Self-Healing Sulfonated Poly(ether ether ketone)-Based Polymer Electrolyte Membrane for Direct Methanol Fuel Cells: Effect of Solvent Content
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Synthesis of Sulfonated Poly(ether ether ketone) (SPEEK)
2.3. Preparation of SPEEK and SPEEK/PVA Blend Membranes
2.4. Characterization
2.4.1. Structural Characterization
2.4.2. Water Uptake and Methanol Uptake
2.4.3. Methanol Permeability
2.4.4. Proton Conductivity
2.4.5. Selectivity
2.4.6. Self-Healing Property
3. Results and Discussion
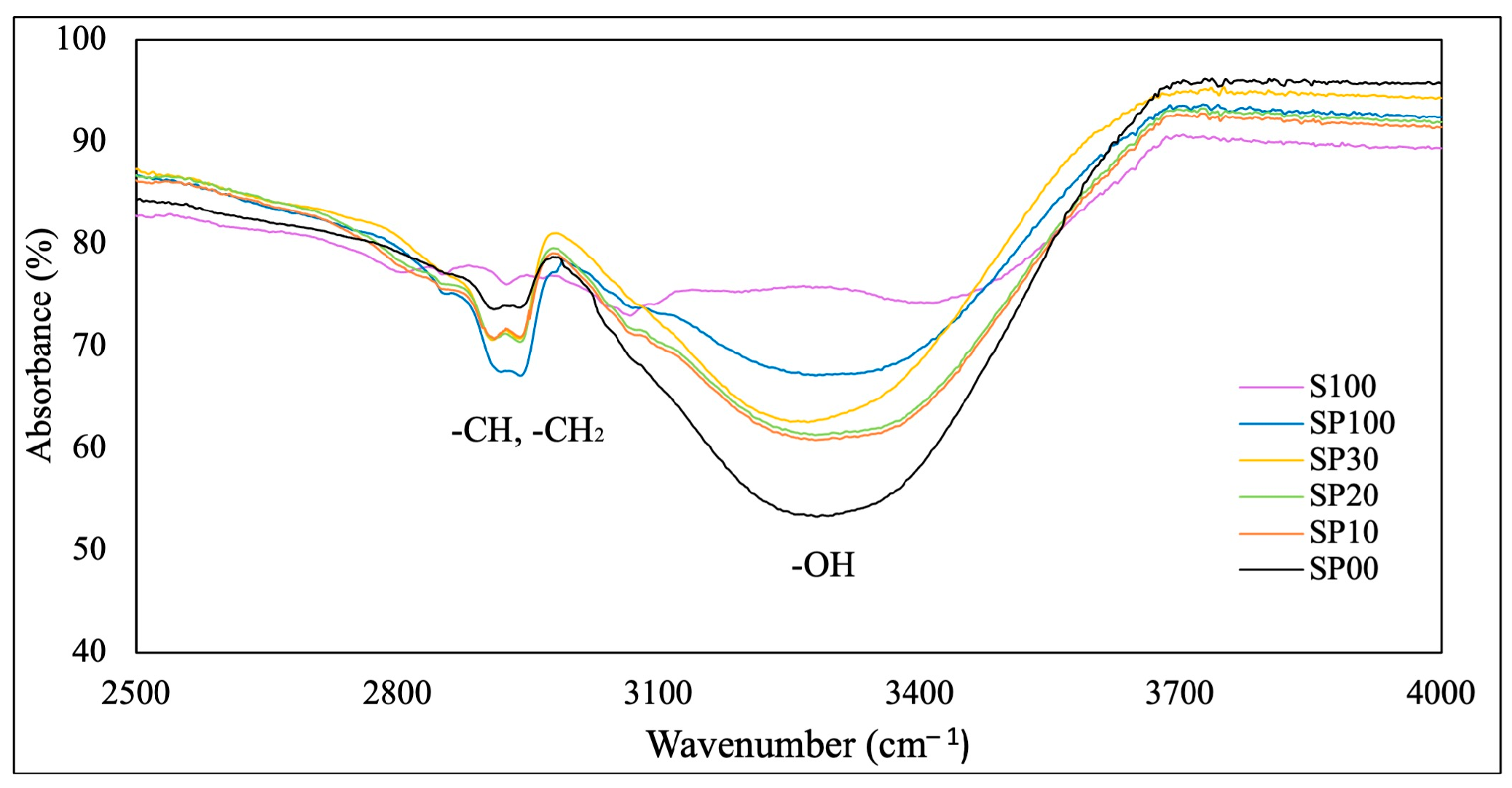
3.1. FTIR
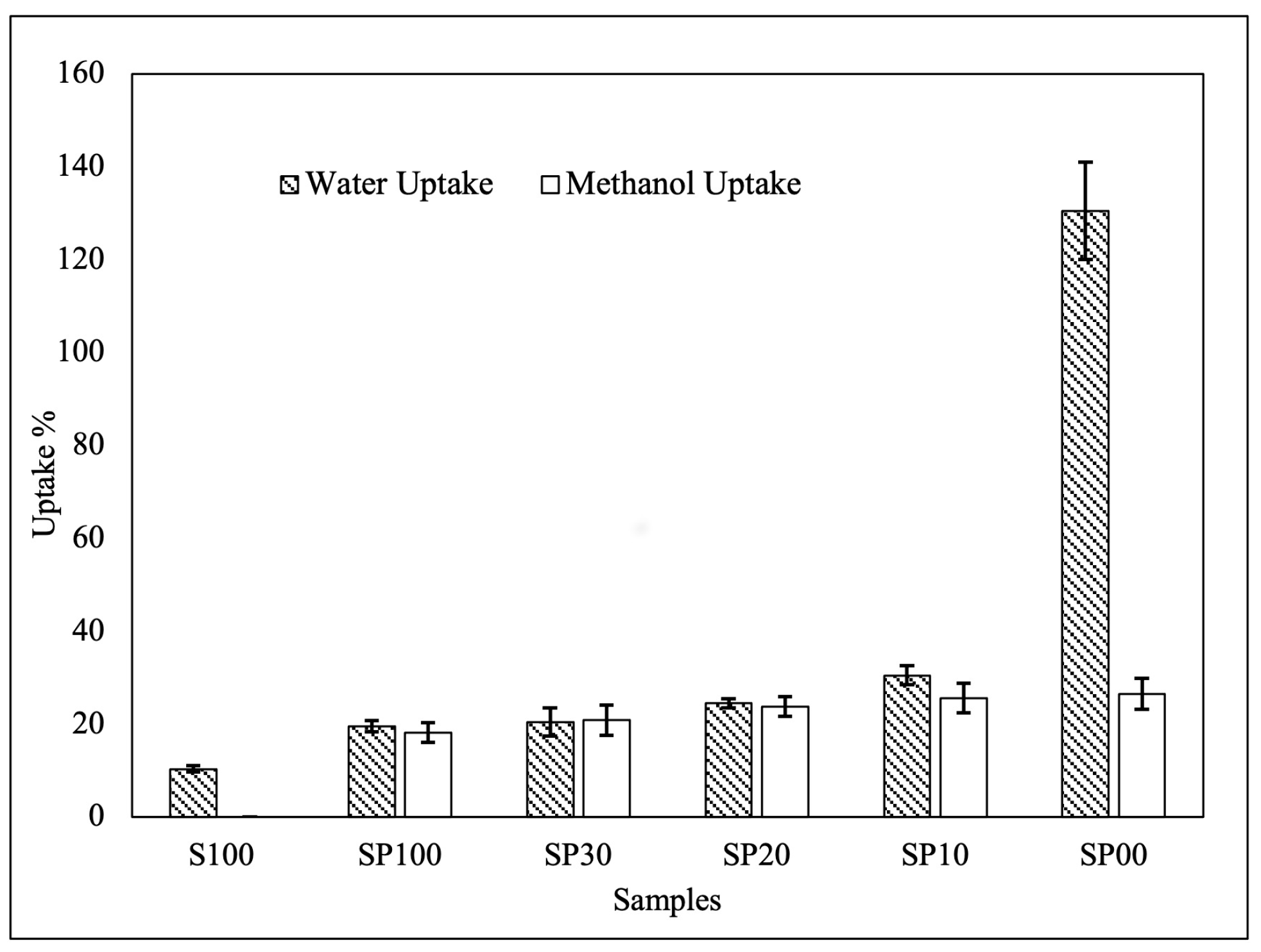
3.2. Water Uptake and Methanol Uptake
3.3. Proton Conductivity
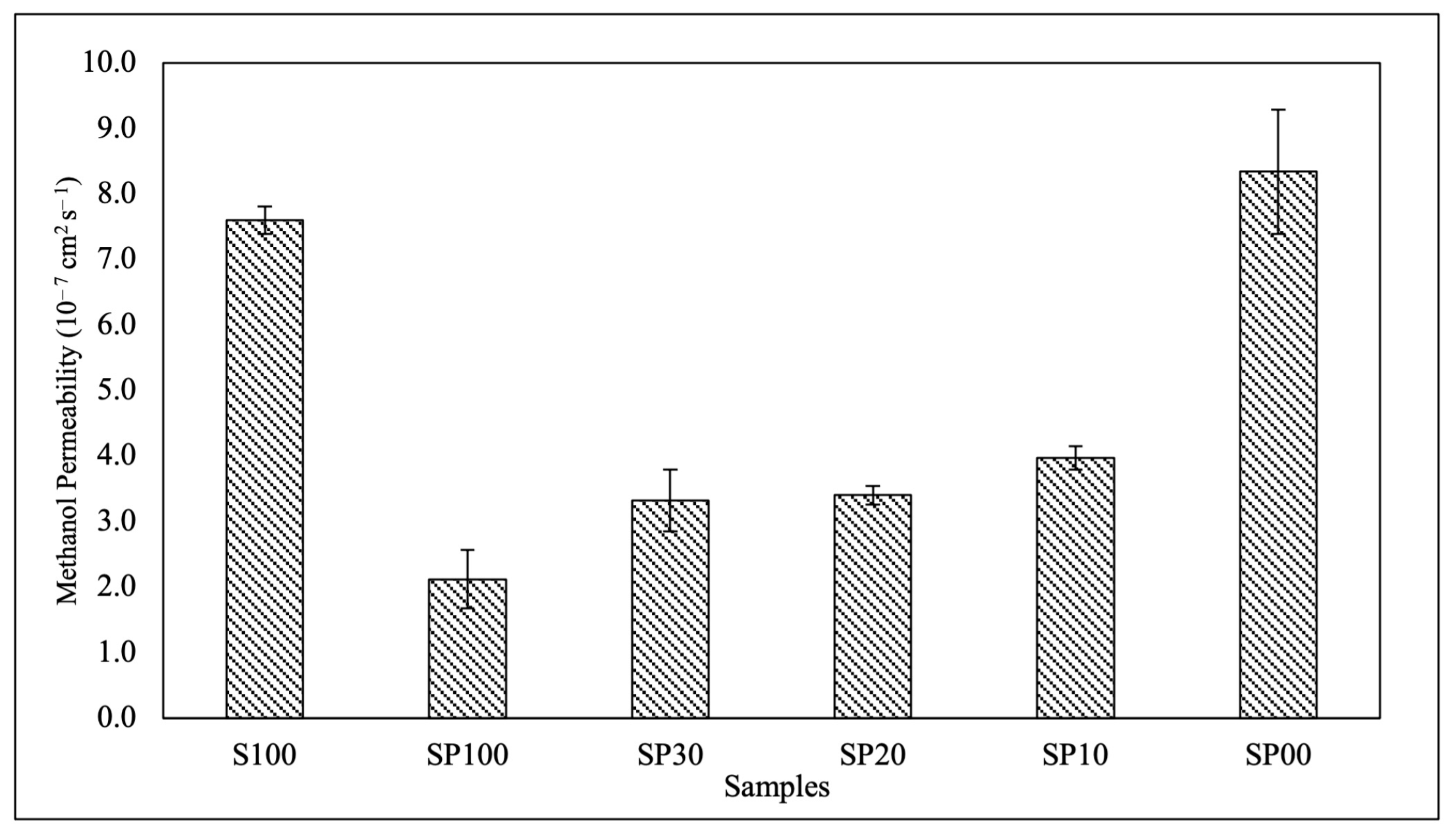
3.4. Methanol Permeability
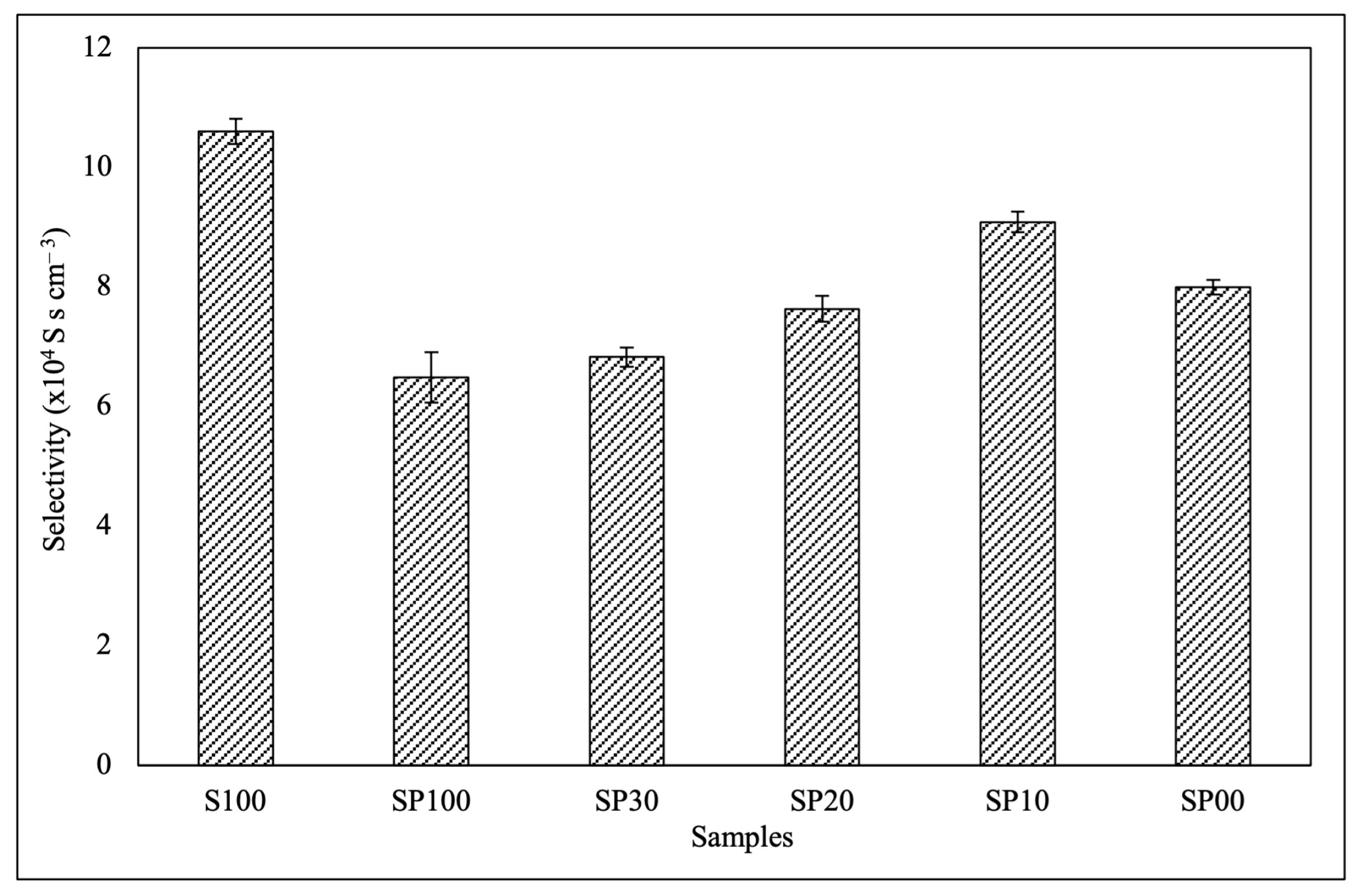
3.5. Membrane Selectivity
3.6. Self-Healing Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrogen Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Purwanto, M.; Widiastuti, N.; Saga, B.H.; Gusmawan, H. Synthesis of Composite Membrane Based Biopolymer Chitosan With Silica From Rice Husk Ash For Direct Methanol Fuel Cell Application. IOP Conf. Ser. Earth Environ. Sci. 2021, 830, 012021. [Google Scholar] [CrossRef]
- Mikhailenko, S.D.; Zaidi, J.; Kaliaguine, S. Sulfonated polyether ether ketone based composite polymer electrolyte membranes. Catal. Today 2001, 67, 225–236. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H.; Thangarasu, S. Modified Cellulose Proton-Exchange Membranes for Direct Methanol Fuel Cells. Polymers 2023, 15, 659. [Google Scholar] [CrossRef] [PubMed]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Mossayebi, Z.; Rowshanzamir, S. Solvent Casting Effects of Sulfonated Poly(ether ether ketone) for Polymer Electrolyte Membrane Fuel Cell. In Proceedings of the 3rd Hydrogen & Fuel Cell Conference (HFCC3), Tehran, Iran, 12–13 May 2015. [Google Scholar]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Yang, J.H.; Bae, Y.C. Methanol Crossover Effect for Direct Methanol Fuel Cells: Applicability of Methanol Activity in Polymer Electrolyte Membrane. J. Electrochem. Soc. 2008, 155, B194–B199. [Google Scholar] [CrossRef]
- Sonpingkam, S.; Pattavarakorn, D. Mechanical Properties of Sulfonated Poly (Ether Ether Ketone) Nanocomposite Membranes. Int. J. Chem. Eng. Appl. 2014, 5, 181–185. [Google Scholar] [CrossRef]
- Tao, X.-Y.; Ma, W.-B.; Han, X.-D.; Zhu, K.-H.; Ye, S.-F.; Sha, H.; Guo, L.; Wei, X.-Y.; Xu, C.; Zhu, S.-G. Preparation and application of self-healing polyvinyl alcohol/bacterial cellulose hydrogel electrolyte. J. Fuel Chem. Technol. 2022, 50, 304–313. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wang, Y.-Z. Synthesis and characterization of epoxy-based semi-interpenetrating polymer networks sulfonated polyimides proton-exchange membranes for direct methanol fuel cell applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2262–2276. [Google Scholar] [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Chong, K.C.; Lai, S.O. A State-of-Art on the Development of Nafion-Based Membrane for Performance Improvement in Direct Methanol Fuel Cells. Membranes 2022, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Li, Z.; Hao, X.; Xiao, M.; Huang, S.; Han, D.; Wang, S.; Meng, Y. Surface-Densified Non-Fluorinated Proton Exchange Membrane Used for Direct Methanol Fuel Cell. J. Electrochem. Soc. 2023, 170, 064502. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly(ether ether ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Wang, Y. Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cell. J. Membr. Sci. 2003, 226, 159–167. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Shahida, S.; Lashari, M.H.; Manzoor, S.; Fernandez, J. SPEEK and SPPO Blended Membranes for Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 263. [Google Scholar] [CrossRef]
- Chikumba, F.T.; Tamer, M.; Akyalçın, L.; Kaytakoğlu, S. The development of sulfonated polyether ether ketone (sPEEK) and titanium silicon oxide (TiSiO4) composite membranes for DMFC applications. Int. J. Hydrogen Energy 2023, 48, 14038–14052. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Park, C.J.; Yoo, D.J. Alleviating the Mechanical and Thermal Degradations of Highly Sulfonated Poly(Ether Ether Ketone) Blocks via Copolymerization with Hydrophobic Unit for Intermediate Humidity Fuel Cells. Polymers 2018, 10, 1346. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly(ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.F.; Mustafa, A. Physicochemical study of poly(ether ether ketone) electrolyte membranes sulfonated with mixtures of fuming sulfuric acid and sulfuric acid for direct methanol fuel cell application. Mater. Sci. Eng. A 2007, 460–461, 475–484. [Google Scholar] [CrossRef]
- Wan Mohd Noral Azman, W.N.E.; Jaafar, J.; Salleh, W.N.W.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Rasdi, F.R.M. Highly selective SPEEK/ENR blended polymer electrolyte membranes for direct methanol fuel cell. Mater. Today Energy 2020, 17, 100427. [Google Scholar] [CrossRef]
- Purnama, H.; Mujiburohman, M.; Hakim, M.F.; Hidayati, N. Preparation and Characterisation of Composite Sulfonated Polyether Ether Ketone for Direct Methanol Fuel Cells. J. Phys. Conf. Ser. 2019, 1295, 012048. [Google Scholar] [CrossRef]
- Chia, M.Y.; Thiam, H.S.; Leong, L.K.; Koo, C.H.; Saw, L.H. Study on improvement of the selectivity of proton exchange membrane via incorporation of silicotungstic acid-doped silica into SPEEK. Int. J. Hydrogen Energy 2020, 45, 22315–22323. [Google Scholar] [CrossRef]
- Niluroutu, N.; Shukla, A.; Dhavale, V.M.; Unni, S.M.; Bhat, S.D. Sulfonated poly(ether ether ketone) reinforced with polystyrene sulfonic acid functionalized micelle templated mesoporous MCM-41 for direct methanol fuel cells. Int. J. Hydrogen Energy 2021, 46, 20640–20649. [Google Scholar] [CrossRef]
- Tie, J.; Liu, H.; Lv, J.; Wang, B.; Mao, Z.; Zhang, L.; Zhong, Y.; Feng, X.; Sui, X.; Xu, H. Multi-responsive, self-healing and adhesive PVA based hydrogels induced by the ultrafast complexation of Fe3+ ions. Soft Matter 2019, 15, 7404–7411. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Lim, Y.S.; Wong, J.; Saw, L.H. Self-sustainable, self-healable sulfonated graphene oxide incorporated nafion/poly(vinyl alcohol) proton exchange membrane for direct methanol fuel cell applications. J. Environ. Chem. Eng. 2023, 11, 111151. [Google Scholar] [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Lim, Y.S.; Wong, J.; Lai, S.O. Freeze-Thawed Nafion-Poly(vinyl alcohol) Self-healing Membranes for Direct Methanol Fuel Cells. Chem. Eng. Technol. 2023, 46, 2600–2607. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Li, Y.; Xing, W.; Sun, J. Self-Healing Proton-Exchange Membranes Composed of Nafion-Poly(vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, e1707146. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Lim, Y.S.; Wong, J. Facile synthesis of nafion based self-healable proton exchange membranes for direct methanol fuel cells. Mater. Today Proc. 2023, in press. [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Lim, Y.S.; Wong, J. Self-healable Nafion-poly(vinyl alcohol)/phosphotungstic acid proton exchange membrane prepared by freezing–thawing method for direct methanol fuel cell. J. Solid State Electrochem. 2023, 27, 1477–1492. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Wang, W.; Sun, J. Self-healing and highly elastic fluorine-free proton exchange membranes comprised of poly(vinyl alcohol) derivative and phytic acid for durable fuel cells. Sci. China Mater. 2020, 63, 1235–1246. [Google Scholar] [CrossRef]
- Yu, F.; Hu, J. Electrochemical Properties of Proton Exchange Membrane I: The Influence of Sulfonation Degree and Solvent. Int. J. Electrochem. Sci. 2016, 11, 724–737. [Google Scholar] [CrossRef]
- Jun, M.-S.; Choi, Y.-W.; Kim, J.-D. Solvent casting effects of sulfonated poly(ether ether ketone) for Polymer electrolyte membrane fuel cell. J. Membr. Sci. 2012, 396, 32–37. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, J.; Wang, Y.; Li, Z.; Zheng, J. A highly stretchable, self-healing, recyclable and interfacial adhesion gel: Preparation, characterization and applications. Chem. Eng. J. 2019, 360, 334–341. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of degree of sulfonation and casting solvent on sulfonated poly(ether ether ketone) membrane for vanadium redox flow battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Zaidi, J. Polymer sulfonation—A versatile route to prepare proton-conducting membrane material for advanced technologies. Arab. J. Sci. Eng. 2003, 28, 183–194. [Google Scholar]
- Ding, J.; Zhao, C.; Wu, K.; Yi, X.; Zheng, Z. Preparation and Properties of PVA/SPEEK/p-ADA Electroactive Composite Membranes. Cailiao Daobao/Mater. Rev. 2018, 32, 2481–2485. [Google Scholar]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Shen, W.; Ao, F.; Ge, X.; Ning, Y.; Wang, L.; Ren, H.; Fan, G. Effects of solvents on electrospun fibers and the biological application of different hydrophilic electrospun mats. Mater. Today Commun. 2022, 30, 103093. [Google Scholar] [CrossRef]
- Rianjanu, A.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Solvent vapor treatment improves mechanical strength of electrospun polyvinyl alcohol nanofibers. Heliyon 2018, 4, e00592. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cho, E.-B.; Kim, D.H. Sulfonated Peek/Mesoporous Benzene-Silica Composite Membranes Operable at Low Humidity. Solid State Ion. 2011, 203, 1–8. [Google Scholar] [CrossRef]
- Robertson, G.; Mikhailenko, S.; Wang, K.; Xing, P.; Guiver, M.; Kaliaguine, S. Casting Solvent Interactions with Sulfonated Poly (Ether Ether Ketone) during Proton Exchange Membrane Fabrication. J. Membr. Sci. 2003, 219, 113–121. [Google Scholar] [CrossRef]
- Sahin, A. The development of Speek/Pva/Teos blend membrane for proton exchange membrane fuel cells. Electrochim. Acta 2018, 271, 127–136. [Google Scholar] [CrossRef]
- Murmu, R.; Roy, D.; Patra, S.C.; Sutar, H.; Senapati, P. Preparation and characterization of the SPEEK/PVA/Silica hybrid membrane for direct methanol fuel cell (DMFC). Polym. Bull. 2022, 79, 2061–2087. [Google Scholar] [CrossRef]
- Vasanth, P.; Kolar, A.K. Experimental Investigation of Heat Loss from a Passive DMFC using Differential Interferometer. Fuel Cells 2018, 18, 195–205. [Google Scholar] [CrossRef]
- Diken, M.; Kizilduman, B.; Kardaş, B.; Doğan, E.; Doğan, M.; Turhan, Y.; Doğan, S. Synthesis, characterization, and their some chemical and biological properties of PVA/PAA/nPS hydrogel nanocomposites: Hydrogel and wound dressing. J. Bioact. Compat. Polym. 2020, 35, 203–215. [Google Scholar] [CrossRef]
- Wu, J.; Wang, F.; Fan, X.; Chu, J.; Cheng, F.; Hu, F.; Liu, H.; Zhang, Q.; Xu, Z.; Gong, C. Phosphoric acid-doped Gemini quaternary ammonium-grafted SPEEK membranes with superhigh proton conductivity and mechanical strength for direct methanol fuel cells. J. Membr. Sci. 2023, 672, 121431. [Google Scholar] [CrossRef]
- Zhao, C.; He, D.; Li, Y.; Xiang, J.; Li, P.; Sue, H.-J. High-performance proton exchange membranes for direct methanol fuel cells based on a SPEEK/polybenzoxazine crosslinked structure. RSC Adv. 2015, 5, 47284–47293. [Google Scholar] [CrossRef]
- Wang, J.; Liao, J.; Yang, L.; Zhang, S.; Huang, X.; Ji, J. Highly compatible acid–base blend membranes based on sulfonated poly(ether ether ketone) and poly(ether ether ketone-alt-benzimidazole) for fuel cells application. J. Membr. Sci. 2012, 415–416, 644–653. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Bi, D.; Lin, H.; Shao, K.; Fu, T.; Zhong, S.; Na, H. Blend membranes based on disulfonated poly(aryl ether ether ketone)s (SPEEK) and poly(amide imide) (PAI) for direct methanol fuel cell usages. Polymer 2007, 48, 3090–3097. [Google Scholar] [CrossRef]
- Wu, H.-L.; Ma, C.-C.; Li, C.-H.; Lee, T.-M.; Chen, C.-Y.; Chiang, C.-L.; Wu, C. Sulfonated poly(ether ether ketone)/poly(amide imide) polymer blends for proton conducting membrane. J. Membr. Sci. 2006, 280, 501–508. [Google Scholar] [CrossRef]
- Zhong, F.; Zeng, Z.; Liu, Y.; Hou, R.; Nie, X.; Jia, Y.; Xi, J.; Liu, H.; Niu, W.; Zhang, F. Modification of sulfonated poly (etherether ketone) composite polymer electrolyte membranes with 2D molybdenum disulfide nanosheet-coated carbon nanotubes for direct methanol fuel cell application. Polymer 2022, 249, 124839. [Google Scholar] [CrossRef]
- Wu, H.; Shen, X.; Xu, T.; Hou, W.; Jiang, Z. Sulfonated poly(ether ether ketone)/amino-acid functionalized titania hybrid proton conductive membranes. J. Power Sources 2012, 213, 83–92. [Google Scholar] [CrossRef]


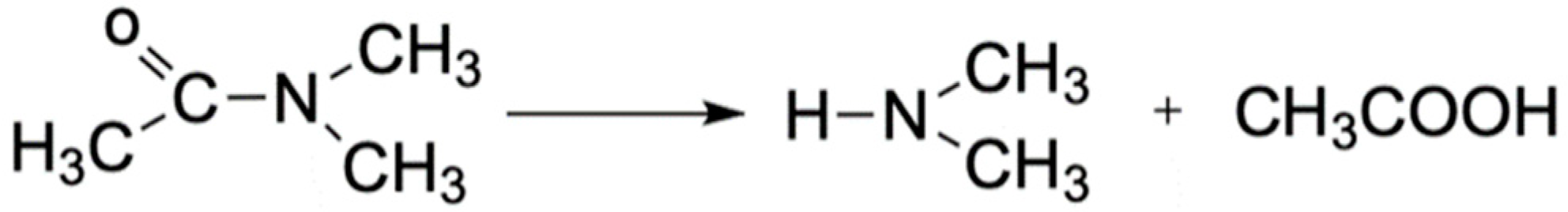

| Sample | DMAc Solution (wt%) |
|---|---|
| S100 | 100 |
| SP100 | 100 |
| SP30 | 30 |
| SP20 | 20 |
| SP10 | 10 |
| SP00 | 0 |
| Sample | S100 | SP100 | SP30 | SP20 | SP10 | SP00 |
|---|---|---|---|---|---|---|
| Recovery % | 7 | 10 | 38 | 61 | 88 | 90 |
| Tensile Strength (MPa) | Original | Damaged | Healed | Recovery (%) |
|---|---|---|---|---|
| S100 | 7.34 | 3.23 | 3.02 | N/A |
| SP10 | 2.43 | 1.01 | 2.02 | 83 |
| Sample | σ (S cm−1) | PM (2 M) (×10−7cm2 s−1) | Φ (×104 S s cm−3) | Ref. |
|---|---|---|---|---|
| SP10 | 0.036 | 3.97 | 5.80 | This work |
| SPEEK-ENR | 0.002 | 0.88 | 2.40 | [24] |
| SPEEK | 0.048 | 7.45 | 6.44 | [26] |
| S-DMEA-BPT-PA | 0.112 | 21.70 | 5.54 | [51] |
| SPEEK-PVDF | 0.001 | 0.65 | 1.22 | [52] |
| SPEEK-PEEK-alt-BI-3 | 0.012 | 1.80 | 6.60 | [53] |
| SPEEK-PAI | 0.040 | 8.47 | 4.72 | [54] |
| SPEEK-PAI | 0.010 | 2.50 | 3.46 | [55] |
| SPEEK-MoS2@CNTs | 0.042 | 5.2 | 3.2 | [56] |
| SPEEK-TiO2 | 0.039 | 8.44 | 4.62 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, M.H.; Thiam, H.S.; Tee, S.F.; Lim, Y.S.; Saw, L.H.; Lai, S.O. Self-Healing Sulfonated Poly(ether ether ketone)-Based Polymer Electrolyte Membrane for Direct Methanol Fuel Cells: Effect of Solvent Content. Polymers 2023, 15, 4641. https://doi.org/10.3390/polym15244641
Tai MH, Thiam HS, Tee SF, Lim YS, Saw LH, Lai SO. Self-Healing Sulfonated Poly(ether ether ketone)-Based Polymer Electrolyte Membrane for Direct Methanol Fuel Cells: Effect of Solvent Content. Polymers. 2023; 15(24):4641. https://doi.org/10.3390/polym15244641
Chicago/Turabian StyleTai, Mae Hwa, Hui San Thiam, Shiau Foon Tee, Yun Seng Lim, Lip Huat Saw, and Soon Onn Lai. 2023. "Self-Healing Sulfonated Poly(ether ether ketone)-Based Polymer Electrolyte Membrane for Direct Methanol Fuel Cells: Effect of Solvent Content" Polymers 15, no. 24: 4641. https://doi.org/10.3390/polym15244641
APA StyleTai, M. H., Thiam, H. S., Tee, S. F., Lim, Y. S., Saw, L. H., & Lai, S. O. (2023). Self-Healing Sulfonated Poly(ether ether ketone)-Based Polymer Electrolyte Membrane for Direct Methanol Fuel Cells: Effect of Solvent Content. Polymers, 15(24), 4641. https://doi.org/10.3390/polym15244641









