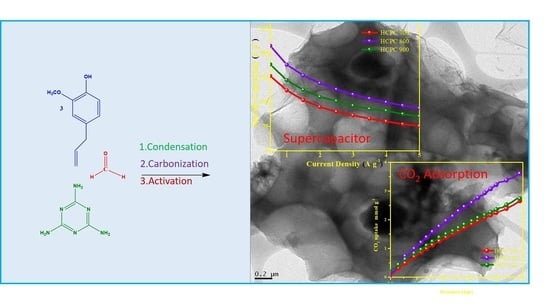
Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture
Abstract
1. Introduction
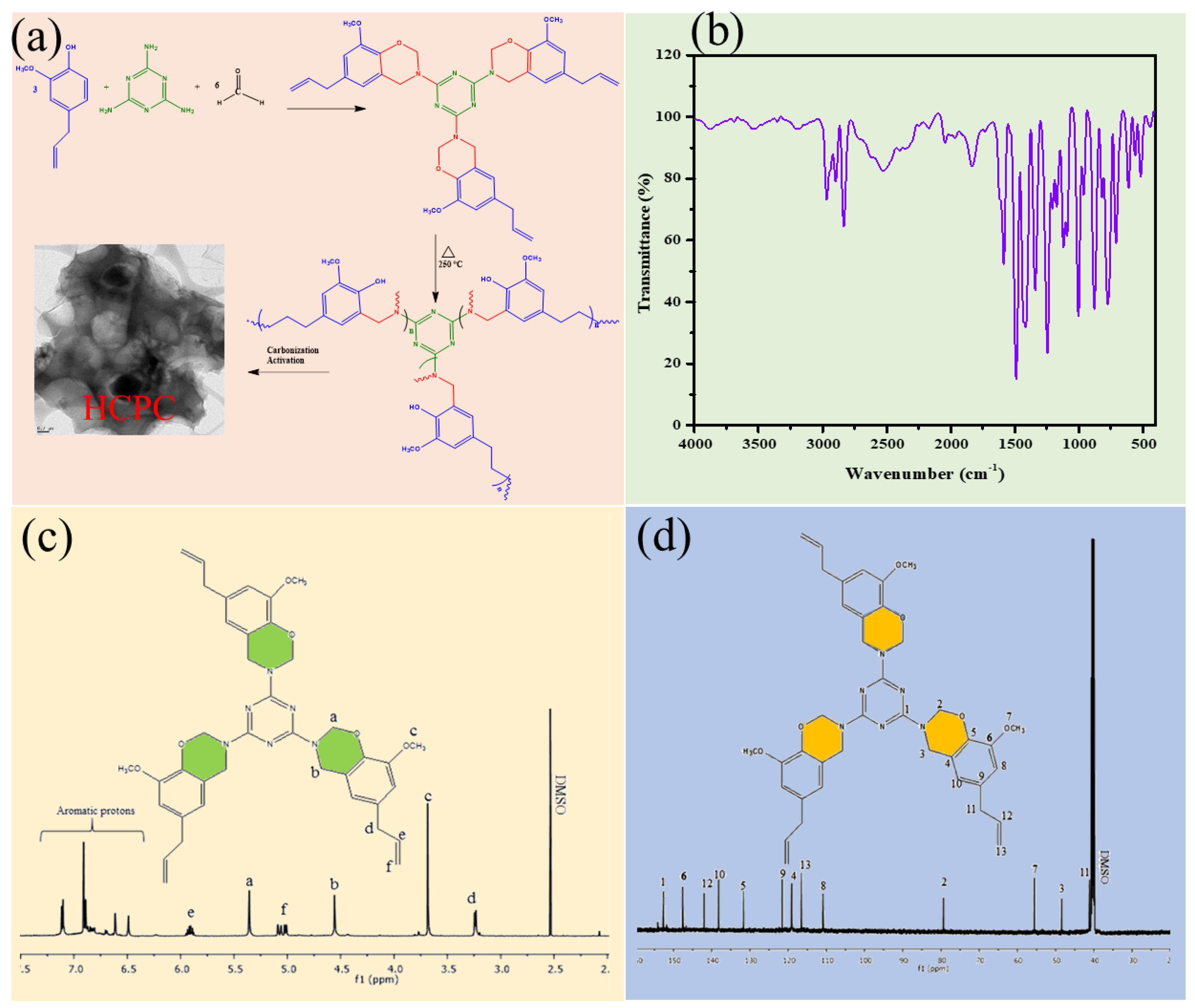
2. Synthesis of Heteroatoms Containing Porous Carbon Sheets via Polybenzoxazine (HCPC)
3. Results and Discussion
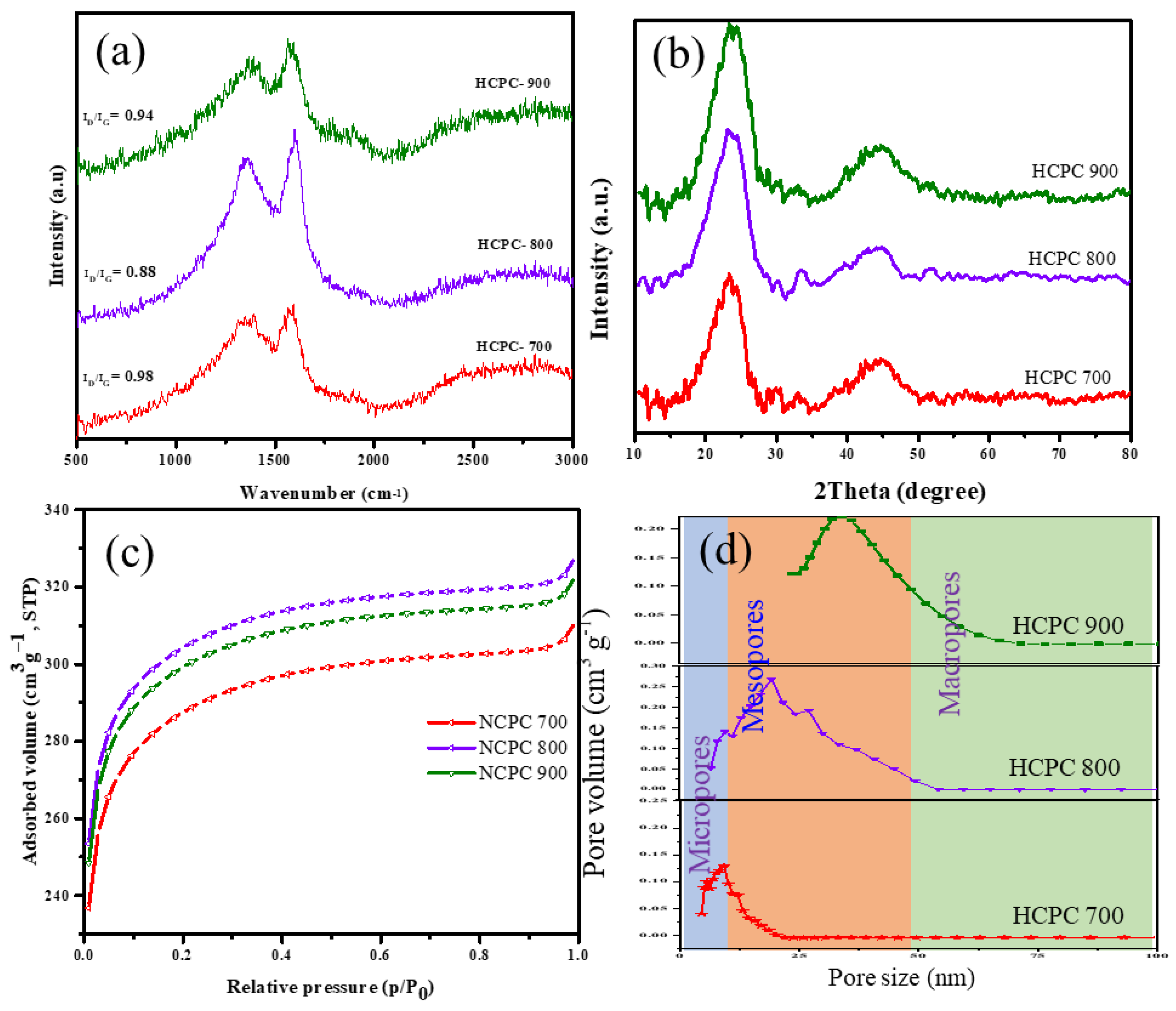
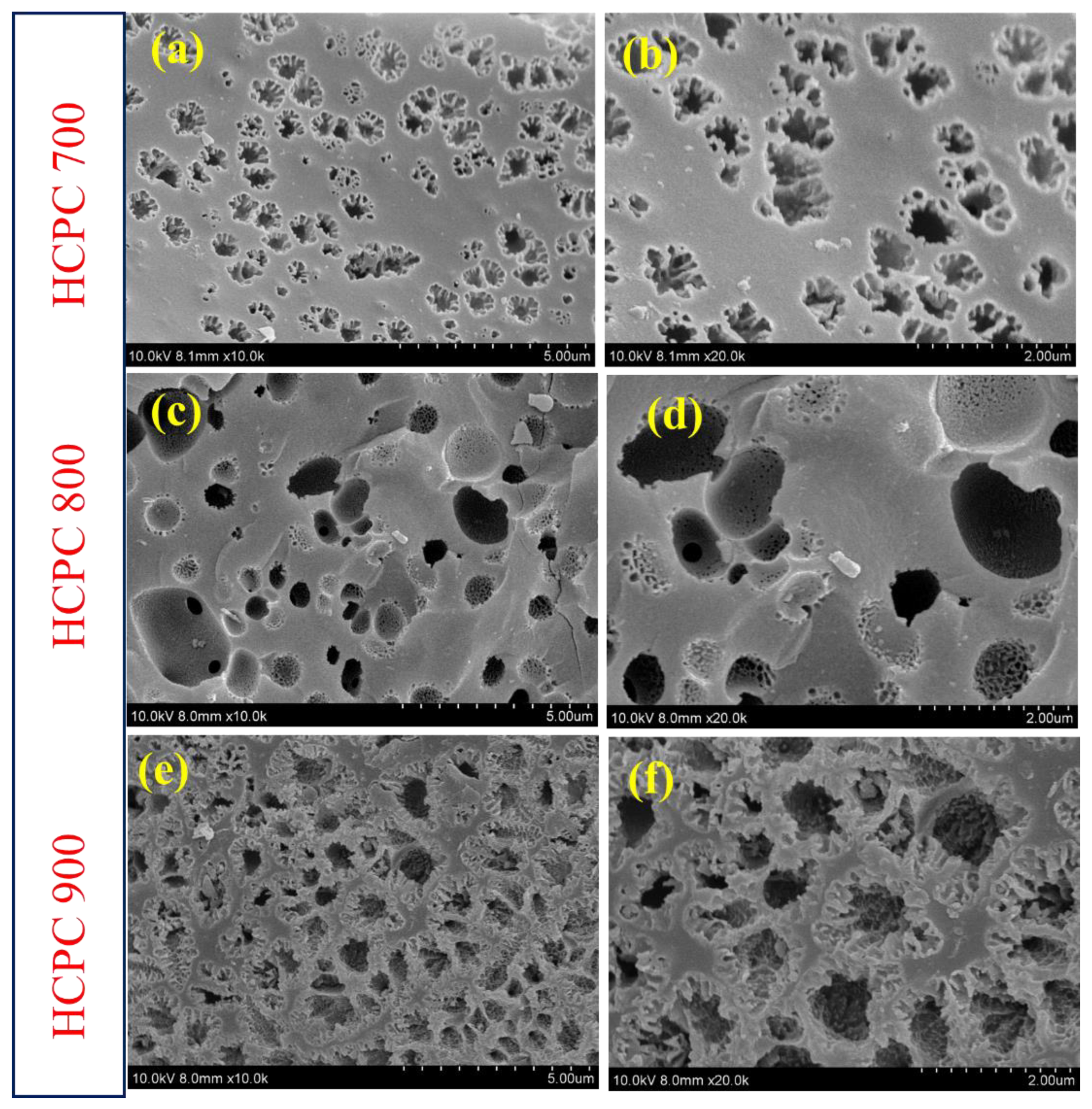
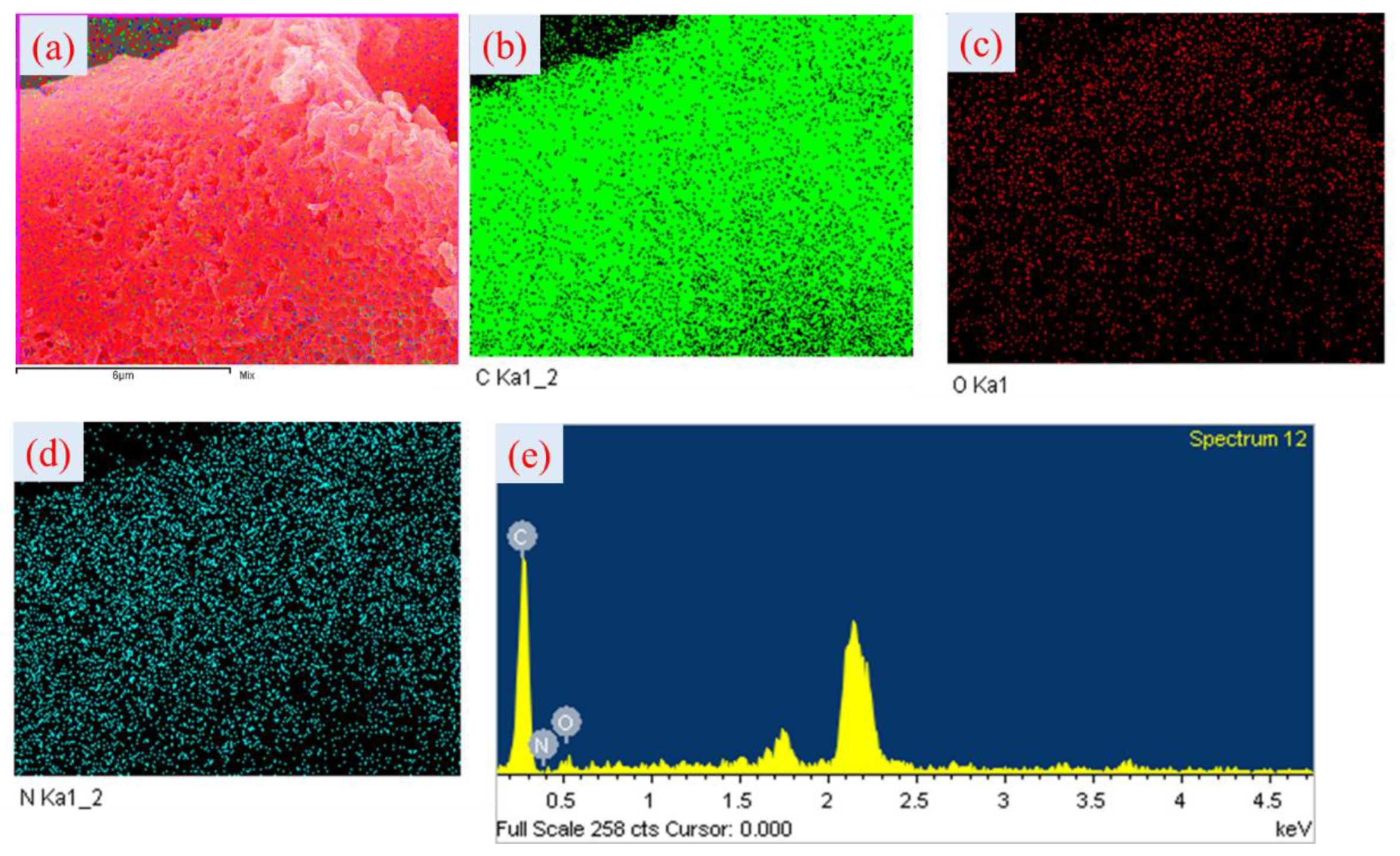
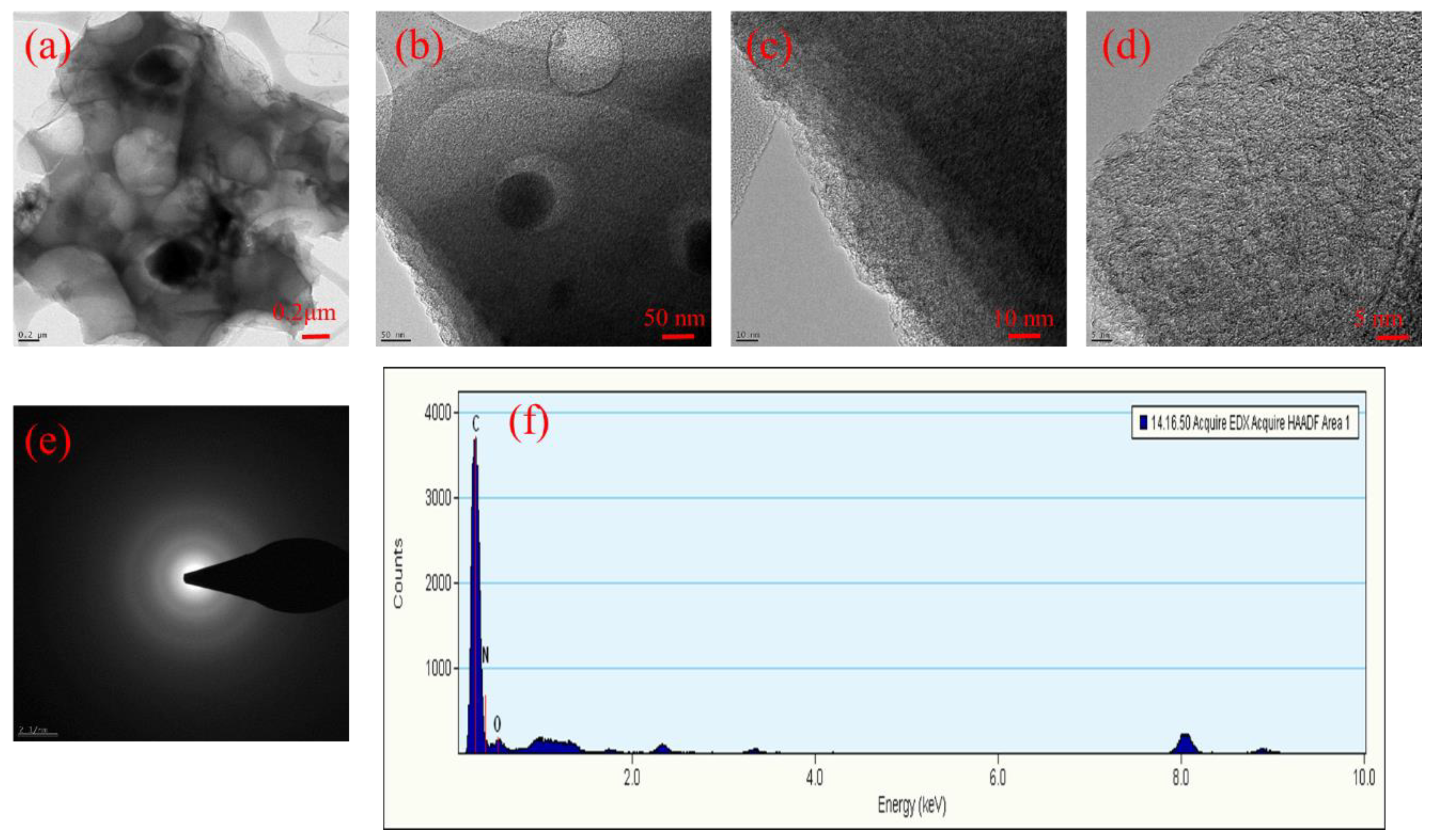
3.1. Structural Properties of Benzoxazine Monomer and HCPCs
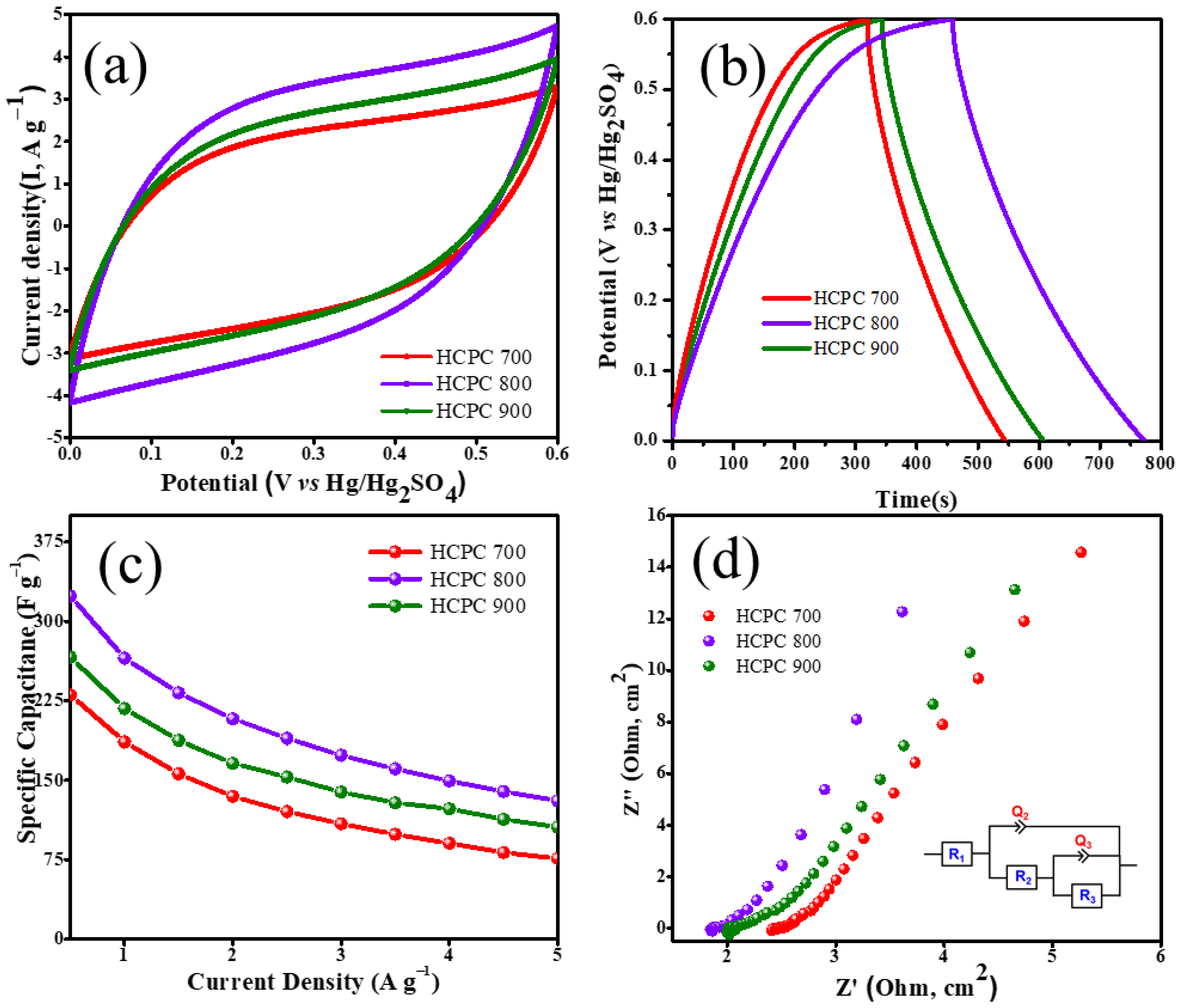
3.2. Electrochemical Measurements
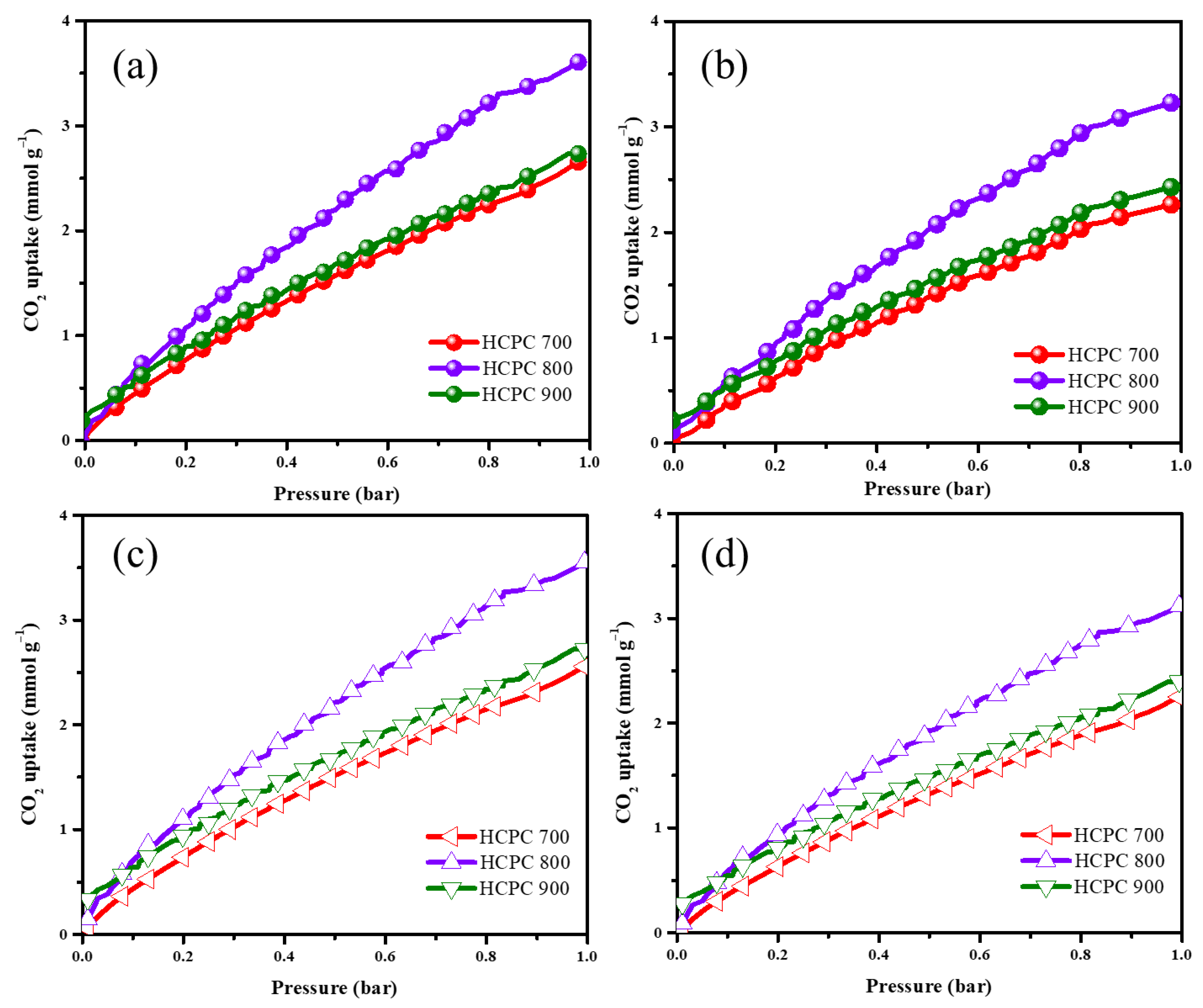
3.3. CO2 Adsorption Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, F.; Poh, K.; Chen, S.; Xu, G.; Wang, D.; Li, Q. Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ. Sci. 2011, 4, 717–724. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.; Ji, H.; Stoller, M.D.; Lai, L.; Murali, S.; McDonnell, S.; Cleveger, B.; Wallace, R.M.; Ruoff, R.S. Nitrogen doping of graphene and its effect on quantum capacitance, and a new insight on the enhanced capacitance of n-doped carbon. Energy Environ. Sci. 2012, 5, 9618–9625. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J.; Wang, X.; Wang, H.; Yang, X. Microorganism-derived heteroatom-doped carbon materials for oxygen reduction and supercapacitors. Adv. Funct. Mater. 2013, 23, 1305–1312. [Google Scholar] [CrossRef]
- Pietrzak, R.; Wachowska, H.; Nowicki, P.; December, R.V.; Re, V.; Recei, M.; March, V. Preparation of nitrogen-enriched activated carbons from brown coal. Energy Fuels 2006, 23, 1275–1280. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Hao, G.; Hao, Y.; Sun, Q.; Zhang, X.; Lu, A. Temperature-programmed precise control over the sizes of carbon nanospheres based on benzoxazine chemistry. J. Am. Chem. Soc. 2011, 133, 15304–15307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C.; Fulvio, P.F.; Mayes, R.T.; Zhamu, A.; Fang, Q.; Chen, G.; Meyer, H.M. Nitrogen-enriched ordered mesoporous carbons through direct pyrolysis in ammonia with enhanced capacitive performance. J. Mater. Chem. A 2013, 1, 7920–7926. [Google Scholar] [CrossRef]
- Nilantha, P.W.; Vindya, S.P.; James, M.R.; Songping, D.H.; Mietek, J. Cysteine-assisted tailoring of adsorption properties and particle size of polymer and carbon spheres. Langmuir 2013, 29, 4032–4038. [Google Scholar]
- Tanaka, S.; Nakao, H.; Mukai, T.; Katayama, Y.; Miyake, Y. An experimental investigation of the ion storage/transfer behavior in an electrical double-layer capacitor by using monodisperse carbon spheres with microporous structure. J. Phys. Chem. C. 2012, 116, 26791–26799. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Significantly increased CO2 adsorption performance of nanostructured templated carbon by tuning surface area and nitrogen doping. J. Phys. Chem. C 2012, 116, 1099–1106. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of n-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Liang, H.; Kong, M.; Guan, Q.; Chen, P.; Al, C.E.T. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 8, 7092–7102. [Google Scholar] [CrossRef]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Pan, C.; Qian, W.; Peng, Y.; Qiu, L.; Yan, F. Nitrogen-doped mesoporous carbons originated from ionic liquids as electrode materials for supercapacitors. J. Mater. Chem. A 2013, 1, 6373–6378. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Perera, V.S.; Park, B.W.; Gao, M.; McGimpsey, G.W.; Huang, S.D.; Jaroniec, M. Graphitic mesoporous carbons with embedded prussian blue-derived iron oxide nanoparticles synthesized by soft templating and low-temperature graphitization. Chem. Mater. 2013, 25, 2803–2811. [Google Scholar] [CrossRef]
- Chen, H.; Sun, F.; Wang, J.; Li, W.; Qiao, W.; Ling, L.; Long, D. Nitrogen doping effects on the physical and chemical properties of mesoporous carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, C.; Chen, G.; Liu, Z.; Ma, M.; Xie, Q.; Zheng, N.; Yao, S. Synthesis of ultrathin nitrogen-doped graphitic carbon nanocages as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 2241–2248. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.Z.; Zhou, M.Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.M. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Zhong, M.; Natesakhawat, S.; Baltrus, J.P.; Luebke, D.; Nulwala, H.; Matyjaszewski, K.; Kowalewski, T. copolymer-templated nitrogen-enriched porous nanocarbons for CO2 capture. Chem. Commun. 2012, 48, 11516–11518. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Tailoring microporosity and nitrogen content in carbons for achieving high uptake of CO2 at ambient conditions. Adsorption 2014, 20, 287–293. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Manoharan, R.K.; Parveen, A.S.; Atchudan, R.; Kim, S.-C. Sustainability and antimicrobial assessments of apigenin based polybenzoxazine film. Polymers 2019, 172, 100–109. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Lee, Y.R.; Kim, S.-C. Polybenzoxazine originated N-doped mesoporous carbon ropes as an electrode material for high-performance supercapacitors. J. Alloys Compd. 2018, 750, 384–391. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Balasubramanian, R.; Parveen, A.S.; Kim, S.-C. Direct synthesis of nitrogen-rich carbon sheets via polybenzoxazine as highly active electrocatalyst for water splitting. Int. J. Hydrogen Energy 2018, 43, 13266–13275. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, C.; Lu, W.; Wang, X.; Yue, H.; Zhang, D.; Xing, Z. A graphitized hierarchical porous carbon as an advanced cathode host for alkali metal-selenium batteries. Chem. Eng. J. 2022, 433, 133527. [Google Scholar] [CrossRef]
- Li, W.; Peng, D.; Huang, W.; Zhang, X.; Hou, Z.; Zhang, W.; Lin, B.; Xing, Z. Adjusting coherence length of expanded graphite by self-activation and its electrochemical implication in potassium ion battery. Carbon 2023, 204, 315–324. [Google Scholar] [CrossRef]
- Cancado, L.G.; Takai, K.; Enoki, T. General equation for the determination of the crystallite size 𝐿𝑎 of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Yang, L.; Ma, L.; Park, C.B.; Gong, P.; Li, G. Electromagnetic wave absorption in graphene nanoribbon nanocomposite foam by multiscale electron dissipation of atomic defects, interfacial polarization and impedance match. Carbon 2023, 205, 159–170. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, J.; Zeng, J.; Tan, S. Preparation of monodisperse carbon nanospheres for electrochemical capacitors. Electrochem. Commun. 2008, 10, 1067–1070. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Gu, Z.; Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources 2013, 236, 285–292. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, M.; Reddeppa, N.; Xu, D.; Jing, Q.-S.; Zha, R. Nitrogen self-doped carbon aerogels derived from trifunctional benzoxazine monomers as ultralight supercapacitor electrodes. Nanoscale 2018, 10, 6549–6557. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Wang, Y.; Ning, G.; Fan, Z.; Wei, T.; Cheng, J.; Zhang, M.; Jing, X. Template synthesis of hollow carbon spheres anchored on carbon nanotubes for high rate performance supercapacitors. Carbon 2013, 52, 209–218. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: Catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Wu, C.; Liu, J.; Wang, H.; Gao, J.; Zhang, Y.; Shu, H. Supercapacitive performance of hierarchical porous carbon microspheres prepared by simple one-pot method. J. Power Sources 2014, 254, 10–17. [Google Scholar] [CrossRef]
- Andreas, H.A.; Conway, B.E. Examination of the double-layer capacitance of an high specific-area C-cloth electrode as titrated from acidic to alkaline pHs. Electrochim. Acta 2006, 51, 6510–6520. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Kerisit, S.; Schwenzer, B.; Vijayakumar, M. Effects of oxygen-containing functional groups on supercapacitor performance. J. Phys. Chem. Lett. 2014, 5, 2330–2334. [Google Scholar] [CrossRef]
- Fujisawa, K.; Cruz-Silva, R.; Yang, K.-S.; Kim, Y.A.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Importance of open, heteroatom-decorated edges in chemically doped-graphene for supercapacitor applications. J. Mater. Chem. A 2014, 2, 9532–9540. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G.; Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G. Nitrogen modification of highly porous carbon for improved supercapacitor performance. J. Mater. Chem. 2012, 22, 9884–9889. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Lee, Y.R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, Z.-Q.; Chen, M.-M.; Wang, C.-Y. Hierarchical porous carbon derived from sulfonated pitch for electrical double layer capacitors. J. Power Sources 2014, 252, 235–243. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, K.-H.; Hu, C.-C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Chen, Z.-G.; Lu, G.Q.; Cheng, H.-M. Synthesis and electrochemical property of boron-doped mesoporous carbon in supercapacitor. Chem. Mater. 2008, 20, 7195–7200. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Karthikeyan, D.; Pandurangan, A.; Lee, Y.R. Synthesis and characterization of graphitic mesoporous carbon using metal–metal oxide by chemical vapor deposition method. Microporous Mesoporous Mater. 2015, 215, 123–132. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Chen, X.; Fu, R. Nitrogen-enriched nanocarbons with a 3-d continuous mesopore structure from polyacrylonitrile for supercapacitor application. J. Phys. Chem. C 2010, 114, 8581–8586. [Google Scholar] [CrossRef]
- Nasini, U.B.; Bairi, V.G.; Ramasahayam, S.K.; Bourdo, S.E.; Viswanathan, T.; Shaikh, A.U. Phosphorous and nitrogen dual heteroatom doped mesoporous carbon synthesized via microwave method for supercapacitor application. J. Power Sources 2014, 250, 257–265. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Zhang, F.; Cao, G.; Chu, M.; Yang, Y. Nitrogen-doped mesoporous carbon derived from biopolymer as electrode material for supercapacitors. J. Electroanal. Chem. 2014, 712, 146–150. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Lee, Y.R. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhao, X.; Wu, L.; Zhang, Q. Graphene-wrapped polyaniline nanowire arrays on nitrogen-doped carbon fabric as novel flexible hybrid electrode materials for high-performance supercapacitor. Langmuir 2014, 30, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Pevida, C.; Drage, T.C.; Snape, C.E. Silica-templated melamine–formaldehyde resin derived adsorbents for CO2 capture. Carbon 2008, 46, 1464–1474. [Google Scholar] [CrossRef]
- Fan, X.Q.; Zhang, L.X.; Zhang, G.B.; Shu, Z.; Shi, J.L. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Sevilla, M.; Vigon, P.V.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Feng, C.; Suna, Y.; Li, K. Synthesis of polybenzoxazine based nitrogen-rich porous carbons for carbon dioxide capture. Nanoscale 2015, 7, 6534–6544. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Kim, S.-C. Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture. Polymers 2023, 15, 1564. https://doi.org/10.3390/polym15061564
Periyasamy T, Asrafali SP, Kim S-C. Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture. Polymers. 2023; 15(6):1564. https://doi.org/10.3390/polym15061564
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, and Seong-Cheol Kim. 2023. "Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture" Polymers 15, no. 6: 1564. https://doi.org/10.3390/polym15061564
APA StylePeriyasamy, T., Asrafali, S. P., & Kim, S.-C. (2023). Heteroatom-Enhanced Porous Carbon Materials Based on Polybenzoxazine for Supercapacitor Electrodes and CO2 Capture. Polymers, 15(6), 1564. https://doi.org/10.3390/polym15061564