Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Commercial Film-Forming Components
2.1.2. pH Regulators
2.1.3. Mucilage Precipitation and Freeze-Drying of Precipitate into Powder
2.2. Preparation of Various Film-Forming Solutions
2.3. Single-Polymer Film Development
2.4. pH Adjustment
2.5. Evaluation of Film Mechanical Properties
Puncture Test Evaluation
2.6. Experimental Design
2.7. Statistical Analysis
3. Results
3.1. Film Concentration
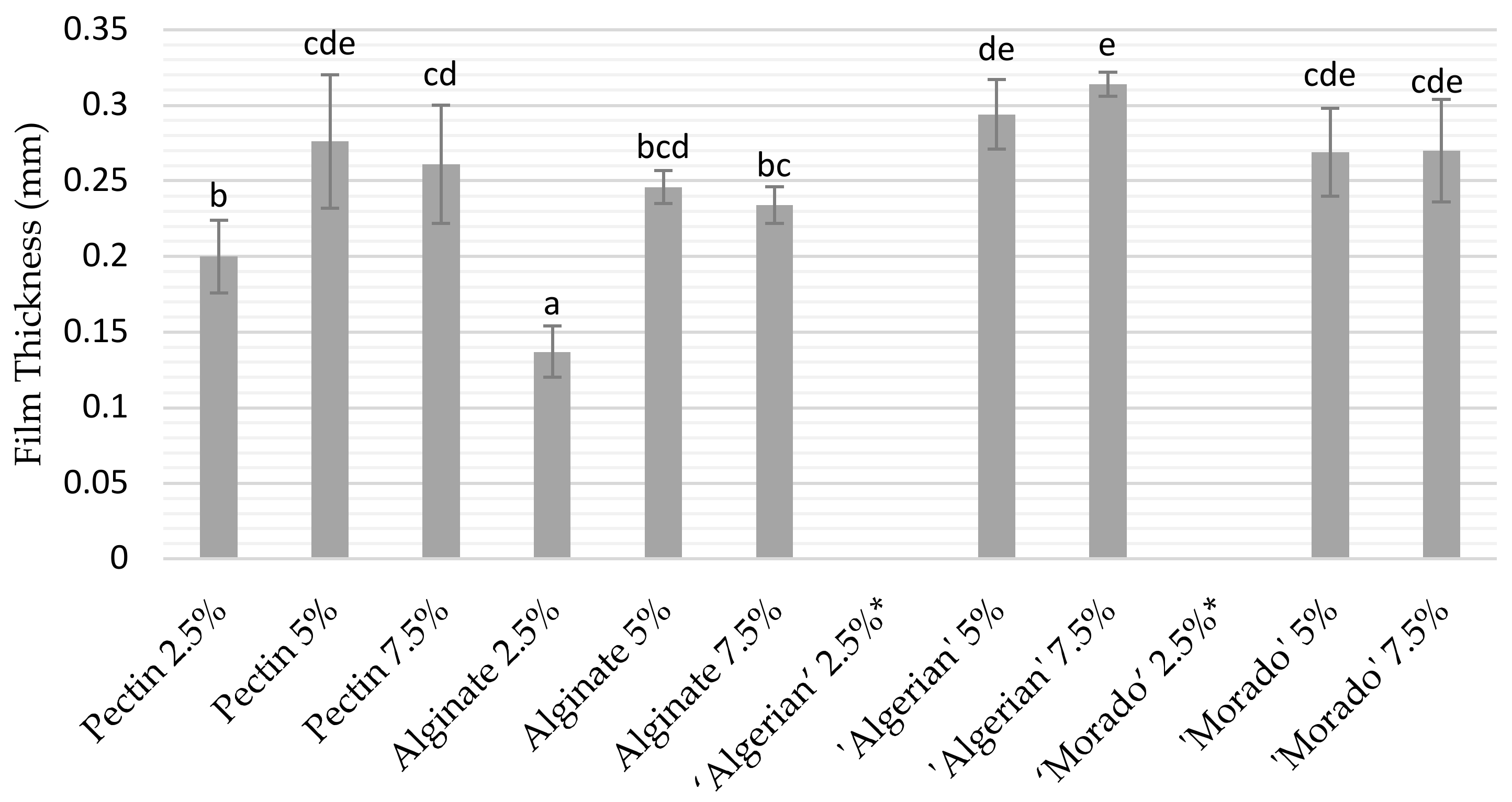
3.1.1. Film Thickness
3.1.2. Tensile Tests
3.1.3. Puncture Tests
3.2. Film pH
3.2.1. Film Thickness and the Influence of pH
3.2.2. pH and Film Tensile Measurements
3.2.3. pH and Film Puncture Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J. Cactus Pear (Opuntia spp.) Crop Applications and Emerging Biopolymer Innovations. In Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2023; pp. 129–134. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and Pectin Composite Films Crosslinked with Ca2+ Ions: Effect of the Plasticizer Concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; Da Silva, M.A.; Kieckbusch, T.G. Natamycin Release from Alginate/Pectin Films for Food Packaging Applications. J. Food Eng. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Development and Characterization of Composite Edible Films Based on Sodium Alginate and Pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Innovative Plasticized Alginate Obtained by Thermo-Mechanical Mixing: Effect of Different Biobased Polyols Systems. Carbohydr. Polym. 2017, 157, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Zawiślak, G.; Maj, G.; Chawla, P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers 2021, 13, 2588. [Google Scholar] [CrossRef]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Damas, M.S.P.; Pereira Junior, V.A.; Nishihora, R.K.; Quadri, M.G.N. Edible Films from Mucilage of Cereus Hildmannianus Fruits: Development and Characterization. J. Appl. Polym. Sci. 2017, 134, 45223. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Effect of Native Mucilage on the Mechanical Properties of Pectin-Based and Alginate-Based Polymeric Films. Coatings 2023, 13, 1611. [Google Scholar] [CrossRef]
- Sandoval, D.C.G.; Sosa, B.L.; Martínez-Ávila, G.C.G.; Fuentes, H.R.; Abarca, V.H.A.; Rojas, R. Formulation and Characterization of Edible Films Based on Organic Mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, Mechanical and Barrier Properties of Bio-Based Composite Films Based on Chitosan and Whey Protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, 347–352. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia ficus-indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lira-Vargas, A.A.; Lira-Vargas, A.A.; Corrales-Garcia, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric Films Based on Cactus (Opuntia ficus-indica) Mucilage Incorporated with Gelatin and Beeswax. J. Prof. Assoc. Cactus Dev. 2014, 16, 51–70. [Google Scholar]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rodríguez-Núñez, J.R. Mucilage from Cladodes of Opuntia Spinulifera Salm-Dyck: Chemical, Morphological, Structural and Thermal Characterization. CYTA J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef]
- Rodríguez-González, F.; Pérez-González, J.; Muñoz-López, C.N.; Vargas-Solano, S.V.; Marín-Santibáñez, B.M. Influence of Age on Molecular Characteristics and Rheological Behavior of Nopal Mucilage. Food Sci. Nutr. 2021, 9, 6776–6785. [Google Scholar] [CrossRef]
- Garfias Silva, V.; Cordova Aguilar, M.S.; Ascanio, G.; Aguayo, J.P.; Pérez-Salas, K.Y.; Susunaga Notario, A.D.C. Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides. Molecules 2022, 27, 5830. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Sáenz, C.; Urzúa, C.C.; Zárate, O. Chemical Characterization of the Mucilage from Fruits of Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacõn-García, L. Extraction and Characterization of Mucilage from Wild Species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant Seed Mucilage as Emerging Biopolymer in Food Industry Applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Du Toit, A.; de Wit, M.; Seroto, K.D.; Fouche, H.; Hugo, A.; Venter, S. Rheological Characterization of Cactus Pear Mucilage for Application in Nutraceutical Food Products. Acta Hortic. 2019, 1247, 63–72. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The Influence of Opuntia ficus-indica Mucilage Edible Coating on the Quality of “Hayward” Kiwifruit Slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Zibaei, R.; Hasanvand, S.; Hashami, Z.; Roshandel, Z.; Rouhi, M.; Guimarães, J.D.T.; Mortazavian, A.M.; Sarlak, Z.; Mohammadi, R. Applications of Emerging Botanical Hydrocolloids for Edible Films: A Review. Carbohydr. Polym. 2021, 256, 117554. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Microstructural and Mechanical Properties of Calcium-Treated Cactus Pear Mucilage (Opuntia spp.), Pectin and Alginate Single-Biopolymer Films. Polymers 2023, 15, 4295. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M. Patent PA153178P A Process for Extracting Mucilage from Opuntia ficus-indica, Aloe barbadensis and Agave americana. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2021. [Google Scholar] [CrossRef]
- Du Toit, A. Selection, Extraction, Characterization and Application of Mucilage from Cactus Pear (Opuntia ficus-indica and Opuntia robusta) Cladodes. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2016. Volume 2. pp. 1–13. [Google Scholar]
- Du Toit, A.; De Wit, M.; Fouché, H.J.; Taljaard, M.; Venter, S.L.; Hugo, A. Mucilage Powder from Cactus Pears as Functional Ingredient: Influence of Cultivar and Harvest Month on the Physicochemical and Technological Properties. J. Food Sci. Technol. 2019, 56, 2404–2416. [Google Scholar] [CrossRef]
- ASTM-D882:2010; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2010.
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of Nopal Mucilage Addition on Physical, Barrier and Mechanical Properties of Citric Pectin-Based Films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.; McHugh, T.H. Edible Films from Pectin: Physical-Mechanical and Antimicrobial Properties—A Review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]

| Treatments | Tensile Strength (MPa) | Elongation at Break % |
|---|---|---|
| Pectin 2.5% | 3.31 ± 0.43 b | 5.59 ± 1.51 a |
| Pectin 5% | 6.41 ± 0.50 c | 14.32 ± 1.88 bc |
| Pectin 7.5% | 6.27 ± 1.11 c | 18.98 ± 5.66 cd |
| Alginate 2.5% | 10.20 ± 1.38 d | 4.91 ± 0.75 a |
| Alginate 5% | 17.57 ± 0.90 e | 7.79 ± 1.03 a |
| Alginate 7.5% | 16.88 ± 0.69 e | 21.99 ± 0.69 d |
| ‘Algerian’ 2.5% * | ||
| ‘Algerian’ 5% | 0.26 + 0.05 a | 33.10 ± 6.10 e |
| ‘Algerian’ 7.5% | 1.17 ± 0.08 a | 49.82 ± 1.53 f |
| ‘Morado’ 2.5% * | ||
| ‘Morado’ 5% | 0.32 ± 0.10 a | 21.58 ± 1.80 d |
| ‘Morado’ 7.5% | 1.36 ± 0.15 a | 47.79 ± 5.84 f |
| Significance level | p < 0.005 | p < 0.005 |
| Treatments | Puncture Force (N) | Distance to Puncture (mm) |
|---|---|---|
| Pectin 2.5% | 25.67 ± 5.51 b | 3.51 ± 0.78 ab |
| Pectin 5% | 31.75 ± 2.38 bc | 4.04 ± 0.38 abc |
| Pectin 7.5% | 47.85 ± 4.17 d | 5.98 ± 0.62 e |
| Alginate 2.5% | 34.47 ± 2.72 c | 4.02 ± 0.23 abc |
| Alginate 5% | 72.17 ± 4.68 e | 5.61 ± 0.37 de |
| Alginate 7.5% | 72.22 ± 8.23 e | 8.63 ± 0.75 f |
| ‘Algerian’ 2.5% * | ||
| ‘Algerian’ 5% | 2.43 + 0.26 a | 4.24 ± 0.63 abc |
| ‘Algerian’ 7.5% | 5.67 ± 0.64 a | 3.09 ± 0.93 a |
| ‘Morado’ 2.5% * | ||
| ‘Morado’ 5% | 1.82 ± 0.21 a | 4.71 + 0.89 bcd |
| ‘Morado’ 7.5% | 8.30 ± 0.70 a | 4.99 ± 0.45 cde |
| Significance level | p < 0.005 | p < 0.005 |
| Treatments/Films | Tensile Strength (MPa) | Elongation at Break % |
|---|---|---|
| Pectin Native pH 3.81 | 6.41 ± 0.50 b | 14.31 ± 1.88 b |
| Pectin (pH 3–3.5) | 11.52 ± 1.06 d | 2.39 ± 0.34 a |
| Pectin (pH9–10) | 8.51 ± 1.30 c | 4.68 ± 1.04 a |
| Alginate Native pH 6.45 | 17.57 ± 0.90 e | 7.79 ± 1.03 a |
| Alginate (pH 3–3.5) | 20.71 ± 0.85 f | 5.38 ± 0.23 a |
| Alginate (pH 9–10) | 17.58 ± 0.73 e | 5.17 ± 0.48 a |
| ‘Algerian’ Native pH 4.9 | 0.262 ± 0.05 a | 33.10 ± 6.10 d |
| ‘Algerian’ (pH 3–3.5) | 1.01 ± 0.15 a | 55.01 ± 6.32 e |
| ‘Algerian’ (pH 9–10) | 0.27 ± 0.09 a | 14.19 ± 0.61 b |
| ‘Morado’ Native pH 4.6 | 0.31 ± 0.10 a | 21.58 ± 1.76 c |
| ‘Morado’ (pH 3–3.5) | 0.71 ± 0.10 a | 36.53 ± 3.69 d |
| ‘Morado’ (pH 9–10) | 0.48 ± 0.03 a | 35.42 ± 5.13 d |
| Significance level | p < 0.005 | p < 0.005 |
| Treatments/Films | Puncture Force (N) | Distance to Puncture (mm) |
|---|---|---|
| Pectin Native pH 3.81 | 31.75 ± 2.38 b | 4.04 ± 0.38 bc |
| Pectin (pH 3–3.5) | 44.19 ± 4.32 c | 2.68 ± 0.48 a |
| Pectin (pH9–10) | 41.95 ± 8.77 c | 3.77 ± 0.83 b |
| Alginate Native pH 6.45 | 72.17 ± 4.68 d | 5.61 + 0.37 de |
| Alginate (pH 3–3.5) | 80.29 ± 2.12 e | 5.00 ± 0.41 cde |
| Alginate (pH 9–10) | 73.78 ± 4.54 de | 5.89 ± 0.49 e |
| ‘Algerian’ Native pH 4.9 | 2.43 ± 0.26 a | 4.24 ± 0.63 bc |
| ‘Algerian’ (pH 3–3.5) | 2.65 ± 0.56 a | 4.17 ± 0.48 bc |
| ‘Algerian’ (pH 9–10) | 2.10 ± 0.67 a | 5.99 ± 0.45 e |
| ‘Morado’ Native pH 4.6 | 1.82 ± 0.21 a | 4.71 ± 0.88 bcd |
| ‘Morado’ (pH 3–3.5) | 3.43 ± 0.34 a | 3.78 ± 0.16 b |
| ‘Morado’ (pH 9–10) | 3.15 ± 0.51 a | 4.22 ± 0.25 bc |
| Significance level | p < 0.005 | p < 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation. Polymers 2023, 15, 4640. https://doi.org/10.3390/polym15244640
Van Rooyen B, De Wit M, Osthoff G, Van Niekerk J, Hugo A. Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation. Polymers. 2023; 15(24):4640. https://doi.org/10.3390/polym15244640
Chicago/Turabian StyleVan Rooyen, Brandon, Maryna De Wit, Gernot Osthoff, Johan Van Niekerk, and Arno Hugo. 2023. "Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation" Polymers 15, no. 24: 4640. https://doi.org/10.3390/polym15244640
APA StyleVan Rooyen, B., De Wit, M., Osthoff, G., Van Niekerk, J., & Hugo, A. (2023). Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation. Polymers, 15(24), 4640. https://doi.org/10.3390/polym15244640








