In Situ Synthesis of Environmentally Friendly Waterborne Polyurethane Extended with Regenerated Cellulose Nanoparticles for Enhanced Mechanical Performances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RCNs
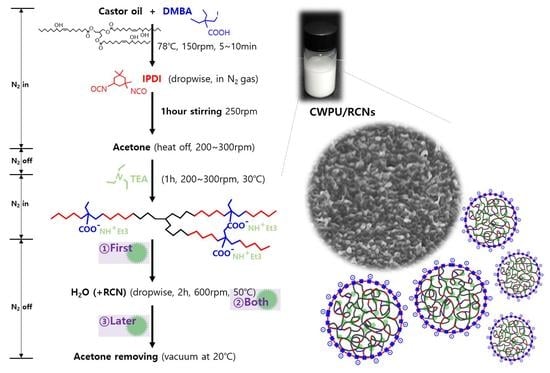
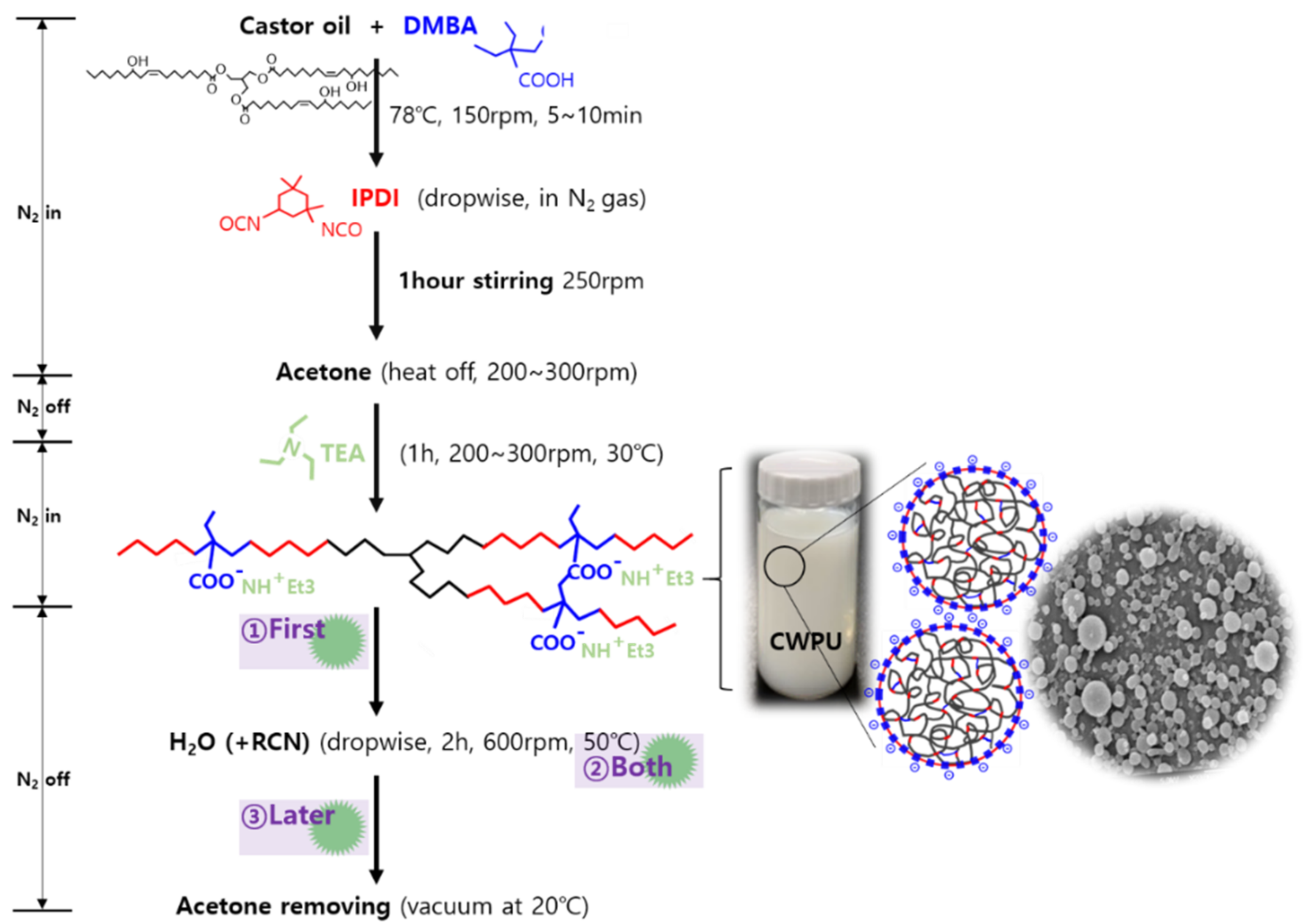
2.3. Preparation of CWPU and CWPU/RCNs Nanocomposite Dispersions and Films
2.4. Analysis of CWPU/RCNs Nanocomposites Dispersions and Films
3. Results
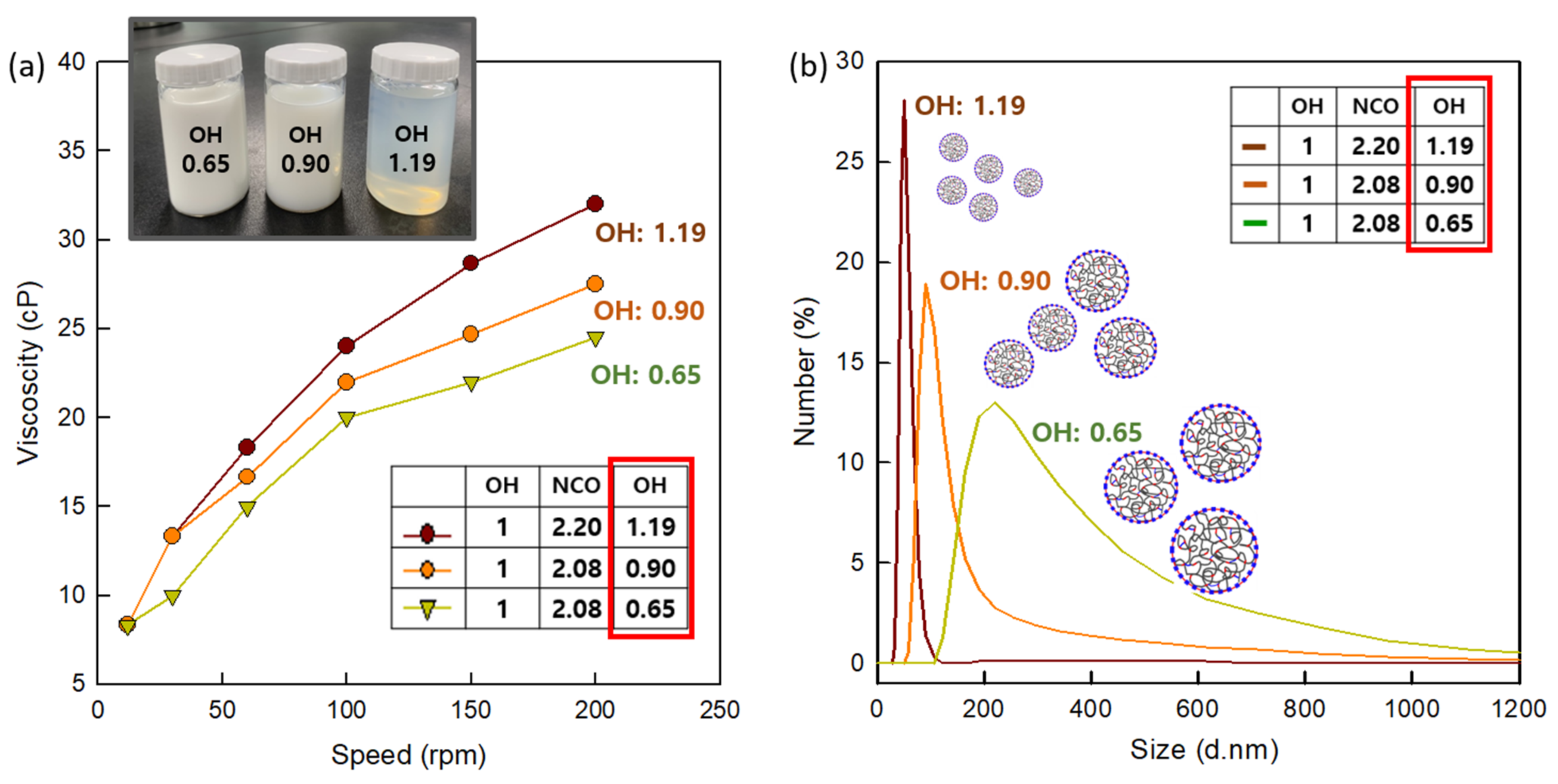
3.1. Properties of CWPU Dispersions According to Emulsifier Ratio
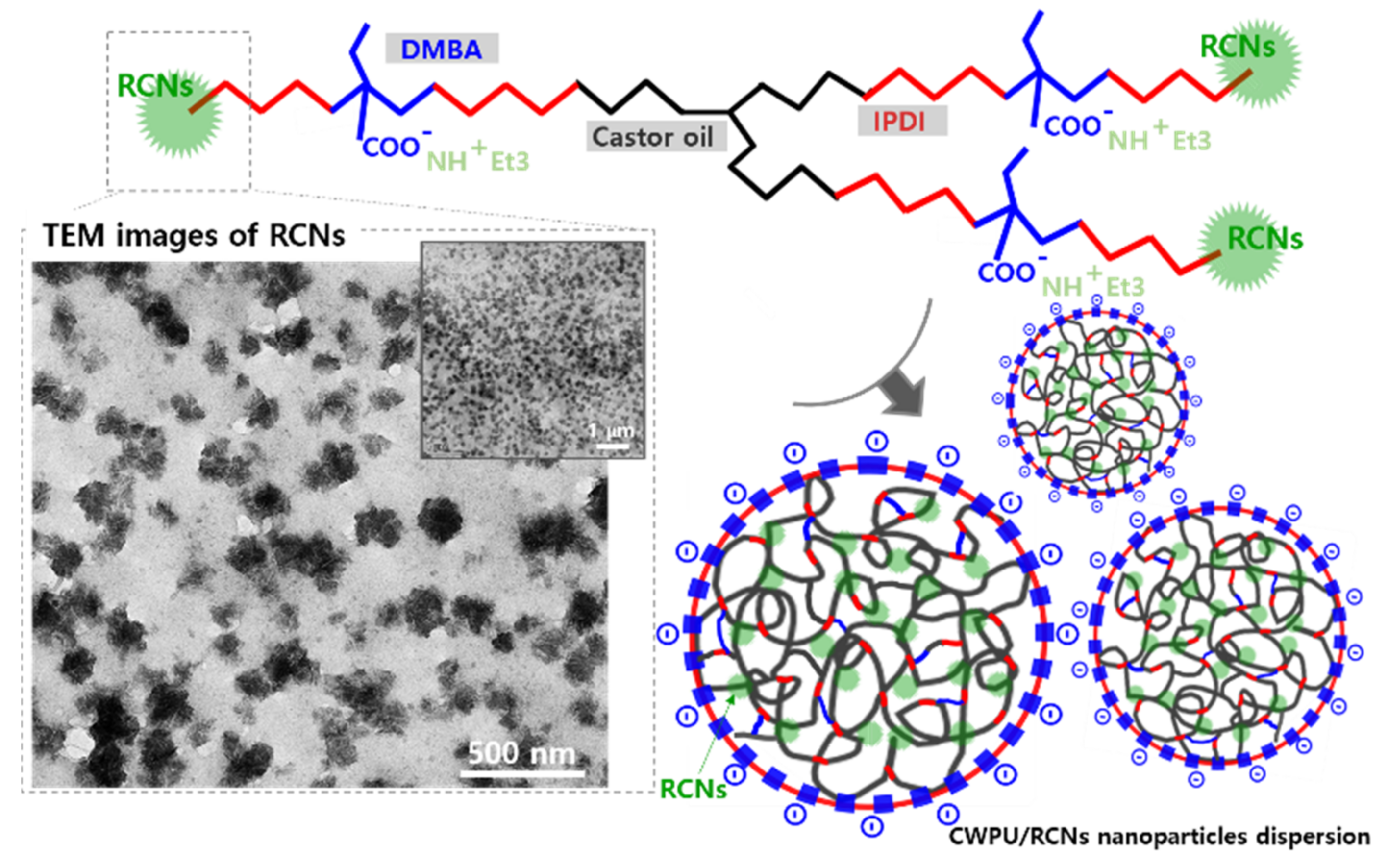
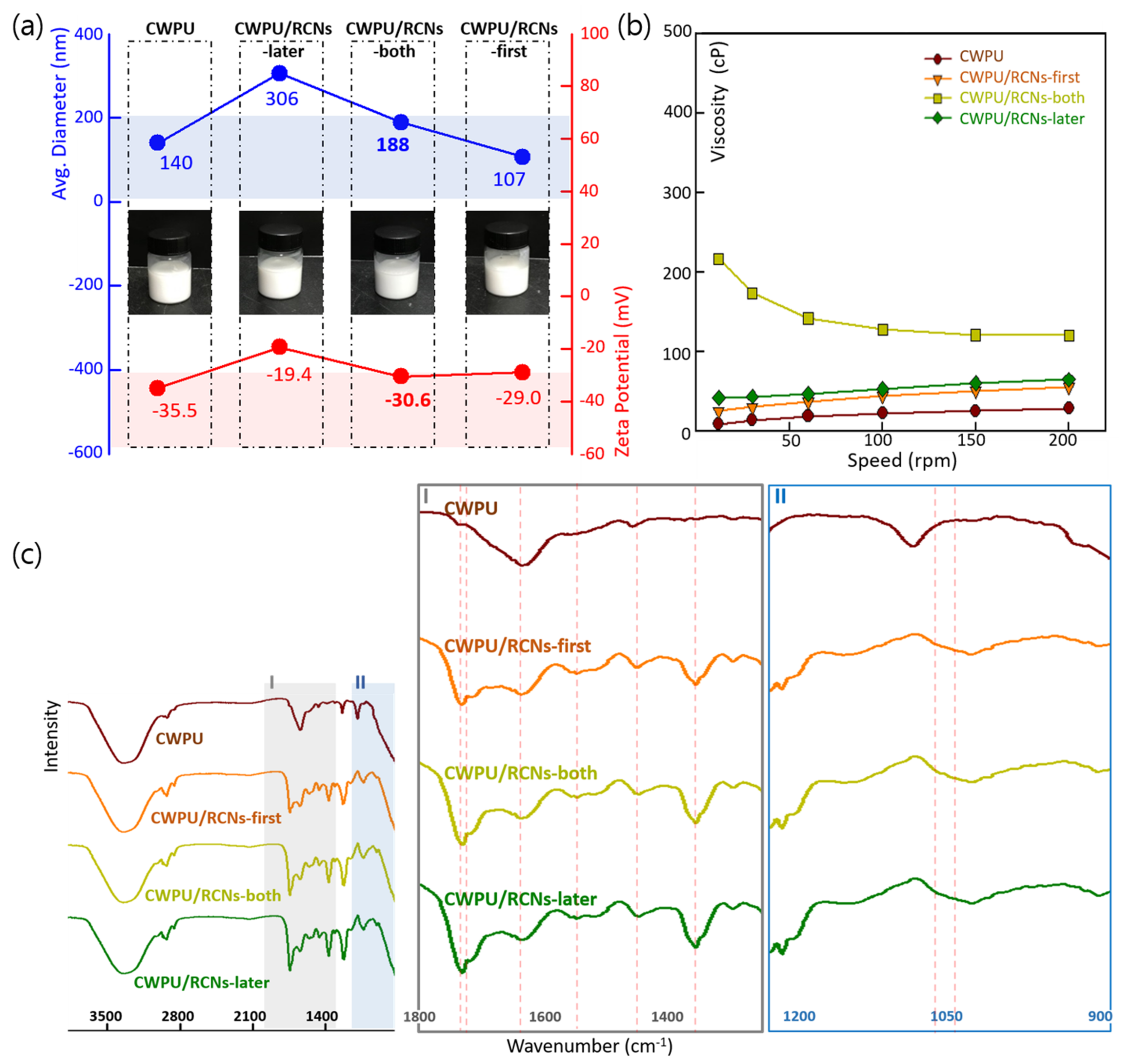
3.2. Characteristics of the CWPU/RCNs Nanocomposites Dispersions and Films According to the Step of Adding RCNs
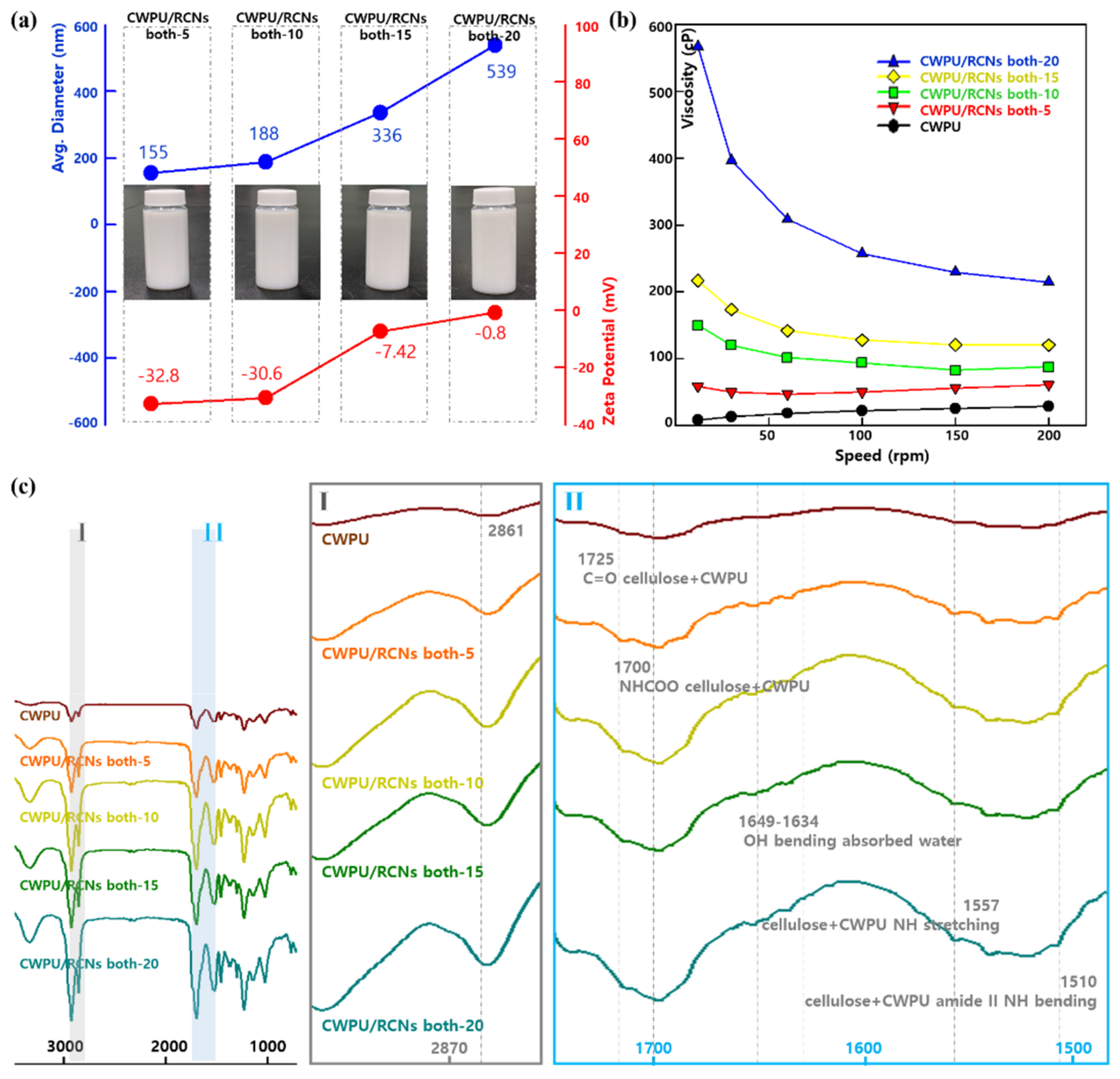
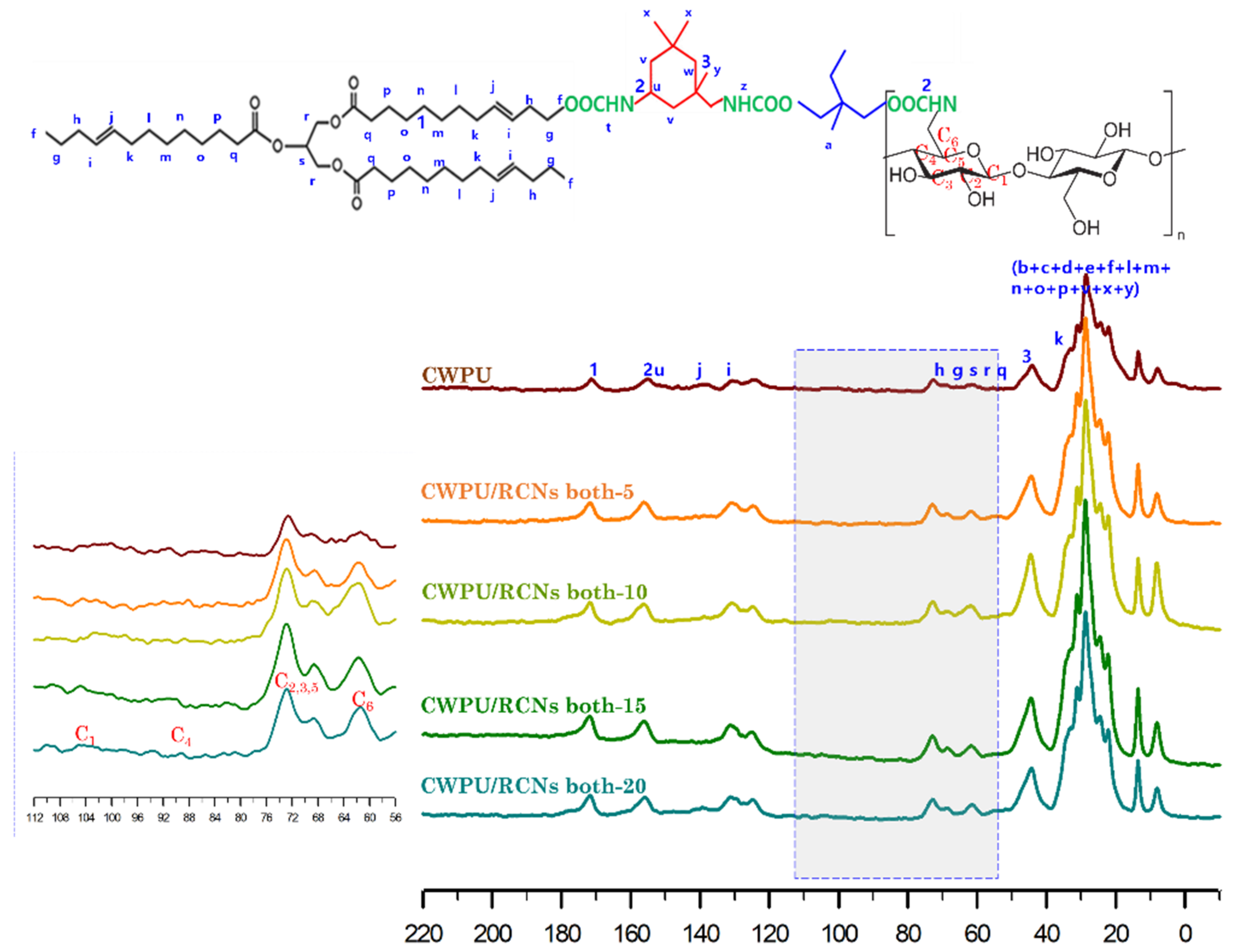
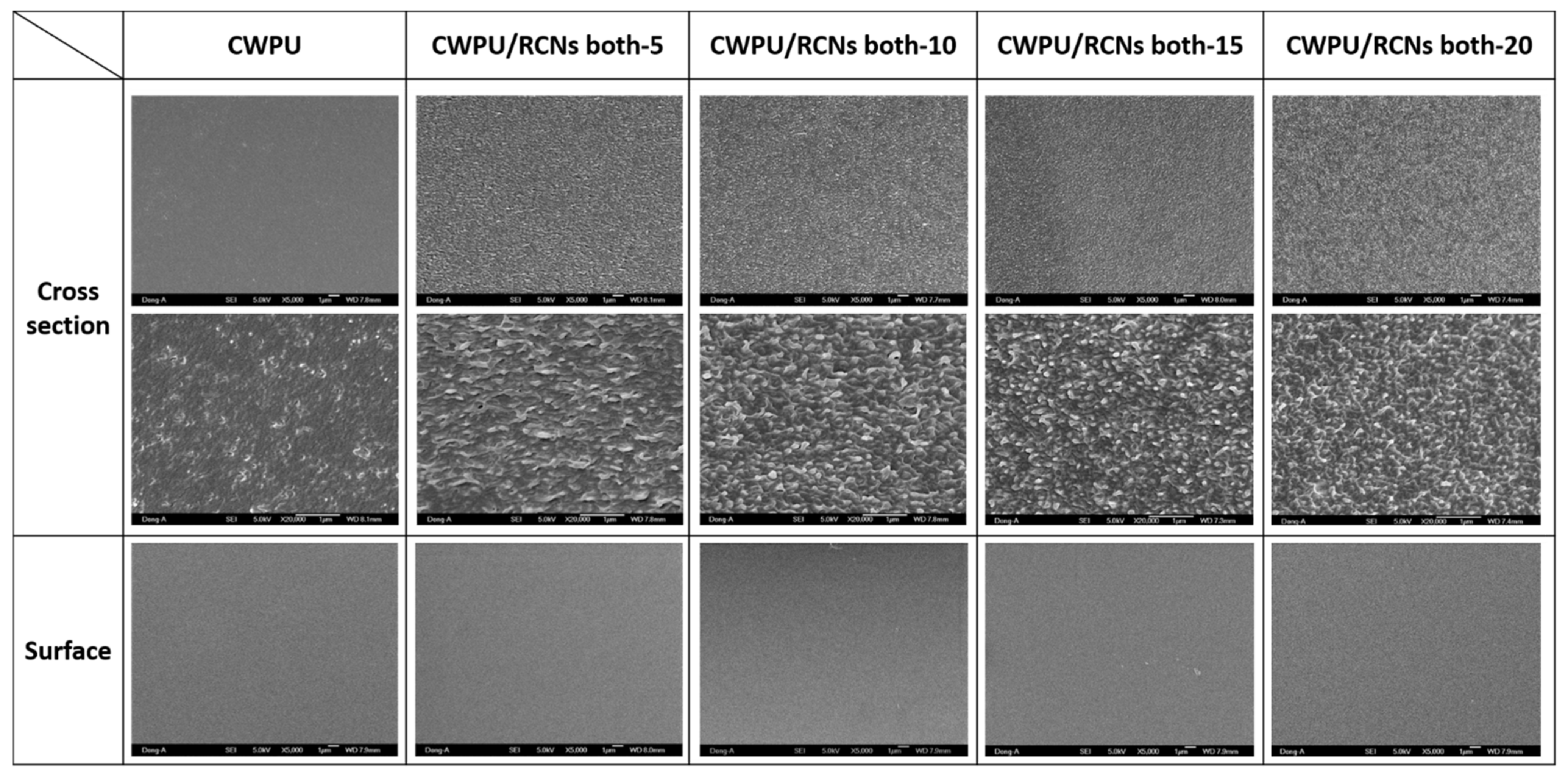
3.3. Characteristics of the CWPURCNs Nanocomposites Dispersions and Films According to RCNs Contents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, Y.; Zhu, Q. The development of polyurethane. Mater. Sci. Mater. Rev. 2017, 1, 1–8. [Google Scholar]
- Maiuolo, L.; Olibito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Tursi, A.; Sposato, C.; Feo, A.; De Nino, A. Synthesis, characterization and mechanical properties of novel bio-based polyurethane foams using cellulose-derived polyol for chain extension and cellulose citrate as a thickener additive. Polymers 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.Q.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnama, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of bioplastics production from starch and lignocellulosic components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: A riview. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M. Advances in waterborne polyurethane-based biomaterials for biomedical applications. Adv. Exp. Med. Biol. 2018, 1077, 251–283. [Google Scholar]
- Xu, D.; Liang, G.; Qi, Y.; Gong, R.; Zhang, X.; Zhang, Y.; Liu, B.; Kong, L.; Dong, X.; Li, Y. Enhancing the mechanical properties of waterborne polyurethane paint by graphene oxide for wood products. Polymers 2022, 14, 5456. [Google Scholar] [CrossRef]
- De Nino, A.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and fast removal of oils from water surfaces via highly olephilic polyurethane composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M.; Lee, J.W. Fabrication of regenerated cellulose nanoparticles/waterborne polyurethane nanocomposites. J. Appl. Polym. Sci. 2018, 135, 46633–46641. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A riview. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of bio-based polyurethane: Perspective on applications and biodegradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Qian, Y.; Dong, F.; Guo, L.; Xu, S.; Liu, H. Tow-component waterborne polyurethane modified with terpene derivative-based polysiloxane for coatings via a thiol-ene click reaction. Ind. Crops Prod. 2021, 171, 113903. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, Q.D.; Min, B.K.; Yi, Y.S.; Choi, C.G. Ti3C2Tx MXene/carbon nanotubes/waterborne polyurethane based composite ink for electromagnetic interference shielding and sheet heater applications. Chem. Eng. J. 2022, 430, 133171. [Google Scholar] [CrossRef]
- Yang, W.; Bai, H.; Jiang, B.; Wang, C.; Ye, W.; Li, Z.; Xu, C.; Wang, X.; Li, Y.F. Flexible and densified graphene/waterborne polyurethane composite film with thermal conducting property for high performance electromagnetic interference shielding. Nano Res. 2022, 15, 9926–9935. [Google Scholar] [CrossRef]
- Rubino, L.; Torrisi, G.; Brambilla, L.; Rubino, L.; Ortenzi, M.A.; Galimberti, M.; Barbera, V. Polyhydroxylated nanosized graphite as multifunctional building block for polyurethane. Polymers 2022, 14, 1159. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, L.; Curtis, J.M. Polyurethane nanocomposites incorporating biobased polyols and reinforced with a low fraction of cellulose nanocrystals. Carbohydr. Polym. 2016, 152, 487–495. [Google Scholar] [CrossRef]
- Saalah, S.; Chuah, A.L.; Aung, M.M.; Salleh, M.Z.; Radiah, D.; Basri, M.; Jusoh, E.R. Waterborne polyurethane dispersions synthesized from Jatropha oil. Ind. Crops Prod. 2015, 64, 194–200. [Google Scholar] [CrossRef]
- Shendi, H.K.; Omrani, I.; Ahmadi, A.; Farhadian, A.; Babnejad, N.; Nabid, M.R. Synthesis and characterization of a novel internal emulsifier derived from sunflower oil for the preparation of waterborne polyurethane and their application in coatings. Prog. Org. Coat. 2017, 105, 303–309. [Google Scholar] [CrossRef]
- Moghadam, P.N.; Yarmohamadi, M.; Hasanzadeh, R.; Nuri, S. Preparation of polyurethane wood adhesives by polyols formulated with polyester polyols based on castor oil. Int. J. Adhes. Adhes. 2016, 68, 273–282. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Bhosale, N.; Shaik, A.; Mandal, S.K. Synthesis and characterization of castor oil based hybrid polymers and their polyurethane-urea/silica coatings. RSC Adv. 2015, 5, 103625–103635. [Google Scholar] [CrossRef]
- Touchet, T.J.; Cosgriff-Hernandez, E.M. Hierarchal structure-property relationships of segmented polyurethanes. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 3–22. [Google Scholar]
- Velankar, S.; Cooper, S.L. Microphase separation and rheological properties of polyurethane melts. 2. Effect of block incompatibility on the microstructure. Macromolecules 2000, 33, 382–394. [Google Scholar] [CrossRef]
- Hsu, S.H.; Tang, C.M.; Tseng, H.J. Biocompatibility of poly(ether)urethane-gold nanocomposites. J. Biomed. Mater. Res. 2006, 79, 759–770. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, M.W.; Shin, E.J. One-pot processing of regenerated cellulose nanoparticles/waterborne polyurehane nanocomposite for eco-friendly polyurethane matrix. Polymers 2019, 11, 356–369. [Google Scholar] [CrossRef]
- Honarkar, H.; Barmar, M.; Mehdi, B. Synthesis, characterization and properties of waterborne polyurethanes based on two different ionic centers. Fibers Polym. 2015, 16, 718–725. [Google Scholar] [CrossRef]
- Rosthauser, J.W.; Nachtkamp, K. Advances in Urethane Science and Technology; RAPRA Technology: Shawbury, UK, 1987; pp. 121–162. [Google Scholar]
- Lu, M.G.; Lee, J.Y.; Shim, M.J.; Kim, S.W. Synthesis and properties of anionic aqueous polyurethane dispersions. J. Appl. Polym. Sci. 2002, 86, 3461–3465. [Google Scholar] [CrossRef]
- Liang, H.; Wang, S.; He, H.; Wang, M.; Liu, L.; Lu, J.; Zhang, Y.; Zhang, C. Aqqueous anionic polyurethane dispersions from castor oil. Ind. Crops Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong nanocomposite reinforcement effects in polyurethane elastomer with low volume fraction of cellulose nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Ewa, G.; Janusz, D. Bio polyetherurethane composites with high content of natural ingredients: Hydroxylated soybean oil based polyol, bio glycol and microcrystalline cellulose. Cellulose 2016, 23, 581–592. [Google Scholar]
- Xu, P.; Luo, Y.; Zhang, P. Interfacial architecting of organic-inorganic hybrid toward mechanically reinforced, fire-resistant and smoke-suppressed polyurethane composites. J. Colloid Interface Sci. 2022, 621, 385–397. [Google Scholar] [CrossRef]
- Adsul, M.; Soni, S.K.; Bhargava, S.K.; Bansal, V. Facile approach for the dispersion of regenerated cellulose in aqueous system in the form of nanoparticles. Biomacromolecules 2012, 13, 2890–2895. [Google Scholar] [CrossRef]
- Cao, X.; Habibi, Y.; Lucia, L.A. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J. Mater. Chem. 2009, 19, 7137–7145. [Google Scholar] [CrossRef]
- Zuki, N.M.; Ismail, N.; Omar, F.M. Evaluation of zeta potential and particle size measurements of multiple coagulants in semiconductor wastewater. In Proceedings of the AIP Conference, Leuven, Belgium, 8–10 April 2019. [Google Scholar]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of suwannee river humic acid. Sci. Total Environ. 2014, 35, 195–201. [Google Scholar] [CrossRef]
- Park, S.K.; Johnson, D.K.; Ishizawa, C.I.; Parilla, P.A.; Davis, M.F. Measuring the crystallinity index of cellulose by solid state 13C nuclear magnetic resonance. Cellulose 2009, 16, 641–647. [Google Scholar] [CrossRef]
- Zuckerstatter, G.; Schild, G.; Wollboldt, P.; Roder, T.; Weber, H.K.; Sixta, H. The elucidation of cellulose supramolecular structure by 13C CP-MAS NMR. Lenzing. Ber. 2009, 87, 38–46. [Google Scholar]
- Haslinger, S.; Hietala, S.; Hummel, M.; Maunu, S.L.; Sixta, H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr. Polym. 2019, 207, 11–16. [Google Scholar] [CrossRef]
- Gao, Z.; Peng, J.; Zhong, T.; Sun, J.; Wang, X.; Yue, C. Biocompatible elastomer of waterborne polyurethane based on castor oil and polyehtylene glycol with cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2068–2075. [Google Scholar] [CrossRef]
- Reddy, M.; Reddy, V. Dynamic mechanical analysis of hemp fiber reinforced polymer matrix composites. Int. J. Eng. Res. Technol. 2014, 3, 137745824. [Google Scholar]


| Sample | Molar Ratio | |||
|---|---|---|---|---|
| OH (Castor Oil) | NCO (IPDI) | OH (DMBA) | RCNs Content (wt% of CWPU) | |
| CWPU 0.65 | 1 | 2.08 | 0.65 | - |
| CWPU 0.90 | 1 | 2.08 | 0.90 | - |
| CWPU 1.19 | 1 | 2.20 | 1.19 | - |
| CWPU/RCNs-5 | 1 | 2.08 | 0.90 | 0.18 |
| CWPU/RCNs-10 | 1 | 2.08 | 0.90 | 0.37 |
| CWPU/RCNs-15 | 1 | 2.08 | 0.90 | 0.58 |
| CWPU/RCNs-20 | 1 | 2.08 | 0.90 | 0.76 |
| Related Group | C=O Stretching of Urethane Group | Cellulose CH Stretching Band | Cellulose CH2 Wagging Vibration | Cellulose C-C Ring Vibration | Cellulose C-O-C Ring Vibration | Hydrogen Bond | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location (cm−1) | 1731 | 1365 | 1317 | 1196 | 1062 | 1055 | ||||||
| Items | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area |
| Samples | ||||||||||||
| CWPU | 3 | 125 | 0.9 | 10 | 1.08 | 19 | 0.45 | 3 | 0.05 | 0.04 | 1.6 | 6 |
| CWPU/RCNs-first | 23 | 781 | 11 | 189 | 2.56 | 39 | 6 | 234 | 4 | 164 | 8 | 220 |
| CWPU/RCNs-both | 22 | 714 | 15 | 262 | 2.27 | 35 | 13 | 517 | 6 | 91 | 9 | 207 |
| CWPU/RCNs-later | 25 | 762 | 17 | 301 | 2.39 | 38 | 15 | 517 | 7 | 131 | 9 | 283 |
| Sample | Hard Segment Content (wt%) 1 | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Toughness (kgf∙mm) |
|---|---|---|---|---|---|
| CWPU | 51.35 | 124.5 ± 4.7 | 15.5 ± 3.2 | 190 ± 15 | 40.5 ± 2.2 |
| CWPU/RCNs-first | 52.23 | 134.5 ± 5.3 | 22.5 ± 2.4 | 230 ± 20 | 48.4 ± 2.7 |
| CWPU/RCNs-both | 52.23 | 139.6 ± 6.5 | 17.2 ± 3.2 | 240 ± 20 | 58.8 ± 3.0 |
| CWPU/RCNs-later | 52.23 | 144.3 ± 7.6 | 17.6 ± 2.7 | 196 ± 25 | 43.5 ± 3.4 |
| Related Group | CH2 Cellulose | C=O Cellulose + CWPU | NHCOO Cellulose + CWPU | OH Bending Absorbed Water | Cellulose + CWPU NH Stretching | Cellulose + CWPU Amide II Bending | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location (cm-1) | 2861 | 1732 | 1700 | 1649 | 1557 | 1510 | ||||||
| Items | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area | Intensity | Area |
| Samples | ||||||||||||
| CWPU | 3.8 | 76 | 7.08 | 273 | 11.9 | 3520 | 4.07 | 102 | 4047 | 70 | 5.92 | 268 |
| CWPU/RCNs both-5 | 11.75 | 239 | 18.72 | 403 | 25.78 | 743 | 9.73 | 123 | 10.08 | 117 | 11.14 | 130 |
| CWPU/RCNs both-10 | 17.46 | 361 | 25.25 | 957 | 35.42 | 1289 | 13.35 | 216 | 13.74 | 237 | 14.75 | 354 |
| CWPU/RCNs both-15 | 19.44 | 396 | 29.80 | 991 | 42.85 | 1426 | 16.12 | 300 | 16.86 | 314 | 17.28 | 308 |
| CWPU/RCNs both-20 | 22.87 | 481 | 33.18 | 1259 | 47.68 | 1678 | 18.13 | 311 | 18.89 | 373 | 19.41 | 349 |
| Sample | Hard Segment Content (wt%)1 | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Toughness (kgf∙mm) | Tg from tan δ (°C) |
|---|---|---|---|---|---|---|
| CWPU | 51.35 | 124.5 ± 4.7 | 15.5 ± 3.2 | 190 ± 15 | 40.5 ± 2.2 | 59.5 |
| CWPU/RCNs both-5 | 51.81 | 133.6 ± 5.3 | 16.0 ± 2.5 | 224 ± 20 | 47.4 ± 3.3 | 62.5 |
| CWPU/RCNs both-10 | 52.23 | 139.6 ± 6.5 | 17.2 ± 3.2 | 240 ± 20 | 58.8 ± 3.0 | 61.4 |
| CWPU/RCNs both-15 | 53.04 | 150.3 ± 5.1 | 18.5 ± 2.3 | 222 ± 15 | 49.4 ± 3.1 | 64.8 |
| CWPU/RCNs both-20 | 53.82 | 155.6 ± 3.6 | 17.1 ± 2.2 | 144 ± 13 | 35.8 ± 3.5 | 65.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.M.; Lee, S.Y.; Lee, S.; Han, S.S.; Shin, E.J. In Situ Synthesis of Environmentally Friendly Waterborne Polyurethane Extended with Regenerated Cellulose Nanoparticles for Enhanced Mechanical Performances. Polymers 2023, 15, 1541. https://doi.org/10.3390/polym15061541
Choi SM, Lee SY, Lee S, Han SS, Shin EJ. In Situ Synthesis of Environmentally Friendly Waterborne Polyurethane Extended with Regenerated Cellulose Nanoparticles for Enhanced Mechanical Performances. Polymers. 2023; 15(6):1541. https://doi.org/10.3390/polym15061541
Chicago/Turabian StyleChoi, Soon Mo, Soo Young Lee, Sunhee Lee, Sung Soo Han, and Eun Joo Shin. 2023. "In Situ Synthesis of Environmentally Friendly Waterborne Polyurethane Extended with Regenerated Cellulose Nanoparticles for Enhanced Mechanical Performances" Polymers 15, no. 6: 1541. https://doi.org/10.3390/polym15061541
APA StyleChoi, S. M., Lee, S. Y., Lee, S., Han, S. S., & Shin, E. J. (2023). In Situ Synthesis of Environmentally Friendly Waterborne Polyurethane Extended with Regenerated Cellulose Nanoparticles for Enhanced Mechanical Performances. Polymers, 15(6), 1541. https://doi.org/10.3390/polym15061541