1. Introduction
In recent years, there has been growing interest in developing products from natural resources. Cellulose, being one of the most prevalent substances on Earth, has garnered significant interest owing to its renewable nature, safety, biodegradability, and biocompatibility. Nanocellulose refers to cellulosic material at the nanoscale, characterized by at least one dimension measuring less than 100 nm. The subject of nanocellulose has gained considerable attention in recent years, and it has been applied in various fields [
1,
2]. As a cellulose material with nanoscale size, nanocellulose has outstanding characteristics including a high aspect ratio, large specific surface area, and high viscosity under certain conditions. Nanocrystalline cellulose, also known as cellulose nanocrystals (CNCs), is a type of nanocellulose with high crystallinity. CNCs are typically produced through the sulfuric acid hydrolysis of cellulose materials, resulting in a width ranging from 5 to 70 nm and a length of 100 to 250 nm, or even extending to several microns [
3]. Having a cellulose content of approximately 42% [
4], soybean stovers are widely available, low-cost sources for the preparation of nanocellulose. So far, cellulose nanofibrils or cellulose nanocrystals have been prepared from soybean straw, soybean residues or soybean hull via mechanical methods [
5] and chemical or enzymatic methods [
6]. However, information is limited regarding the potential application of soybean nanocellulose, especially in the formulation of emulsion-based products.
Emulsions can generally be stabilized by a single emulsifier or emulsion stabilizer. However, many commercial emulsion-based products are formulated with multiple emulsifiers or emulsion stabilizers, including surfactants, polymers and particles [
7]. Mixed emulsifier systems may exhibit synergistic effects on emulsion properties and may be cheaper than a single emulsifier [
7]. Surfactants and polymers can simultaneously concentrate at the oil–water interface, making the emulsion interface layer more compact and enhancing the emulsion stability [
8,
9]. Synergistic effects for the stabilization of emulsions have been found between polymers and surfactants, such as hydroxypropylmethyl cellulose and sodium dodecylsulfate [
8], as well as maltodextrin and Tween 80 [
9]. The combination of polymers and surfactants could result in more stable emulsions than those stabilized by either a polymer or surfactant [
8,
10].
CNCs have been shown to be Pickering emulsion stabilizers [
11]. CNCs can adsorb at the oil–water interface, particularly along its less polar crystalline phase. However, they exhibit only intermediate wettability and do not form micelle-like aggregates, raising certain concerns [
12]. The hydrophilicity of CNCs led to poor emulsification performance and limited their application [
13]. The addition of salt or lowering pH were shown to be effective in increasing the emulsion phase of CNC-stabilized emulsions [
14], however, information is limited on the impact of NaCl on the thermodynamic stability of CNC-stabilized Pickering emulsions. In addition, knowledge is lacking regarding the influence of a mixed emulsifier system (NaCl + CNC and surfactants) on emulsion properties. We hypothesized that by incorporating salt and small molecular weight surfactants with CNCs, a stable nanoemulsion could be formulated. This study explored the influence of NaCl on the characteristics of a Pickering emulsion stabilized by CNCs, along with its synergistic impact when combined with a nonionic surfactant (such as Tween 80) on the stabilization of oil-in-water emulsions. In this study, we highlight the synergistic effects of salted CNCs and Tween 80 in enhancing thermodynamic stability of oil-in-water emulsions loaded with lemongrass essential oil. The results of this study open avenues for the prospective use of CNCs in conjunction with other surfactants in the formulation of stable emulsion products. Moreover, essential oil encapsulation finds applications in various domains, encompassing areas such as food, agriculture, cosmetics, medicine, and packaging.
2. Materials and Methods
Soybean stover was obtained from Iowa State University Research Farms (Boone, IA, USA) and reduced to a 1/8″ size using a knife mill. Lemongrass essential oil (Cymbopogon schoenanthus Oil, 100%, reagent grade) was obtained from Spectrum Chemical MFG Corp (New Brunswick, NJ). Tween 80 (P1754) (Sigma-Aldrich, Inc. (St. Louis, MO, USA), sulfuric acids (95–98%) (chemPUR, Karlsruhe, Germany), glacial acetic acids (Fisher scientific, Hampton, NH, USA), sodium hydroxide (Macron Fine Chemicals, Center Valley, PA, USA), sodium chlorite (Beantown chemical, Hudson, NH, USA), sodium chloride (VWR International, Radnor, PA, USA) and dialysis tubing (Cellulose, 12–14 kDa MWCO) (Chemical MFG Corp, New Brunswick, NJ, USA) were used in this study.
2.1. Production of Cellulose Nanocrystals from Soybean Stover
Soybean stover was washed, dried and ground with similar procedures as described in our previous publication [
15]. The removal of hemicellulose and lignin from soybean stover were performed by using alkaline and bleaching treatment with similar procedures as described in our previous publication [
15]. In particular, soybean stover underwent treatment using a 4 wt% sodium hydroxide solution with a ratio of 1 part solid to 20 parts liquid. This process occurred at 95 °C in a water bath (Boekel Scientific, Feasterville-Trevose, PA, USA) with agitation at 120 rpm for a duration of 6 h. The alkaline-treated soybean stover was then washed with distilled (DI) water until the affluent ran clear, followed by drying at 75 °C for 24 h, grinding and passing through a 40-mesh sieve. Following the bleaching process, 3 wt% sodium chlorite and 3 wt% acetic acid were employed with a ratio of 1 part solid to 20 parts liquid. This treatment took place at 80 °C in a water bath with agitation at 80 rpm for 2 h. The bleached sample underwent washing until the effluent pH approached that of DI water. The bleaching treatment was reiterated, followed by drying, grinding, and sieving as previously detailed. The bleached soybean stover then underwent ball milling (Fritsch pulverisette, FRITSCH Milling and Sizing, Inc., Pittsboro, NC, USA) for 10 min at maximum speed to further decrease the particle size.
The treated soybean stover then underwent sulfuric acid (64 wt%) hydrolysis with a ratio of 1 part solid to 20 parts liquid at 45 °C for 75 min in a water bath with agitation at 120 rpm. The reaction was quenched by adding 10-fold cold water, followed by centrifugation at 13,689×
g (Sorvall Evolution RC centrifuge, Waltham, MA, USA) for a duration of 10 min at 4 °C. The pellet underwent a repeated washing and centrifugation process, followed by dialysis until the pH of the dialysate closely matched that of water. The suspension after dialysis was then subjected to sonication using a 500 W ultrasonicator (Fisher Scientific, Hampton, NH, USA) for 30 min at 100% amplitude in pulse mode (5 s on/2 s off) in an ice bath. Subsequently, the suspension underwent centrifugation (Eppendorf 5430R, Enfield, CT, USA) at 7745×
g for a duration of 20 min at 4 °C, followed by collection of the supernatant. The obtained CNC suspension was kept at 4 °C for subsequent utilization. The color of the soybean stover following each treatment was assessed with a colorimeter (3nh, Shenzhen THREENH Technology Co., Ltd., Shenzhen, China). The step yields and overall yields of the soybean stover sample following each treatment were determined using the following equations.
where
n = 1, 2, 3, 4.
2.2. Characterization of CNC
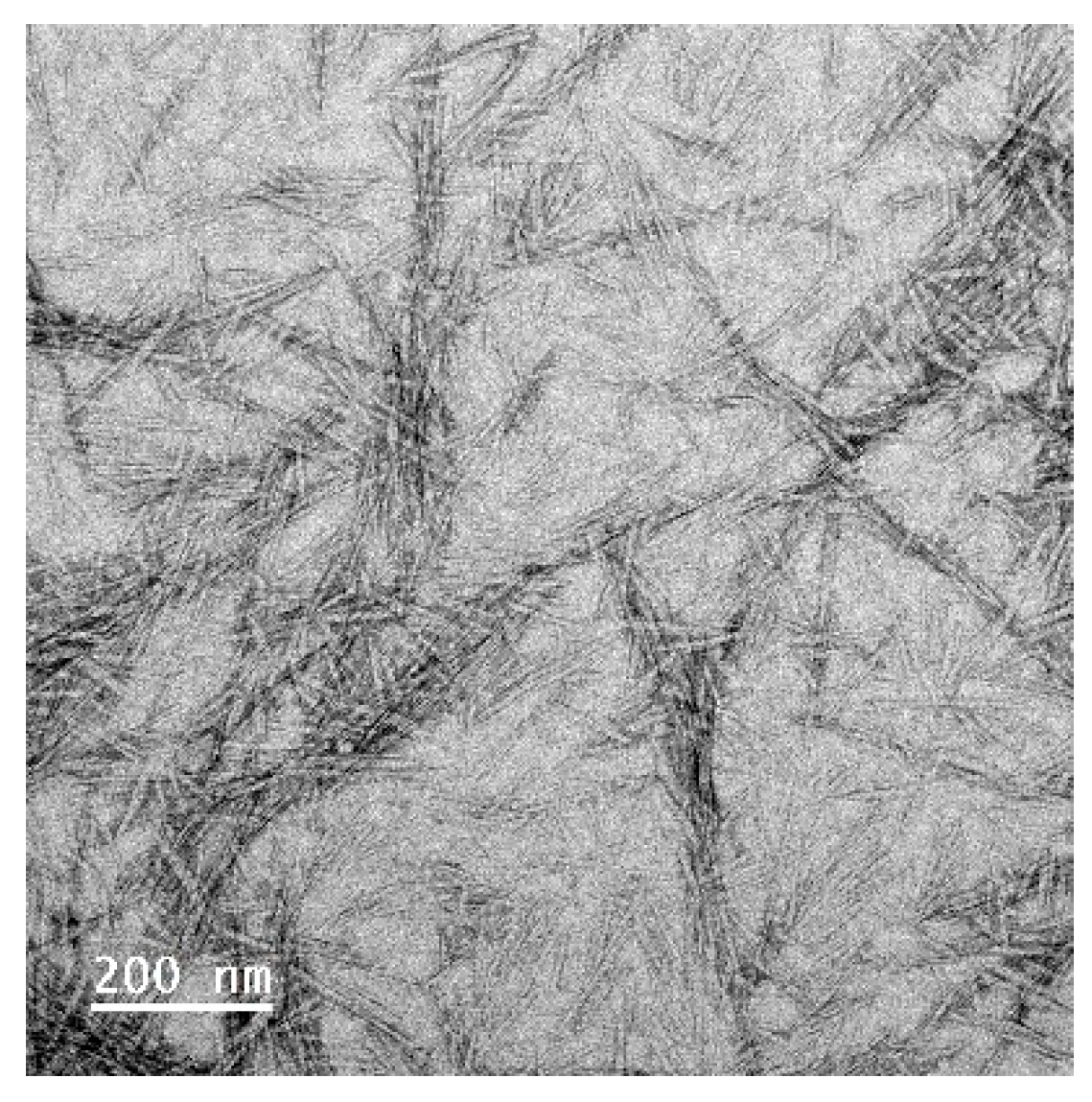
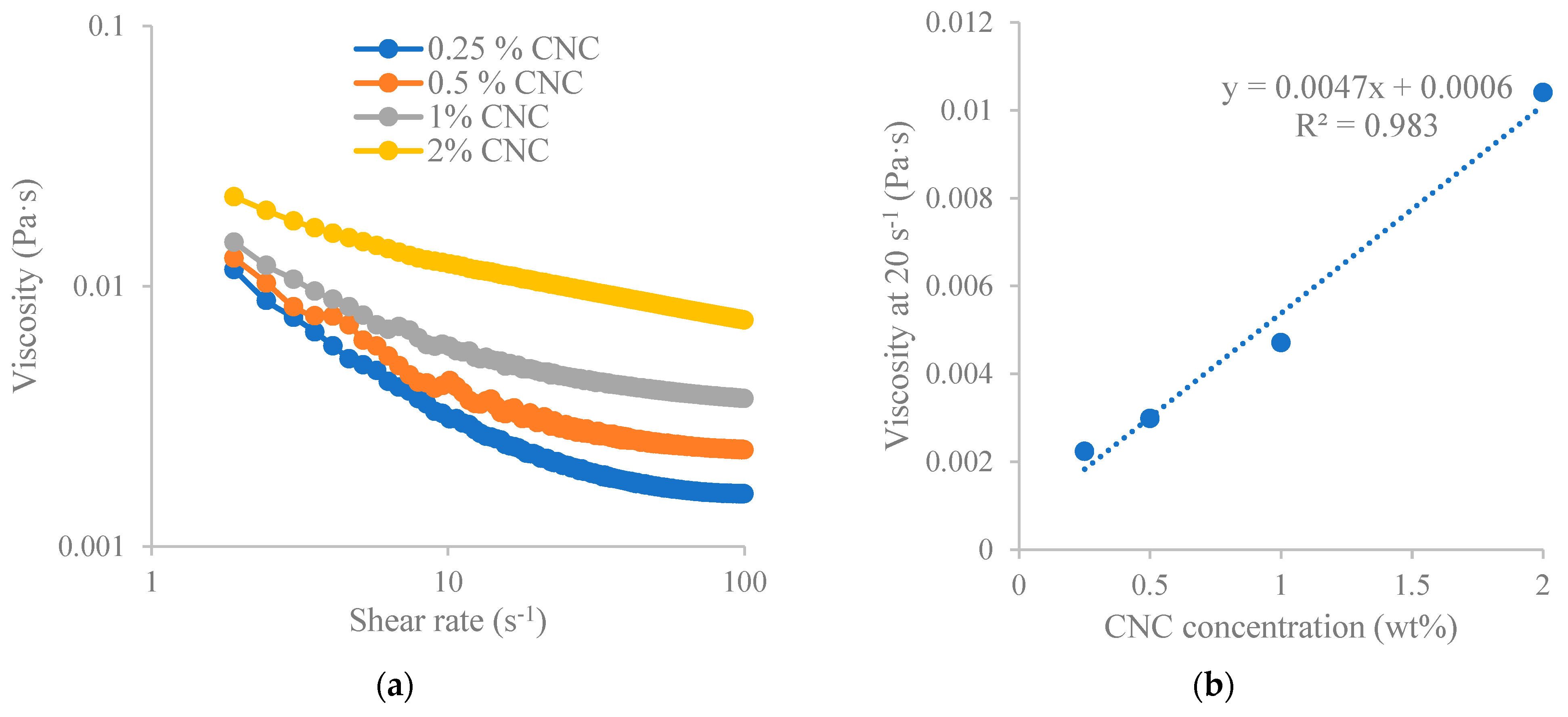
The as-prepared soybean CNC suspension was imaged utilizing a JEM-2100 scanning/transmission electron microscope (STEM) (JEOL Ltd., Akishima, Tokyo) with a voltage of 200 kV. The length and width of CNCs were assessed from the images using an Image J 1.53t software (Wayne Rasband and contributors, National Institutes of Health, Washington, DC, USA). The average particle size, polydispersity index, and zeta potential of diluted CNC sample (0.1 wt%) were assessed with a Zetasizer Nano ZS instrument (Malvern Instruments Ltd., Worcestershire, UK). The material refractive index is 1.50 while the dispersant refractive index is 1.33. The rheological behavior of CNCs at varied concentrations was assessed using a Discovery HR-2 rheometer (TA instruments, Newcastle, DE, USA) fitted with a DIN Concentric Cylinder featuring bob and cup geometry (bob diameter = 28 mm, cup diameter = 30.4 mm) according to the method we established previously [
15]. Samples were loaded and maintained at 37 °C for 5 min, followed by a flow ramp test at 37 °C spanning a 180-s duration (sampling interval of 1 s/pt) and a range of shear rate from 1 to 100 s
−1. Viscosity readings versus shear rate were recorded. Three tests were performed for each sample.
2.3. Preparation and Characterization of CNC-Stabilized Pickering Emulsion
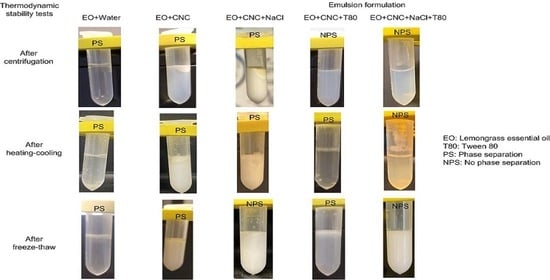
CNC dispersion with varied concentrations was introduced into lemongrass essential oil (EO) while employing magnetic stirring; subsequently, ultrasonication was performed using a 500 W ultrasonicator at 60% amplitude (5 s on/2 s off) for 5 min. The emulsion comprised 5 wt% EO and specific concentrations of CNCs (0, 0.5 and 1.0 wt%). The thermodynamic stability of emulsion samples was then tested via centrifugation test, freeze–thaw test, heating–cooling test and room temperature storage test. The behavior of sample phase separation (if any) was recorded after each test. Centrifugation test was performed by subjecting the emulsion samples to a centrifugal force of 10,000 rpm (Eppendorf 5418, Enfield, CT, USA) for a duration of 15 min at 25 °C. The freeze–thaw tests comprised two cycles, with each cycle involving storage at −20 °C for 48 h followed by 25 °C for 48 h. Samples under heating–cooling test were subjected to six cycles of sample storage at 4 °C for 24 h followed by 45 °C for 24 h. Additionally, emulsion samples were stored at room temperature (shielded from light) and monitored for any potential phase separation over time.
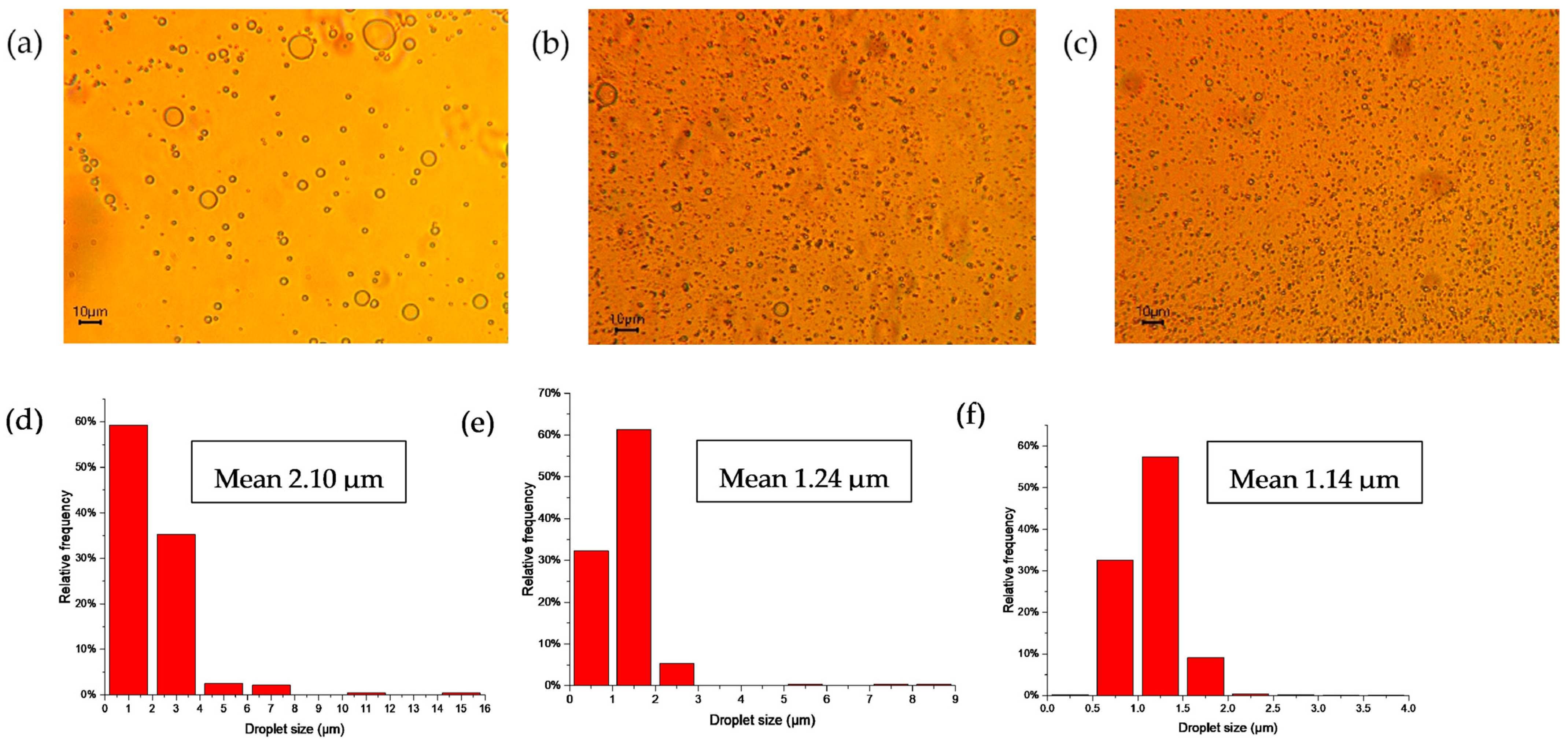
Droplet size of CNC-stabilized Pickering emulsions was measured by using an optical microscope (50W Halogen Trinocular Microscope, AmScope, United Scope LLC, Irvine, CA, USA) right after the preparation of the sample. Specifically, an undiluted sample of 1 µL was applied to a slide, covered with a coverslip, and images were captured under a 40× objective magnification. The determination of emulsion droplet size was performed using an Image J 1.53t software (Wayne Rasband and contributors, National Institutes of Health, Washington, DC, USA).
2.4. Effect of NaCl on CNC and CNC-Stabilized Pickering Emulsion
In order to determine the effect of NaCl on CNCs, CNC suspension (1 wt%) in the presence of various concentrations of NaCl (1 mM, 10 mM, and 40 mM) (i.e., salted CNC) was prepared and characterized. Specifically, CNCs and NaCl were mixed and sonicated as shown in
Section 2.3. Particle size and zeta potential of salted CNCs were determined by the zetasizer to reflect the effect of NaCl on hydrodiameter and surface charge of CNCs. All experiments were conducted in triplicate at 23 °C. The refractive indices of the material and dispersant are 1.50 and 1.33, respectively. Before measurement, samples were diluted with deionized water at a ratio of 1:100. Viscosity of salted CNCs was also determined with the similar method as described above but at 23 °C.
Emulsions were formulated with 5% EO, 1 wt% CNC, and varied concentrations of NaCl (1 mM, 10 mM, and 40 mM). The emulsion was prepared via sonication (using the same parameters as above) to obtain Pickering emulsion. The stability of salted CNC-stabilized Pickering emulsion was also tested as described above. Particle size and zeta potential of salted CNC-stabilized Pickering emulsion were determined by utilizing the Zetasizer. The samples underwent dilution with water, following the procedures outlined earlier.
The stability of emulsion against creaming was assessed through the creaming index using the formula below.
where
represents the volume of the serum layer and
represents the overall volume of the emulsion sample.
Encapsulation efficiency of the emulsion sample after centrifugation was also determined by the equation below [
16].
where
represents the fraction of volume occupied by the encapsulated emulsion, and
represents the overall volume of the emulsion sample.
2.5. Preparation and Characterization of Emulsions Stabilized with CNC and Tween 80
Emulsion samples containing both CNC and a low molecular weight surfactant (namely, Tween 80) were also formulated. The emulsions loaded with essential oil comprised 5 wt% lemongrass EO, 10 wt% Tween 80 and varying concentrations of CNCs (0, 0.25, 0.5, and 1 wt%). Emulsions were created by gradually introducing Tween 80 to EO with stirring. Subsequently, the water phase containing CNCs was added while stirring, following that, ultrasonication was applied using the same procedures as above. Thermodynamic stability, particle size and zeta potential of the emulsions were tested as above.
Emulsion samples were also prepared with salted CNCs and Tween 80. The emulsions contained 5 wt% EO, 10 wt% Tween 80, 1 wt% CNC and varying concentrations of NaCl (0, 1, 10, and 40 mM). The size of particles, zeta potential and thermodynamic stability of the emulsions were also assessed.
2.6. Statistical Analysis
All experiments were conducted with at least triplicate measurements. Statistical significance (α = 0.05) was evaluated using Duncan’s tests with SAS/STAT software (SAS Institute Inc., Cary, NC, USA). Samples labeled with distinct letters exhibited significant differences (Duncan, p < 0.05) when compared to each other.