Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems
Abstract
1. Introduction
We dedicate this article to the late Professor Andrzej Dworak, who made tremendous contributions to the development of research on the self-organization processes of amphiphilic polymers and stimuli-responsive materials and their practical use in modern medicine and pharmacy. He was the author of many original papers as well as fundamental review papers, which provide valuable information and inspiration for future generations of chemists undertaking research in this fascinating area of science.
2. The Principles of ATRP
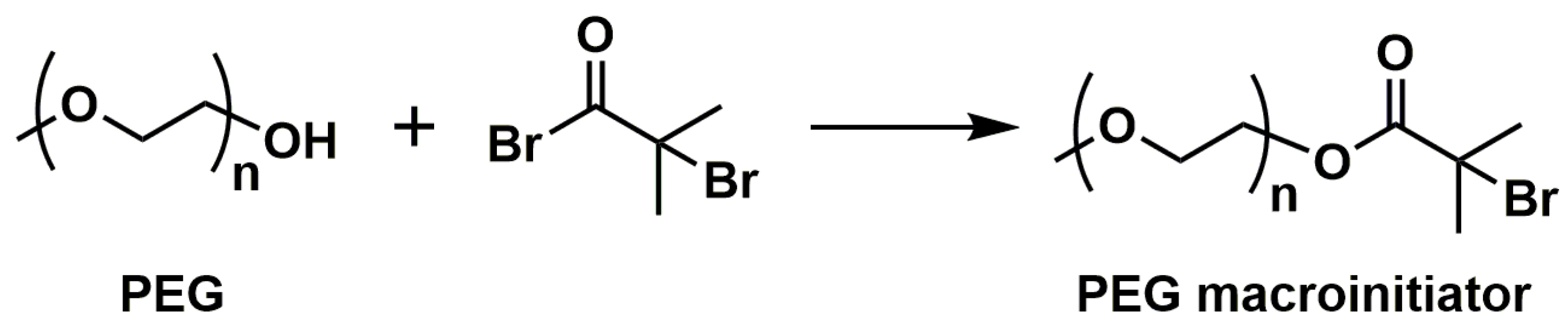
3. DDSs Based on Linear Block Copolymers
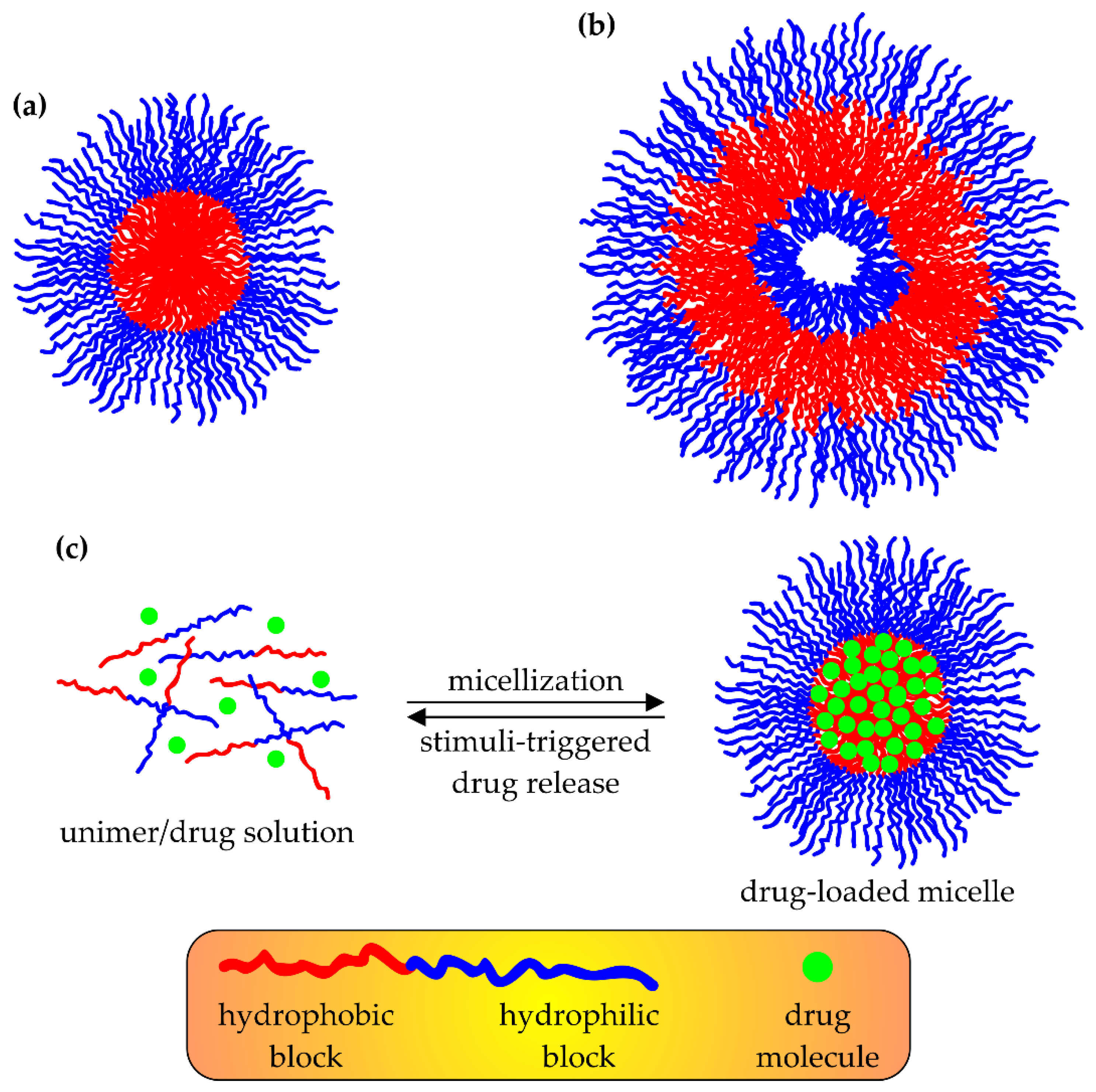
3.1. Smart DDSs Based on Micelles
3.1.1. Drug Release Induced by Protonation
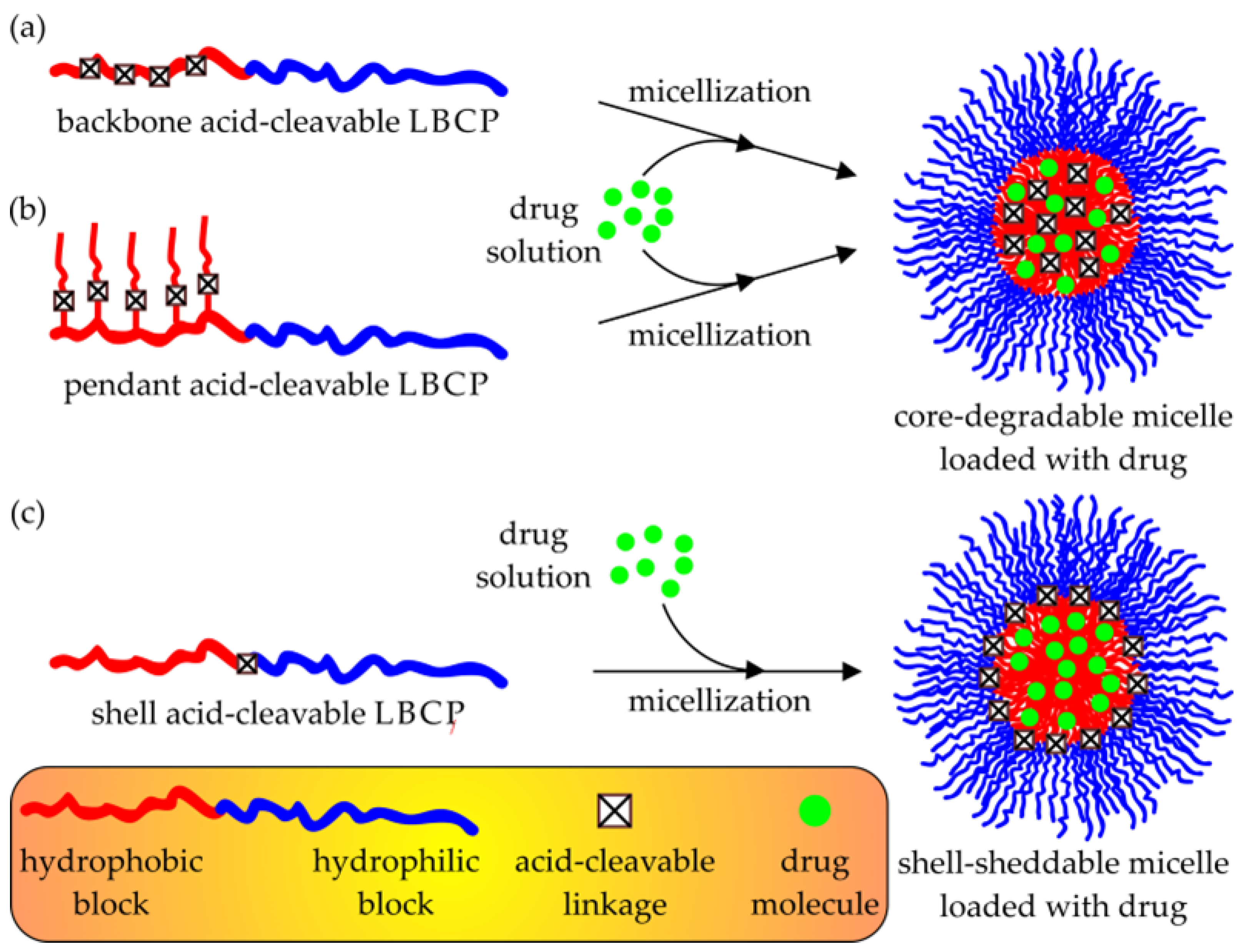
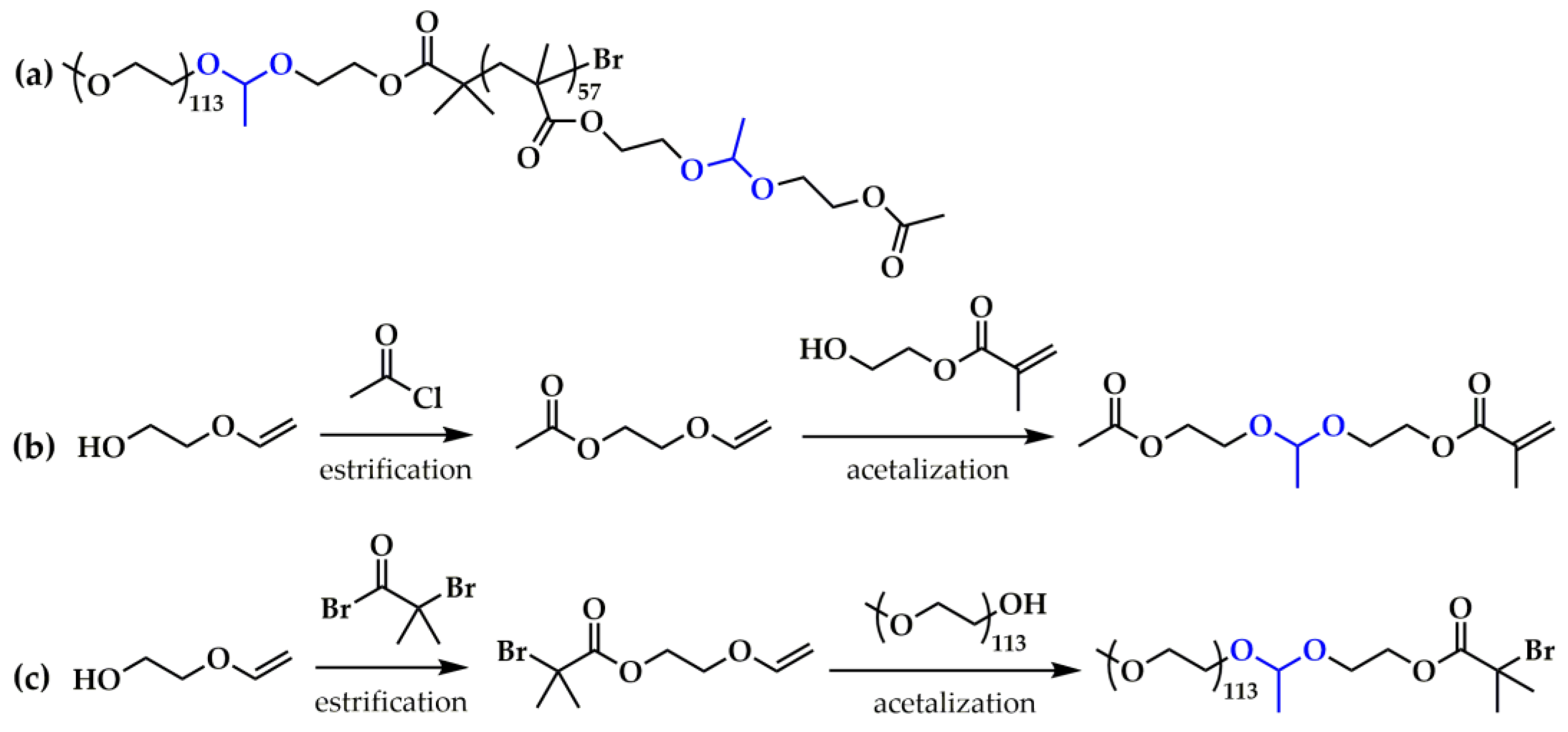
3.1.2. Drug Release Induced by Acid Bond Cleavage
3.1.3. Redox and Dual Redox/pH-Sensitive Systems
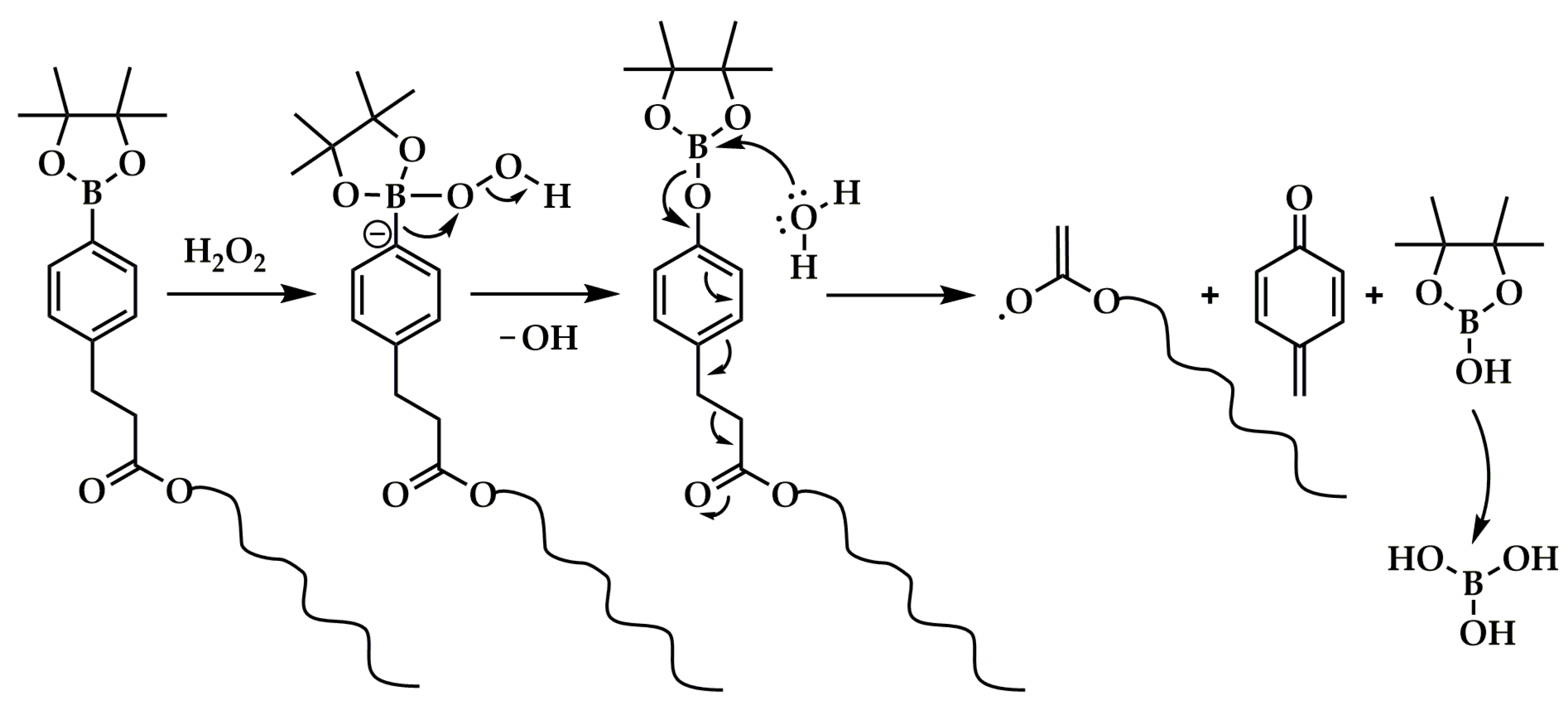
Boronate-Bearing Oxidation-Responsive Systems
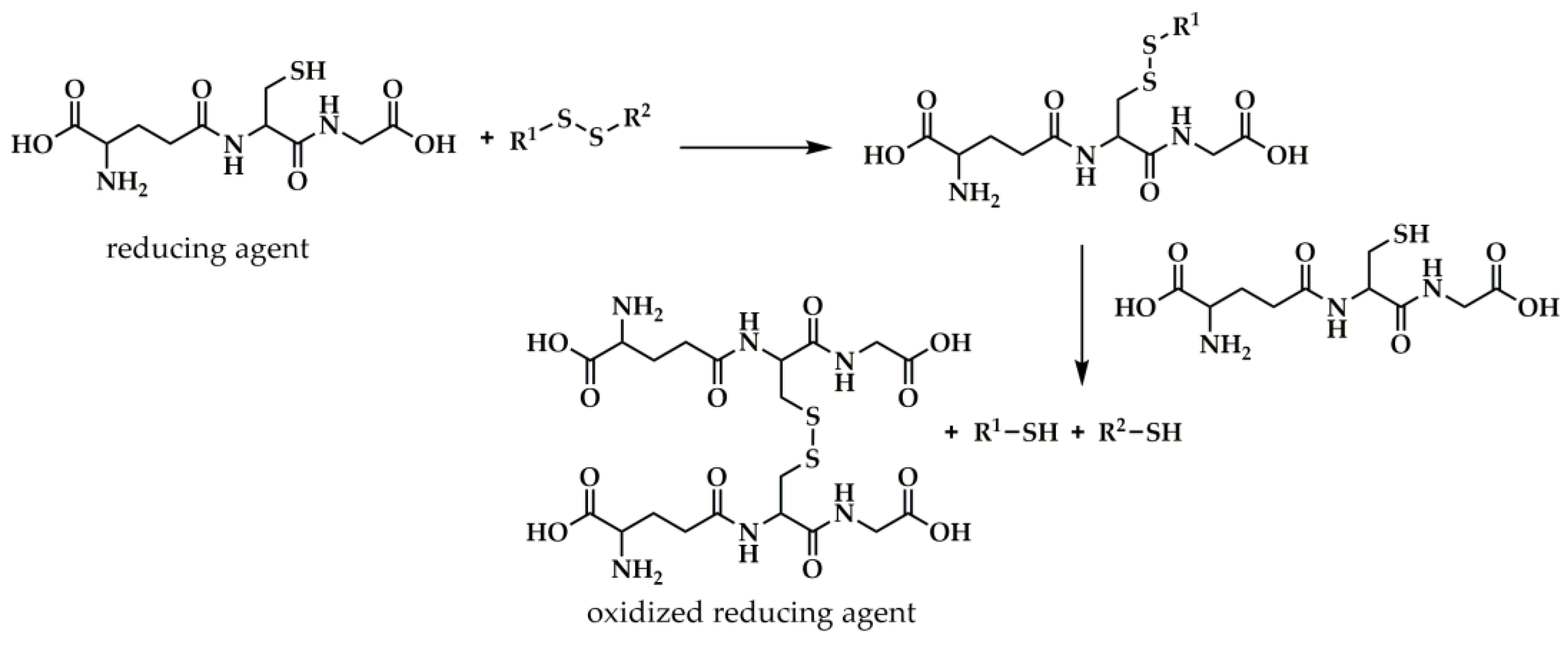
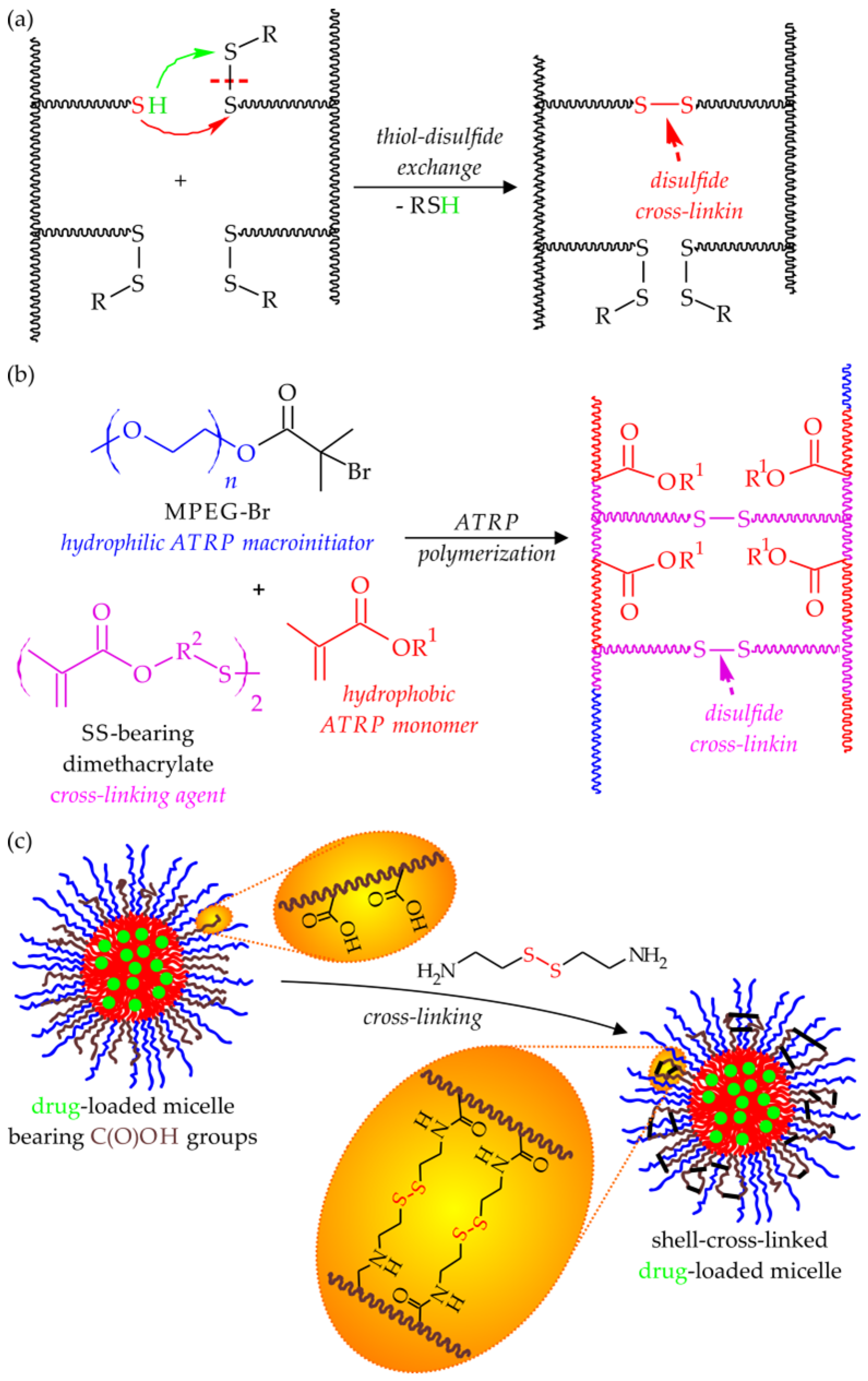
Disulfide-Bearing Reduction-Responsive Systems
3.1.4. Micelles Responsive to External Stimuli
3.2. Smart DDSs Based on Polymersomes
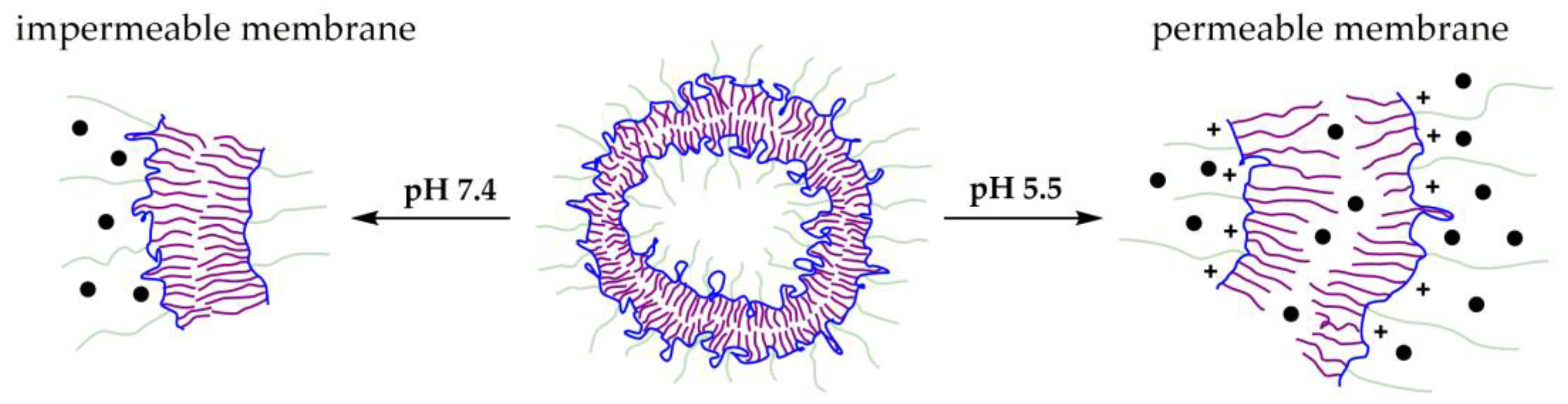
3.2.1. pH-Triggered Drug Release
3.2.2. Miscellaneous Systems
3.3. Polyplexes
3.3.1. Nucleic Acid Delivery
3.3.2. Simultaneous Nucleic Acid and Drug Delivery
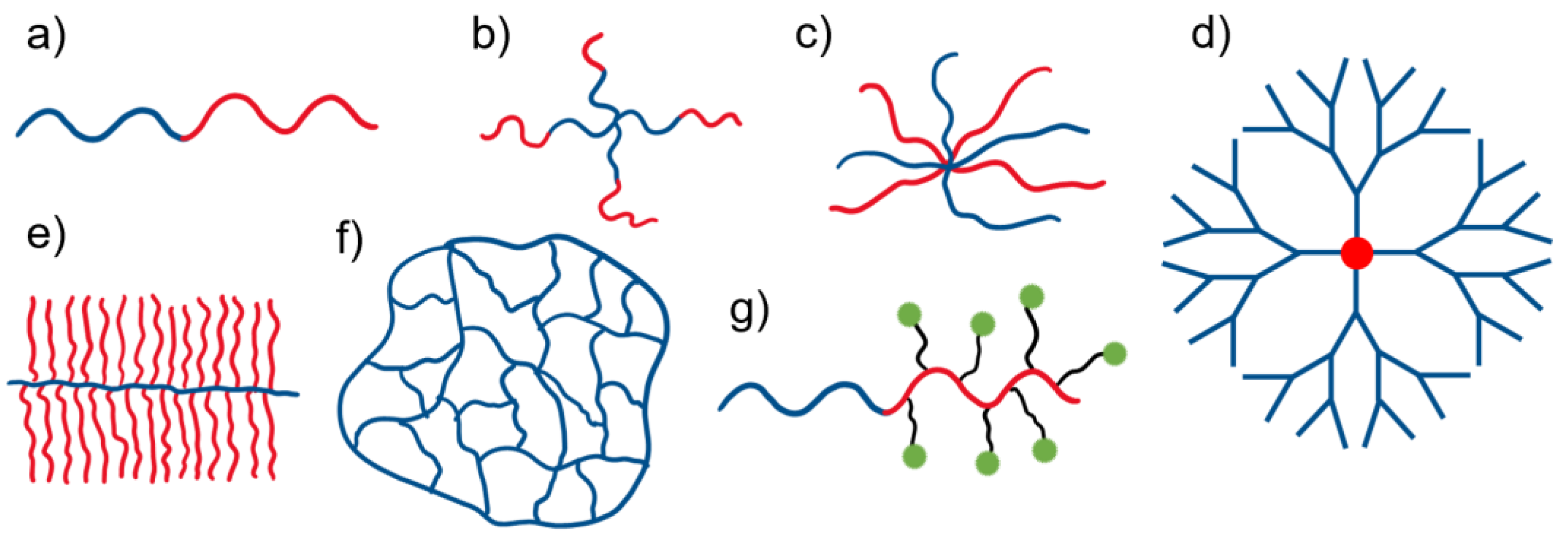
4. Branched Copolymers in DDSs
4.1. Polymer Stars
4.2. Polymer Combs and Brushes
5. Smart DDSs Based on Nanoparticles Coated with Polymers Obtained via ATRP
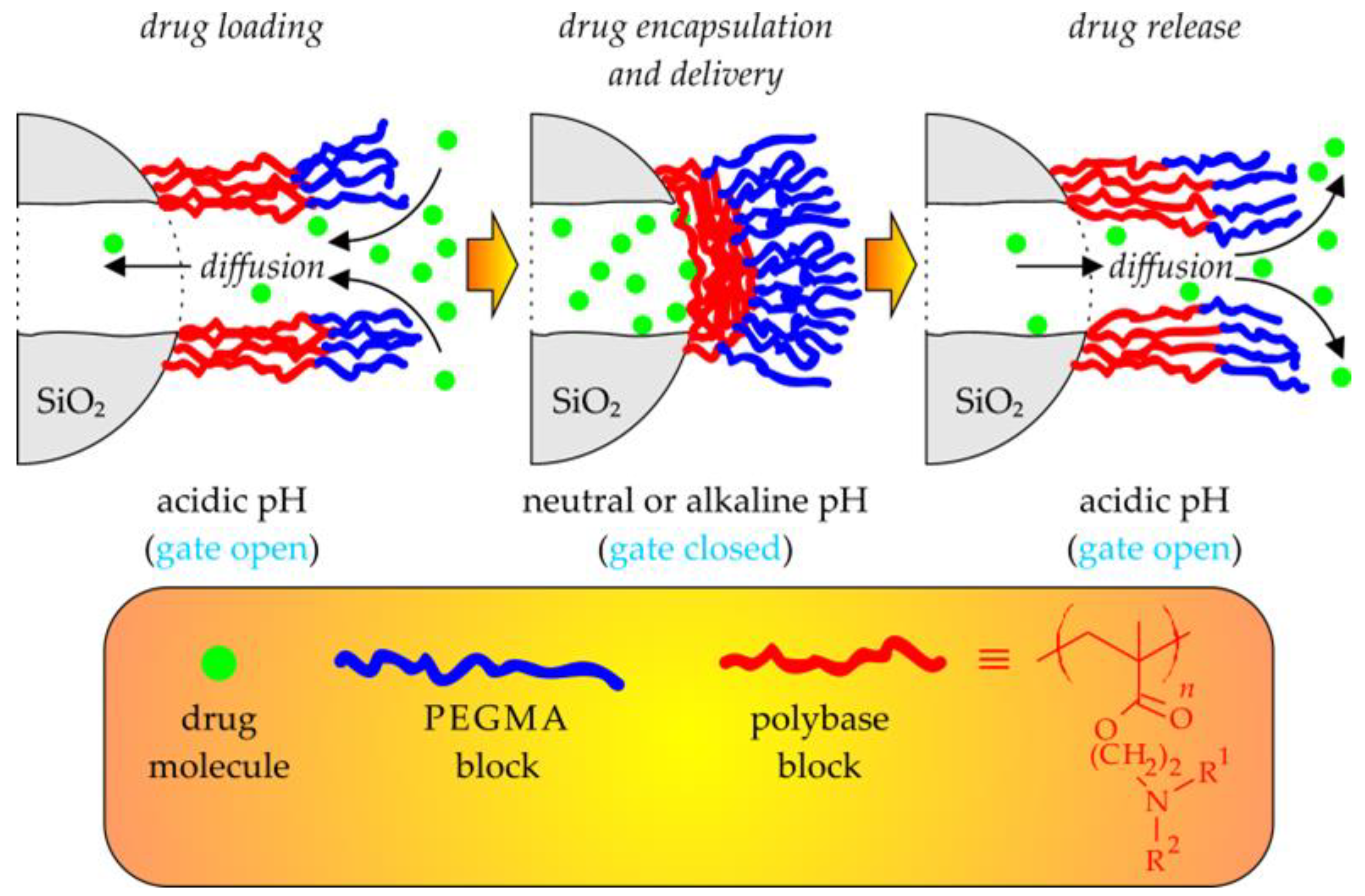
5.1. Metal Oxide-Based Nanocarriers
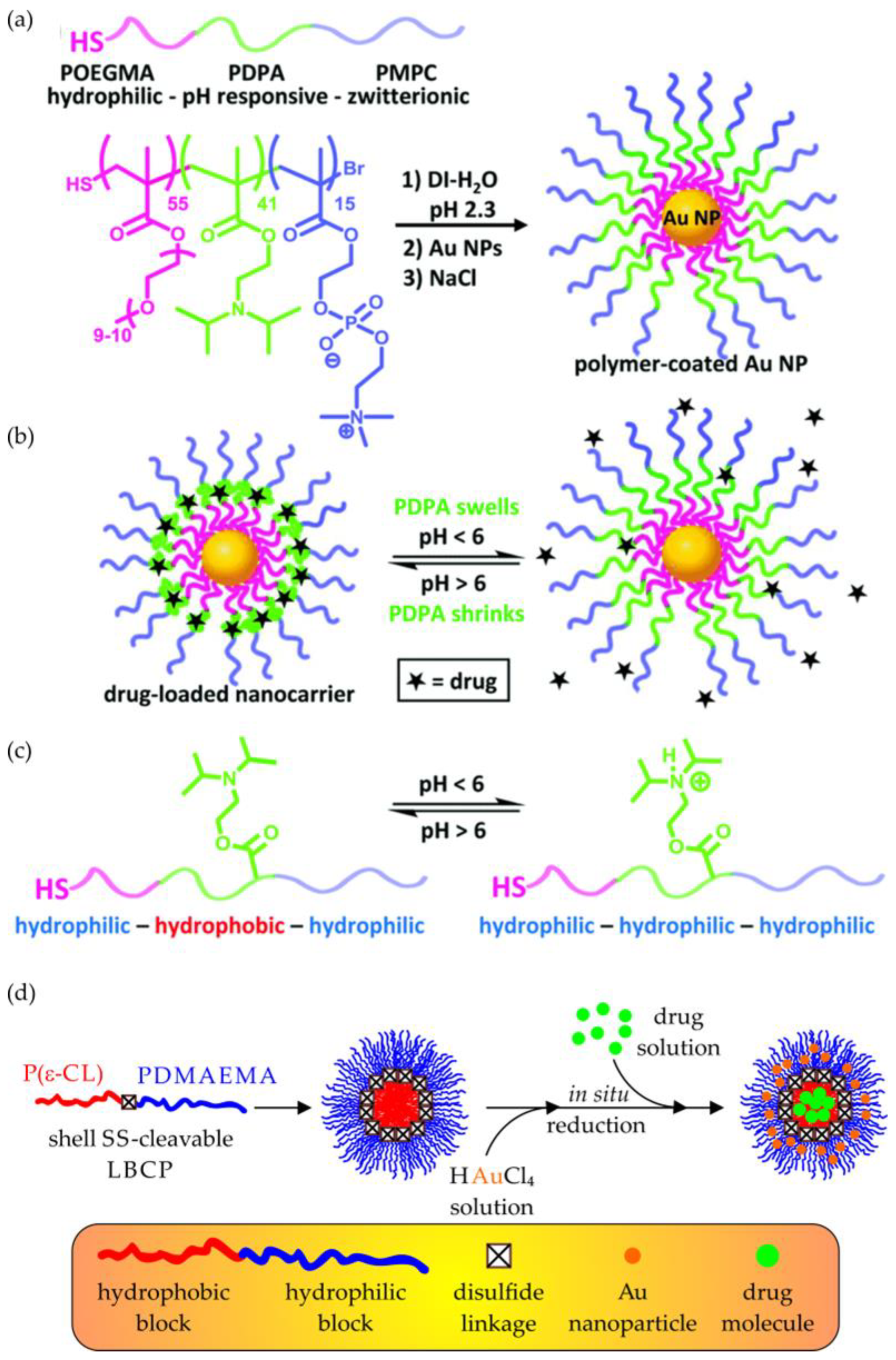
5.2. Metal-Based Nanocarriers
6. Bioconjugates
6.1. Protein–Polymer Conjugates
6.2. Drug–Polymer Conjugates
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Widder, K.J. Controlled Release Delivery Systems; Wiley Online Library: New York, NY, USA; Marcel Dekker, Inc.: New York, NY, USA, 1983. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. Columb. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug. Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.C. Targeted drug conjugates: Principles and progress. Adv. Drug Deliv. Rev. 2001, 53, 171–216. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Vicent, M.J. Polymer-drug conjugates: Current status and future trends. Front. Biosci.-Landmark 2008, 13, 2744–2756. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961. [Google Scholar] [CrossRef]
- Goodman, L.S.; Gilman, A.G. Goodman & Gilman’s Pharmacological Basis of Therapeutics, 13th ed.; McGraw-Hill Education LLC: New York, NY, USA, 2017. [Google Scholar]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for drug delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Wei, H.; Zhuo, R.-X.; Zhang, X.-Z. Design and development of polymeric micelles with cleavable links for intracellular drug delivery. Prog. Polym. Sci. 2013, 38, 503–535. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular design and preparation of polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Anglin, E.J.; Cheng, L.; Freeman, W.R.; Sailor, M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Fouassier, J.; Allonas, X.; Lalevée, J. Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Corbin, D.A.; Miyake, G.M. Photoinduced organocatalyzed atom transfer radical polymerization (O-ATRP): Precision polymer synthesis using organic photoredox catalysis. Chem. Rev. 2021, 122, 1830–1874. [Google Scholar] [CrossRef]
- Kreutzer, J. Atom-transfer radical polymerization: New method breathes life into ATRP. Nat. Rev. Chem. 2018, 2, 0111. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Atilla Tasdelen, M.; Yagci, Y. Photoinitiated atom transfer radical polymerization: Current status and future perspectives. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2878–2888. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. Atom transfer radical polymerization (ATRP). In Fundamentals of Conrolled/Living Radical Polymerization; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 287–357. [Google Scholar] [CrossRef]
- Ayres, N. Atom transfer radical polymerization: A robust and versatile route for polymer synthesis. Polym. Rev. 2011, 51, 138–162. [Google Scholar] [CrossRef]
- Pintauer, T.; Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 2008, 37, 1087–1097. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” atom transfer radical polymerization: From process design to preparation of well-defined environmentally friendly polymeric materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef]
- Faucher, S.; Zhu, S. Fundamentals and development of high-efficiency supported catalyst systems for atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 553–565. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. Environmentally benign atom transfer radical polymerization: Towards “green” processes and materials. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5098–5112. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Beers, K.L. The first dive into the mechanism and kinetics of ATRP. Macromolecules 2020, 53, 1115–1118. [Google Scholar] [CrossRef]
- Curran, D.P. The Design and Application of Free Radical Chain Reactions in Organic Synthesis. Part 1. Synthesis 2002, 1998, 417–439. [Google Scholar] [CrossRef]
- Hawker, C.J.; Barclay, G.G.; Orellana, A.; Dao, J.; Devonport, W. Initiating systems for nitroxide-mediated “living” free radical polymerizations: Synthesis and evaluation. Macromolecules 1996, 29, 5245–5254. [Google Scholar] [CrossRef]
- Goto, A.; Sato, K.; Tsujii, Y.; Fukuda, T.; Moad, G.; Rizzardo, E.; Thang, S.H. Mechanism and kinetics of RAFT-based living radical polymerizations of styrene and methyl methacrylate. Macromolecules 2001, 34, 402–408. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Li, J.-J.; Wang, T.-T.; Wu, Y.-Y.; Luo, Z.-H. Precision Polymer Synthesis by Controlled Radical Polymerization: Fusing the progress from Polymer Chemistry and Reaction Engineering. Prog. Polym. Sci. 2022, 101555. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Fundamentals of Atom Transfer Radical Polymerization. In Handbook of Radical Polymerization; Matyjaszewski, K.D., Thomas, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 523–628. [Google Scholar] [CrossRef]
- Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: A Mechanistic Perspective. J. Am. Chem. Soc. 2022, 144, 15413–15430. [Google Scholar] [CrossRef]
- Fung, A.K.; Coote, M.L. A mechanistic perspective on atom transfer radical polymerization. Polym. Int. 2021, 70, 918–926. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Matyjaszewski, K. The effect of ligands on copper-mediated atom transfer radical polymerization. In Transition Metal Catalysis in Macromolecular Design; ACS Publications: Washington, DC, USA, 2000; pp. 207–223. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Göbelt, B.; Paik, H.-j.; Horwitz, C.P. Tridentate nitrogen-based ligands in Cu-based ATRP: A structure− activity study. Macromolecules 2001, 34, 430–440. [Google Scholar] [CrossRef]
- Chen, X.P.; Qiu, K.Y. ‘Living’radical polymerization of styrene with AIBN/FeCl3/PPh3 initiating system via a reverse atom transfer radical polymerization process. Polym. Int. 2000, 49, 1529–1533. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Matyjaszewski, K. Iron catalysts in atom transfer radical polymerization. Molecules 2020, 25, 1648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xiao, G.; Yan, D. Synthesis of aromatic polyethersulfone-based graft copolyacrylates via ATRP catalyzed by FeCl2/isophthalic acid. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2943–2950. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Iron-catalyzed atom transfer radical polymerization. Polym. Chem. 2015, 6, 1660–1687. [Google Scholar] [CrossRef]
- Plichta, A.; Li, W.; Matyjaszewski, K. ICAR ATRP of styrene and methyl methacrylate with Ru (Cp*) Cl (PPh3) 2. Macromolecules 2009, 42, 2330–2332. [Google Scholar] [CrossRef]
- He, D.; Noh, S.K.; Lyoo, W.S. In situ-generated Ru (III)-mediated ATRP from the polymeric Ru (III) complex in the absence of activator generation agents. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4594–4602. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Lai, Q.; Yang, X.; Luo, P.; Zhang, B.; Zhang, X.; Wei, Y. Recent development and advances on fabrication and biomedical applications of Ga-based liquid metal micro/nanoparticles. Compos. Part B Eng. 2022, 110384. [Google Scholar] [CrossRef]
- Noto, N.; Saito, S. Arylamines as More Strongly Reducing Organic Photoredox Catalysts than fac-[Ir (ppy) 3]. ACS Catal. 2022, 12, 15400–15415. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, L.; Cheng, Z.; Zhu, X. Activators generated by electron transfer for atom transfer radical polymerization: Recent advances in catalyst and polymer chemistry. Polym. Chem. 2012, 3, 2685–2697. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Tang, W.; Matyjaszewski, K. Successful chain extension of polyacrylate and polystyrene macroinitiators with methacrylates in an ARGET and ICAR ATRP. Macromolecules 2007, 40, 6464–6472. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, J.; Cheng, Z.; Zhu, X. Iron-Mediated ICAR ATRP of Styrene and Methyl Methacrylate in the Absence of Thermal Radical Initiator. Macromol. Rapid Commun. 2010, 31, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Wang, Y.; Zhong, M.; Krys, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Reversible-deactivation radical polymerization in the presence of metallic copper. A critical assessment of the SARA ATRP and SET-LRP mechanisms. Macromolecules 2013, 46, 8749–8772. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Magenau, A.; Gennaro, A.; Strandwitz, N.C. Electrochemically mediated atom transfer radical polymerization. Science 2017, 332, 81–84. [Google Scholar] [CrossRef]
- Mohapatra, H.; Kleiman, M.; Esser-Kahn, A.P. Mechanically controlled radical polymerization initiated by ultrasound. Nat. Chem. 2017, 9, 135–139. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Pan, X.; Fu, L.; Lathwal, S.; Olszewski, M.; Yan, J.; Enciso, A.E.; Wang, Z.; Xia, H. Ultrasonication-induced aqueous atom transfer radical polymerization. ACS Macro Lett. 2018, 7, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Soly, S.; Mistry, B.; Murthy, C. Photo-mediated metal-free atom transfer radical polymerization: Recent advances in organocatalysts and perfection towards polymer synthesis. Polym. Int. 2022, 71, 159–168. [Google Scholar] [CrossRef]
- Dworakowska, S.; Lorandi, F.; Gorczyński, A.; Matyjaszewski, K. Toward Green Atom Transfer Radical Polymerization: Current Status and Future Challenges. Adv. Sci. 2022, 2106076. [Google Scholar] [CrossRef]
- Berbigier, J.F.; Teixeira Alves Duarte, L.G.; Zawacki, M.F.; de Araujo, B.B.; Moura Santos, C.d.; Atvars, T.D.Z.; Gonçalves, P.F.B.; Petzhold, C.L.; Rodembusch, F.S. ATRP initiators based on proton transfer benzazole dyes: Solid-state photoactive polymer with very large Stokes shift. ACS Appl. Polym. Mater. 2020, 2, 1406–1416. [Google Scholar] [CrossRef]
- Qi, X.; Yan, H.; Li, Y. ATRP-based synthesis of a pH-sensitive amphiphilic block polymer and its self-assembled micelles with hollow mesoporous silica as DOX carriers for controlled drug release. RSC Adv. 2021, 11, 29986–29996. [Google Scholar] [CrossRef]
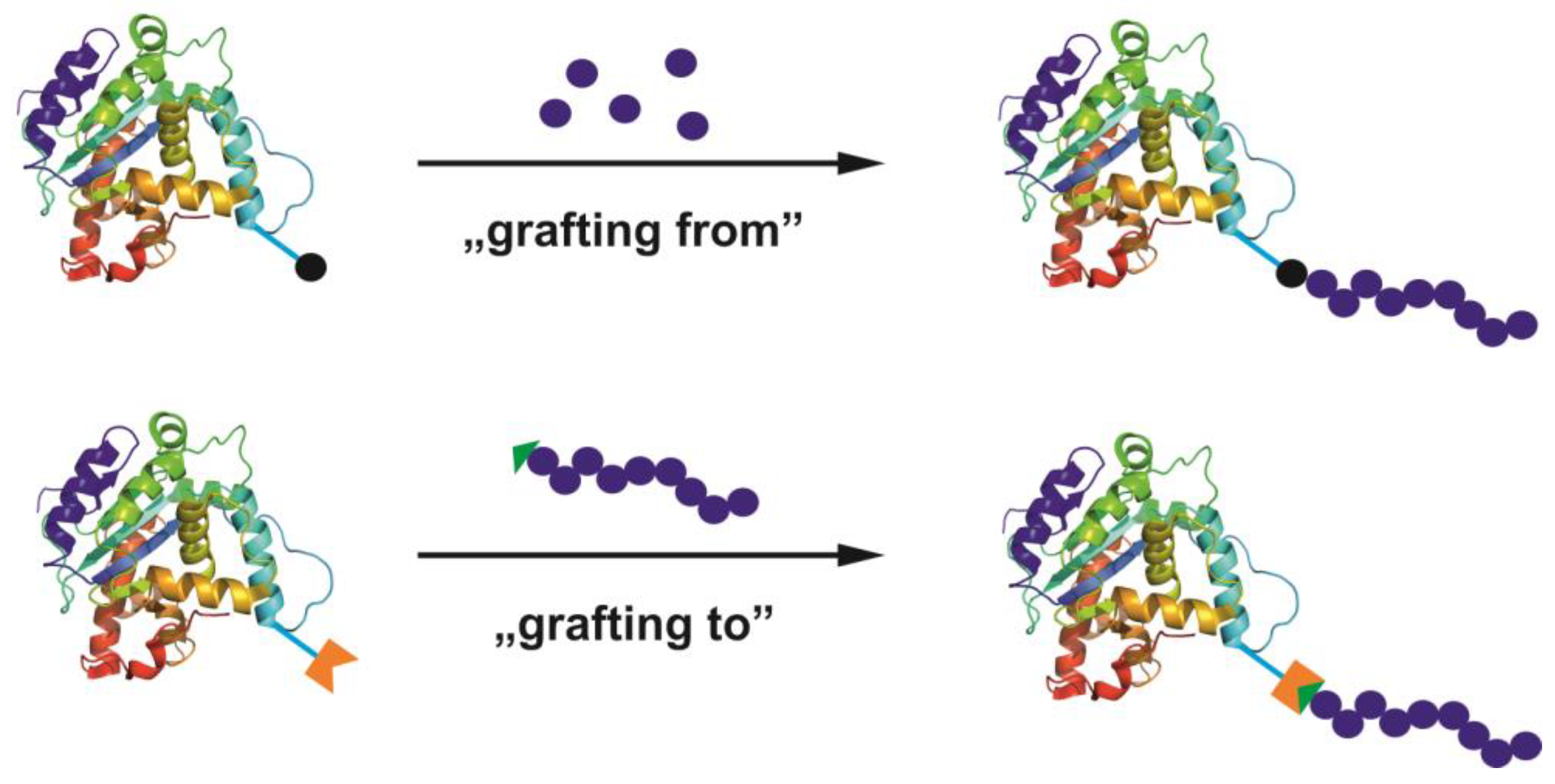
- Sumerlin, B.S. Proteins as initiators of controlled radical polymerization: Grafting-from via ATRP and RAFT. ACS Macro Lett. 2012, 1, 141–145. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McRae, S.; Parelkar, S.; Emrick, T. Polymeric Phosphorylcholine−Camptothecin Conjugates Prepared by Controlled Free Radical Polymerization and Click Chemistry. Bioconjugate Chem. 2009, 20, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Mei, L.; Rui, B.; Jin-Jun, Q.; Fen, H.; Yan, X.; Chen, Z.; Yun, Z. Coumarin end-capped polystyrene by ATRP and photodimerization reaction. Polym. Bull. 2006, 57, 139–149. [Google Scholar] [CrossRef]
- Mansfeld, U.; Pietsch, C.; Hoogenboom, R.; Becer, C.R.; Schubert, U.S. Clickable initiators, monomers and polymers in controlled radical polymerizations—A prospective combination in polymer science. Polym. Chem. 2010, 1, 1560–1598. [Google Scholar] [CrossRef]
- Coad, B.R.; Styan, K.E.; Meagher, L. One step ATRP initiator immobilization on surfaces leading to gradient-grafted polymer brushes. ACS Appl. Mater. Interfaces 2014, 6, 7782–7789. [Google Scholar] [CrossRef]
- Ślusarczyk, K.; Flejszar, M.; Chmielarz, P. Less is more: A review of μL-scale of SI-ATRP in polymer brushes synthesis. Polymer 2021, 233, 124212. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Boyce, J.R.; Shirvanyants, D.; Sheiko, S.S.; Ivanov, D.A.; Qin, S.; Börner, H.; Matyjaszewski, K. Multiarm molecular brushes: Effect of the number of arms on the molecular weight polydispersity and surface ordering. Langmuir 2004, 20, 6005–6011. [Google Scholar] [CrossRef]
- Nasrullah, M.J.; Vora, A.; Webster, D.C. Block copolymer synthesis via a combination of ATRP and RAFT using click chemistry. Macromol. Chem. Phys. 2011, 212, 539–549. [Google Scholar] [CrossRef]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.-S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Basbug Alhan, H. Linear block copolymer synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef]
- Plichta, A.; Jaskulski, T.; Lisowska, P.; Macios, K.; Kundys, A. Elastic polyesters improved by ATRP as reactive epoxy-modifiers of PLA. Polymer 2015, 72, 307–316. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Block copolymer solution self-assembly: Recent advances, emerging trends, and applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Plichta, A.; Zhong, M.; Li, W.; Elsen, A.M.; Matyjaszewski, K. Tuning dispersity in diblock copolymers using ARGET ATRP. Macromol. Chem. Phys. 2012, 213, 2659–2668. [Google Scholar] [CrossRef]
- Listak, J.; Jia, X.; Plichta, A.; Zhong, M.; Matyjaszewski, K.; Bockstaller, M.R. Effect of block molecular weight distribution on the structure formation in block copolymer/homopolymer blends. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 106–116. [Google Scholar] [CrossRef]
- Listak, J.; Jakubowski, W.; Mueller, L.; Plichta, A.; Matyjaszewski, K.; Bockstaller, M.R. Effect of symmetry of molecular weight distribution in block copolymers on formation of “metastable” morphologies. Macromolecules 2008, 41, 5919–5927. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Mendonça, P.V.; Simões, S.; Serra, A.C.; Coelho, J.F. Amphiphilic well-defined degradable star block copolymers by combination of ring-opening polymerization and atom transfer radical polymerization: Synthesis and application as drug delivery carriers. J. Polym. Sci. 2021, 59, 211–229. [Google Scholar] [CrossRef]
- Mühlebach, A.; Gaynor, S.G.; Matyjaszewski, K. Synthesis of amphiphilic block copolymers by atom transfer radical polymerization (ATRP). Macromolecules 1998, 31, 6046–6052. [Google Scholar] [CrossRef]
- Hua, M.; Kaneko, T.; Liu, X.-Y.; Chen, M.-q.; Akashi, M. Successful ATRP syntheses of amphiphilic block copolymers poly (styrene-block-N, N-dimethylacrylamide) and their self-assembly. Polym. J. 2005, 37, 59–64. [Google Scholar] [CrossRef]
- Wei, H.; Perrier, S.; Dehn, S.; Ravarian, R.; Dehghani, F. One-pot ATRP synthesis of a triple hydrophilic block copolymer with dual LCSTs and its thermo-induced association behavior. Soft Matter 2012, 8, 9526–9528. [Google Scholar] [CrossRef]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-assembly of amphiphilic compounds as a versatile tool for construction of nanoscale drug carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef]
- He, W.; Jiang, H.; Zhang, L.; Cheng, Z.; Zhu, X. Atom transfer radical polymerization of hydrophilic monomers and its applications. Polym. Chem. 2013, 4, 2919–2938. [Google Scholar] [CrossRef]
- Colombani, O.; Ruppel, M.; Schubert, F.; Zettl, H.; Pergushov, D.V.; Müller, A.H. Synthesis of poly (n-butyl acrylate)-block-poly (acrylic acid) diblock copolymers by ATRP and their micellization in water. Macromolecules 2007, 40, 4338–4350. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef]
- Prasad, P.V.; Purkayastha, K.; Sharma, U.; Barik, M. Ph-sensitive nanomedicine for treating gynaecological cancers. J. Womans Reprod. Health 2020, 2, 35. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L. Synthesis, self-assembly and pH sensitivity of PDEAEMA–PEG–PDEAEMA triblock copolymer micelles for drug delivery. React. Funct. Polym. 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Gu, X.; Wang, G. Self-assembly, pH-responsibility and controlled release of doxorubicin of PDEAEMA-PEG-PDEAEMA triblock copolymers: Effects of PEG length. J. Polym. Res. 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Biswas, D.; An, S.Y.; Li, Y.; Wang, X.; Oh, J.K. Intracellular delivery of colloidally stable core-cross-linked triblock copolymer micelles with glutathione-responsive enhanced drug release for cancer therapy. Mol. Pharm. 2017, 14, 2518–2528. [Google Scholar] [CrossRef]
- Zeng, Z.; Wei, Z.; Ma, L.; Xu, Y.; Xing, Z.; Niu, H.; Wang, H.; Huang, W. pH-Responsive nanoparticles based on ibuprofen prodrug as drug carriers for inhibition of primary tumor growth and metastasis. J. Mater. Chem. B 2017, 5, 6860–6868. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, T.; Dong, H.; Xu, J.; Wang, D.; Tong, H.; Ji, X.; Sun, B.; Zhu, M.; Jiang, X. Novel block glycopolymers prepared as delivery nanocarriers for controlled release of bortezomib. Colloid Polym. Sci. 2018, 296, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, W.; Xiao, J.; Yuan, C.; Chen, Y.; Guo, J.; Yue, H.; Zhu, D.; Lin, W.; Tang, S. pH-sensitive mixed micelles assembled from PDEAEMA-PPEGMA and PCL-PPEGMA for doxorubicin delivery: Experimental and DPD simulations study. Pharmaceutics 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Zhang, Z.; Ji, Y. Responsive polymeric drug delivery systems for combination anticancer therapy: Experimental design and computational insights. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1221–1239. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. The Ph paradigm in cancer. Eur. J. Clin. Nutr. 2020, 74, 14–19. [Google Scholar] [CrossRef]
- Kumar, R.; Santa Chalarca, C.F.; Bockman, M.R.; Bruggen, C.V.; Grimme, C.J.; Dalal, R.J.; Hanson, M.G.; Hexum, J.K.; Reineke, T.M. Polymeric delivery of therapeutic nucleic acids. Chem. Rev. 2021, 121, 11527–11652. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Sardon, H.; Mecerreyes, D. Current trends in redox polymers for energy and medicine. Prog. Polym. Sci. 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, W.; Wang, H.; Wang, J.; Zhang, L. PDEAEMA-based pH-sensitive amphiphilic pentablock copolymers for controlled anticancer drug delivery. RSC Adv. 2016, 6, 68018–68027. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Yuan, X.; Wang, J.; Zhang, L. Folic acid grafted and tertiary amino based pH-responsive pentablock polymeric micelles for targeting anticancer drug delivery. Mater. Sci. Eng. C 2018, 82, 1–9. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, J.; Xiao, W.; Lin, W.; Chen, J.; Chen, Q.; Zhang, L.; Zhang, C.; Guo, J. Fabrication of PDEAEMA-based pH-responsive mixed micelles for application in controlled doxorubicin release. RSC Adv. 2017, 7, 27564–27573. [Google Scholar] [CrossRef]
- Li, Y.; Yu, A.; Li, L.; Zhai, G. The development of stimuli-responsive polymeric micelles for effective delivery of chemotherapeutic agents. J. Drug Target. 2018, 26, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Jazani, A.M.; Oh, J.K. Development and disassembly of single and multiple acid-cleavable block copolymer nanoassemblies for drug delivery. Polym. Chem. 2020, 11, 2934–2954. [Google Scholar] [CrossRef]
- Hu, X.; Jazani, A.M.; Oh, J.K. Recent advances in development of imine-based acid-degradable polymeric nanoassemblies for intracellular drug delivery. Polymer 2021, 230, 124024. [Google Scholar] [CrossRef]
- Gannimani, R.; Walvekar, P.; Naidu, V.R.; Aminabhavi, T.M.; Govender, T. Acetal containing polymers as pH-responsive nano-drug delivery systems. J. Control. Release 2020, 328, 736–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Dong, B.-T.; Du, F.-S.; Li, Z.-C. Degradable thermoresponsive polyesters by atom transfer radical polyaddition and click chemistry. Macromolecules 2012, 45, 8580–8587. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Ji, R.; Huang, X.-N.; Du, F.-S.; Zhang, R.; Liang, D.-H.; Li, Z.-C. Polymersomes from dual responsive block copolymers: Drug encapsulation by heating and acid-triggered release. Biomacromolecules 2013, 14, 1555–1563. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, X.; Wang, Y.; Liu, F.; Peng, J.; Zhao, X.; Yang, H.; Ma, L.; Wang, B.; Chang, C. Fabrication of acidic pH-cleavable polymer for anticancer drug delivery using a dual functional monomer. Biomacromolecules 2018, 19, 3874–3882. [Google Scholar] [CrossRef]
- Khorsand, B.; Oh, J.K. pH-responsive destabilization and facile bioconjugation of new hydroxyl-terminated block copolymer micelles. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1620–1629. [Google Scholar] [CrossRef]
- Cao, H.; Chen, C.; Xie, D.; Chen, X.; Wang, P.; Wang, Y.; Song, H.; Wang, W. A hyperbranched amphiphilic acetal polymer for pH-sensitive drug delivery. Polym. Chem. 2018, 9, 169–177. [Google Scholar] [CrossRef]
- Song, C.-C.; Su, C.-C.; Cheng, J.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Toward tertiary amine-modulated acid-triggered hydrolysis of copolymers containing pendent ortho ester groups. Macromolecules 2013, 46, 1093–1100. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, X.; Liu, J.; Zhang, J.; Deng, L.; Liu, J.; Dong, A. Synergistic dual-pH responsive copolymer micelles for pH-dependent drug release. Nanoscale 2016, 8, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef]
- Patil, S.S.; Wadgaonkar, P.P. Temperature and pH dual stimuli responsive PCL-b-PNIPAA m block copolymer assemblies and the cargo release studies. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1383–1396. [Google Scholar] [CrossRef]
- Babikova, D.; Kalinova, R.; Momekova, D.; Ugrinova, I.; Momekov, G.; Dimitrov, I. Multifunctional polymer nanocarrier for efficient targeted cellular and subcellular anticancer drug delivery. ACS Biomater. Sci. Eng. 2019, 5, 2271–2283. [Google Scholar] [CrossRef]
- Jazani, A.M.; Shetty, C.; Movasat, H.; Bawa, K.K.; Oh, J.K. Imidazole-Mediated Dual Location Disassembly of Acid-Degradable Intracellular Drug Delivery Block Copolymer Nanoassemblies. Macromol. Rapid Commun. 2021, 42, 2100262. [Google Scholar] [CrossRef]
- Hao, Y.; He, J.; Li, S.; Liu, J.; Zhang, M.; Ni, P. Synthesis of an acid-cleavable and fluorescent amphiphilic block copolymer as a combined delivery vector of DNA and doxorubicin. J. Mater. Chem. B 2014, 2, 4237–4249. [Google Scholar] [CrossRef]
- Babikova, D.; Kalinova, R.; Zhelezova, I.; Momekova, D.; Konstantinov, S.; Momekov, G.; Dimitrov, I. Functional block copolymer nanocarriers for anticancer drug delivery. RSC Adv. 2016, 6, 84634–84644. [Google Scholar] [CrossRef]
- Kamenova, K.; Grancharov, G.; Kortenova, V.; Petrov, P.D. Redox-Responsive Crosslinked Mixed Micelles for Controllable Release of Caffeic Acid Phenethyl Ester. Pharmaceutics 2022, 14, 679. [Google Scholar] [CrossRef]
- Mirhadi, E.; Mashreghi, M.; Maleki, M.F.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, Y. Recent advances in redox-responsive nanoparticles for combined cancer therapy. Nanoscale Adv. 2022, 4, 3504–3516. [Google Scholar] [CrossRef]
- Zhang, M.; Song, C.-C.; Ji, R.; Qiao, Z.-Y.; Yang, C.; Qiu, F.-Y.; Liang, D.-H.; Du, F.-S.; Li, Z.-C. Oxidation and temperature dual responsive polymers based on phenylboronic acid and N-isopropylacrylamide motifs. Polym. Chem. 2016, 7, 1494–1504. [Google Scholar] [CrossRef]
- Zhang, M.; Song, C.-C.; Su, S.; Du, F.-S.; Li, Z.-C. ROS-activated ratiometric fluorescent polymeric nanoparticles for self-reporting drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 7798–7810. [Google Scholar] [CrossRef] [PubMed]
- Stubelius, A.; Lee, S.; Almutairi, A. The chemistry of boronic acids in nanomaterials for drug delivery. Acc. Chem. Res. 2019, 52, 3108–3119. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-C.; Ji, R.; Du, F.-S.; Liang, D.-H.; Li, Z.-C. Oxidation-accelerated hydrolysis of the ortho ester-containing acid-labile polymers. ACS Macro Lett. 2013, 2, 273–277. [Google Scholar] [CrossRef]
- Hermanson, G. The Reactions of Bioconjugation. In Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 229–258. [Google Scholar] [CrossRef]
- Oh, J.K. Disassembly and tumor-targeting drug delivery of reduction-responsive degradable block copolymer nanoassemblies. Polym. Chem. 2019, 10, 1554–1568. [Google Scholar] [CrossRef]
- Ko, N.R.; Oh, J.K. Glutathione-triggered disassembly of dual disulfide located degradable nanocarriers of polylactide-based block copolymers for rapid drug release. Biomacromolecules 2014, 15, 3180–3189. [Google Scholar] [CrossRef]
- Chan, N.; An, S.Y.; Oh, J.K. Dual location disulfide degradable interlayer-crosslinked micelles with extended sheddable coronas exhibiting enhanced colloidal stability and rapid release. Polym. Chem. 2014, 5, 1637–1649. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Huang, S.-J.; Huang, X.-S.; Wu, Y.-T.; Chen, H.-Y.; Lo, Y.-L.; Wang, L.-F. The synthesis and comparison of poly (methacrylic acid)–poly (ε-caprolactone) block copolymers with and without symmetrical disulfide linkages in the center for enhanced cellular uptake. RSC Adv. 2016, 6, 75092–75103. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Huang, X.-S.; Chen, H.-Y.; Huang, Y.-C.; Liao, Z.-X.; Wang, L.-F. ROP and ATRP fabricated redox sensitive micelles based on PCL-SS-PMAA diblock copolymers to co-deliver PTX and CDDP for lung cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111443. [Google Scholar] [CrossRef]
- Li, S.-X.; Liu, L.; Zhang, L.-J.; Wu, B.; Wang, C.-X.; Zhou, W.; Zhuo, R.-X.; Huang, S.-W. Synergetic enhancement of antitumor efficacy with charge-reversal and reduction-sensitive polymer micelles. Polym. Chem. 2016, 7, 5113–5122. [Google Scholar] [CrossRef]
- Ko, N.R.; Cheong, J.; Noronha, A.; Wilds, C.J.; Oh, J.K. Reductively-sheddable cationic nanocarriers for dual chemotherapy and gene therapy with enhanced release. Colloids Surf. B Biointerfaces 2015, 126, 178–187. [Google Scholar] [CrossRef]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Modulated morphologies and tunable thiol-responsive shedding of aqueous block copolymer aggregates. RSC Adv. 2012, 2, 8079–8086. [Google Scholar] [CrossRef]
- An, S.Y.; Hong, S.H.; Tang, C.; Oh, J.K. Rosin-based block copolymer intracellular delivery nanocarriers with reduction-responsive sheddable coronas for cancer therapy. Polym. Chem. 2016, 7, 4751–4760. [Google Scholar] [CrossRef]
- Zhang, Q.; Aleksanian, S.; Noh, S.M.; Oh, J.K. Thiol-responsive block copolymer nanocarriers exhibiting tunable release with morphology changes. Polym. Chem. 2013, 4, 351–359. [Google Scholar] [CrossRef]
- Khorsand, B.; Lapointe, G.; Brett, C.; Oh, J.K. Intracellular drug delivery nanocarriers of glutathione-responsive degradable block copolymers having pendant disulfide linkages. Biomacromolecules 2013, 14, 2103–2111. [Google Scholar] [CrossRef]
- Kumar, P.; Behl, G.; Kaur, S.; Yadav, N.; Liu, B.; Chhikara, A. Tumor microenvironment responsive nanogels as a smart triggered release platform for enhanced intracellular delivery of doxorubicin. J. Biomater. Sci. Polym. Ed. 2021, 32, 385–404. [Google Scholar] [CrossRef]
- Tian, K.; Jia, X.; Zhao, X.; Liu, P. pH/Reductant Dual-Responsive Core-Cross-Linked Micelles via Facile in Situ ATRP for Tumor-Targeted Delivery of Anticancer Drug with Enhanced Anticancer Efficiency. Mol. Pharm. 2016, 13, 2683–2690. [Google Scholar] [CrossRef]
- Chan, N.; Khorsand, B.; Aleksanian, S.; Oh, J.K. A dual location stimuli-responsive degradation strategy of block copolymer nanocarriers for accelerated release. Chem. Commun. 2013, 49, 7534–7536. [Google Scholar] [CrossRef]
- Huang, Y.; Moini Jazani, A.; Howell, E.P.; Oh, J.K.; Moffitt, M.G. Controlled Microfluidic Synthesis of Biological Stimuli-Responsive Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2019, 12, 177–190. [Google Scholar] [CrossRef]
- Huang, Y.; Jazani, A.M.; Howell, E.P.; Reynolds, L.A.; Oh, J.K.; Moffitt, M.G. Microfluidic Shear Processing Control of Biological Reduction Stimuli-Responsive Polymer Nanoparticles for Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 5069–5083. [Google Scholar] [CrossRef]
- Jazani, A.M.; Oh, J.K. Dual location, dual acidic pH/reduction-responsive degradable block copolymer: Synthesis and investigation of ketal linkage instability under ATRP conditions. Macromolecules 2017, 50, 9427–9436. [Google Scholar] [CrossRef]
- Shetty, C.; Noronha, A.; Pontarelli, A.; Wilds, C.J.; Oh, J.K. Dual-location dual-acid/glutathione-degradable cationic micelleplexes through hydrophobic modification for enhanced gene silencing. Mol. Pharm. 2020, 17, 3979–3989. [Google Scholar] [CrossRef]
- Akimoto, J.; Ito, Y.; Okano, T.; Nakayama, M. Controlled aggregation behavior of thermoresponsive polymeric micelles by introducing hydrophilic segments as corona components. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1695–1704. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, X.; Huo, H.; Zhang, Q.; Qu, F.; Chen, F. Preparation of quadruple responsive polymeric micelles combining temperature-, pH-, redox-, and UV-responsive behaviors and its application in controlled release system. J. Appl. Polym. Sci. 2018, 135, 46675. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Lei, L.; Yang, H.; Lei, Z. Synthesis of light and dual-redox triple-stimuli-responsive core-crosslinked micelles as nanocarriers for controlled release. J. Appl. Polym. Sci. 2019, 136, 47946. [Google Scholar] [CrossRef]
- Yuan, W.; Guo, W. Ultraviolet light-breakable and tunable thermoresponsive amphiphilic block copolymer: From self-assembly, disassembly to re-self-assembly. Polym. Chem. 2014, 5, 4259–4267. [Google Scholar] [CrossRef]
- Jazani, A.M.; Oh, J.K. Synthesis of multiple stimuli-responsive degradable block copolymers via facile carbonyl imidazole-induced postpolymerization modification. Polym. Chem. 2022, 13, 4557–4568. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef]
- Trombino, S.; Curcio, F.; Cassano, R. Polymersomes as a promising vehicle for controlled drug delivery. In Stimuli-Responsive Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 351–366. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Vieira, R.P. Advances in atom-transfer radical polymerization for drug delivery applications. Eur. Polym. J. 2019, 115, 45–58. [Google Scholar] [CrossRef]
- Hasannia, M.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of block copolymers used in polymersome fabrication: Application in drug delivery. J. Control. Release 2022, 341, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ramezani, M.; Abnous, K.; Alibolandi, M. Biocompatible polymersomes-based cancer theranostics: Towards multifunctional nanomedicine. Int. J. Pharm. 2017, 519, 287–303. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, D.S. Biodegradable and pH-sensitive polymersome with tuning permeable membrane for drug delivery carrier. Chem. Comm. 2010, 46, 4481–4483. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Adami, R.; Reverchon, E.; Ferretti, A.M.; Ponti, A.; Lepretti, M.; Caputo, I.; Izzo, L. pH-sensitive polymersomes: Controlling swelling via copolymer structure and chemical composition. J. Drug Target. 2017, 25, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Sun, M.; Yang, B.; Xiao, J.; Du, J. Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy. J. Control. Release 2020, 322, 81–94. [Google Scholar] [CrossRef]
- Miele, Y.; Mingotaud, A.-F.; Caruso, E.; Malacarne, M.C.; Izzo, L.; Lonetti, B.; Rossi, F. Hybrid giant lipid vesicles incorporating a PMMA-based copolymer. Biochim. Biophys. Acta BBA Gen. Subj. 2021, 1865, 129611. [Google Scholar] [CrossRef]
- Dadhwal, S.; Lee, A.; Goswami, S.K.; Hook, S.; Gamble, A.B. Synthesis and formulation of self-immolative PEG-aryl azide block copolymers and click-to-release reactivity with trans-cyclooctene. J. Polym. Sci. 2021, 59, 646–658. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric nanomaterials: Recent developments, properties and medical applications. In Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Müller, A.H.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef]
- Ita, K. Polyplexes for gene and nucleic acid delivery: Progress and bottlenecks. Eur. J. Pharm. Sci. 2020, 150, 105358. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Pettit, D.K. Biodegradable polymers for protein and peptide drug delivery. Bioconjugate Chem. 1995, 6, 332–351. [Google Scholar] [CrossRef] [PubMed]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef] [PubMed]
- Machtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. Part A 2019, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- De Ávila Gonçalves, S.; Vieira, R.P. Current status of ATRP-based materials for gene therapy. React. Funct. Polym. 2020, 147, 104453. [Google Scholar] [CrossRef]
- Xu, F.; Yang, W. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099–1131. [Google Scholar] [CrossRef]
- Fliervoet, L.A.; van Nostrum, C.F.; Hennink, W.E.; Vermonden, T. Balancing hydrophobic and electrostatic interactions in thermosensitive polyplexes for nucleic acid delivery. Multifunct. Mater. 2019, 2, 024002. [Google Scholar] [CrossRef]
- Fliervoet, L.A.; Zhang, H.; van Groesen, E.; Fortuin, K.; Duin, N.J.; Remaut, K.; Schiffelers, R.M.; Hennink, W.E.; Vermonden, T. Local release of siRNA using polyplex-loaded thermosensitive hydrogels. Nanoscale 2020, 12, 10347–10360. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Cao, D.; Zhang, M.; Ni, P. Galactosylated reduction and pH dual-responsive triblock terpolymer Gal-PEEP-a-PCL-ss-PDMAEMA: A multifunctional carrier for the targeted and simultaneous delivery of doxorubicin and DNA. Polym. Chem. 2014, 5, 5124–5138. [Google Scholar] [CrossRef]
- Wang, X.; Liow, S.S.; Wu, Q.; Li, C.; Owh, C.; Li, Z.; Loh, X.J.; Wu, Y.L. Codelivery for Paclitaxel and Bcl-2 Conversion Gene by PHB-PDMAEMA Amphiphilic Cationic Copolymer for Effective Drug Resistant Cancer Therapy. Macromol. Biosci. 2017, 17, 1700186. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Gou, P.; Zuo, M.; Li, X.; Zhu, W.; Shen, Z. Amphiphilic seven-arm star triblock copolymers with diverse morphologies in aqueous solution induced by crystallization and pH. Chem. Res. Chin. Univ. 2018, 34, 132–137. [Google Scholar] [CrossRef]
- Huang, B.; Chen, M.; Zhou, S.; Wu, L. Synthesis and properties of clickable A(B-b-C)20 miktoarm star-shaped block copolymers with a terminal alkyne group. Polym. Chem. 2015, 6, 3913–3917. [Google Scholar] [CrossRef]
- Zhang, Y.; Bradley, M.; Geng, J. Photo-controlled one-pot strategy for the synthesis of asymmetric three-arm star polymers. Polym. Chem. 2019, 10, 4769–4773. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Y.-Y.; Niu, L.-Y.; Zhu, J. Self-assemblies of the six-armed star triblock ABC copolymer: pH-tunable morphologies and drug release. Polym. Chem. 2015, 6, 2934–2944. [Google Scholar] [CrossRef]
- Huang, L.-m.; Li, L.-d.; Shang, L.; Zhou, Q.-h.; Lin, J. Preparation of pH-sensitive micelles from miktoarm star block copolymers by ATRP and their application as drug nanocarriers. React. Funct. Polym. 2016, 107, 28–34. [Google Scholar] [CrossRef]
- Neugebauer, D.; Odrobińska, J.; Bielas, R.; Mielańczyk, A. Design of systems based on 4-armed star-shaped polyacids for indomethacin delivery. New J. Chem. 2016, 40, 10002–10011. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Z.; Chen, C.; Allcock, H.R. UV-cleavable unimolecular micelles: Synthesis and characterization toward photocontrolled drug release carriers. Polym. Chem. 2013, 4, 1115–1125. [Google Scholar] [CrossRef]
- Mendrek, B.; Fus, A.; Klarzyńska, K.; Sieroń, A.L.; Smet, M.; Kowalczuk, A.; Dworak, A. Synthesis, Characterization and Cytotoxicity of Novel Thermoresponsive Star Copolymers of N,N′-Dimethylaminoethyl Methacrylate and Hydroxyl-Bearing Oligo(Ethylene Glycol) Methacrylate. Polymers 2018, 10, 1255. [Google Scholar] [CrossRef]
- Zheng, A.; Xue, Y.; Wei, D.; Guan, Y.; Xiao, H. Amphiphilic star block copolymers as gene carrier Part I: Synthesis via ATRP using calix[4]resorcinarene-based initiators and characterization. Mater. Sci. Eng. C 2013, 33, 519–526. [Google Scholar] [CrossRef]
- Mendrek, B.; Sieroń, Ł.; Żymełka-Miara, I.; Binkiewicz, P.; Libera, M.; Smet, M.; Trzebicka, B.; Sieroń, A.L.; Kowalczuk, A.; Dworak, A. Nonviral Plasmid DNA Carriers Based on N,N′-Dimethylaminoethyl Methacrylate and Di(ethylene glycol) Methyl Ether Methacrylate Star Copolymers. Biomacromolecules 2015, 16, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Averick, S.E.; Paredes, E.; Wegner, K.; Averick, A.; Jurga, S.; Das, S.R.; Matyjaszewski, K. Star Polymers with a Cationic Core Prepared by ATRP for Cellular Nucleic Acids Delivery. Biomacromolecules 2013, 14, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Yilmaz, G. One-Pot Synthesis of Star Copolymers by the Combination of Metal-Free ATRP and ROP Processes. Polymers 2019, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- Mielańczyk, A.; Kupczak, M.; Klymenko, O.; Mielańczyk, Ł.; Arabasz, S.; Madej, K.; Neugebauer, D. The structure–self-assembly relationship in PDMAEMA/polyester miktoarm stars. Polym. Chem. 2022, 13, 4763–4775. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, S.-Z.; Luo, Y.-L. Thermosensitive t-PLA-b-PNIPAAm tri-armed star block copolymer nanoscale micelles for camptothecin drug release. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4429–4439. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Liu, Y.; Cao, J.; Feng, T.; Chen, Y.; Luo, X. Synthesis and evaluation of star-shaped poly(ϵ-caprolactone)-poly(2-hydroxyethyl methacrylate) as potential anticancer drug delivery carriers. J. Biomater. Sci. Polym. Ed. 2013, 24, 741–757. [Google Scholar] [CrossRef]
- Xiong, D.; Yao, N.; Gu, H.; Wang, J.; Zhang, L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 2017, 114, 161–172. [Google Scholar] [CrossRef]
- Yuan, H.; Chi, H.; Yuan, W. A star-shaped amphiphilic block copolymer with dual responses: Synthesis, crystallization, self-assembly, redox and LCST–UCST thermoresponsive transition. Polym. Chem. 2016, 7, 4901–4911. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Kupczak, M.; Burek, M.; Mielańczyk, Ł.; Klymenko, O.; Wandzik, I.; Neugebauer, D. Functional (mikto)stars and star-comb copolymers from d-gluconolactone derivative: An efficient route for tuning the architecture and responsiveness to stimuli. Polymer 2018, 146, 331–343. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Lang, M. Synthesis and self-assembly behavior of six-armed block copolymers with pH- and thermo-responsive properties. Macromol. Res. 2011, 19, 113–121. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, P.; Liu, T.; Xin, J.; Zhang, J. Biobased miktoarm star copolymer from soybean oil, isosorbide, and caprolactone. J. Appl. Polym. Sci. 2020, 137, 48281. [Google Scholar] [CrossRef]
- Milner, S.T. Polymer brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef]
- Bousquet, A.; Boyer, C.; Davis, T.P.; Stenzel, M.H. Electrostatic assembly of functional polymer combs onto gold nanoparticle surfaces: Combining RAFT, click and LbL to generate new hybrid nanomaterials. Polym. Chem. 2010, 1, 1186–1195. [Google Scholar] [CrossRef]
- Peng, S.; Bhushan, B. Smart polymer brushes and their emerging applications. RSC Adv. 2012, 2, 8557–8578. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Wu, Z.; Chen, H. Chemical surface modification of polymeric biomaterials for biomedical applications. Macromol. Rapid Commun. 2020, 41, 1900430. [Google Scholar] [CrossRef]
- Celentano, W.; Ordanini, S.; Bruni, R.; Marocco, L.; Medaglia, P.; Rossi, A.; Buzzaccaro, S.; Cellesi, F. Complex poly(ε-caprolactone)/poly(ethylene glycol) copolymer architectures and their effects on nanoparticle self-assembly and drug nanoencapsulation. Eur. Polym. J. 2021, 144, 110226. [Google Scholar] [CrossRef]
- Tu, X.Y.; Meng, C.; Zhang, X.L.; Jin, M.G.; Zhang, X.S.; Zhao, X.Z.; Wang, Y.F.; Ma, L.W.; Wang, B.Y.; Liu, M.Z. Fabrication of Reduction-Sensitive Amphiphilic Cyclic Brush Copolymer for Controlled Drug Release. Macromol. Biosci. 2018, 18, 1800022. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Kumar, A.; Lale, S.V.; Mahajan, S.; Choudhary, V.; Koul, V. ROP and ATRP fabricated dual targeted redox sensitive polymersomes based on pPEGMA-PCL-ss-PCL-pPEGMA triblock copolymers for breast cancer therapeutics. ACS Appl. Mater. Interfaces 2015, 7, 9211–9227. [Google Scholar] [CrossRef]
- Nehate, C.; Moothedathu Raynold, A.A.; Haridas, V.; Koul, V. Comparative assessment of active targeted redox sensitive polymersomes based on pPEGMA-SS-PLA diblock copolymer with marketed nanoformulation. Biomacromolecules 2018, 19, 2549–2566. [Google Scholar] [CrossRef]
- Zhao, P.; Deng, M.; Yang, Y.; Zhang, J.; Zhang, Y. Synthesis and Self-Assembly of Thermoresponsive Biohybrid Graft Copolymers Based on a Combination of Passerini Multicomponent Reaction and Molecular Recognition. Macromol. Rapid Commun. 2021, 42, 2100424. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.R.; Wang, X.; Li, Z.; Lyu, J.; Wang, W.; Vieira, R.P. A new nano hyperbranched β-pinene polymer: Controlled synthesis and nonviral gene delivery. Colloids Surf. B Biointerfaces 2023, 222, 113032. [Google Scholar] [CrossRef] [PubMed]
- Newland, B.; Tai, H.; Zheng, Y.; Velasco, D.; Di Luca, A.; Howdle, S.M.; Alexander, C.; Wang, W.; Pandit, A. A highly effective gene delivery vector–hyperbranched poly(2-(dimethylamino)ethyl methacrylate) from in situ deactivation enhanced ATRP. Chem. Comm. 2010, 46, 4698–4700. [Google Scholar] [CrossRef] [PubMed]
- Flejszar, M.; Chmielarz, P.; Smenda, J.; Wolski, K. Following principles of green chemistry: Low ppm photo-ATRP of DMAEMA in water/ethanol mixture. Polymer 2021, 228, 123905. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
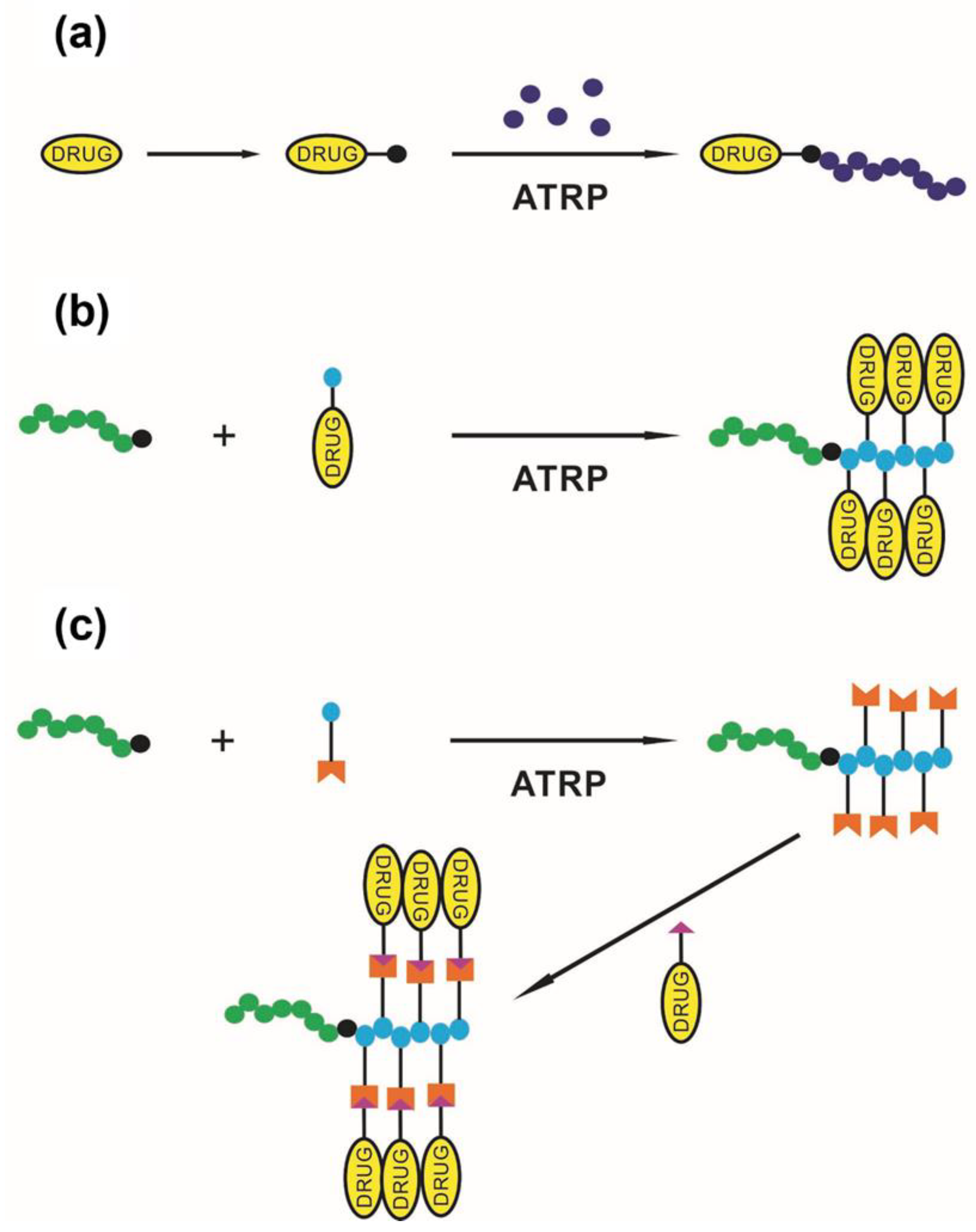
- Cohen-Karni, D.; Kovaliov, M.; Li, S.; Jaffee, S.; Tomycz, N.D.; Averick, S. Fentanyl initiated polymers prepared by atrp for targeted delivery. Bioconjugate Chem. 2017, 28, 1251–1259. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo (ethylene glycol)(meth) acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Li, S.; Cohen-Karni, D.; Kallick, E.; Edington, H.; Averick, S. Post-polymerization functionalization of epoxide-containing copolymers in trifluoroethanol for synthesis of polymer-drug conjugates. Polymer 2016, 99, 59–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Wojnilowicz, M.; Pan, S.; Ju, Y.; Zhang, W.; Liu, J.; Zhuo, R.; Jiang, X. Acid-sensitive poly (β-cyclodextrin)-based multifunctional supramolecular gene vector. Polym. Chem. 2018, 9, 450–462. [Google Scholar] [CrossRef]
- Nehate, C.; Moothedathu Raynold, A.A.; Koul, V. ATRP fabricated and short chain polyethylenimine grafted redox sensitive polymeric nanoparticles for codelivery of anticancer drug and siRNA in cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 39672–39687. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khoddami, E.; Nabiyan, A.; Rezaei, B. Study the role of poly (diethyl aminoethyl methacrylate) as a modified and grafted shell for TiO2 and ZnO nanoparticles, application in flutamide delivery. React. Funct. Polym. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Alfhaid, L.H.; Ahamed, M.; El-Toni, A.M.; Alswieleh, A.M. Poly (oligo (ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly (2-(diethylamino) ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier. Polymers 2021, 13, 823. [Google Scholar] [CrossRef]
- Alswieleh, A.M.; Beagan, A.M.; Alsheheri, B.M.; Alotaibi, K.M.; Alharthi, M.D.; Almeataq, M.S. Hybrid mesoporous silica nanoparticles grafted with 2-(tert-butylamino) ethyl methacrylate-b-poly (ethylene glycol) methyl ether methacrylate diblock brushes as drug nanocarrier. Molecules 2020, 25, 195. [Google Scholar] [CrossRef] [PubMed]
- Alswieleh, A.M.; Alshahrani, M.M.; Alzahrani, K.E.; Alghamdi, H.S.; Niazy, A.A.; Alsilme, A.S.; Beagan, A.M.; Alsheheri, B.M.; Alghamdi, A.A.; Almeataq, M.S. Surface modification of pH-responsive poly (2-(tert-butylamino) ethyl methacrylate) brushes grafted on mesoporous silica nanoparticles. Des. Monomers Polym. 2019, 22, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, W.; Xiao, H.; Chen, Y.; Chen, M.; Li, J. Intelligent drug delivery system based on mesoporous silica nanoparticles coated with an ultra-pH-sensitive gatekeeper and poly (ethylene glycol). ACS Macro Lett. 2016, 5, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yuan, X.; Lin, W.; Cai, C.; Zhang, L. pH-responsive controlled release of mesoporous silica nanoparticles capped with Schiff base copolymer gatekeepers: Experiment and molecular dynamics simulation. Colloids Surf. B Biointerfaces 2019, 176, 394–403. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.; Mao, L.; Xu, D.; Wan, Q.; Zeng, G.; Shi, Y.; Wen, Y.; Zhang, X.; Wei, Y. Preparation and controlled drug delivery applications of mesoporous silica polymer nanocomposites through the visible light induced surface-initiated ATRP. Appl. Surf. Sci. 2017, 412, 571–577. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic acid-terminated poly (2-diethyl amino ethyl methacrylate) brush-gated magnetic mesoporous nanoparticles as a smart drug delivery system. Polymers 2020, 13, 59. [Google Scholar] [CrossRef]
- He, X.; Wu, X.; Cai, X.; Lin, S.; Xie, M.; Zhu, X.; Yan, D. Functionalization of magnetic nanoparticles with dendritic–linear–brush-like triblock copolymers and their drug release properties. Langmuir 2012, 28, 11929–11938. [Google Scholar] [CrossRef]
- Ellis, E.; Zhang, K.; Lin, Q.; Ye, E.; Poma, A.; Battaglia, G.; Loh, X.J.; Lee, T.-C. Biocompatible pH-responsive nanoparticles with a core-anchored multilayer shell of triblock copolymers for enhanced cancer therapy. J. Mater. Chem. B 2017, 5, 4421–4425. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, X.; Peng, S.; Gu, H.; Zhang, L. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloids Surf. B: Biointerfaces 2018, 163, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Lin, W.; Zhang, X.; Gu, H.; Zhang, L. Amphiphilic β-cyclodextrin-based star-like block copolymer unimolecular micelles for facile in situ preparation of gold nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Sinha, P.; Fresta, M.; Ferrari, M. Drug Delivery Systems. In Encyclopedia of Medical Devices and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Huang, Y.; Cole, S.P.C.; Cai, T.; Cai, Y. Applications of nanoparticle drug delivery systems for the reversal of multidrug resistance in cancer (Review). Oncol. Lett. 2016, 12, 11–15. [Google Scholar] [CrossRef]
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.; Minko, T. Polymer–drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359–397. [Google Scholar] [CrossRef]
- Neeraj Agrawal, R.; Alok Mukerji, A.J. Polymeric Prodrugs: Recent Achievements and General Strategies. J. Antivir. Antiretrovir. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional Supramolecular Polymers for Biomedical Applications. Adv. Mater. 2015, 27, 498–526. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Liu, S.; Maheshwari, R.; Kiick, K.L. Polymer-Based Therapeutics. Macromolecules 2009, 42, 3–13. [Google Scholar] [CrossRef]
- Parveen, S.; Arjmand, F.; Tabassum, S. Clinical developments of antitumor polymer therapeutics. RSC Adv. 2019, 9, 24699–24721. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: Billion Times More Active Catalysts and New Initiation Systems. Macromol. Rapid Commun. 2019, 40, 1800616. [Google Scholar] [CrossRef]
- Briand, V.A.; Kumar, C.V.; Kasi, R.M. Protein-Polymer Conjugates. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, F.; Chen, Y.; Bai, J.; Gao, W. Synthesis of well-defined protein–polymer conjugates for biomedicine. Polymer 2015, 66, A1–A10. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C. Site-Specific Conjugation of Polymers to Proteins. Biomacromolecules 2018, 19, 1804–1825. [Google Scholar] [CrossRef]
- Kovaliov, M.; Cohen-Karni, D.; Burridge, K.A.; Mambelli, D.; Sloane, S.; Daman, N.; Xu, C.; Guth, J.; Kenneth Wickiser, J.; Tomycz, N.; et al. Grafting strategies for the synthesis of active DNase I polymer biohybrids. Eur. Polym. J. 2018, 107, 15–24. [Google Scholar] [CrossRef]
- Silva, A.R.P.; Guimarães, M.S.; Rabelo, J.; Belén, L.H.; Perecin, C.J.; Farías, J.G.; Santos, J.H.P.M.; Rangel-Yagui, C.O. Recent advances in the design of antimicrobial peptide conjugates. J. Mater. Chem. B 2022, 10, 3587–3600. [Google Scholar] [CrossRef]
- Pelegri-O’Day, E.M.; Maynard, H.D. Controlled Radical Polymerization as an Enabling Approach for the Next Generation of Protein–Polymer Conjugates. Acc. Chem. Res. 2016, 49, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Kaupbayeva, B.; Boye, S.; Munasinghe, A.; Murata, H.; Matyjaszewski, K.; Lederer, A.; Colina, C.M.; Russell, A.J. Molecular Dynamics-Guided Design of a Functional Protein–ATRP Conjugate That Eliminates Protein–Protein Interactions. Bioconjug. Chem. 2021, 32, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, D.; Heredia, K.L.; Fish, B.A.; Maynard, H.D. Cysteine-Reactive Polymers Synthesized by Atom Transfer Radical Polymerization for Conjugation to Proteins. J. Am. Chem. Soc. 2004, 126, 15372–15373. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Graeff-Armas, L.; Ross, M.; Goldner, W. 35—Hypercalcemia. In Abeloff’s Clinical Oncology, 6th ed.; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 565–571.e1. [Google Scholar] [CrossRef]
- Sayers, C.T.; Mantovani, G.; Ryan, S.M.; Randev, R.K.; Keiper, O.; Leszczyszyn, O.I.; Blindauer, C.; Brayden, D.J.; Haddleton, D.M. Site-specific N-terminus conjugation of poly(mPEG1100) methacrylates to salmon calcitonin: Synthesis and preliminary biological evaluation. Soft Matter 2009, 5, 3038–3046. [Google Scholar] [CrossRef]
- Baker, S.L.; Kaupbayeva, B.; Lathwal, S.; Das, S.R.; Russell, A.J.; Matyjaszewski, K. Atom Transfer Radical Polymerization for Biorelated Hybrid Materials. Biomacromolecules 2019, 20, 4272–4298. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Sasaki, M.; Nabil, A.; Iijima, M.; Ebara, M. Temperature Responsive Polymer Conjugate Prepared by “Grafting from” Proteins toward the Adsorption and Removal of Uremic Toxin. Molecules 2022, 27, 1051. [Google Scholar] [CrossRef]
- Cummings, C.S.; Fein, K.; Murata, H.; Ball, R.L.; Russell, A.J.; Whitehead, K.A. ATRP-grown protein-polymer conjugates containing phenylpiperazine selectively enhance transepithelial protein transport. J. Control. Release 2017, 255, 270–278. [Google Scholar] [CrossRef]
- Trzebicka, B.; Robak, B.; Trzcinska, R.; Szweda, D.; Suder, P.; Silberring, J.; Dworak, A. Thermosensitive PNIPAM-peptide conjugate—Synthesis and aggregation. Eur. Polym. J. 2013, 49, 499–509. [Google Scholar] [CrossRef]
- Cohen-Karni, D.; Kovaliov, M.; Ramelot, T.; Konkolewicz, D.; Graner, S.; Averick, S. Grafting challenging monomers from proteins using aqueous ICAR ATRP under bio-relevant conditions. Polym. Chem. 2017, 8, 3992–3998. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu Catalyst in Water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Moncalvo, F.; Lacroce, E.; Franzoni, G.; Altomare, A.; Fasoli, E.; Aldini, G.; Sacchetti, A.; Cellesi, F. Selective Protein Conjugation of Poly(glycerol monomethacrylate) and Poly(polyethylene glycol methacrylate) with Tunable Topology via Reductive Amination with Multifunctional ATRP Initiators for Activity Preservation. Macromolecules 2022, 55, 7454–7468. [Google Scholar] [CrossRef]
- Kaupbayeva, B.; Murata, H.; Rule, G.S.; Matyjaszewski, K.; Russell, A.J. Rational Control of Protein–Protein Interactions with Protein-ATRP-Generated Protease-Sensitive Polymer Cages. Biomacromolecules 2022, 23, 3831–3846. [Google Scholar] [CrossRef]
- Baker, S.L.; Munasinghe, A.; Murata, H.; Lin, P.; Matyjaszewski, K.; Colina, C.M.; Russell, A.J. Intramolecular Interactions of Conjugated Polymers Mimic Molecular Chaperones to Stabilize Protein–Polymer Conjugates. Biomacromolecules 2018, 19, 3798–3813. [Google Scholar] [CrossRef]
- Li, P.; Sun, M.; Xu, Z.; Liu, X.; Zhao, W.; Gao, W. Site-Selective in Situ Growth-Induced Self-Assembly of Protein–Polymer Conjugates into pH-Responsive Micelles for Tumor Microenvironment Triggered Fluorescence Imaging. Biomacromolecules 2018, 19, 4472–4479. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, M.; Sun, J.; Hu, J.; Wang, Z.; Guo, J.; Gao, W. Polymerization Induced Self-Assembly of a Site-Specific Interferon α-Block Copolymer Conjugate into Micelles with Remarkably Enhanced Pharmacology. J. Am. Chem. Soc. 2018, 140, 10435–10438. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.L.; Munasinghe, A.; Kaupbayeva, B.; Rebecca Kang, N.; Certiat, M.; Murata, H.; Matyjaszewski, K.; Lin, P.; Colina, C.M.; Russell, A.J. Transforming protein-polymer conjugate purification by tuning protein solubility. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Lu, D.; Ge, J.; Liu, Z. Uniform polymer–protein conjugate by aqueous AGET ATRP using protein as a macroinitiator. Acta Biomater. 2011, 7, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Averick, S.E.; Bazewicz, C.G.; Woodman, B.F.; Simakova, A.; Mehl, R.A.; Matyjaszewski, K. Protein–polymer hybrids: Conducting ARGET ATRP from a genetically encoded cleavable ATRP initiator. Eur. Polym. J. 2013, 49, 2919–2924. [Google Scholar] [CrossRef]
- Kovaliov, M.; Cheng, C.; Cheng, B.; Averick, S. Grafting-from lipase: Utilization of a common amino acid residue as a new grafting site. Polym. Chem. 2018, 9, 4651–4659. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; Lathwal, S.; Enciso, A.E.; Simakova, A.; Das, S.R.; Russell, A.J.; Matyjaszewski, K. Synthesis of Polymer Bioconjugates via Photoinduced Atom Transfer Radical Polymerization under Blue Light Irradiation. ACS Macro Lett. 2018, 7, 1248–1253. [Google Scholar] [CrossRef]
- Olszewski, M.; Jeong, J.; Szczepaniak, G.; Li, S.; Enciso, A.; Murata, H.; Averick, S.; Kapil, K.; Das, S.R.; Matyjaszewski, K. Sulfoxide-Containing Polyacrylamides Prepared by PICAR ATRP for Biohybrid Materials. ACS Macro Lett. 2022, 11, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Charan, H.; Kinzel, J.; Glebe, U.; Anand, D.; Garakani, T.M.; Zhu, L.; Bocola, M.; Schwaneberg, U.; Böker, A. Grafting PNIPAAm from β-barrel shaped transmembrane nanopores. Biomaterials 2016, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Elvira, C.; Gallardo, A.; Roman, J.; Cifuentes, A. Covalent Polymer-Drug Conjugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.H. The Fentanyl Story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef]
- Stanley, T.H. The History of Opioid Use in Anesthetic Delivery. In The Wondrous Story of Anesthesia; Eger Ii, E.I., Saidman, L.J., Westhorpe, R.N., Eds.; Springer: New York, NY, USA, 2014; pp. 641–659. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Yang, J.; Ma, G.; Zhang, Z.; Zhang, X. Dual sensitive and temporally controlled camptothecin prodrug liposomes codelivery of siRNA for high efficiency tumor therapy. Biomaterials 2014, 35, 9731–9745. [Google Scholar] [CrossRef]
- Mei, C.; Lei, L.; Tan, L.-M.; Xu, X.-J.; He, B.-M.; Luo, C.; Yin, J.-Y.; Li, X.; Zhang, W.; Zhou, H.-H.; et al. The role of single strand break repair pathways in cellular responses to camptothecin induced DNA damage. Biomed. Pharmacother. 2020, 125, 109875. [Google Scholar] [CrossRef]
- Fan, X.; Lin, X.; Ruan, Q.; Wang, J.; Yang, Y.; Sheng, M.; Zhou, W.; Kai, G.; Hao, X. Research progress on the biosynthesis and metabolic engineering of the anti-cancer drug camptothecin in Camptotheca acuminate. Ind. Crops Prod. 2022, 186, 115270. [Google Scholar] [CrossRef]
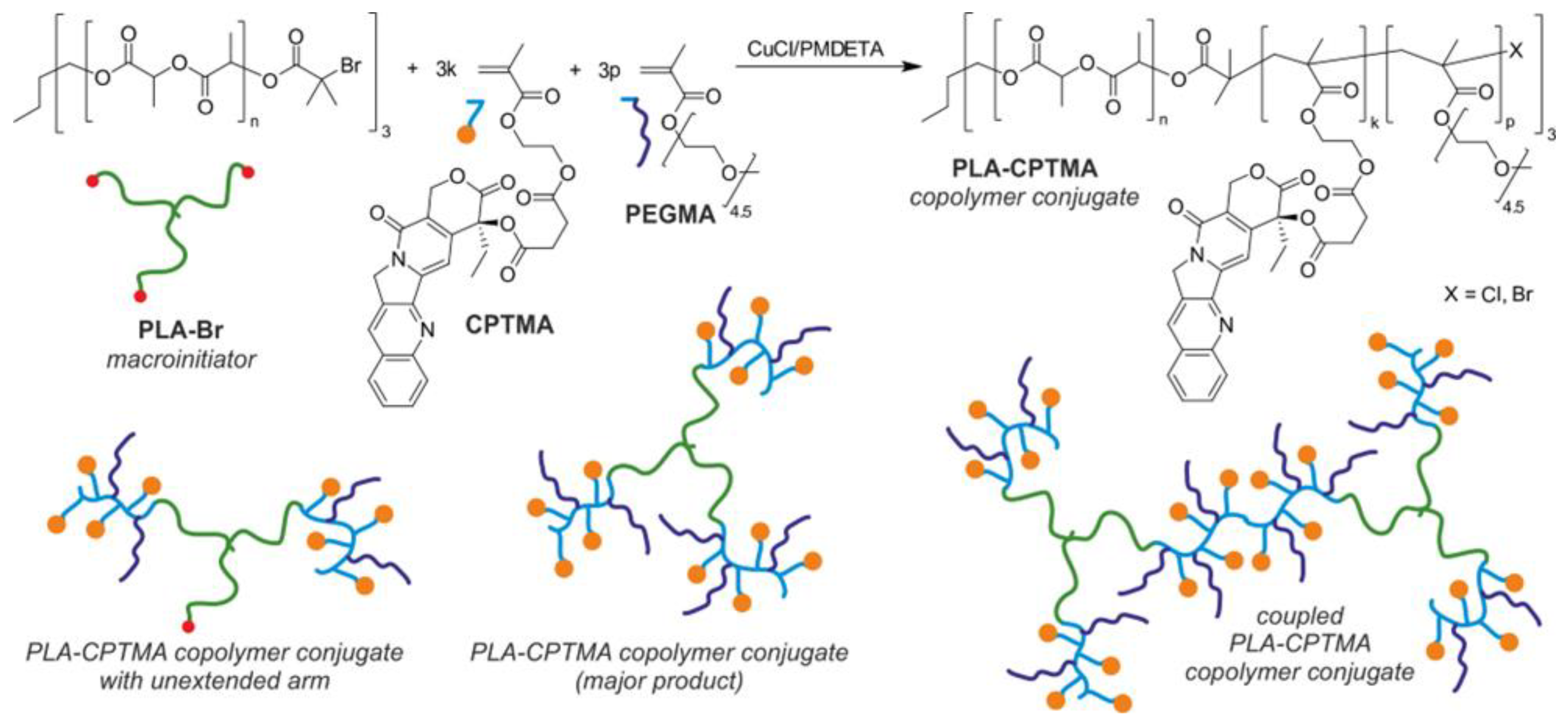
- Plichta, A.; Kowalczyk, S.; Kamiński, K.; Wasyłeczko, M.; Więckowski, S.; Olędzka, E.; Nałęcz-Jawecki, G.; Zgadzaj, A.; Sobczak, M. ATRP of Methacrylic Derivative of Camptothecin Initiated with PLA toward Three-Arm Star Block Copolymer Conjugates with Favorable Drug Release. Macromolecules 2017, 50, 6439–6450. [Google Scholar] [CrossRef]
- Gao, Y.-E.; Bai, S.; Shi, X.; Hou, M.; Ma, X.; Zhang, T.; Xiao, B.; Xue, P.; Kang, Y.; Xu, Z. Irinotecan delivery by unimolecular micelles composed of reduction-responsive star-like polymeric prodrug with high drug loading for enhanced cancer therapy. Colloids Surf. B: Biointerfaces 2018, 170, 488–496. [Google Scholar] [CrossRef]
- Chen, W.; Shah, L.A.; Yuan, L.; Siddiq, M.; Hu, J.; Yang, D. Polymer–paclitaxel conjugates based on disulfide linkers for controlled drug release. RSC Adv. 2014, 5, 7559–7566. [Google Scholar] [CrossRef]
- Dong, Y.; Du, P.; Pei, M.; Liu, P. Design, postpolymerization conjugation and self-assembly of a di-block copolymer-based prodrug for tumor intracellular acid-triggered DOX release. J. Mater. Chem. B 2019, 7, 5640–5647. [Google Scholar] [CrossRef] [PubMed]
- Odrobińska, J.; Neugebauer, D. Retinol derivative as bioinitiator in the synthesis of hydroxyl-functionalized polymethacrylates for micellar delivery systems. Express Polym. Lett. 2019, 13, 806–817. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Synthesis of Riboflavin-Based Macromolecules through Low ppm ATRP in Aqueous Media. Macromol. Chem. Phys. 2020, 221, 1900496. [Google Scholar] [CrossRef]
- Plichta, A.; Kowalczyk, S.; Olędzka, E.; Sobczak, M.; Strawski, M. Effect of structural factors on release profiles of camptothecin from block copolymer conjugates with high load of drug. Int. J. Pharm. 2018, 538, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, Q.; Hong, C.-Y.; Pan, C.-Y. Unimolecular micelles of camptothecin-bonded hyperbranched star copolymers via β-thiopropionate linkage: Synthesis and drug delivery. J. Mater. Chem. B 2015, 4, 141–151. [Google Scholar] [CrossRef]
- Bai, S.; Jia, D.; Ma, X.; Liang, M.; Xue, P.; Kang, Y.; Xu, Z. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact. Mater. 2021, 6, 2894–2904. [Google Scholar] [CrossRef]
- Bai, S.; Gao, Y.-E.; Ma, X.; Shi, X.; Hou, M.; Xue, P.; Kang, Y.; Xu, Z. Reduction stimuli-responsive unimolecular polymeric prodrug based on amphiphilic dextran-framework for antitumor drug delivery. Carbohydr. Polym. 2018, 182, 235–244. [Google Scholar] [CrossRef]
- Bai, S.; Hou, M.; Shi, X.; Chen, J.; Ma, X.; Gao, Y.-E.; Wang, Y.; Xue, P.; Kang, Y.; Xu, Z. Reduction-active polymeric prodrug micelles based on α-cyclodextrin polyrotaxanes for triggered drug release and enhanced cancer therapy. Carbohydr. Polym. 2018, 193, 153–162. [Google Scholar] [CrossRef]
- Chen, X.; Parelkar, S.S.; Henchey, E.; Schneider, S.; Emrick, T. PolyMPC–Doxorubicin Prodrugs. Bioconjugate Chem. 2012, 23, 1753–1763. [Google Scholar] [CrossRef]
- Lale, S.V.; Ravindran Girija, A.; Aravind, A.; Kumar, D.S.; Koul, V. AS1411 Aptamer and Folic Acid Functionalized pH-Responsive ATRP Fabricated pPEGMA–PCL–pPEGMA Polymeric Nanoparticles for Targeted Drug Delivery in Cancer Therapy. Biomacromolecules 2014, 15, 1737–1752. [Google Scholar] [CrossRef]
- Wang, H.; Xu, F.; Li, D.; Liu, X.; Jin, Q.; Ji, J. Bioinspired phospholipid polymer prodrug as a pH-responsive drug delivery system for cancer therapy. Polym. Chem. 2013, 4, 2004–2010. [Google Scholar] [CrossRef]
- Pelras, T.; Duong, H.T.T.; Kim, B.J.; Hawkett, B.S.; Müllner, M. A ‘grafting from’ approach to polymer nanorods for pH-triggered intracellular drug delivery. Polymer 2017, 112, 244–251. [Google Scholar] [CrossRef]
- Rickert, E.L.; Trebley, J.P.; Peterson, A.C.; Morrell, M.M.; Weatherman, R.V. Synthesis and Characterization of Bioactive Tamoxifen-Conjugated Polymers. Biomacromolecules 2007, 8, 3608–3612. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Mitschang, F.; Agarwal, S. Biocompatible Drug Delivery System for Photo-Triggered Controlled Release of 5-Fluorouracil. Biomacromolecules 2011, 12, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Schmidt, V.; Borsali, R. Nanocontainers Formed by Self-Assembly of Poly(ethylene oxide)-b-poly(glycerol monomethacrylate)−Drug Conjugates. Macromolecules 2007, 40, 2148–2157. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Wolski, K. Riboflavin-induced metal-free ATRP of (meth) acrylates. Eur. Polym. J. 2020, 140, 110055. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition–fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef]
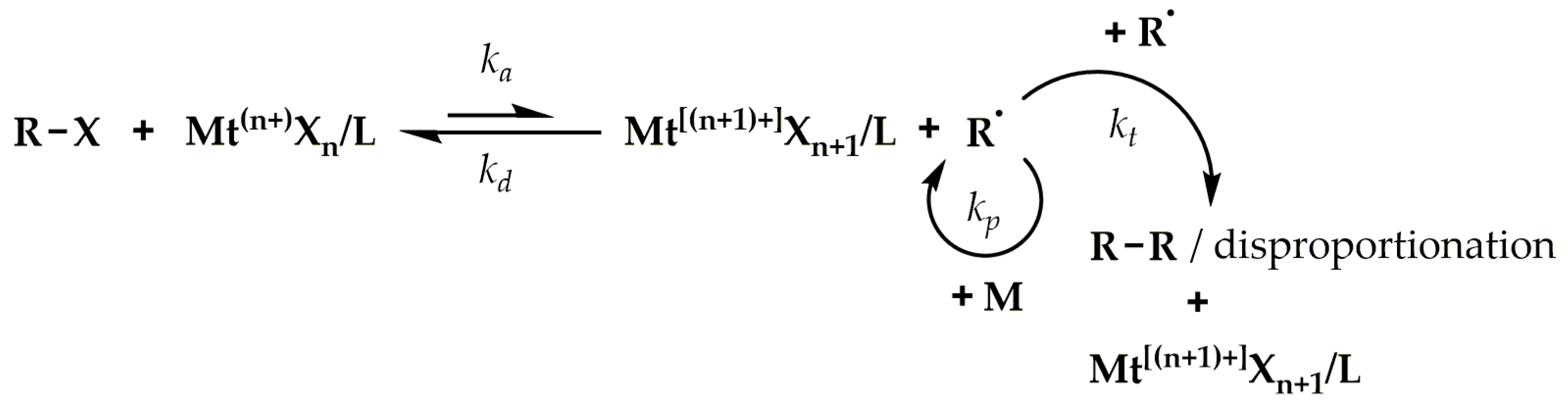
| Encapsulated Drug | Monomers Used | The Variant of the ATRP Technique | Applications | Ref |
|---|---|---|---|---|
| PTX | MMA, DMAEMA | normal | pH-triggered drug release | [163] |
| DOX·HCl | MEMA, DEAEMA | normal | Ultrasound-triggered drug release | [164] |
| - | MMA, DMAEMA | ARGET | Gateway to stimuli-responsive giant hybrid vesicle DDS | [165] |
| - | ABOC, FBOC | normal | Gateway for pH-triggered DDS | [166] |
| Encapsulated Drug | Nucleic Acid Used | Monomers Used | Applications | Ref |
|---|---|---|---|---|
| - | pDNA | NIPAM | Thermosensitive nucleic acid delivery | [176] |
| - | siRNA | NIPAM | Hydrogel-aided nucleic acid delivery | [177] |
| DOX | DNA | DMAEMA | Redox-triggered drug and nucleic acid release | [178] |
| PTX | pDNA | DMAEMA | Bcl-2 targeted drug and nucleic acid delivery | [179] |
| Encapsulated Drug/Nucleic Acid | Monomers Used | The Variant of the ATRP Technique | Applications | Ref |
|---|---|---|---|---|
| DEX | PEGMA | normal | Tuning molecular architecture for tailoring drug-releasing properties | [206] |
| DOX | OEGMA | normal | Redox-triggered drug release | [207] |
| DOX | PEGMA | normal | Redox-triggered drug release | [209,210] |
| - | ABMA | normal | Gateway for thermos-responsive drug release | [211] |
| pDNA | EGDMA, DMAEMA, β-Pinene | DE/AGET | Nucleic acid delivery | [212] |
| siRNA | OEOMA, GMA | ARGET | MOR-targeted nucleic acid delivery | [216] |
| pDNA | GMA | normal | pH-triggered nucleic acid delivery | [219] |
| DOX, siRNA | PEGMA | normal | Redox-triggered drug and nucleic acid release | [220] |
| Type of Protein | Type of Monomers 1 | The Variant of the ATRP Technique Used | Applications and Conclusions | Ref. |
|---|---|---|---|---|
| Chymotrypsin | CBMA, AMA | normal | Modifying the structure by adding a polymer significantly increased protein stability and reduced protein–protein interactions. | [253] |
| Chymotrypsin-α Trypsin | CBMA PEGMA | normal | Protein–polymer conjugates, which can exist as a prodrug until the activator is introduced, can be used in enzyme-based biosensors and drug delivery for cancer treatment. | [264] |
| Chymotrypsin-α | CBMA PEGMA 3-SPMA DMAEMA | normal | Covalently attached synthetic polymers are able to modulate protein folding, emulating molecular chaperones. | [265] |
| Human serum albumin | DPA | normal | Promising as a new class of tumor microenvironment responsive nanocarriers for improved tumor imaging and therapy. | [266] |
| Interferon-α | HPMAPEGMA | normal | Promising next-generation technology that will significantly improve the pharmacological performance of therapeutic proteins with a short circulating half-life. | [267] |
| Lysozyme | CBMA PEGMA | normal | The covalent attachment of polymers to a protein can significantly change the protein solubility, which can be adjusted by changing the polymer type, grafting density, and polymer length. Polymer attachment increases the resistance to unfavorable environments and the thermostability of the protein. | [268] |
| Horseradish peroxidase | ACR | AGET | The resulting conjugates essentially retained the catalytic properties of the protein and showed significantly improved thermal stability to high temperature and trypsin digestion. | [269] |
| Green fluorescent protein | PEGMA | ARGET | The protein retained its bio-fluorescent properties during the process, indicating the utility of ARGET ATRP for the preparation of protein–polymer conjugates. | [270] |
| Lipase | DMAPAA | ICAR | A ubiquitous class of amino acid residues can be modified by ATRP initiators without affecting enzyme activity. This new amino acid modification strategy can be applied to other enzymes, providing access to new biohybrid modification schemes. | [271] |
| Bovine serum albumin | OEOMA | Photo | The first example of photo-ATRP using blue LED irradiation in an aquatic environment. Compared to more energetic light sources, blue light is more friendly to biological systems and allows enzymes to survive and maintain their structure and functions. | [272] |
| Bovine serum albumin | MSEAM | PICAR | A new sulfoxide-functional acrylamide monomer was synthesized as an alternative to PEG in some biomedical applications. It was used in the PICAR ATRP process under biologically relevant conditions without degassing the reaction mixture. | [273] |
| β-barrel transmembrane | NIPAM | SARA | The first example of the use of a transmembrane protein in the production of conjugates by the “grafting from” strategy, using ATRP techniques. Thanks to the preserved pore geometry, transmembrane protein–polymer conjugates can be used as building blocks of functional polymer membranes, drug and gene carriers, and nanoreactors. | [274] |
| Strategy | (Co)Polymers | Active Substance | Synthesis Techniques | Ref. |
|---|---|---|---|---|
| Active substance as an ATRP initiator | Poly(carboxybetaine) | CPT | ATRP | [278] |
| Poly(methacryloyloxyethyl phosphorylcholine) | CPT | ATRP | [68] | |
| Poly(oligo(ethylene oxide) methacrylate)-b-(glycidyl methacrylate) | Fentanyl | AGET ATRP | [216] | |
| Poly(di(ethylene glycol) methyl ether methacrylate) Poly(di(ethylene glycol) methyl ether methacrylate)-b-poly(methyl methacrylate) | Inositol (vitamin B8) | ARGET ATRP, SARA ATRP, seATRP | [183] | |
| Poly(methyl methacrylate-co-2-hydroxyethyl methacrylate) | Retinol (vitamin A) | ATRP | [285] | |
| Poly(n-butyl acrylate) Poly(methyl methacrylate) Poly(N-isopropylacrylamide) Poly(N-isopropylacrylamide)-b-poly(oligo(ethylene glycol) acrylate) Poly(N-isopropylacrylamide)-b-poly(2-hydroxyethyl acrylate) | Riboflavin (vitamin B2) | ARGET ATRP, Metal-free ATRP, Photo ATRP, seATRP | [286] | |
| Active substance as (meth)acrylate monomer | Poly(lactic acid)-b-poly(camptothecin mono-2-(methacryloyloxy)ethyl succinate) Poly(lactic acid)-b-poly(camptothecin mono-2-(methacryloyloxy)ethyl succinate-co- poly(ethylene glycol) methyl ether methacrylate) | CPT | ATRP | [281,287] |
| Poly(hydroxypropyl methacrylate-co-Methacryloyloxy-3-thiohexanoyl camptothecin-co-2-(2′-Bromoisobutyryloxy)ethyl-2′’-methacryloyl oxyethyl disulfide) Poly(hydroxypropyl methacrylate-co-Methacryloyloxy-3-thiohexanoyl camptothecin-co-2-(2′-Bromoisobutyryloxy)ethyl-2′’-methacryloyl oxyethyl disulfide)(poly(poly(ethylene glycol) methyl ether methacrylate)) | CPT | ATRP | [288] | |
| Cellulose-g-poly(methacrylate derivative of camptothecin)-b- poly(ethylene glycol) methyl ether methacrylate) | CPT | ATRP | [289] | |
| Dextran-poly(methacrylate derivative of camptothecin)-b-poly(ethylene glycol) methyl ether methacrylate) | CPT | ATRP | [290] | |
| α-cyclodextrin- poly(ethylene glycol) polyrotaxanes-poly(methacrylate derivative of camptothecin)-b-poly(ethylene glycol) methyl ether methacrylate) | CPT | ATRP | [291] | |
| Poly(ethylene glycol)-b-poly(2-([2-4-(2-methylpropil)phenyl]propionyl]oxy)ethyl methacrylate | Ibuprofen | ATRP | [96] | |
| β-cyclodextrin-poly(methacrylate derivative of irinotecan-co-poly(ethylene glycol) methyl ether methacrylate) | Irinotecan | ATRP | [282] | |
| Post-polymerization conjugation | Poly(methacryloyloxyethyl phosphorylcholine)-graft-camptothecin | CPT | ATRP, Click Chemistry | [68] |
| Poly(glycidyl methacrylate) Poly(poly(ethylene glycol) methyl ether methacrylate-co-glycidyl methacrylate) | Ciprofloxacin | AGET ATRP, ICAR ATRP, ROP, Click Chemistry | [218] | |
| Poly(methacryloyloxyethyl phosphorylcholine)-graft-doxorubicin Poly(methacryloyloxyethyl phosphorylcholine-co-2-tert-butoxy-2-oxoethyl methacrylate) | DOX | ATRP, Click Chemistry, Acylhydrazine formation | [292] | |
| Poly(poly(ethylene glycol) methacrylate)−b- poly(caprolactone)−b-poly(poly(ethylene glycol) methacrylate) | DOX | ATRP, Acylhydrazine formation | [293] | |
| Poly(methacryloyloxyethyl phosphorylcholine)-b-poly(2-methoxy-2-oxoethyl methacrylate) | DOX | ATRP, Acylhydrazine formation | [294] | |
| Poly(ethylene oxide)-b-poly(glycidyl methacrylate) | DOX | ATRP, Imine formation | [284] | |
| Poly(2-(2-bromoisobutyryloxy)ethyl methacrylate)-co-poly[poly(ethylene glycol) methacrylate-co-3-vinyl benzaldehyde] | DOX | ATRP, Imine formation | [295] | |
| Poly(methacrylic acid) | Estradiol Tamoxifen | ATRP, N-alkylation of amines with carboxylic acid | [296] | |
| Poly(ethylene oxide)-b-poly- (n-butyl methacrylate-co-4-methyl-[7-(methacryloyl)-oxyethyloxy]coumarin)) | 5-fluorouracil | ATRP, Photochemically induced [2 + 2] cycloaddition reaction | [297] | |
| Poly(ethylene oxide)-b-poly(glycerol monomethacrylate) | Indomethacin | ATRP, Steglich esterification | [298] | |
| Poly(ethylene glycol)-b-poly(2-(trimethylsilyloxyl) ethyl methacrylate) | Paclitaxel | ATRP, Esterification | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk-Łagodzińska, M.; Plichta, A.; Dębowski, M.; Kowalczyk, S.; Iuliano, A.; Florjańczyk, Z. Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems. Polymers 2023, 15, 1234. https://doi.org/10.3390/polym15051234
Szewczyk-Łagodzińska M, Plichta A, Dębowski M, Kowalczyk S, Iuliano A, Florjańczyk Z. Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems. Polymers. 2023; 15(5):1234. https://doi.org/10.3390/polym15051234
Chicago/Turabian StyleSzewczyk-Łagodzińska, Matylda, Andrzej Plichta, Maciej Dębowski, Sebastian Kowalczyk, Anna Iuliano, and Zbigniew Florjańczyk. 2023. "Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems" Polymers 15, no. 5: 1234. https://doi.org/10.3390/polym15051234
APA StyleSzewczyk-Łagodzińska, M., Plichta, A., Dębowski, M., Kowalczyk, S., Iuliano, A., & Florjańczyk, Z. (2023). Recent Advances in the Application of ATRP in the Synthesis of Drug Delivery Systems. Polymers, 15(5), 1234. https://doi.org/10.3390/polym15051234









