootman, D.; Demer, J.L. Biomechanical and Morphologic Effects of Collagen Cross-Linking in Human Tarsus. Transl. Vis. Sci. Technol. 2019, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Suzuki, S.; Sabat, N.; Rayner, C.L.; Harkin, D.G.; Chirila, T.V. Further Investigations on the Crosslinking of Tarsal Collagen as a Treatment for Eyelid Laxity: Optimizing the Procedure in Animal Tissue. Ophthal. Plast. Reconstr. Surg. 2019, 35, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Makuloluwa, A.K.; Hamill, K.J.; Rauz, S.; Bosworth, L.; Haneef, A.; Romano, V.; Williams, R.L.; Dartt, D.A.; Kaye, S.B. The Conjunctival Extracellular Matrix, Related Disorders and Development of Substrates for Conjunctival Restoration. Ocul. Surf. 2023, 28, 322–335. [Google Scholar] [CrossRef]
- Eidet, J.; Dartt, D.; Utheim, T. Concise Review: Comparison of Culture Membranes Used for Tissue Engineered Conjunctival Epithelial Equivalents. J. Funct. Biomater. 2015, 6, 1064–1084. [Google Scholar] [CrossRef]
- Swamynathan, S.K.; Wells, A. Conjunctival Goblet Cells: Ocular Surface Functions, Disorders That Affect Them, and the Potential for Their Regeneration. Ocul. Surf. 2020, 18, 19–26. [Google Scholar] [CrossRef]
- Dietrich, J.; Garreis, F.; Paulsen, F. Pathophysiology of Meibomian Glands—An Overview. Ocul. Immunol. Inflamm. 2021, 29, 803–810. [Google Scholar] [CrossRef]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian Gland Disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Fujioka, J.K.; Goodyear, E.; Tran, S.D. Polymers and Biomaterials for Posterior Lamella of the Eyelid and the Lacrimal System. Polymers 2024, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fu, R.; Ji, Q.; Liu, C.; Yang, J.; Yin, X.; Oranges, C.M.; Li, Q.; Huang, R.-L. Surgical Strategies for Eyelid Defect Reconstruction: A Review on Principles and Techniques. Ophthalmol. Ther. 2022, 11, 1383–1408. [Google Scholar] [CrossRef] [PubMed]
- Vaca, E.E.; Surek, C.; Klosowiak, J.; Dumanian, G.A.; Alghoul, M.S. Neurotized Free Platysma Flap for Functional Eyelid Reconstruction: A Cadaveric Study of Anatomical Feasibility. Plast. Reconstr. Surg. 2020, 145, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.M.; Vahdani, K. Lower Eyelid Reconstruction: A New Classification Incorporating the Vertical Dimension. Plast. Reconstr. Surg. 2020, 145, 877e–878e. [Google Scholar] [CrossRef] [PubMed]
- Segal, K.L.; Nelson, C.C. Periocular Reconstruction. Facial Plast. Surg. Clin. N. Am. 2019, 27, 105–118. [Google Scholar] [CrossRef]
- Fin, A.; De Biasio, F.; Lanzetta, P.; Mura, S.; Tarantini, A.; Parodi, P.C. Posterior Lamellar Reconstruction: A Comprehensive Review of the Literature. Orbit 2019, 38, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lewis, K.; Alghoul, M.S. Comparison of Efficacy and Complications Among Various Spacer Grafts in the Treatment of Lower Eyelid Retraction: A Systematic Review. Aesthet. Surg. J. 2017, 37, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.; Krakauer, M.; Nunery, W.R.; Aakalu, V.K. Advancements in the Repair of Large Upper Eyelid Defects: A 10-Year Review. Orbit 2021, 40, 470–480. [Google Scholar] [CrossRef]
- Tenland, K.; Berggren, J.; Engelsberg, K.; Bohman, E.; Dahlstrand, U.; Castelo, N.; Lindstedt, S.; Sheikh, R.; Malmsjö, M. Successful Free Bilamellar Eyelid Grafts for the Repair of Upper and Lower Eyelid Defects in Patients and Laser Speckle Contrast Imaging of Revascularization. Ophthal. Plast. Reconstr. Surg. 2021, 37, 168–172. [Google Scholar] [CrossRef]
- Pham, C.M.; Heinze, K.D.; Mendes-Rufino-Uehara, M.; Setabutr, P. Single-Stage Repair of Large Full Thickness Lower Eyelid Defects Using Free Tarsoconjunctival Graft and Transposition Flap: Experience and Outcomes. Orbit 2022, 41, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ominato, J.; Oyama, T.; Cho, H.; Shiozaki, N.; Eguchi, K.; Fukuchi, T. Evaluation of the Postoperative Course of East Asian Eyelid Reconstruction with Free Tarsoconjunctival Graft Transplantation: A Japanese Single-Centre Retrospective Study. JPRAS Open 2022, 33, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Hishmi, A.M.; Koch, K.R.; Matthaei, M.; Bölke, E.; Cursiefen, C.; Heindl, L.M. Modified Hughes Procedure for Reconstruction of Large Full-Thickness Lower Eyelid Defects Following Tumor Resection. Eur. J. Med. Res. 2016, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, J.; Ferguson, R.; Ng, S.G.J. Eyelid Reconstruction Using the “Hughes” Tarsoconjunctival Advancement Flap: Long-Term Outcomes in 122 Consecutive Cases over a 13-Year Period. Orbit 2017, 36, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, D.; Mehmet, B.; Ceyda, B.; Sibel, Ö. Management of the Large Upper Eyelid Defects with Cutler-Beard Flap. J. Ophthalmol. 2014, 2014, 424546. [Google Scholar] [CrossRef] [PubMed]
- Toft, P.B. Reconstruction of Large Upper Eyelid Defects with a Free Tarsal Plate Graft and a Myocutaneous Pedicle Flap plus a Free Skin Graft. Orbit 2016, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.J.; Jamell, G.A. Complications of Tarsoconjunctival Grafts. Ophthal. Plast. Reconstr. Surg. 1996, 12, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yazici, B.; Ozturker, C.; Cetin Efe, A. Reconstruction of Large Upper Eyelid Defects With Bilobed Flap and Tarsoconjunctival Graft. Ophthal. Plast. Reconstr. Surg. 2020, 36, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Rajak, S.N.; Malhotra, R.; Selva, D. The ‘over-the-Top’ Modified Cutler–Beard Procedure for Complete Upper Eyelid Defect Reconstruction. Orbit 2019, 38, 133–136. [Google Scholar] [CrossRef]
- Yan, Y.; Ji, Q.; Fu, R.; Liu, C.; Yang, J.; Yin, X.; Li, Q.; Huang, R. Biomaterials and Tissue Engineering Strategies for Posterior Lamellar Eyelid Reconstruction: Replacement or Regeneration? Bioeng. Transl. Med. 2023, 8, e10497. [Google Scholar] [CrossRef]
- Grixti, A.; Malhotra, R. Oral Mucosa Grafting in Periorbital Reconstruction. Orbit 2018, 37, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ogi, H.; Yanagibayashi, S.; Yoshida, R.; Takikawa, M.; Nishijima, A.; Kiyosawa, T. Eyelid Reconstruction Using Oral Mucosa and Ear Cartilage Strips as Sandwich Grafting. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1301. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.; Bertelmann, E. Oral Mucosal Grafts: Old Technique in New Light. Ophthalmic Res. 2013, 50, 91–98. [Google Scholar] [CrossRef]
- Yue, H.; Tian, L.; Bi, Y.; Qian, J. Hard Palate Mucoperiosteal Transplantation for Defects of the Upper Eyelid: A Pilot Study and Evaluation. Ophthal. Plast. Reconstr. Surg. 2020, 36, 469–474. [Google Scholar] [CrossRef]
- Weinberg, D.A.; Tham, V.; Hardin, N.; Antley, C.; Cohen, A.J.; Hunt, K.; Glasgow, B.J.; Baylis, H.I.; Shorr, N.; Goldberg, R.A. Eyelid Mucous Membrane Grafts: A Histologic Study of Hard Palate, Nasal Turbinate, and Buccal Mucosal Grafts. Ophthal. Plast. Reconstr. Surg. 2007, 23, 211–216. [Google Scholar] [CrossRef]
- Keçeci, Y.; Bali, Z.U.; Ahmedov, A.; Yoleri, L. Angular Artery Island Flap for Eyelid Defect Reconstruction. J. Plast. Surg. Hand Surg. 2020, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Eser, C.; Kesiktaş, E.; Gencel, E.; Tabakan, İ.; Yavuz, M. Total or Near-Total Lower Eyelid Defect Reconstruction Using Malar Myocutaneous Bridge and Nasojugal Flaps and Septal Chondromucosal Graft. Ophthal. Plast. Reconstr. Surg. 2016, 32, 225–229. [Google Scholar] [CrossRef]
- Pushker, N.; Modaboyina, S.; Meel, R.; Agrawal, S. Auricular Skin-Cartilage Sandwich Graft Technique for Full-Thickness Eyelid Reconstruction. Indian J. Ophthalmol. 2022, 70, 1404. [Google Scholar] [CrossRef]
- Suga, H.; Ozaki, M.; Narita, K.; Kurita, M.; Shiraishi, T.; Ohura, N.; Takushima, A.; Harii, K. Comparison of Nasal Septum and Ear Cartilage as a Graft for Lower Eyelid Reconstruction. J. Craniofac. Surg. 2016, 27, 305–307. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Witt, J.; Borrelli, M.; Mertsch, S.; Geerling, G.; Spaniol, K.; Schrader, S. Evaluation of Plastic-Compressed Collagen for Conjunctival Repair in a Rabbit Model. Tissue Eng. Part A 2019, 25, 1084–1095. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Q.; Guo, Q.; Chae, J.; Fan, X.; Elisseeff, J.H.; Grant, M.P. Vitrified Collagen-Based Conjunctival Equivalent for Ocular Surface Reconstruction. Biomaterials 2014, 35, 7398–7406. [Google Scholar] [CrossRef]
- Drechsler, C.C.; Kunze, A.; Kureshi, A.; Grobe, G.; Reichl, S.; Geerling, G.; Daniels, J.T.; Schrader, S. Development of a Conjunctival Tissue Substitute on the Basis of Plastic Compressed Collagen: Plastic Compressed Collagen Gels as a Conjunctival Tissue Substitute. J. Tissue Eng. Regen. Med. 2017, 11, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; O’Connor, A.J.; Milne, I.; Biswas, D.; Casson, R.; Wood, J.; Selva, D. Development of Macroporous Chitosan Scaffolds for Eyelid Tarsus Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Feng, X.; Zheng, H.; Feng, Z.; Fu, Z.; Gao, C.; Ye, J. A Tarsus Construct of a Novel Branched Polyethylene with Good Elasticity for Eyelid Reconstruction in Vivo. Regen. Biomater. 2020, 7, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Doherty, K.G.; Hsuan, J.D.; Cray, S.P.; D’Sa, R.A.; Pineda Molina, C.; Badylak, S.F.; Williams, R.L. Material Characterisation and Stratification of Conjunctival Epithelial Cells on Electrospun Poly(ε-Caprolactone) Fibres Loaded with Decellularised Tissue Matrices. Pharmaceutics 2021, 13, 318. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, W.; Hu, Y.; Chen, J.; Shao, C.; Fan, X.; Fu, Y. Electrospun Collagen/Poly(L-Lactic Acid-co-ε-caprolactone) Scaffolds for Conjunctival Tissue Engineering. Exp. Ther. Med. 2017, 14, 4141–4147. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, S.; Yu, F.; Gong, D.; Lin, J.; Yao, Q.; Fu, Y. Insight into Levofloxacin Loaded Biocompatible Electrospun Scaffolds for Their Potential as Conjunctival Substitutes. Carbohydr. Polym. 2021, 269, 118341. [Google Scholar] [CrossRef]
- Mavrikakis, I.; Francis, N.; Poitelea, C.; Parkin, B.; Brittain, P.; Olver, J. Medpor® Lower Eyelid Spacer: Does It Biointegrate? Orbit 2009, 28, 58–62. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Liu, X.; Zhang, H. Applications and Recent Developments of Hydrogels in Ophthalmology. ACS Biomater. Sci. Eng. 2023, 9, 5968–5984. [Google Scholar] [CrossRef]
- Nguyen, J.; Werner, L. Intraocular Lenses for Cataract Surgery. In Webvision: The Organization of the Retina and Visual System; University of Utah Health Sciences Center: Utah, Idaho, 2017; ISBN NBK481726. [Google Scholar]
- Vacalebre, M.; Frison, R.; Corsaro, C.; Neri, F.; Santoro, A.; Conoci, S.; Anastasi, E.; Curatolo, M.C.; Fazio, E. Current State of the Art and Next Generation of Materials for a Customized IntraOcular Lens According to a Patient-Specific Eye Power. Polymers 2023, 15, 1590. [Google Scholar] [CrossRef]
- Xu, J.-W.; Li, H.-N.; Hu, D.-F.; Zhang, X.-B.; Wang, W.; Ji, J.; Xu, Z.-K.; Yao, K. Intraocular Lens with Mussel-Inspired Coating for Preventing Posterior Capsule Opacification via Photothermal Effect. ACS Appl. Bio. Mater. 2021, 4, 3579–3586. [Google Scholar] [CrossRef]
- Khader, A.; Fahoum, A.A. INTRAOCULAR LENS BIOMATERIALS FOR CATARACT SURGERY. Semicond. Optoelectron. 2023, 42, 492–501. [Google Scholar]
- De Giacinto, C.; Porrelli, D.; Turco, G.; Pastore, M.R.; D’Aloisio, R.; Tognetto, D. Surface Properties of Commercially Available Hydrophobic Acrylic Intraocular Lenses: Comparative Study. J. Cataract. Refract. Surg. 2019, 45, 1330–1334. [Google Scholar] [CrossRef]
- Özyol, P.; Özyol, E.; Karel, F. Biocompatibility of Intraocular Lenses. Türk Oftalmol. Derg. 2017, 47, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, X.; Tang, J.; Han, Y.; Lin, Q. Drug-Eluting Hydrophilic Coating Modification of Intraocular Lens via Facile Dopamine Self-Polymerization for Posterior Capsular Opacification Prevention. ACS Biomater. Sci. Eng. 2021, 7, 1065–1073. [Google Scholar] [CrossRef]
- Yang, C.-J.; Huang, W.-L.; Yang, Y.; Kuan, C.-H.; Tseng, C.-L.; Wang, T.-W. Zwitterionic Modified and Freeze-Thaw Reinforced Foldable Hydrogel as Intraocular Lens for Posterior Capsule Opacification Prevention. Biomaterials 2024, 309, 122593. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.A.; Piller, I.; Henkel, F.; Fromme, R.; Lieleg, O. Multifunctional Glycoprotein Coatings Improve the Surface Properties of Highly Oxygen Permeable Contact Lenses. Biomater. Adv. 2023, 145, 213233. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.A.; Wittmann, B.; Fromme, R.; Lieleg, O. Highly Transparent Covalent Mucin Coatings Improve the Wettability and Tribology of Hydrophobic Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 28024–28033. [Google Scholar] [CrossRef]
- Naik, K.; Du Toit, L.C.; Ally, N.; Choonara, Y.E. Advances in Polysaccharide- and Synthetic Polymer-Based Vitreous Substitutes. Pharmaceutics 2023, 15, 566. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.E.G.; Cui, H.; Ballios, B.G.; Ing, S.; Yan, P.; Wolfer, J.; Wright, T.; Dang, M.; Gan, N.Y.; Cooke, M.J.; et al. Stable Oxime-Crosslinked Hyaluronan-Based Hydrogel as a Biomimetic Vitreous Substitute. Biomaterials 2021, 271, 120750. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V.; Ortin-Martinez, A.; Tsai, E.L.S.; Amin, A.N.; Wallace, V.; Shoichet, M.S. Controlled Release Strategy Designed for Intravitreal Protein Delivery to the Retina. J. Control. Release 2019, 293, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Han, X.; Zhong, Y.; Kam, H.T.; Qiao, D.; Chen, Z.; Chan, K.W.Y.; Chong, W.P.; Chen, J. Regulatory T Cell Intravitreal Delivery Using Hyaluronan Methylcellulose Hydrogel Improves Therapeutic Efficacy in Experimental Autoimmune Uveitis. Biomater. Adv. 2023, 151, 213496. [Google Scholar] [CrossRef] [PubMed]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of Silk-Hyaluronic Acid Composite Hydrogels towards Vitreous Humor Substitutes. Biomaterials 2020, 233, 119729. [Google Scholar] [CrossRef]
- Choi, G.; An, S.H.; Choi, J.-W.; Rho, M.S.; Park, W.C.; Jeong, W.J.; Cha, H.J. Injectable Alginate-Based in Situ Self-Healable Transparent Hydrogel as a Vitreous Substitute with a Tamponading Function. Biomaterials 2024, 305, 122459. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid–Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Kiani Shahvandi, M.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D Printed Hydrogels for Ocular Wound Healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef]
- Nosrati, H.; Ashrafi-Dehkordi, K.; Alizadeh, Z.; Sanami, S.; Banitalebi-Dehkordi, M. Biopolymer-Based Scaffolds for Corneal Stromal Regeneration: A Review. Polym. Med. 2020, 50, 57–64. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber Reinforced GelMA Hydrogel to Induce the Regeneration of Corneal Stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Du, X.; Li, W.; Wang, Q.; He, D.; Yuan, J. Natural Polymer-Derived Photocurable Bioadhesive Hydrogels for Sutureless Keratoplasty. Bioact. Mater. 2022, 8, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, R.; Liu, C.; Fang, H.; Yang, W.; Wang, Y.; Xian, Y.; Zhang, K.; He, Y.; Zhou, X. A “T.E.S.T.” Hydrogel Bioadhesive Assisted by Corneal Cross-Linking for in Situ Sutureless Corneal Repair. Bioact. Mater. 2023, 25, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Shirzaei Sani, E.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless Repair of Corneal Injuries Using Naturally Derived Bioadhesive Hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef]
- Khalil, I.A.; Saleh, B.; Ibrahim, D.M.; Jumelle, C.; Yung, A.; Dana, R.; Annabi, N. Ciprofloxacin-Loaded Bioadhesive Hydrogels for Ocular Applications. Biomater. Sci. 2020, 8, 5196–5209. [Google Scholar] [CrossRef]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-Based Bioinks for Hard Tissue Engineering Applications: A Comprehensive Review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in Vitro Characterization of Cross-Linked Collagen–Gelatin Hydrogel Using EDC/NHS for Corneal Tissue Engineering Applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef]
- Islam, M.M.; Chivu, A.; AbuSamra, D.B.; Saha, A.; Chowdhuri, S.; Pramanik, B.; Dohlman, C.H.; Das, D.; Argüeso, P.; Rajaiya, J.; et al. Crosslinker-Free Collagen Gelation for Corneal Regeneration. Sci. Rep. 2022, 12, 9108. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Rafat, M.; Moustardas, P.; Mukwaya, A.; Tabe, S.; Bellisario, M.; Peebo, B.; Lagali, N. A Double-Crosslinked Nanocellulose-Reinforced Dexamethasone-Loaded Collagen Hydrogel for Corneal Application and Sustained Anti-Inflammatory Activity. Acta Biomater. 2023, 172, 234–248. [Google Scholar] [CrossRef]
- Tummala, G.K.; Lopes, V.R.; Mihranyan, A.; Ferraz, N. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Qu, M.; Backman, L.J.; Zhang, A.; Liu, H.; Zhang, X.; Zhou, Q.; Danielson, P. Sustained Release of TPCA-1 from Silk Fibroin Hydrogels Preserves Keratocyte Phenotype and Promotes Corneal Regeneration by Inhibiting Interleukin-1 β Signaling. Adv. Healthc. Mater. 2020, 9, 2000591. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Hu, Y.; Shi, H.; Bao, Z.; Wu, Y.; Jiang, J.; Li, X. Biofunctional Peptide-Click PEG-Based Hydrogels as 3D Cell Scaffolds for Corneal Epithelial Regeneration. J. Mater. Chem. B 2022, 10, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Lace, R.; Duffy, G.L.; Gallagher, A.G.; Doherty, K.G.; Maklad, O.; Wellings, D.A.; Williams, R.L. Characterization of Tunable Poly-ε-Lysine-Based Hydrogels for Corneal Tissue Engineering. Macromol. Biosci. 2021, 21, 2100036. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Lace, R.; Carserides, C.; Gallagher, A.G.; Wellings, D.A.; Williams, R.L.; Levis, H.J. Poly-ε-Lysine Based Hydrogels as Synthetic Substrates for the Expansion of Corneal Endothelial Cells for Transplantation. J. Mater. Sci. Mater. Med. 2019, 30, 102. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Melin, M.; Jiang, K.; Trossbach, M.; Badadamath, B.; Langer, K.; Winkeljann, B.; Lieleg, O.; Hong, J.; Joensson, H.N.; et al. Immune-Modulating Mucin Hydrogel Microdroplets for the Encapsulation of Cell and Microtissue. Adv. Funct. Mater. 2021, 31, 2105967. [Google Scholar] [CrossRef]
- Islam, F.; Wong, S.Y.; Li, X.; Arafat, M.T. Pectin and Mucin Modified Cellulose-Based Superabsorbent Hydrogel for Controlled Curcumin Release. Cellulose 2022, 29, 5207–5222. [Google Scholar] [CrossRef]
- Barik, D.; Kundu, K.; Dash, M. Montmorillonite Stabilized Chitosan- Co -Mucin Hydrogel for Tissue Engineering Applications. RSC Adv. 2021, 11, 30329–30342. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Ahmad, H.; Lin, G.; Carbonneau, M.; Tran, S.D. Mesenchymal Stem Cell-Derived Exosomes in Ophthalmology: A Comprehensive Review. Pharmaceutics 2023, 15, 1167. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Carbonneau, M.; Marchand, M.; Tran, S.D. Advancements in Wearable and Implantable Intraocular Pressure Biosensors for Ophthalmology: A Comprehensive Review. Micromachines 2023, 14, 1915. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Garg, A.; Ahmad, M.Z.; Alqahtani, A.A.; Walbi, I.A.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Vichare, R.; Garner, I.; Paulson, R.J.; Tzekov, R.; Sahiner, N.; Panguluri, S.K.; Mohapatra, S.; Mohapatra, S.S.; Ayyala, R.; Sneed, K.B.; et al. Biofabrication of Chitosan-Based Nanomedicines and Its Potential Use for Translational Ophthalmic Applications. Appl. Sci. 2020, 10, 4189. [Google Scholar] [CrossRef]
- Ameeduzzafar; Ali, J.; Bhatnagar, A.; Kumar, N.; Ali, A. Chitosan Nanoparticles Amplify the Ocular Hypotensive Effect of Cateolol in Rabbits. Int. J. Biol. Macromol. 2014, 65, 479–491. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/Hydroxyethyl Cellulose Inserts for Sustained-Release of Dorzolamide for Glaucoma Treatment: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2019, 570, 118662. [Google Scholar] [CrossRef]
- Akulo, K.A.; Adali, T.; Moyo, M.T.G.; Bodamyali, T. Intravitreal Injectable Hydrogels for Sustained Drug Delivery in Glaucoma Treatment and Therapy. Polymers 2022, 14, 2359. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Suzuki, S.; Shadforth, A.M.A.; McLenachan, S.; Zhang, D.; Chen, S.-C.; Walshe, J.; Lidgerwood, G.E.; Pébay, A.; Chirila, T.V.; Chen, F.K.; et al. Optimization of Silk Fibroin Membranes for Retinal Implantation. Mater. Sci. Eng. C 2019, 105, 110131. [Google Scholar] [CrossRef] [PubMed]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic Acid-Coated Chitosan Nanoparticles as Targeted-Carrier of Tamoxifen against MCF7 and TMX-Resistant MCF7 Cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Desai, A.R.; Maulvi, F.A.; Pandya, M.M.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Co-Delivery of Timolol and Hyaluronic Acid from Semi-Circular Ring-Implanted Contact Lenses for the Treatment of Glaucoma: in vitro and in vivo Evaluation. Biomater. Sci. 2018, 6, 1580–1591. [Google Scholar] [CrossRef]
- Egbu, R.; Brocchini, S.; Khaw, P.T.; Awwad, S. Antibody Loaded Collapsible Hyaluronic Acid Hydrogels for Intraocular Delivery. Eur. J. Pharm. Biopharm. 2018, 124, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Anand, A.; Huang, C.-C.; Lai, J.-Y. Unveiling the Power of Gabapentin-Loaded Nanoceria with Multiple Therapeutic Capabilities for the Treatment of Dry Eye Disease. ACS Nano 2023, 17, 25118–25135. [Google Scholar] [CrossRef] [PubMed]
- Pinho Tavares, F.D.; Fernandes, R.S.; Bernardes, T.F.; Bonfioli, A.A.; Carneiro Soares, E.J. Dry Eye Disease. Semin. Ophthalmol. 2010, 25, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Chen, W.T.; Chu-Bédard, Y.-K.; Patel, G.; Tran, S.D. Management of Sjogren’s Dry Eye Disease—Advances in Ocular Drug Delivery Offering a New Hope. Pharmaceutics 2022, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, L.; Shi, H.; Xu, C.; Liu, M.; Li, Q.; Zheng, L.; Chi, H.; Wang, M.; Liu, Z.; et al. Effectiveness of an Ocular Adhesive Polyhedral Oligomeric Silsesquioxane Hybrid Thermo-Responsive FK506 Hydrogel in a Murine Model of Dry Eye. Bioact. Mater. 2022, 9, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Kim, H.T.; Koh, J.W. New Biodegradable Drug Delivery System for Patients with Dry Eye. Korean J. Ophthalmol. 2021, 35, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Long-Acting Mucoadhesive Thermogels for Improving Topical Treatments of Dry Eye Disease. Mater. Sci. Eng. C 2020, 115, 111095. [Google Scholar] [CrossRef] [PubMed]
- Nizami, A.A.; Gulani, A.C. Cataract. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, K.; Lee, G.; Lee, S.; Park, C.Y. Advances in Ophthalmic Drug Delivery Technology for Postoperative Management after Cataract Surgery. Expert Opin. Drug Deliv. 2022, 19, 945–964. [Google Scholar] [CrossRef] [PubMed]
- Ongkasin, K.; Masmoudi, Y.; Tassaing, T.; Le-Bourdon, G.; Badens, E. Supercritical Loading of Gatifloxacin into Hydrophobic Foldable Intraocular Lenses—Process Control and Optimization by Following in Situ CO2 Sorption and Polymer Swelling. Int. J. Pharm. 2020, 581, 119247. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, D.; Chen, W.; Chen, H.; Yang, C.; Li, X.; Yang, C.; Lin, H.; Chen, S.; Hu, N.; et al. Liquid-like Layer Coated Intraocular Lens for Posterior Capsular Opacification Prevention. Appl. Mater. Today 2021, 23, 100981. [Google Scholar] [CrossRef]
- Qie, J.; Wen, S.; Han, Y.; Liu, S.; Shen, L.; Chen, H.; Lin, Q. A Polydopamine-Based Photodynamic Coating on the Intraocular Lens Surface for Safer Posterior Capsule Opacification Conquering. Biomater. Sci. 2022, 10, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Song, Y.; Lim, H.; Lee, S.H.; Lee, H.K.; Lee, E.; Choi, B.G.; Lee, J.J.; Im, S.G.; Lee, K.G. Antibacterial Nanopillar Array for an Implantable Intraocular Lens. Adv. Healthc. Mater. 2020, 9, 2000447. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ma, Z.; Liu, J.; Yin, N.; Lei, S.; Zhang, X.; Li, X.; Zhang, Y.; Kong, J. Thermoresponsive GenisteinNLC-Dexamethasone-Moxifloxacin Multi Drug Delivery System in Lens Capsule Bag to Prevent Complications after Cataract Surgery. Sci. Rep. 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, Y.; Sun, J.; Deng, Y.; Lu, Y. Improved Treatment on Ocular Inflammation with Rationally Designed Thermoresponsive Nanocomposite Formulation. Adv. Ther. 2021, 4, 2100088. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chang, Y.-F.; Ko, Y.-C.; Liu, C.J. Development of a Dual Delivery of Levofloxacin and Prednisolone Acetate via PLGA Nanoparticles/Thermosensitive Chitosan-Based Hydrogel for Postoperative Management: An in-Vitro and Ex-Vivo Study. Int. J. Biol. Macromol. 2021, 180, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Preparation, in Vitro Characterization and Pharmacokinetic Study of Coenzyme Q10 Long-Circulating Liposomes. Drug Res. 2018, 68, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, C.; Muhemaitia, P. Impediment of Selenite-Induced Cataract in Rats by Combinatorial Drug Laden Liposomal Preparation. Libyan J. Med. 2019, 14, 1548252. [Google Scholar] [CrossRef] [PubMed]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent Developments in Natural Biopolymer Based Drug Delivery Systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef]
- Syed, M.H.; Zahari, M.A.K.M.; Khan, M.M.R.; Beg, M.D.H.; Abdullah, N. An Overview on Recent Biomedical Applications of Biopolymers: Their Role in Drug Delivery Systems and Comparison of Major Systems. J. Drug Deliv. Sci. Technol. 2023, 80, 104121. [Google Scholar] [CrossRef]
- Burhan, A.M.; Klahan, B.; Cummins, W.; Andrés-Guerrero, V.; Byrne, M.E.; O’Reilly, N.J.; Chauhan, A.; Fitzhenry, L.; Hughes, H. Posterior Segment Ophthalmic Drug Delivery: Role of Muco-Adhesion with a Special Focus on Chitosan. Pharmaceutics 2021, 13, 1685. [Google Scholar] [CrossRef]
- Yan, R.; Xu, L.; Wang, Q.; Wu, Z.; Zhang, H.; Gan, L. Cyclosporine A Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Cationic Nanoparticles and Drug-Core Mucus Penetrating Nanoparticles. Mol. Pharm. 2021, 18, 4290–4298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Fang, L.; Cao, F. Multifunctional Carboxymethyl Chitosan Derivatives-Layered Double Hydroxide Hybrid Nanocomposites for Efficient Drug Delivery to the Posterior Segment of the Eye. Acta Biomater. 2020, 104, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan–Polycaprolactone Core–Shell Microparticles for Sustained Delivery of Bevacizumab. Mol. Pharm. 2020, 17, 2570–2584. [Google Scholar] [CrossRef]
- Savin, C.-L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan Grafted-Poly(Ethylene Glycol) Methacrylate Nanoparticles as Carrier for Controlled Release of Bevacizumab. Mater. Sci. Eng. C 2019, 98, 843–860. [Google Scholar] [CrossRef]
- Llabot, J.M.; Luis De Redin, I.; Agüeros, M.; Dávila Caballero, M.J.; Boiero, C.; Irache, J.M.; Allemandi, D. In Vitro Characterization of New Stabilizing Albumin Nanoparticles as a Potential Topical Drug Delivery System in the Treatment of Corneal Neovascularization (CNV). J. Drug Deliv. Sci. Technol. 2019, 52, 379–385. [Google Scholar] [CrossRef]
- Luis De Redín, I.; Boiero, C.; Recalde, S.; Agüeros, M.; Allemandi, D.; Llabot, J.M.; García-Layana, A.; Irache, J.M. In Vivo Effect of Bevacizumab-Loaded Albumin Nanoparticles in the Treatment of Corneal Neovascularization. Exp. Eye Res. 2019, 185, 107697. [Google Scholar] [CrossRef]
- Abdi, F.; Arkan, E.; Mansouri, K.; Shekarbeygi, Z.; Barzegari, E. Interactions of Bevacizumab with Chitosan Biopolymer Nanoparticles: Molecular Modeling and Spectroscopic Study. J. Mol. Liq. 2021, 339, 116655. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective Effect of Melatonin Loaded in Ethylcellulose Nanoparticles Applied Topically in a Retinal Degeneration Model in Rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Sarkar, G.; Saha, N.R.; Das, B.; Bhattacharyya, A.; Das, S.; Mishra, R.; Roy, I.; Chattoapadhyay, A.; Ghosh, S.K.; et al. Effect of Cellulose Nanocrystals on the Performance of Drug Loaded in Situ Gelling Thermo-Responsive Ophthalmic Formulations. Int. J. Biol. Macromol. 2019, 124, 235–245. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, W.; Zhang, R.; Shen, S.; Wu, M.; Zhong, S.; Zhang, Y.; Cui, X. Fabrication of Carboxymethyl Cellulose-Based Thermo-Sensitive Hydrogels and Inhibition of Corneal Neovascularization. Int. J. Biol. Macromol. 2024, 261, 129933. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody Biopolymer Conjugate in Retinal Disorders. Ther. Adv. Ophthalmol. 2021, 13, 251584142110277. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol Chitosan/Oxidized Hyaluronic Acid Hydrogel Film for Topical Ocular Delivery of Dexamethasone and Levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chang, Y.; Ko, Y.; Liu, C.J. Sustained Release of Levofloxacin from Thermosensitive Chitosan-based Hydrogel for the Treatment of Postoperative Endophthalmitis. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 8–13. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Oh, Y.; Hartsock, M.J.; Xia, S.; Kim, Y.-C.; Ding, Z.; Meng, T.; Eberhart, C.G.; Ensign, L.M.; et al. Controlled Release of Corticosteroid with Biodegradable Nanoparticles for Treating Experimental Autoimmune Uveitis. J. Control. Release 2019, 296, 68–80. [Google Scholar] [CrossRef]
- Guo, D.; Li, Q.; Sun, Y.; Guo, J.; Zhao, Q.; Yin, X.; Wei, H.; Wu, S.; Bi, H. Evaluation of Controlled-Release Triamcinolone Acetonide-Loaded mPEG-PLGA Nanoparticles in Treating Experimental Autoimmune Uveitis. Nanotechnology 2019, 30, 165702. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Girgis, G.N.; El-Dahan, M.S. Chitosan-Coated PLGA Nanoparticles for Enhanced Ocular Anti-Inflammatory Efficacy of Atorvastatin Calcium. Int. J. Nanomed. 2020, 15, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent Advances in Intraocular Sustained-Release Drug Delivery Devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef]
- Butt, F.; Devonport, H. Treatment of Non-Infectious Posterior Uveitis with Dexamethasone Intravitreal Implants in a Real-World Setting. Clin. Ophthalmol. 2023, 17, 601–611. [Google Scholar] [CrossRef]
- Berkenstock, M.K.; Mir, T.A.; Khan, I.R.; Burkholder, B.M.; Chaon, B.C.; Shifera, A.S.; Thorne, J.E. Effectiveness of the Dexamethasone Implant in Lieu of Oral Corticosteroids in Intermediate and Posterior Uveitis Requiring Immunosuppression. Ocul. Immunol. Inflamm. 2022, 30, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Yang, L.; Bai, F.; Liu, T.; Liu, X. Intravitreal Dexamethasone Implant for Noninfectious Uveitis in Chinese Patients. Int. Ophthalmol. 2022, 42, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Hanumunthadu, D. Inflammatory Eye Disease: An Overview of Clinical Presentation and Management. Clin. Med. 2022, 22, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ravi, P.R.; Mir, S.I.; Rawat, P.S. Optimization and in Vivo Evaluation of Triamcinolone Acetonide Loaded in Situ Gel Prepared Using Reacted Tamarind Seed Xyloglucan and Kappa-Carrageenan for Ocular Delivery. Int. J. Biol. Macromol. 2023, 233, 123533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Wang, Q.; Bai, J.; McAlinden, C.; Skiadaresi, E.; Zhang, J.; Pan, L.; Mei, C.; Zeng, Z.; et al. Hydrogel Eye Drops as a Non-Invasive Drug Carrier for Topical Enhanced Adalimumab Permeation and Highly Efficient Uveitis Treatment. Carbohydr. Polym. 2021, 253, 117216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, X.; Zhao, L.; Zhang, W.; Zhu, S.; Ma, J. Chitosan Nanoparticles to Enhance the Inhibitory Effect of Natamycin on Candida Albicans. J. Nanomater. 2021, 2021, 6644567. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, X.; Hanna, E.; Su, S.Y.; Moreno, A.; Gunn, B.; Frank, S.J.; Ferrarotto, R.; Ning, J.; Esmaeli, B. Orbital and Periocular Complications in Patients with Sinonasal Tumours with Orbital Invasion. Br. J. Ophthalmol. 2023, 108, 465–470. [Google Scholar] [CrossRef]
- Gushchina, M.B.; Tereshchenko, A.V.; Yuzhakova, N.S.; Gavrilova, N.A.; Trifanenkova, I.G.; Plakhotniy, M.A. Morphological Evaluation of Orbital Implants in Postenucleation Reconstruction (an Experimental Study). Vestn. Oftalmol. 2022, 138, 70. [Google Scholar] [CrossRef]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Das, S.; Saha, D.; Majumdar, S.; Giri, L. Imaging Methods for the Assessment of a Complex Hydrogel as an Ocular Drug Delivery System for Glaucoma Treatment: Opportunities and Challenges in Preclinical Evaluation. Mol. Pharm. 2022, 19, 733–748. [Google Scholar] [CrossRef]

| Polymer | Advantages | Disadvantages/Limitation | Applications | References |
|---|---|---|---|---|
| Gelatin methacrylate (GelMA) | Excellent biocompatibility, good mechanical stability, cell adhesive properties, MMP-degradable | May require modification for enhanced properties, cost |
| [128,129,130,131,132] |
| Collagen-based hydrogels | Natural component of the cornea, high biocompatibility, mimics corneal structure | Limited mechanical strength, potential for immunogenicity |
| [134,135,137] |
| Silk fibrin | Biocompatibility, rapid gelation, tunable degradability, transparency | Potential variability in degradation rate, source-dependent |
| [139] |
| Peptide-based, Poly-ε-lysine (pεK) | Mimics native ECM, porosity, transparency, swell-capability | Requires cross-linking for desired properties |
| [140,141,142] |
| Nanocellulose | Abundant, Biocompatible, High mechanical strength, Sustainable | Requires chemical modification for enhanced properties, Potential issues with long-term stability |
| [137,138] |
| Mucin | Abundant, biocompatible, natural component in the eye | Lack of transparency in some formulations, not widely studied for ocular use |
| [143,144,145] |
| Polymer | Advantages/Advancements | Disadvantages/Limitations | Applications | References |
|---|---|---|---|---|
| Chitosan |
|
|
| [143,180,181,182,191,192] |
| Human serum albumin nanoparticles |
|
|
| [183,184,185] |
| Cellulose and its derivatives |
|
|
| [187,188,189] |
| Phosphorylcholine polymer |
|
|
| [190] |
| Glycol chitosan-oxidized hyaluronic acid hydrogel |
|
|
| [191] |
| Poly(lactic-co-glycolic acid) (PLGA) nanoparticles |
|
|
| [193,194,195] |
| PLGA-based drug-eluting implants (ozurdex, dexycu) |
|
|
| [196,197,198,199,200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Khan, S.; Liao, Z.; Marchand, M.; Tran, S.D. Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems. Polymers 2024, 16, 1717. https://doi.org/10.3390/polym16121717
Wu KY, Khan S, Liao Z, Marchand M, Tran SD. Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems. Polymers. 2024; 16(12):1717. https://doi.org/10.3390/polym16121717
Chicago/Turabian StyleWu, Kevin Y., Sameer Khan, Zhuoying Liao, Michael Marchand, and Simon D. Tran. 2024. "Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems" Polymers 16, no. 12: 1717. https://doi.org/10.3390/polym16121717
APA StyleWu, K. Y., Khan, S., Liao, Z., Marchand, M., & Tran, S. D. (2024). Biopolymeric Innovations in Ophthalmic Surgery: Enhancing Devices and Drug Delivery Systems. Polymers, 16(12), 1717. https://doi.org/10.3390/polym16121717









