Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane
Abstract
:1. Introduction
2. Methodology
2.1. Chemicals and Reagents
2.2. Experimental Equipment
2.3. Experimental Method
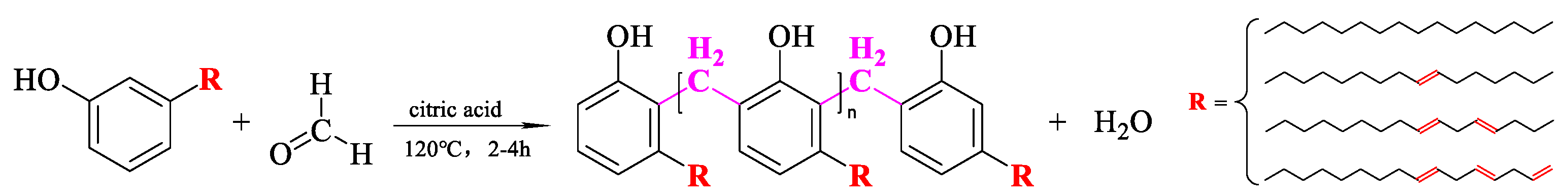
2.3.1. Synthesis and Purification of Cardanol Formaldehyde Oligomer
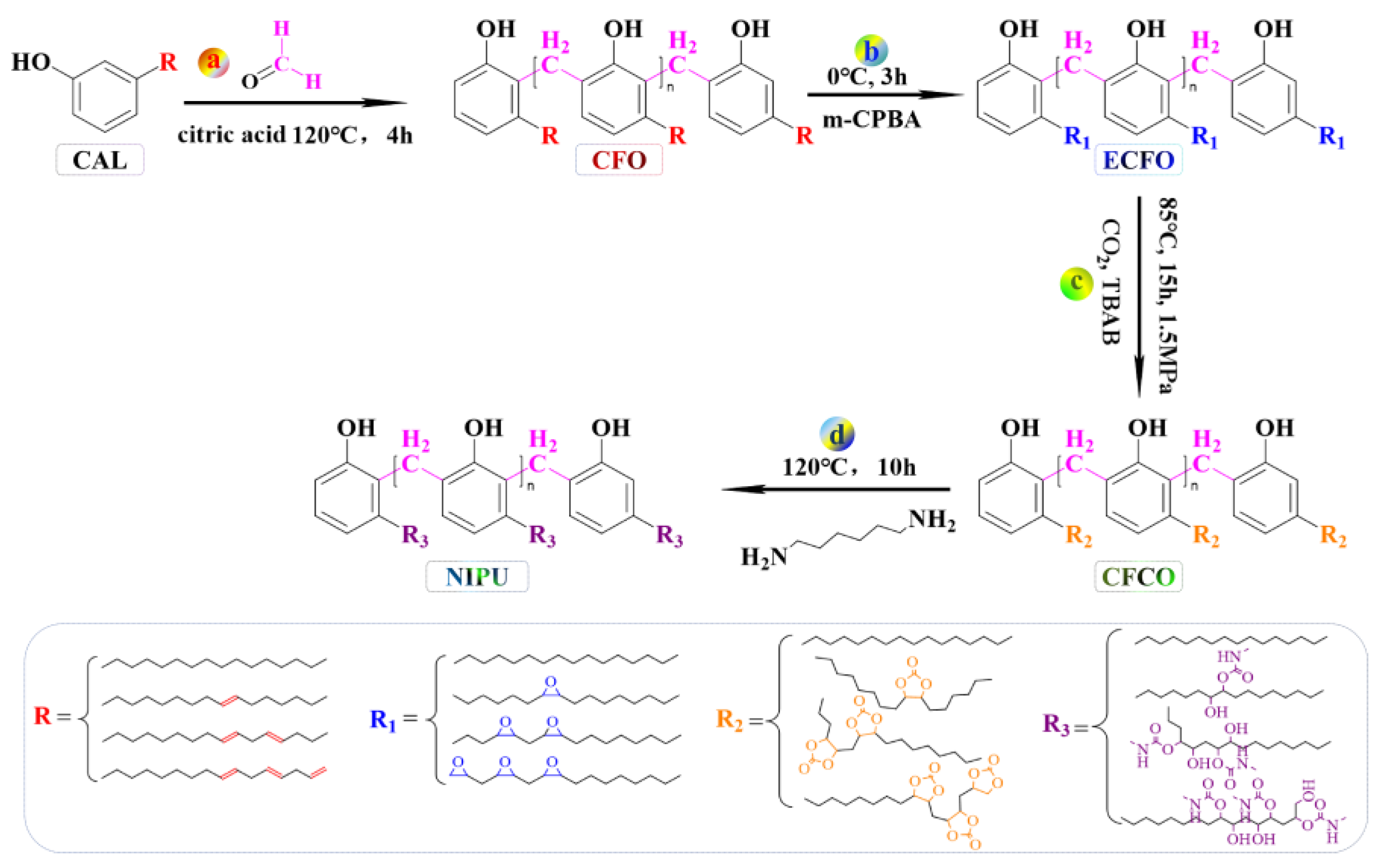
2.3.2. Synthesis and Purification of Epoxidized Cardanol Formaldehyde Oligomer (ECFO)

2.3.3. Synthesis of Cardanol Formaldehyde Cyclocarbonate Oligomer (CFCO)

2.3.4. Synthesis of Cardanol-Based Non-Isocyanate Polyurethane

2.3.5. Characterization of the Prepared Samples: Analysis Method
3. Results and Discussion
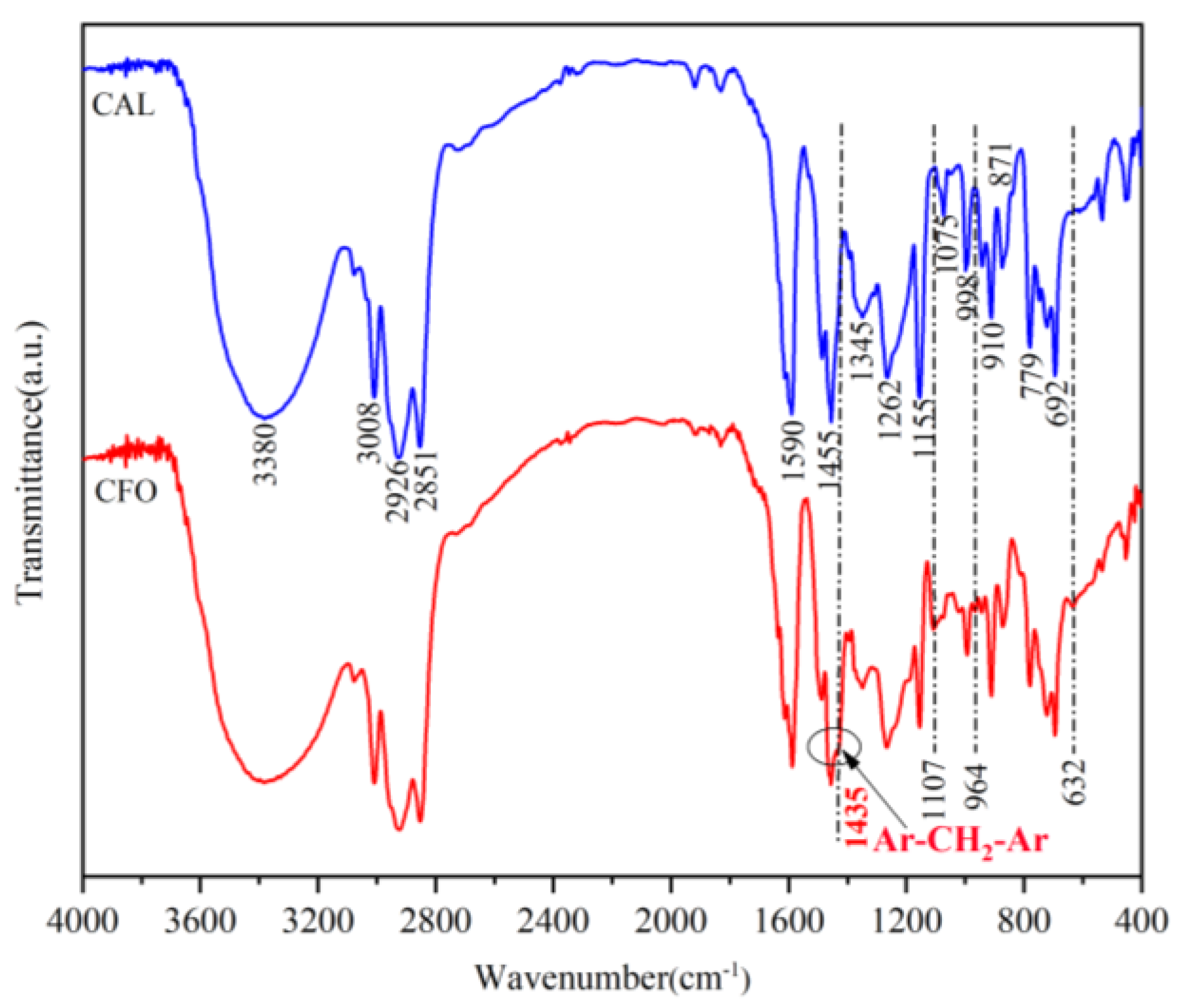
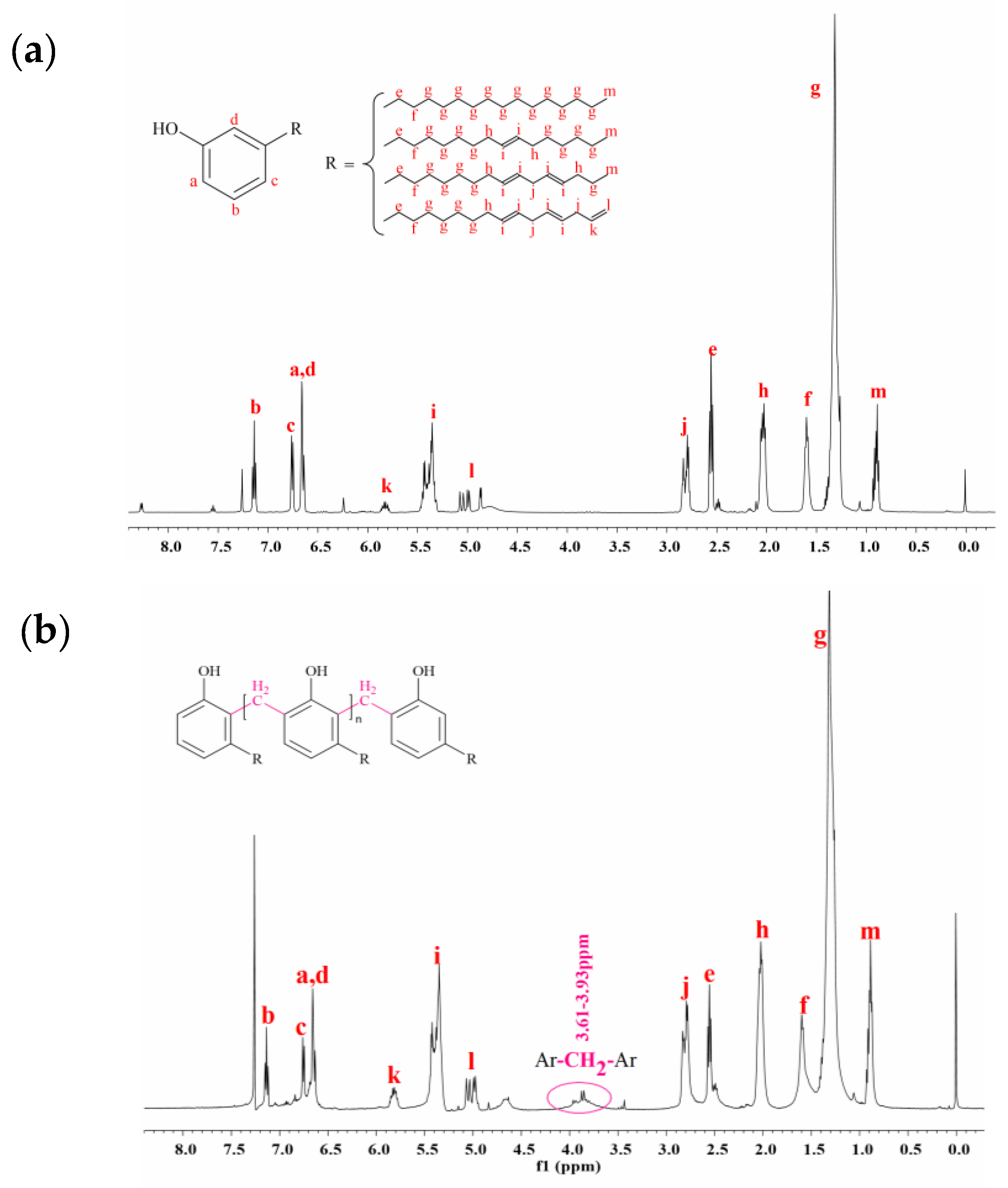
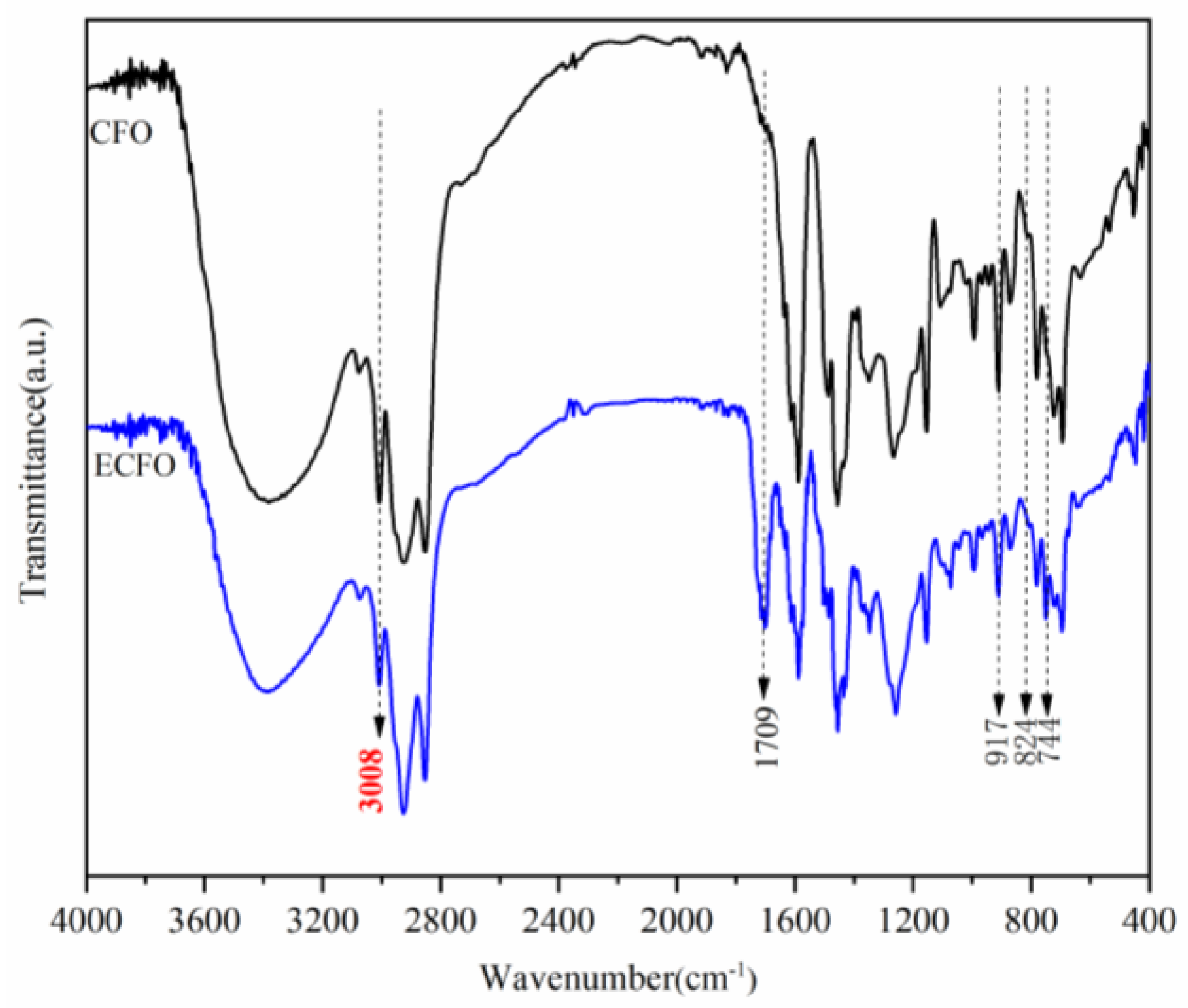
3.1. Synthesis and Characterization of Cardanol Formaldehyde Oligomer (CFO)
3.2. Synthesis and Characterization of Epoxidized Cardanol Formaldehyde Oligomer (ECFO)
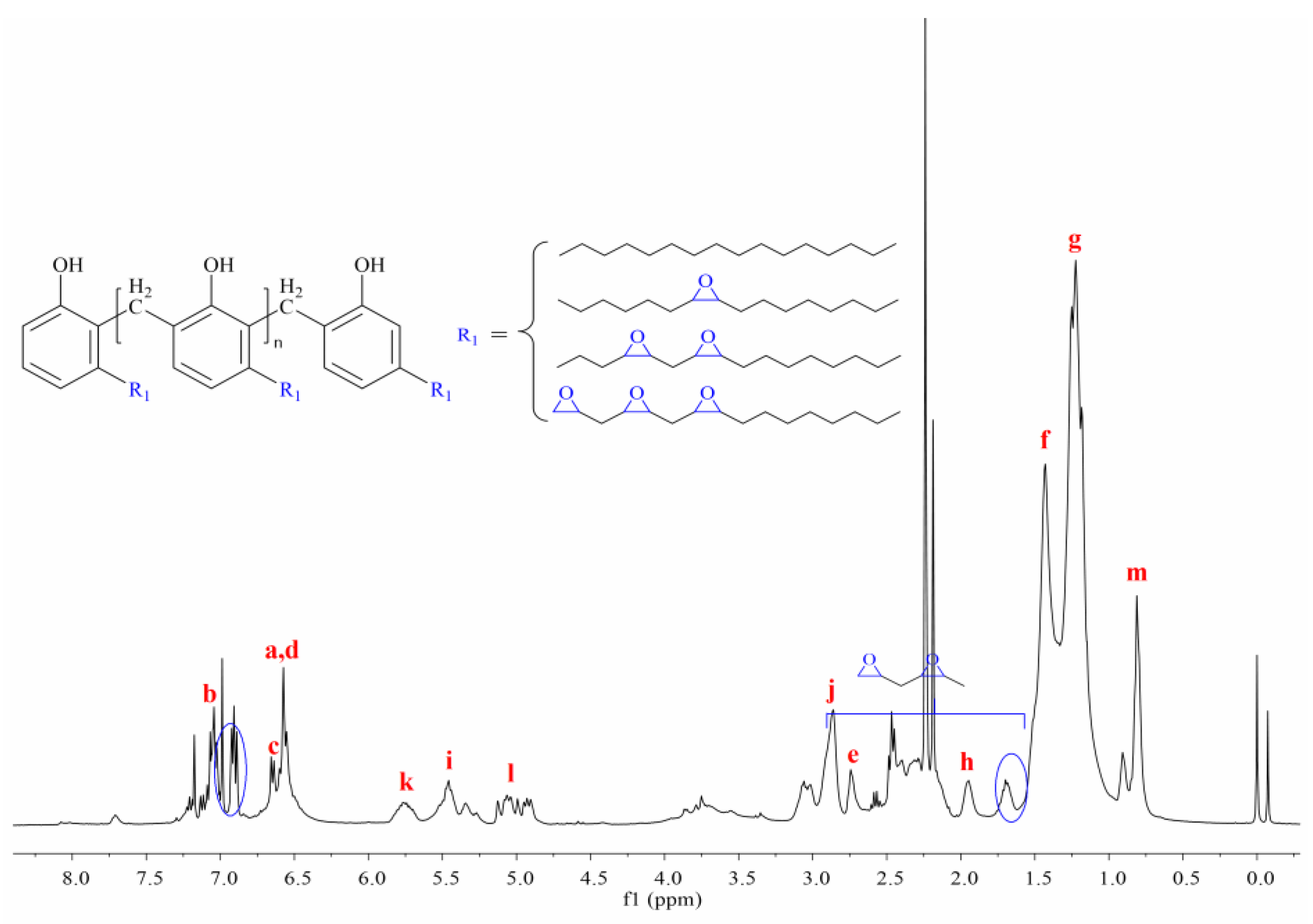
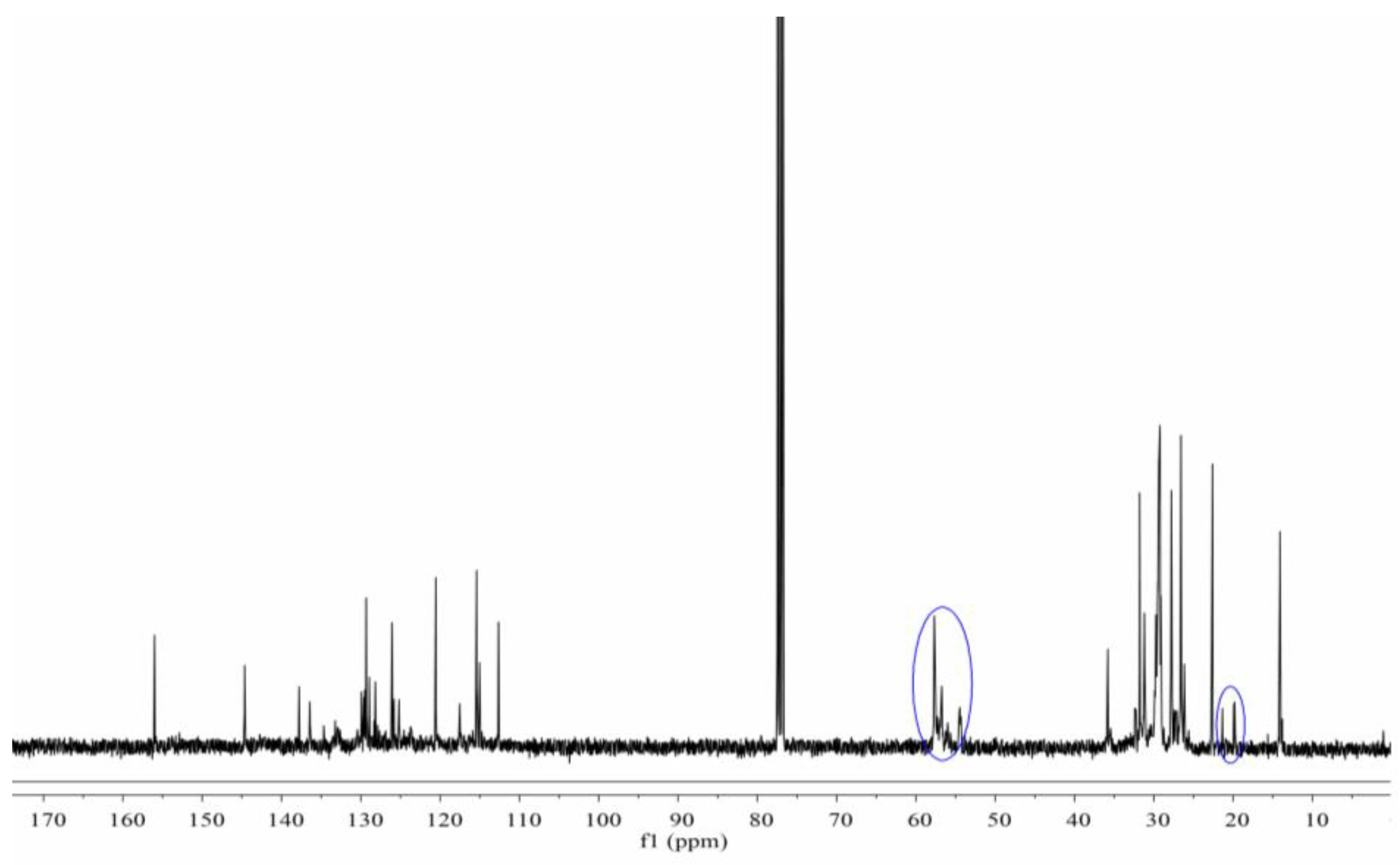
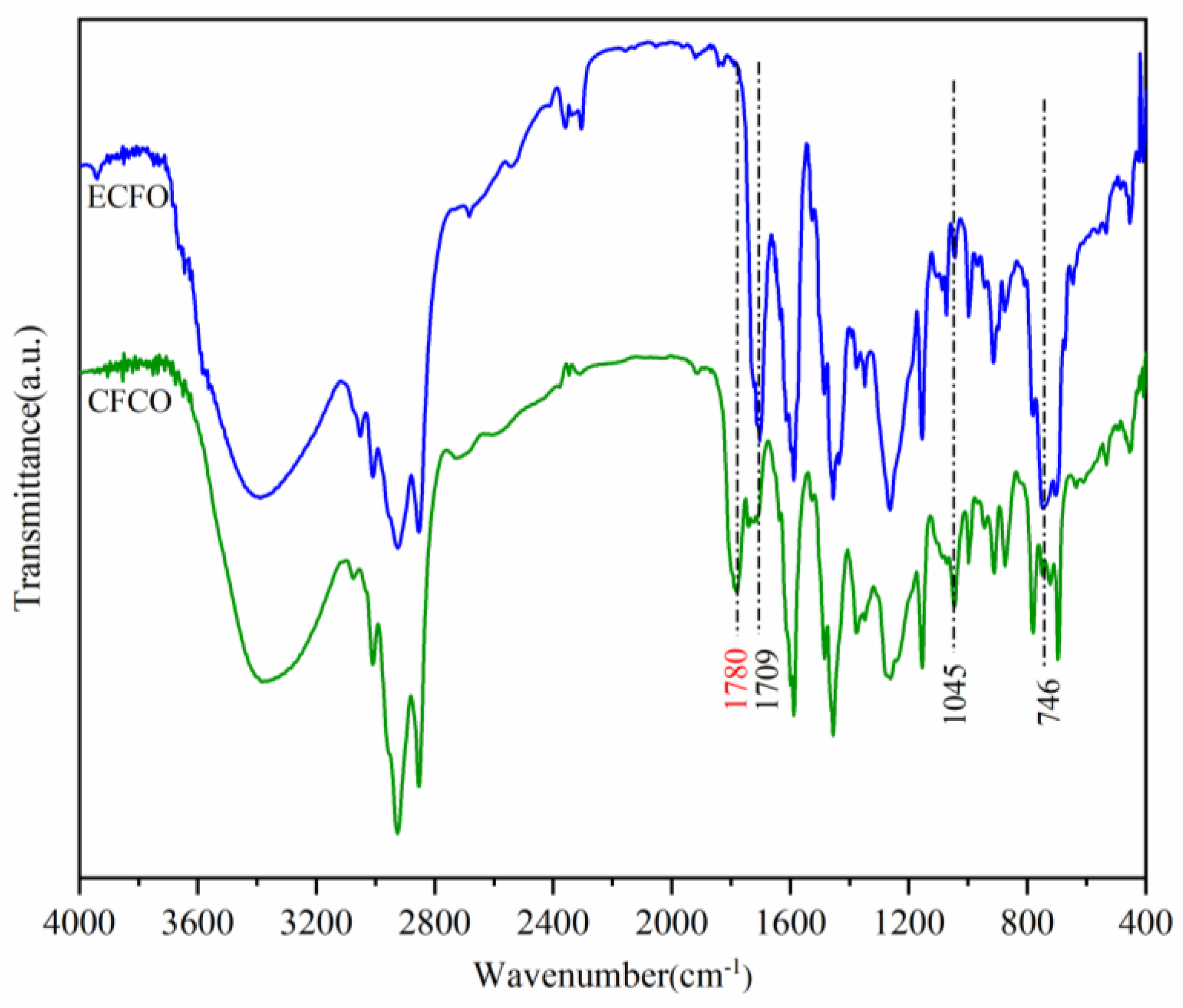
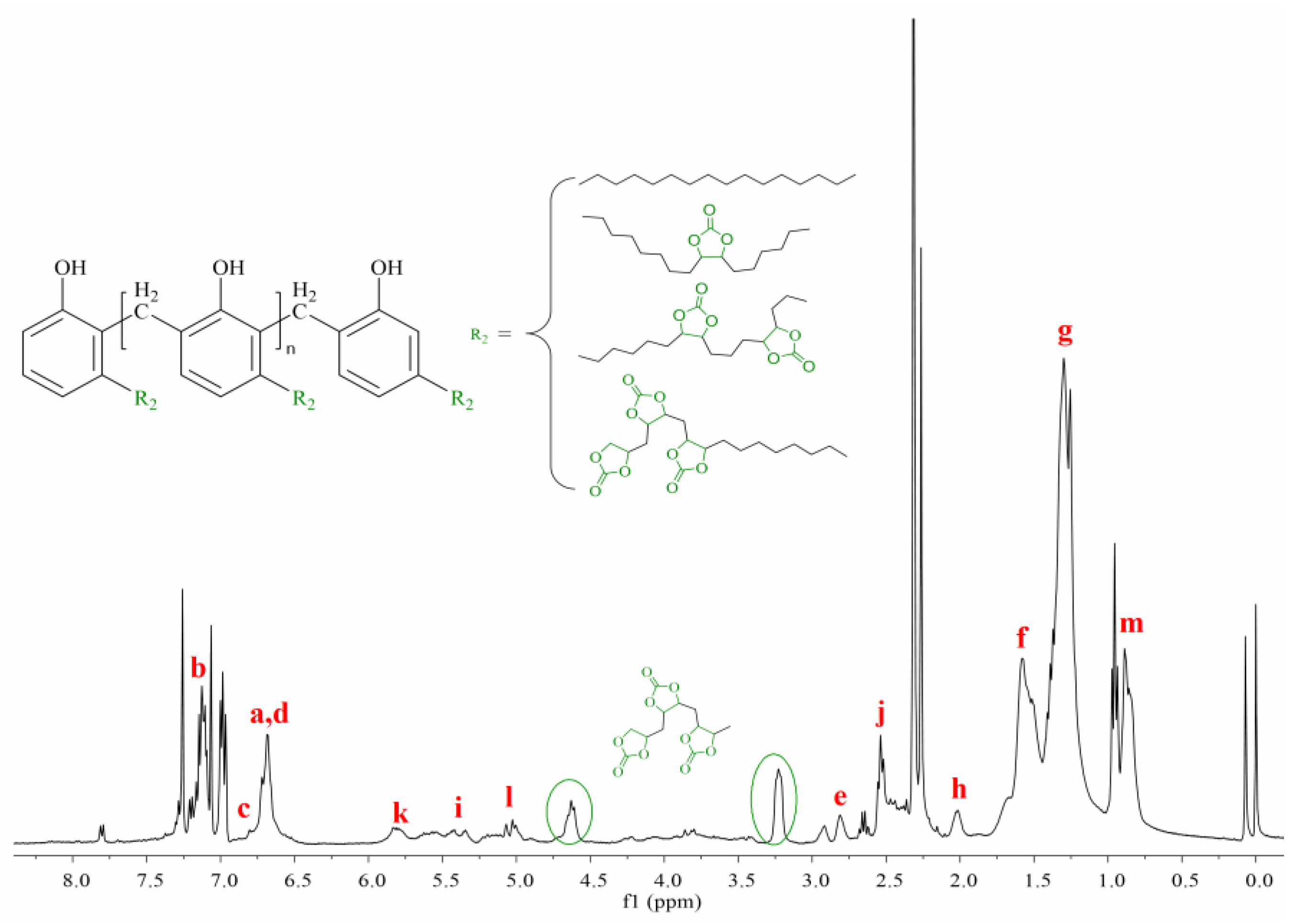
3.3. Synthesis and Characterization of Cardanol Formaldehyde Cyclocarbonate Oligomer (CFCO)
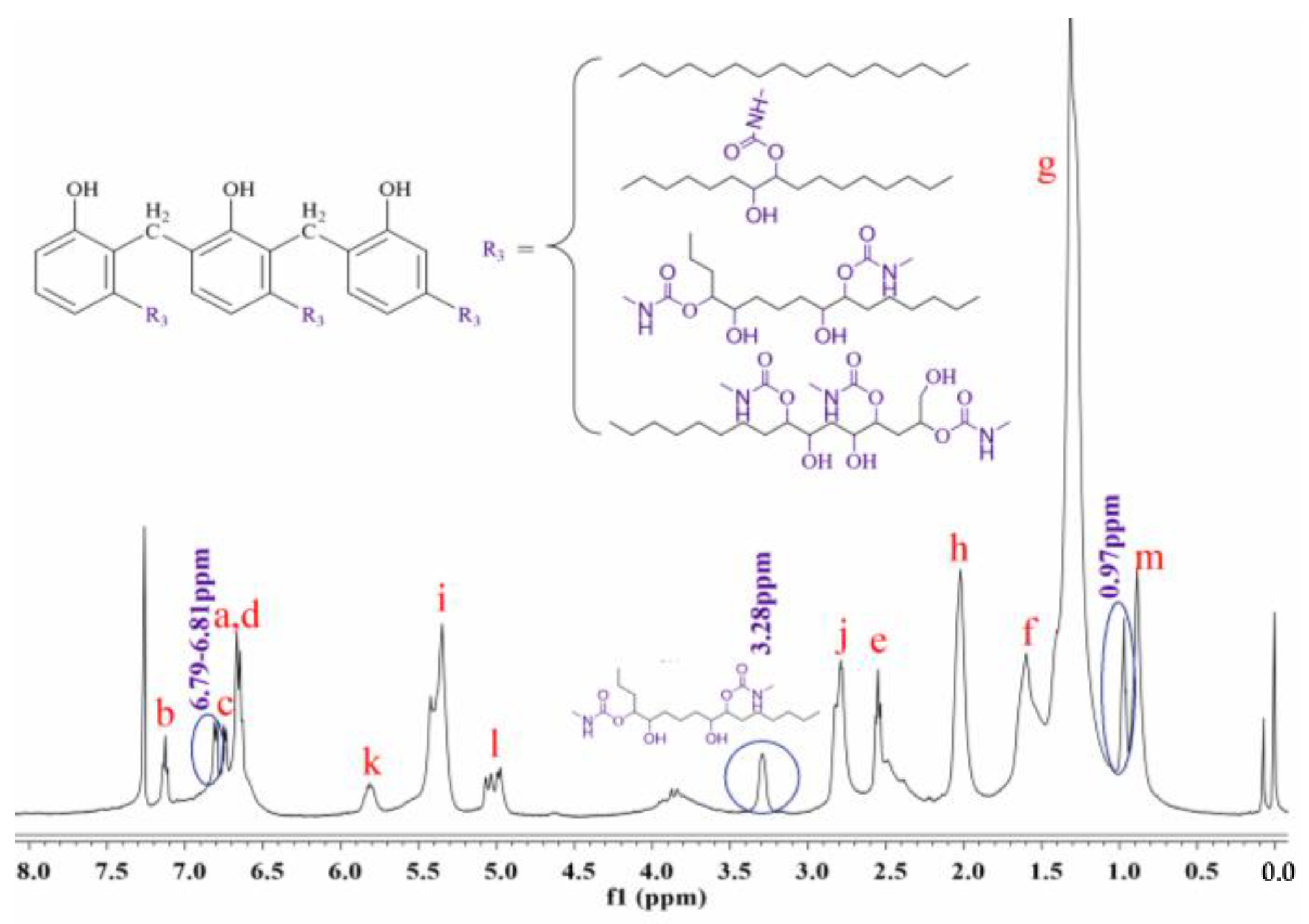
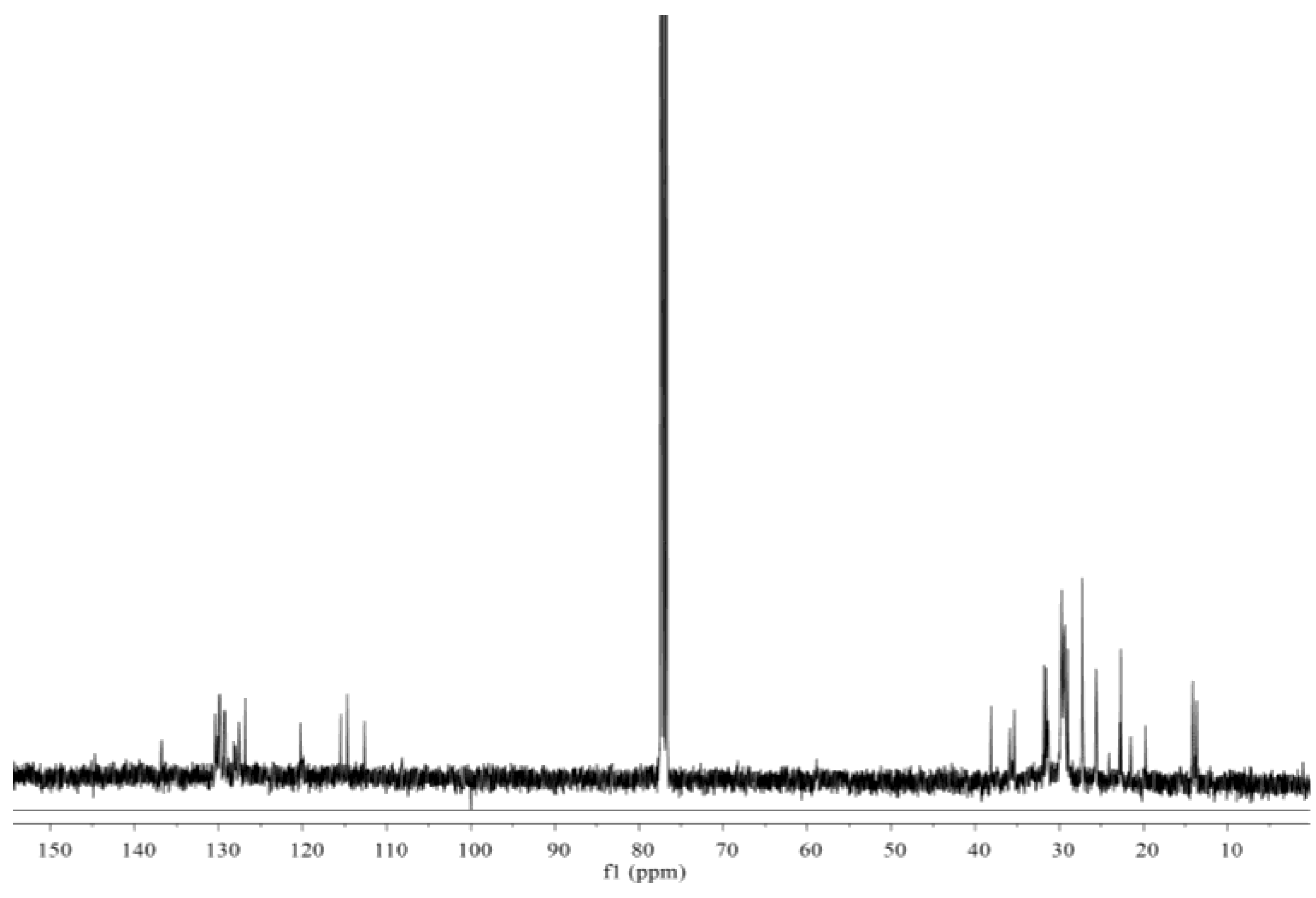
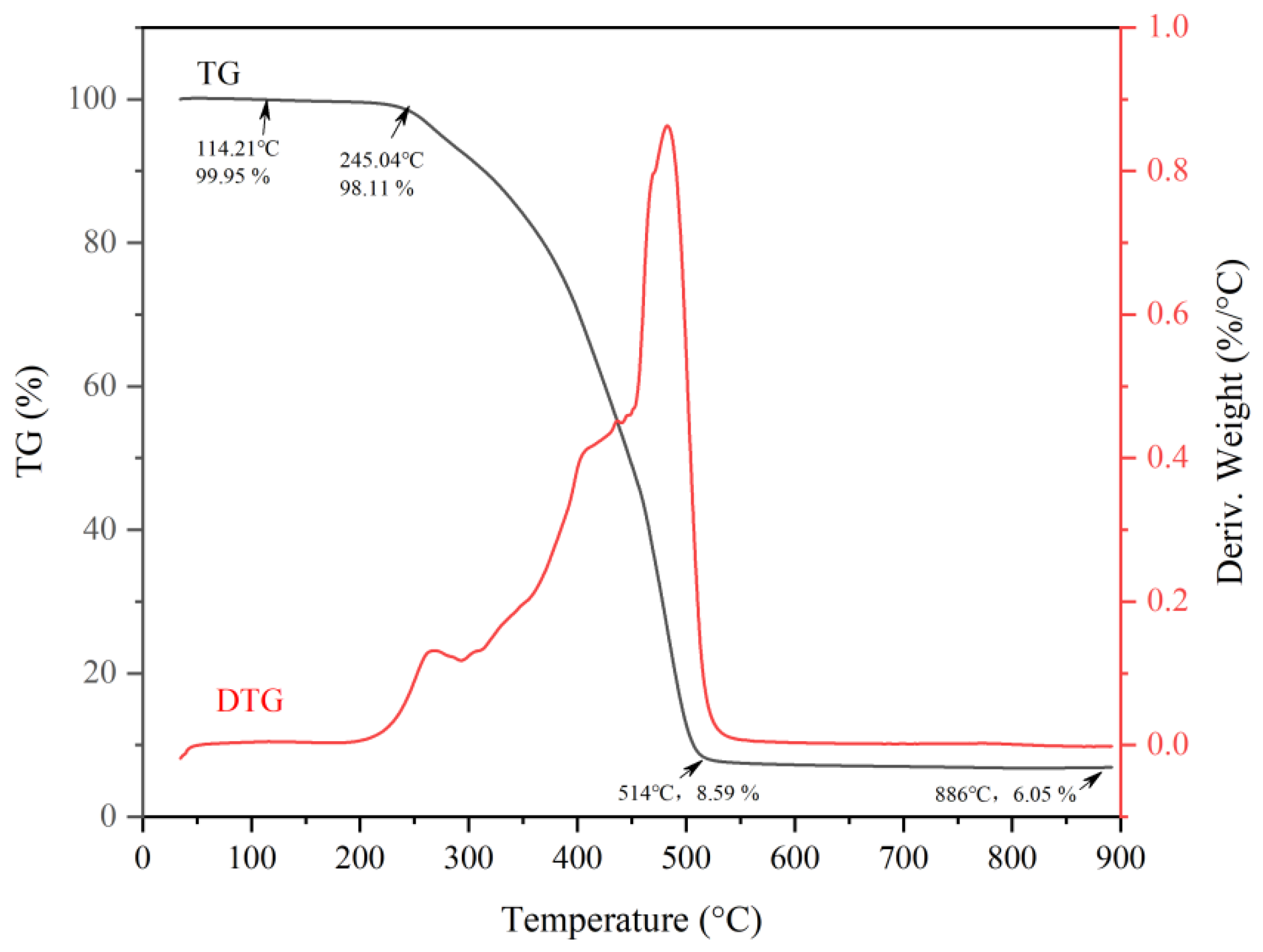
3.4. Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, J.; Li, X.; Wang, F.; Jiang, S.; Kang, M.; Wang, J.; Li, Q.; Wang, Z. Non-Isocyanate Polyurethane/Epoxy Hybrid Materials with Different and Controlled Architectures Prepared from a CO2-Sourced Monomer and Epoxy via an Environmentally-Friendly Route. RSC Adv. 2017, 7, 28841–28852. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- El Khezraji, S.; Chaib, M.; Thakur, S.; Raihane, M.; Lopez-Manchado, M.A.; Verdejo, R.; Lahcini, M. Synthesis of Novel Non-Isocyanate Polyurethane/Functionalized Boron Nitride Composites. Polymers 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497. [Google Scholar] [CrossRef]
- Smith, D.L.; Rodriguez-Melendez, D.; Cotton, S.M.; Quan, Y.; Wang, Q.; Grunlan, J.C. Non-Isocyanate Polyurethane Bio-Foam with Inherent Heat and Fire Resistance. Polymers 2022, 14, 5019. [Google Scholar] [CrossRef]
- Li, X.; Nie, X.; Chen, J.; Wang, Y. Preparation of Epoxidized Cardanol Butyl Ether as a Novel Renewable Plasticizer and Its Application for Poly(Vinyl Chloride): Epoxidized Cardanol Butyl Ether as Novel Renewable Plasticizer. Polym. Int. 2017, 66, 443–449. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Beniah, G.; Fortman, D.J.; Heath, W.H.; Dichtel, W.R.; Torkelson, J.M. Non-Isocyanate Polyurethane Thermoplastic Elastomer: Amide-Based Chain Extender Yields Enhanced Nanophase Separation and Properties in Polyhydroxyurethane. Macromolecules 2017, 50, 4425–4434. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Y.; Zhang, X.; Li, J. Eugenol-Based Non-Isocyanate Polyurethane and Polythiourethane. Iran. Polym. J. 2017, 26, 821–831. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A. Preparation of Novel CNSL-Based Urethane Polyol via Nonisocyanate Route: Curing with Melamine-Formaldehyde Resin and Structure-Property Relationship. J. Appl. Polym. Sci. 2015, 132, 41391. [Google Scholar] [CrossRef]
- Lee, P.S.; Jung, S.M. Single-catalyst Reactions from Depolymerization to Repolymerization: Transformation of Polyethylene Terephthalate to Polyisocyanurate Foam with Deep Eutectic Solvents. J. Appl. Polym. Sci. 2022, 139, e53205. [Google Scholar] [CrossRef]
- Paparella, A.N.; Perrone, S.; Salomone, A.; Messa, F.; Cicco, L.; Capriati, V.; Perna, F.M.; Vitale, P. Use of Deep Eutectic Solvents in Plastic Depolymerization. Catalysts 2023, 13, 1035. [Google Scholar] [CrossRef]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Soy-Based Polyurethanes by Nonisocyanate Route. J. Appl. Polym. Sci. 2008, 108, 3867–3875. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J. Bio-Based Elastic Polyurethane for Controlled-Release Urea Fertilizer: Fabrication, Properties, Swelling and Nitrogen Release Characteristics. J. Clean. Prod. 2019, 209, 528–537. [Google Scholar] [CrossRef]
- Grzęda, D.; Węgrzyk, G.; Nowak, A.; Idaszek, J.; Szczepkowski, L.; Ryszkowska, J. Cytotoxic Properties of Polyurethane Foams for Biomedical Applications as a Function of Isocyanate Index. Polymers 2023, 15, 2754. [Google Scholar] [CrossRef]
- Ruan, M.; Luan, H.; Wang, G.; Shen, M. Bio-Polyols Synthesized from Bio-Based 1,3-Propanediol and Applications on Polyurethane Reactive Hot Melt Adhesives. Ind. Crops Prod. 2019, 128, 436–444. [Google Scholar] [CrossRef]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, G.; Li, Z.; Liu, X.; Chen, Z.; Ariyasivam, S. Cardanol-Based Oligomers with “Hard Core, Flexible Shell” Structures: From Synthesis to UV Curing Applications. Green Chem. 2015, 17, 3319–3325. [Google Scholar] [CrossRef]
- Briou, B.; Caillol, S.; Robin, J.; Lapinte, V. Cardanol-Based and Formaldehyde-Free Flexible Phenolic Networks. Eur. J. Lipid Sci. Technol. 2018, 120, 1800175. [Google Scholar] [CrossRef]
- Kim, Y.H.; An, E.S.; Park, S.Y.; Song, B.K. Enzymatic Epoxidation and Polymerization of Cardanol Obtained from a Renewable Resource and Curing of Epoxide-Containing Polycardanol. J. Mol. Catal. B Enzym. 2007, 45, 39–44. [Google Scholar] [CrossRef]
- Devi, A.; Srivastava, D. Studies on the Blends of Cardanol-Based Epoxidized Novolac Type Phenolic Resin and Carboxyl-Terminated Polybutadiene (CTPB), I. Mater. Sci. Eng. A 2007, 458, 336–347. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Synthesis of Bio Based Low Temperature Curable Liquid Epoxy, Benzoxazine Monomer System from Cardanol: Thermal and Viscoelastic Properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Raden Siti Amirah, H.; Ahmad Faiza, M.; Samsuri, A. Synthesis and Characterization of Non-Isocyanate Polyurethane from Epoxidized Linoleic Acid—A Preliminary Study. AMR 2013, 812, 73–79. [Google Scholar] [CrossRef]
- Rios Yepes, Y.; Quintero, C.; Osorio Meléndez, D.; Daniliuc, C.G.; Martínez, J.; Rojas, R.S. Cyclic Carbonates from CO2 and Epoxides Catalyzed by Tetra- and Pentacoordinate Amidinate Aluminum Complexes. Organometallics 2019, 38, 469–478. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Zhang, G.; Kodama, K.; Yasutake, M.; Hirose, T. Synthesis of Cyclic Carbonates from CO 2 and Epoxides Catalyzed by Low Loadings of Benzyl Bromide/DMF at Ambient Pressure. Chem. Commun. 2014, 50, 14813–14816. [Google Scholar] [CrossRef]
- Devi, A.; Srivastava, D. Cardanol-Based Novolac-Type Phenolic Resins. I. A Kinetic Approach. J. Appl. Polym. Sci. 2006, 102, 2730–2737. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Huang, J.; Cai, W.; Song, L.; Hu, Y. Intrinsically Anti-Flammable and Self-Toughened Phosphorylated Cardanol-Derived Novolac Epoxy Thermosets. Ind. Crops Prod. 2021, 166, 113496. [Google Scholar] [CrossRef]
- Sultania, M.; Rai, J.S.P.; Srivastava, D. Studies on the Synthesis and Curing of Epoxidized Novolac Vinyl Ester Resin from Renewable Resource Material. Eur. Polym. J. 2010, 46, 2019–2032. [Google Scholar] [CrossRef]
- Mythili, C.V.; Retna, A.M.; Gopalakrishnan, S. Synthesis, Mechanical, Thermal and Chemical Properties of Polyurethanes Based on Cardanol. Bull. Mater. Sci. 2004, 27, 235–241. [Google Scholar] [CrossRef]
- Natarajan, M.; Murugavel, S.C. Synthesis, Spectral and Thermal Degradation Kinetics of Novolac Resins Derived from Cardanol. High Perform. Polym. 2013, 25, 685–696. [Google Scholar] [CrossRef]
- Campaner, P.; D’Amico, D.; Longo, L.; Stifani, C.; Tarzia, A. Cardanol-Based Novolac Resins as Curing Agents of Epoxy Resins. J. Appl. Polym. Sci. 2009, 114, 3585–3591. [Google Scholar] [CrossRef]
- Shibata, M.; Itakura, Y.; Watanabe, H. Bio-Based Thermosetting Resins Composed of Cardanol Novolac and Bismaleimide. Polym. J. 2013, 45, 758–765. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, P. Self-curable Epoxide Resins Based on Cardanol for Use in Surface Coatings. Pigment. Resin Technol. 2011, 40, 311–333. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Knothe, G.; Nie, X.; Jiang, J. Synthesis of Epoxidized Cardanol and Its Antioxidative Properties for Vegetable Oils and Biodiesel. ACS Sustain. Chem. Eng. 2016, 4, 901–906. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Qin, Y.; Li, Y. A Novel 2,5-Furandicarboxylic Acid-Based Bis(Cyclic Carbonate) for the Synthesis of Biobased Non-Isocyanate Polyurethanes. RSC Adv. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Sessini, V.; Thai, C.N.; Amorín, H.; Jiménez, R.; Samuel, C.; Caillol, S.; Cornil, J.; Hoyas, S.; Barrau, S.; Dubois, P.; et al. Solvent-Free Design of Biobased Non-Isocyanate Polyurethanes with Ferroelectric Properties. ACS Sustain. Chem. Eng. 2021, 9, 14946–14958. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A Review on the Production, Properties and Applications of Non-Isocyanate Polyurethane: A Greener Perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Loureiro, T.; Dip, R.M.M.; Lucas, E.; Spinelli, L. Cardanol Polymerization Under Acid Conditions By Addition And Condensation Reactions. J. Polym. Environ. 2018, 26, 555–566. [Google Scholar] [CrossRef]


| Peak No | Mp g/mol | Mn g/mol | Mw g/mol | Mz g/mol | Mz+1 g/mol | Mv g/mol | PD g/mol |
|---|---|---|---|---|---|---|---|
| 1 | 5237 | 5131 | 6360 | 7857 | 9433 | 6155 | 1.24 |
| 2 | 384 | 354 | 466 | 614 | 775 | 447 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, B.; Zhao, Y.; Lu, S.; Fan, D.; Wang, S.; Liu, J.; Tang, T.; Li, S. Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane. Polymers 2023, 15, 4683. https://doi.org/10.3390/polym15244683
Li Y, Zhang B, Zhao Y, Lu S, Fan D, Wang S, Liu J, Tang T, Li S. Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane. Polymers. 2023; 15(24):4683. https://doi.org/10.3390/polym15244683
Chicago/Turabian StyleLi, Yanan, Bin Zhang, Yuzhuo Zhao, Shuai Lu, Donglei Fan, Song Wang, Jie Liu, Tao Tang, and Sanxi Li. 2023. "Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane" Polymers 15, no. 24: 4683. https://doi.org/10.3390/polym15244683
APA StyleLi, Y., Zhang, B., Zhao, Y., Lu, S., Fan, D., Wang, S., Liu, J., Tang, T., & Li, S. (2023). Synthesis and Characterization of Cardanol-Based Non-Isocyanate Polyurethane. Polymers, 15(24), 4683. https://doi.org/10.3390/polym15244683







