Morphologies of Comb-like Polyacrylic Acid/Polyacrylate Copolymers as Functions of the Degree of Derivatization with n-C22H45 Side Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
3. Results and Discussion
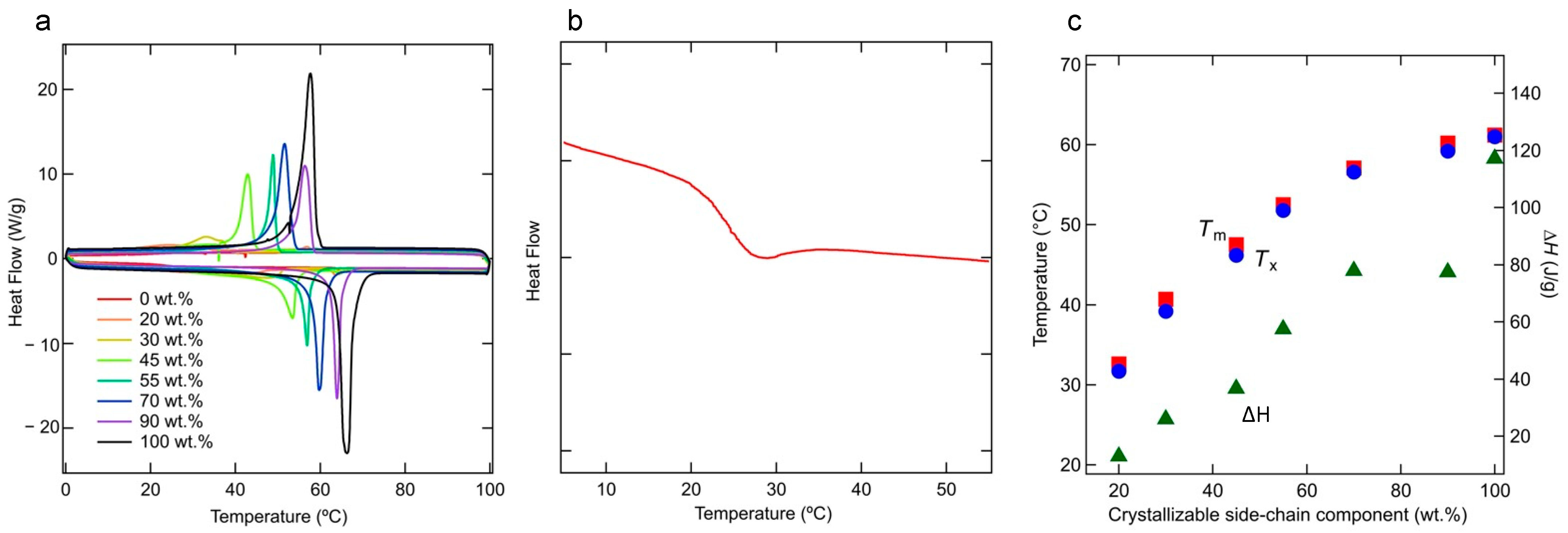
3.1. Thermodynamic Phenomena
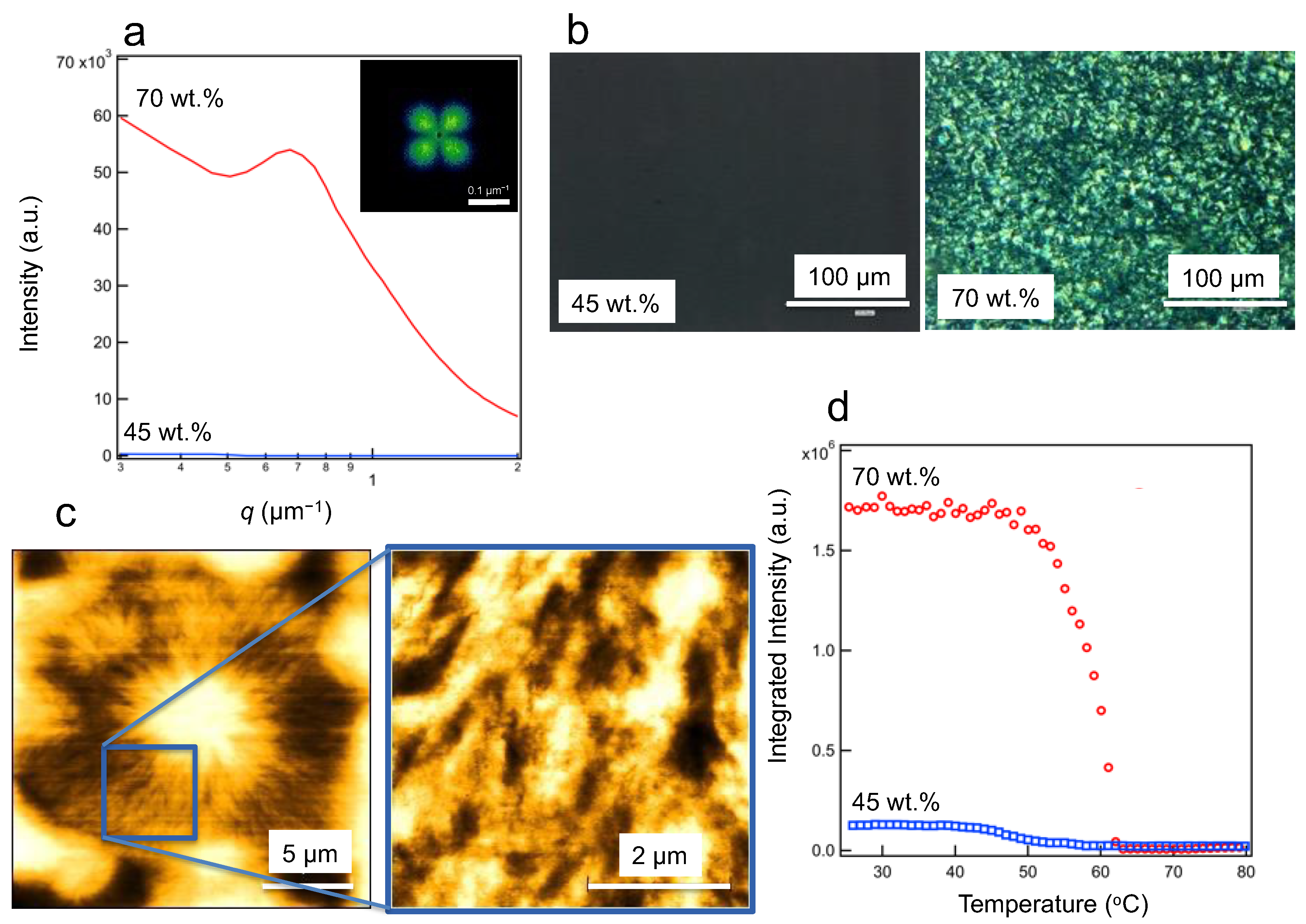
3.2. High-Ordered Structures at the Submicron and Micron Scales
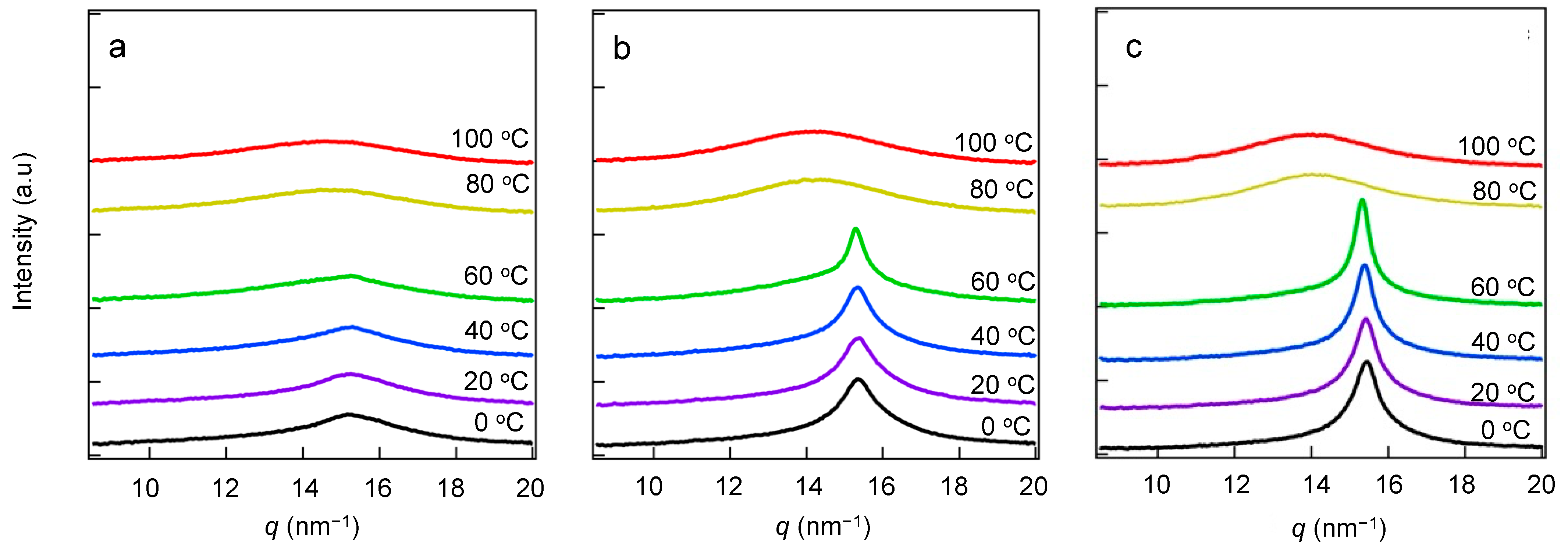
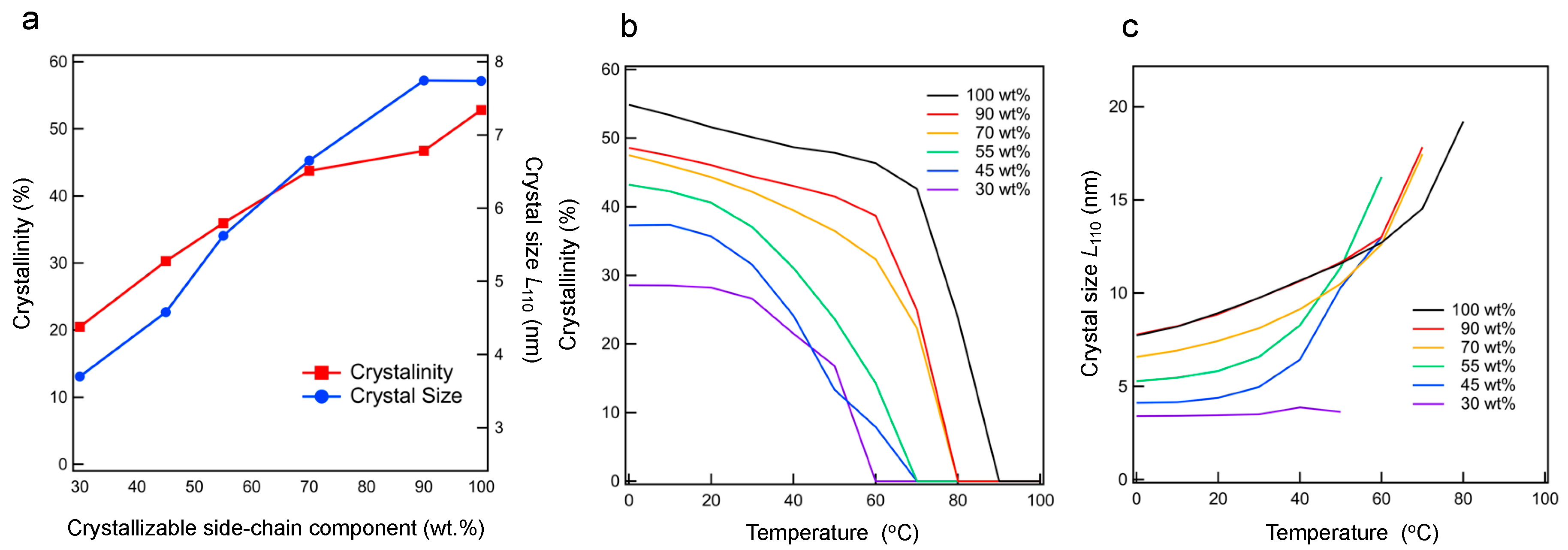
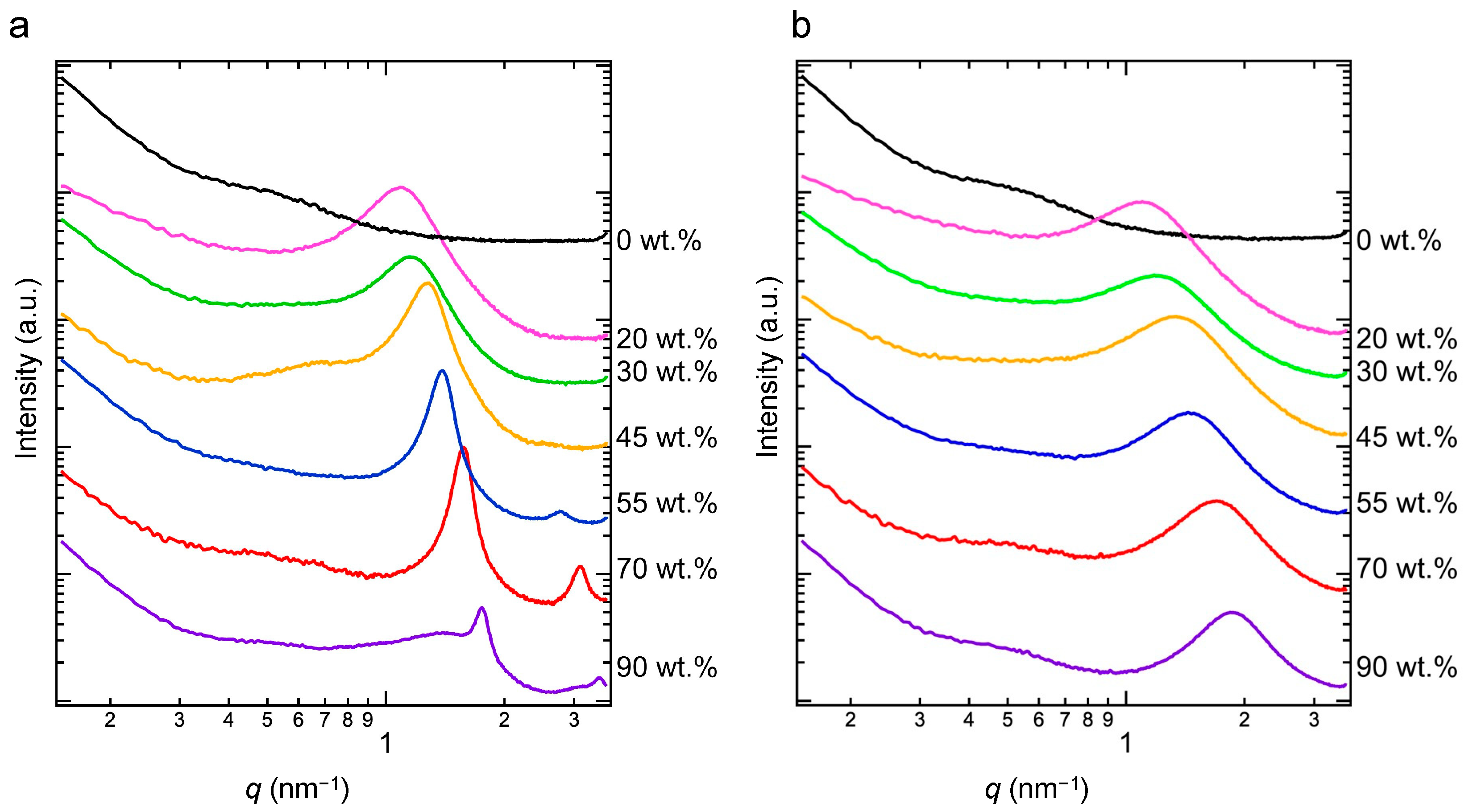
3.3. Crystal Lattice Determined via WAXS
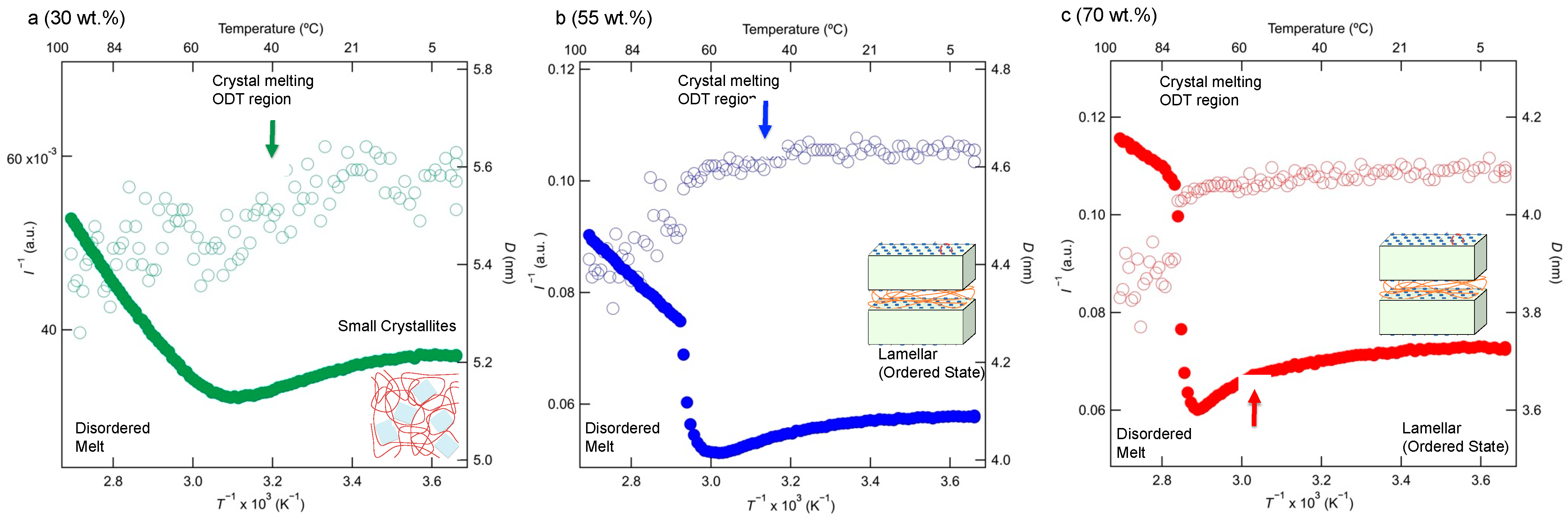
3.4. Sub-Micrometer-Scale Structure Determined via In Situ SAXS
3.5. Modeling the Large-Scale Structures of the Grafted Copolymers with Crystallizable Side-Chain Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, H.; Zhao, Y.; Dong, X.; Zhou, Y.; Wang, D. Frustrated crystallisation and hierarchical self-assembly behaviour of comb-like polymers. Chem. Soc. Rev. 2013, 42, 2075–2099. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kang, D.A.; Park, J.T.; Kim, K.C.; Kim, J.H. Synthesis, structure and gas separation properties of ethanol-soluble, amphiphilic POM-PBHP comb copolymers. Polymer 2019, 180, 121700. [Google Scholar] [CrossRef]
- Liang, H.; Wang, Z.; Sheiko, S.S.; Dobrynin, A.V. Comb and bottlebrush graft copolymers in a melt. Macromolecules 2019, 52, 3942–3950. [Google Scholar] [CrossRef]
- Mao, H.; Wang, H.; Li, J.; Zhang, L.; Shi, J.; Shi, H. Side-chain crystallization and segment packing of poly(isobutylene-alt-maleic anhydride)-g-alkyl alcohol comb-like polymers. Polymer 2020, 202, 122721. [Google Scholar] [CrossRef]
- Andrikopoulos, K.; Vlassopoulos, D.; Voyiatzis, G.A.; Yiannopoulos, Y.D.; Kamitsos, E.I. Molecular orientation of hairy-rod polyesters: Effects of side chain length. Macromolecules 1998, 31, 5465–5473. [Google Scholar] [CrossRef]
- Babur, T.; Gupta, G.; Beiner, M. About different packing states of alkyl groups in comb-like polymers with rigid backbones. Soft Matter 2016, 12, 8093–8097. [Google Scholar] [CrossRef] [PubMed]
- Danke, V.; Gupta, G.; Huth, H.; Schawe, J.E.K.; Beiner, M. Polymorphic states and phase transitions in a comb-like polymer having a rigid polyester backbone and flexible side chains. Thermochim. Acta 2019, 677, 162–168. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Kikuta, T.; Kasabou, K.; Yokota, K. Main-chain flexibility and sidechain crystallization of widely spaced comb-like polymers. Polym. J. 1998, 20, 693–698. [Google Scholar] [CrossRef]
- Meng, J.; Tang, X.; Zhang, Z.; Zhang, X.; Shi, H. Fabrication and Properties of poly(polyethylene glycol octadecyl ether methacrylate). Thermochim. Acta 2013, 574, 116–120. [Google Scholar] [CrossRef]
- Imai, S.; Arakawa, M.; Nakanishi, Y.; Takenaka, M.; Aoki, H.; Ouchi, M.; Terashima, T. Water-assisted microphase separation of cationic random copolymers into sub-5 nm lamellar materials and thin films. Macromolecules 2022, 55, 9113–9125. [Google Scholar] [CrossRef]
- Greenberg, S.A.; Alfrey, T. Side Chain Crystallization of n-alkyl polymethacrylates and polyacrylates1. J. Am. Chem. Soc. 1954, 76, 6280–6285. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.; Yin, Y.; Liu, G.; Zhang, X.; Wang, D. Chain packing and phase transition of N-hexacosylated polyethyleneimine comb-like polymer: A combined investigation by synchrotron X-ray scattering and FTIR spectroscopy. Polymer 2013, 54, 6261–6266. [Google Scholar] [CrossRef]
- Gopi, S.; Kontopoulou, M.; Ramsay, B.A.; Ramsay, J.A. Manipulating the structure of medium-chain-length polyhydroxyalkanoate (MCL-PHA) to enhance thermal properties and crystallization kinetics. Int. J. Biol. Macromol. 2018, 119, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.; Pfukwa, R.; Maiz, J.; Müller, A.J.; Klumperman, B. Synthesis, structure, and crystallization behavior of amphiphilic heteroarm molecular brushes with crystallizable poly(ethylene oxide) and n-alkyl side chains. Macromolecules 2020, 53, 1585–1595. [Google Scholar] [CrossRef]
- Ebata, K.; Hashimoto, Y.; Yamamoto, S.; Mitsuishi, M.; Nagano, S.; Matsui, J. Nanophase separation of poly(N-alkyl acrylamides): The dependence of the formation of lamellar structures on their alkyl side chains. Macromolecules 2019, 52, 9773–9780. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, Y.; Jiang, S.; Xin, J.H.; Rottstegge, J.; Xu, D.; Wang, D. Order-disorder transition in Eicosylated polyethyleneimine comblike polymers. Polymer 2007, 48, 2762–2767. [Google Scholar] [CrossRef]
- Xu, H.; Gao, Y.; Li, J.; Wang, H.; Shi, H. Thermal performance and phase transformation of S-alkylated poly(vinyl chloride) comb-like polymers. Polymer 2018, 153, 362–368. [Google Scholar] [CrossRef]
- Tekin, E.; Egbe, D.A.M.; Kranenburg, J.M.; Ulbricht, C.; Rathgeber, S.; Birckner, E.; Rehmann, N.; Meerholz, K.; Schubert, U.S. Effect of side chain length variation on the optical properties of PPE-PPV hybrid polymers. Chem. Mater. 2008, 20, 2727–2735. [Google Scholar] [CrossRef]
- Zheng, W.; Levon, K.; Laakso, J.; Oesterholm, J.E. Characterization and solid-state properties of processable N-alkylated polyanilines in the neutral state. Macromolecules 1994, 27, 7754–7768. [Google Scholar] [CrossRef]
- Lee, J.L.; Pearce, E.M.; Kwei, T.K. Side-chain crystallization in alkyl-substituted semiflexible polymers. Macromolecules 1997, 30, 6877–6883. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhao, Y.; Zhang, X.Q.; Zhou, Y.; Xu, Y.H.; Zhou, S.R.; Wang, D.J.; Han, C.C.; Xu, D.F. Packing mode and conformational transition of alkyl side chains in N-alkylated poly(p-benzamide) comb-like polymer. Polymer 2004, 45, 6299–6307. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhao, Y.; Zhang, X.Q.; Jiang, S.C.; Wang, D.J.; Han, C.C.; Xu, D.F. Phase transition and conformational variation of N-alkylated branched poly(ethyleneimine) comblike polymer. Macromolecules 2004, 37, 9933–9940. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, H.F.; Zhao, Y.; Men, Y.F.; Jiang, S.C.; Rottstegge, J.; Wang, D.J. Confined crystallization and phase transition in semi-rigid chitosan containing long chain alkyl groups. CrystEngComm 2011, 13, 561–567. [Google Scholar] [CrossRef]
- Li, L.J.; Shi, H.F.; Zhang, X.X. Preparation and characterization of Octadecylated poly(vinyl alcohol) polymers. Adv. Mater. Res. 2012, 482–484, 1921–1924. [Google Scholar] [CrossRef]
- Greene, L.; Phan, L.X.; Schmitt, E.E.; Mohr, J.M. Side-chain crystallizable polymers for temperature-activated controlled release. In Polymeric Delivery Systems; American Chemical Society: Washington, DC, USA, 1993; pp. 244–256. [Google Scholar] [CrossRef]
- Okada, M.; Nakano, S.; Yamashita, K.; Kawahara, S.; Matsui, S. Direct patterning on side chain crystalline polymer by thermal nanoimprinting using mold without antisticking layer. Microelectron. Eng. 2011, 88, 2084–2087. [Google Scholar] [CrossRef]
- Shimizu, H. Adhesive bonding technologies in production engineering. Recent trend on intelligent material. J. Jpn Soc. Precis. Engi 1998, 64, 210–213. [Google Scholar] [CrossRef]
- Sun, H. Recent progress in low temperature nanoimprint lithography. Microsyst. Technol. 2015, 21, 1–7. [Google Scholar] [CrossRef]
- Yang, S.; Bak, J.Y.; Yoon, S.M.; Ryu, M.K.; Oh, H.; Hwang, C.S.; Kim, G.H.; Park, S.K.; Jang, J. Low-temperature processed flexible in–Ga–Zn–O thin-film transistors exhibiting high electrical performance. IEEE Electron Dev. Lett. 2011, 32, 1692–1694. [Google Scholar] [CrossRef]
- Min, S.Y.; Cho, W.J. Resistive switching characteristic improvement in a single-walled carbon nanotube random network embedded hydrogen silsesquioxane thin films for flexible memristors. Int. J. Mol. Sci. 2021, 22, 3390. [Google Scholar] [CrossRef]
- Min, J.G.; Cho, W.J. Chitosan-based flexible memristors with embedded carbon nanotubes for neuromorphic electronics. Micromachines 2021, 12, 1259. [Google Scholar] [CrossRef]
- Greene, L.C.; Meyers, P.A.; Springer, J.T.; Banks, P.A. Biological evaluation of pesticides released from temperature-responsive microcapsules. J. Agric. Food Chem. 1992, 40, 2274–2278. [Google Scholar] [CrossRef]
- Johnson, G.A.; Hicks, D.H.; Stewart, R.F.; Duan, X. Use of temperature-responsive polymer seed coating to control seed germination. Acta Hortic. 1999, 504, 229–236. [Google Scholar] [CrossRef]
- Kim, D.; Seo, J. A review: Breathable films for packaging applications. Trends Food Sci. Technol. 2018, 76, 15–27. [Google Scholar] [CrossRef]
- Parrinello, C.A.; Bounds, C.O.; Liveri, M.L.T.; Pojman, J.A. Thermal frontal polymerization with a thermally released redox catalyst. J. Polym. Sci. A Polym. Chem. 2012, 50, 2337–2343. [Google Scholar] [CrossRef]
- Matsuba, G.; Fujiyama, Y.; Shimeki, T.; Ishii, M.; Nanchi, M.; Kato, T.; Kawahara, S. The effect of tackifier on temperature-sensitive adhesives with polymers of crystallizable side-chain. J. Soc. Mater. Sci. Jpn 2019, 68, 20–25. [Google Scholar] [CrossRef]
- Igarashi, N.; Watanabe, Y.; Shinohara, Y.; Inoko, Y.; Matsuba, G.; Okuda, H.; Mori, T.; Ito, K. Upgrade of the small angle X-ray scattering beamlines at the photon factory. J. Phys. Conf. S. 2011, 272, 012026. [Google Scholar] [CrossRef]
- Takagi, H.; Igarashi, N.; Mori, T.; Saijo, S.; Ohta, H.; Nagatani, Y.; Kosuge, T.; Shimizu, N. Upgrade of small angle X-ray scattering beamline BL-6A at the photon factory. AIP Conf. Proc. 2016, 1741, 030018. [Google Scholar] [CrossRef]
- Nishida, K.; Ogawa, H.; Matsuba, G.; Konishi, T.; Kanaya, T. A high-resolution small-angle light scattering instrument for soft matter studies. J. Appl. Crystallogr. 2008, 41, 723–728. [Google Scholar] [CrossRef]
- Zhao, Y.; Hayasaka, K.; Matsuba, G.; Ito, H. In situ observations of flow-induced precursors during shear flow. Macromolecules 2013, 46, 172–178. [Google Scholar] [CrossRef]
- Miwa, Y.; Nagahama, T.; Sato, H.; Tani, A.; Takeya, K. Intermolecular interaction of tetrabutylammonium and tetrabutylphosphonium salt hydrates by low-frequency Raman observation. Molecules 2022, 27, 4743. [Google Scholar] [CrossRef]
- Domańska, U.; Łachwa, J.; Morawski, P.; Malanowski, S.K. Phase equilibria and volumetric properties in binary mixtures containing branched chain ethers (methyl 1,1-dimethylethyl ether or ethyl 1,1-dimethylethyl ether or methyl 1,1-dimethylpropyl ether or ethyl 1,1-dimethylpropyl ether). J. Chem. Eng. Data 1999, 44, 974–984. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor pressures and vaporization enthalpies of the n-alkanes from C21 to C30 at T = 298.15 K by correlation gas chromatography. J. Chem. Eng. Data 2004, 49, 77–85. [Google Scholar] [CrossRef]
- Stein, R.S.; Rhodes, M.B. Photographic light scattering by polyethylene films. J. Appl. Phys. 1960, 31, 1873–1874. [Google Scholar] [CrossRef]
- Stein, R.S.; Wilson, P.R. Scattering of light by polymer films possessing correlated orientation fluctuations. J. Appl. Phys. 1962, 33, 1914–1922. [Google Scholar] [CrossRef]
- Inomata, K.; Sakamaki, Y.; Nose, T.; Sasaki, S. Solid-state structure of comb-like polymers having n-octadecyl side chains I. Cocrystallization of side chain with n-octadecanoic acid. Polym. J. 1996, 28, 986–991. [Google Scholar] [CrossRef][Green Version]
- Mondieig, D.; Rajabalee, F.; Metivaud, V.; Oonk, H.A.J.; Cuevas-Diarte, M.A. n-alkane binary molecular alloys. Chem. Mater. 2004, 16, 786–798. [Google Scholar] [CrossRef]
- Cholakova, D.; Denkov, N. Rotator phases in alkane systems: In bulk, surface layers and micro/nano-confinements. Adv. Colloid Interface Sci. 2019, 269, 7–42. [Google Scholar] [CrossRef]
- Danke, V.; Beiner, M.; Saalwächter, K.; Schäfer, M. Structure and dynamics in a polymorphic nanophase-separated stiff comblike polymer. Macromolecules 2019, 52, 6943–6952. [Google Scholar] [CrossRef]
- Cholakova, D.; Tsvetkova, K.; Tcholakova, S.; Denkov, N. Rheological properties of rotator and crystalline phases of alkanes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127926. [Google Scholar] [CrossRef]
- Nandan, B.; Lee, C.; Chen, H.; Chen, W. Molecular architecture effect on the microphase separations in supramolecular comb−coil complexes of polystyrene—Block—Poly(2-vinylpyridine) with dodecylbenzenesulfonic acid: (AB) n A n block−arm star copolymer. Macromolecules 2005, 38, 10117–10126. [Google Scholar] [CrossRef]
- Huh, J.; Ikkala, O.; ten Brinke, G. Correlation hole effect in comblike copolymer systems obtained by hydrogen bonding between homopolymers and end-functionalized oligomers. Macromolecules 1997, 30, 1828–1835. [Google Scholar] [CrossRef]
- Chen, H.-L.; Hsiao, M.-S. Self-assembled mesomorphic complexes of branched poly(ethylenimine) and Dodecylbenzenesulfonic acid. Macromolecules 1999, 32, 2967–2973. [Google Scholar] [CrossRef]
- Sakurai, S.; Mori, K.; Okawara, A.; Kimishima, K.; Hashimoto, T. Evaluation of segmental interaction by small-angle x-ray scattering based on the random-phase approximation for asymmetric, polydisperse triblock copolymers. Macromolecules 1992, 25, 2679–2691. [Google Scholar] [CrossRef]
- Russell, T.P.; Hjelm, R.P., Jr.; Seeger, P.A. Temperature dependence of the interaction parameter of polystyrene and poly(methyl methacrylate). Macromolecules 1990, 23, 890–893. [Google Scholar] [CrossRef]
- Leibler, L. Theory of microphase separation in block copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Leibler, L.; Benoit, H. Theory of correlations in partly labelled homopolymer melts. Polymer 1981, 22, 195–201. [Google Scholar] [CrossRef]
- Staropoli, M.; Raba, A.; Hövelmann, C.H.; Krutyeva, M.; Allgaier, J.; Appavou, M.-S.; Keiderling, U.; Stadler, F.J.; Pyckhout-Hintzen, W.; Wischnewski, A.; et al. Hydrogen bonding in a reversible comb polymer architecture: A microscopic and macroscopic investigation. Macromolecules 2016, 49, 5692–5703. [Google Scholar] [CrossRef][Green Version]
- Floudas, G.; Hadjichristidis, N.; Iatrou, H.; Pakula, T.; Fischer, E.W. Microphase separation in model 3-miktoarmstar copolymers (simple graft and terpolymers). 1. Statics and kinetics. Macromolecules 1994, 27, 7735–7746. [Google Scholar] [CrossRef]
- Claudy, P.; Letoffe, J.M. Phase transitions in even n-alkanes CnH2n+2, n = 16–28. Characterization by differential calorimetric analysis and by thermooptical analysis. Effect of deuteration. Calorim. Anal. Therm. 1991, 22, 281. [Google Scholar]
- Schaufele, R.F. Chain Shortening in Polymethylene Liquids. J. Chem. Phys. 1968, 49, 4168. [Google Scholar] [CrossRef]
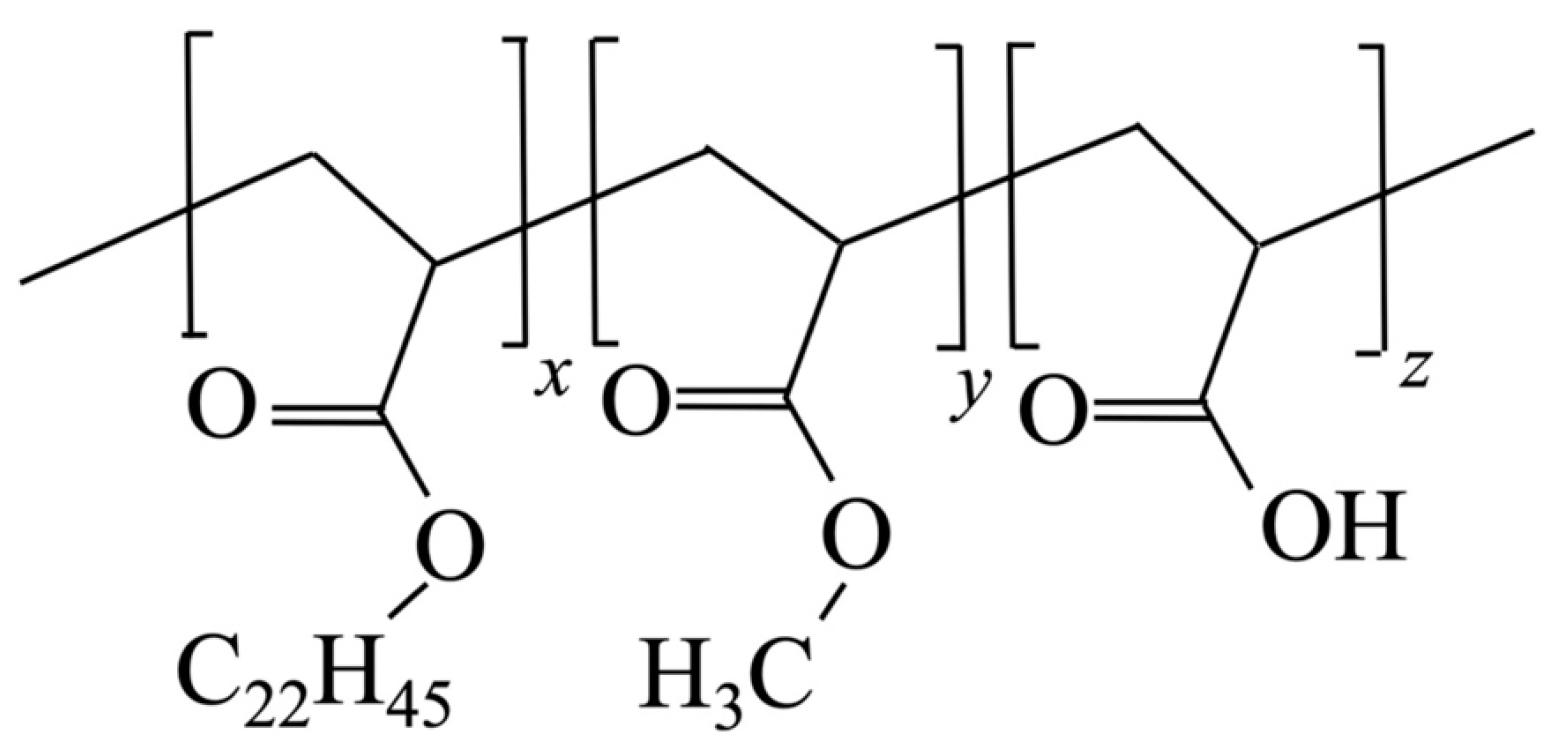


| Sample | Monomer (wt.%) with Side-Chain C22H45/CH3/H | Crystallizable Side Chain (mol%) |
|---|---|---|
| 0 wt.% | 0/95/5 | 0 |
| 20 wt.% | 20/75/5 | 5.3 |
| 30 wt.% | 30/65/5 | 8.7 |
| 45 wt.% | 45/50/5 | 15 |
| 55 wt.% | 55/40/5 | 21 |
| 70 wt.% | 70/25/5 | 34 |
| 90 wt.% | 90/5/5 | 65 |
| 100 wt.% | 100/0/0 | 100 |
| Sample | Correlation Length at 0 °C [nm] | Correlation Length at 100 °C [nm] |
|---|---|---|
| 20 wt.% | 5.92 ± 0.05 | 6.16 ± 0.07 |
| 30 wt.% | 5.58 ± 0.07 | 5.44 ± 0.08 |
| 45 wt.% | 5.08 ± 0.01 | 4.83 ± 0.08 |
| 55 wt.% | 4.62 ± 0.02 | 4.34 ± 0.07 |
| 70 wt.% | 4.09 ± 0.02 | 3.85 ± 0.07 |
| 90 wt.% | 3.59 ± 0.02 | 3.33 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, T.; Ishii, M.; Sato, H.; Matsuba, G. Morphologies of Comb-like Polyacrylic Acid/Polyacrylate Copolymers as Functions of the Degree of Derivatization with n-C22H45 Side Chains. Polymers 2023, 15, 4663. https://doi.org/10.3390/polym15244663
Okada T, Ishii M, Sato H, Matsuba G. Morphologies of Comb-like Polyacrylic Acid/Polyacrylate Copolymers as Functions of the Degree of Derivatization with n-C22H45 Side Chains. Polymers. 2023; 15(24):4663. https://doi.org/10.3390/polym15244663
Chicago/Turabian StyleOkada, Tomoya, Mizuho Ishii, Harumi Sato, and Go Matsuba. 2023. "Morphologies of Comb-like Polyacrylic Acid/Polyacrylate Copolymers as Functions of the Degree of Derivatization with n-C22H45 Side Chains" Polymers 15, no. 24: 4663. https://doi.org/10.3390/polym15244663
APA StyleOkada, T., Ishii, M., Sato, H., & Matsuba, G. (2023). Morphologies of Comb-like Polyacrylic Acid/Polyacrylate Copolymers as Functions of the Degree of Derivatization with n-C22H45 Side Chains. Polymers, 15(24), 4663. https://doi.org/10.3390/polym15244663










