Fabrication of Flexible Films for Supercapacitors Using Halloysite Nano-Clay Incorporated Poly(lactic acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
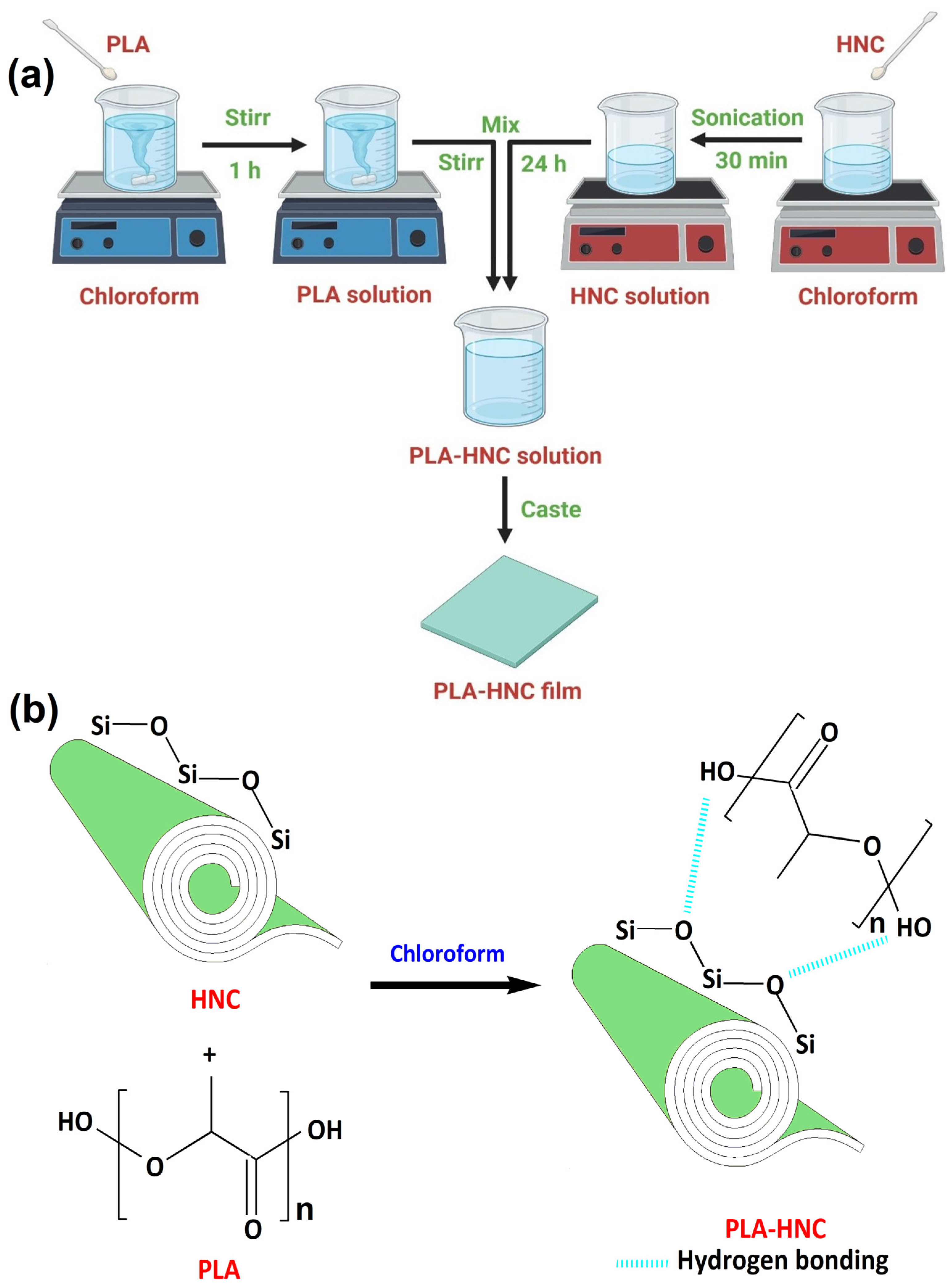
2.2. Preparation of PLA–HNC Films
2.3. Fabrication of the SC Device
2.4. Electrochemical Characterizations of PLA–HNC Films
3. Results
3.1. The Physico-Chemical Characterization of PLA–HNC Films
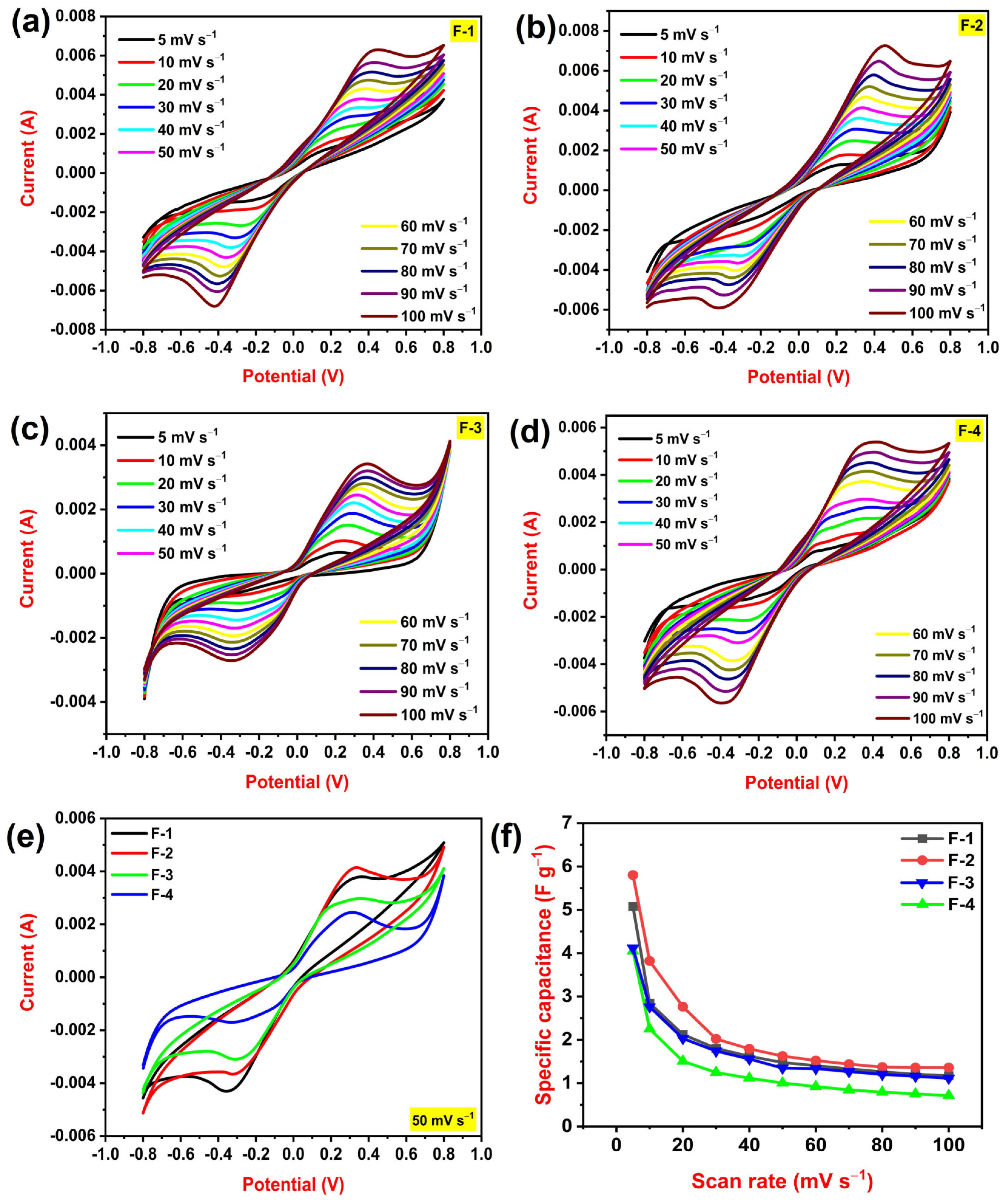
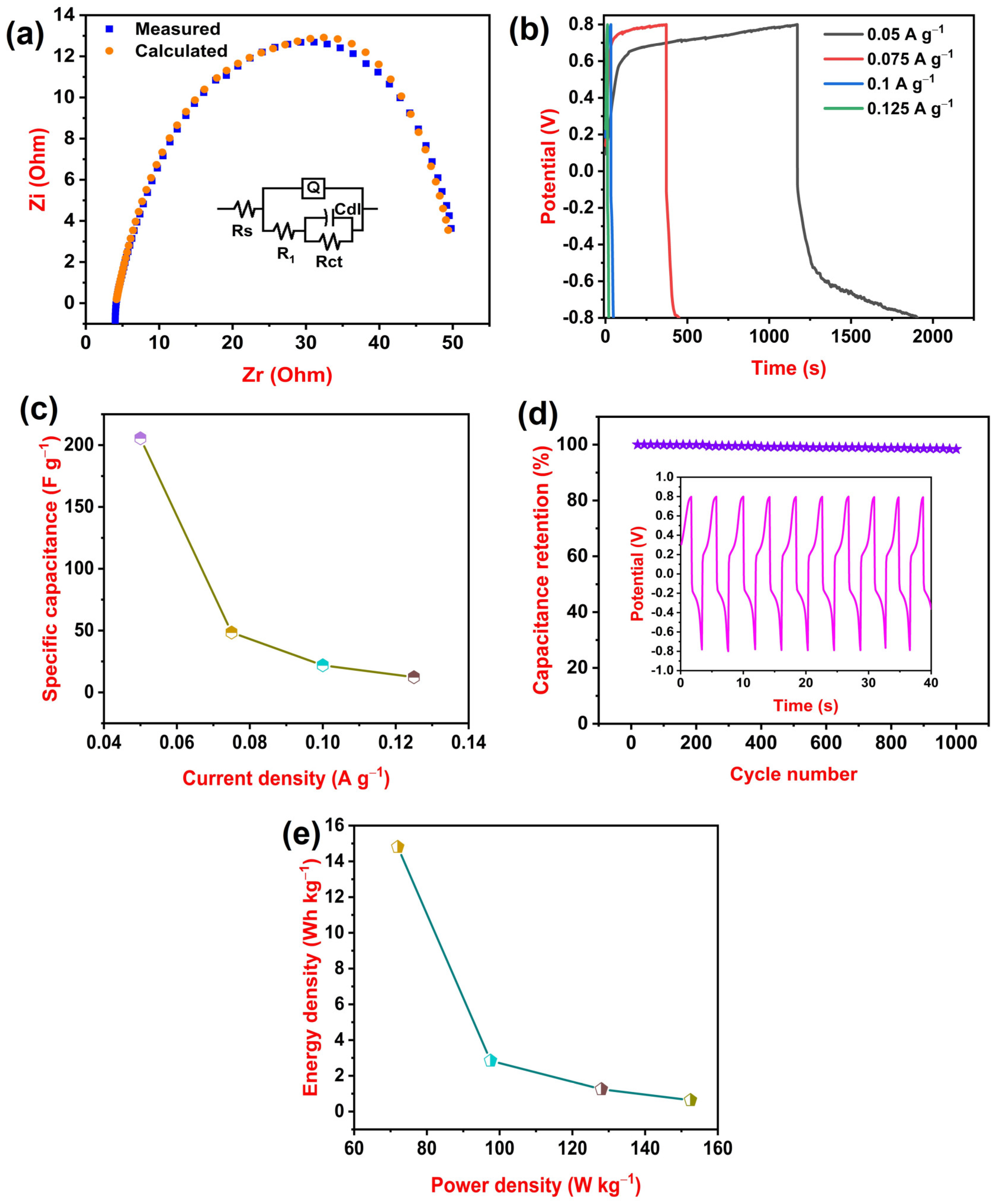
3.2. Electrochemical Performances of PLA–HNC Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ordowich, C.; Chase, J.; Steele, D.; Malhotra, R.; Harada, M.; Makino, K. Applying Learning Curves to Modeling Future Coal and Gas Power Generation Technologies. Energy Fuels 2012, 26, 753–766. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible Supercapacitors Based on Paper Substrates: A New Paradigm for Low-Cost Energy Storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef] [PubMed]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Laelabadi, K.G.; Moradian, R.; Manouchehri, I. Facile Method of Fabricating Interdigitated and Sandwich Electrodes for High-Performance and Flexible Reduced Graphene Oxide@Polyaniline Nanocomposite Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 6697–6710. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Vargun, E.; Ozaltin, K.; Fei, H.; Harea, E.; Vilčáková, J.; Kazantseva, N.; Saha, P. Biodegradable Porous Polylactic Acid Film as a Separator for Supercapacitors. J. Appl. Polym. Sci. 2020, 137, 49270. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of Materials and Fabrication Methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A.; Ogbu, J.; Ndem, J.; Nwuzor, I.C. Recently Emerging Advancements in Halloysite Nanotubes Polymer Nanocomposites. Compos. Interfaces 2019, 26, 751–824. [Google Scholar] [CrossRef]
- Yendluri, R.; Otto, D.P.; De Villiers, M.M.; Vinokurov, V.; Lvov, Y.M. Application of Halloysite Clay Nanotubes as a Pharmaceutical Excipient. Int. J. Pharm. 2017, 521, 267–273. [Google Scholar] [CrossRef]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Kumar Panda, B. Halloysite Nanotubes and Applications: A Review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Ağtaş, M.; Dilaver, M.; Koyuncu, I. Halloysite Nanoclay Doped Ceramic Membrane Fabrication and Evaluation of Textile Wastewater Treatment Performance. Process. Saf. Environ. Prot. 2021, 154, 72–80. [Google Scholar] [CrossRef]
- Sadjadi, S. Halloysite-Based Hybrids/Composites in Catalysis. Appl. Clay Sci. 2020, 189, 105537. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in Food and Beverage Packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The Application of Halloysite Tubule Nanoclay in Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Pavliňáková, V.; Fohlerová, Z.; Pavliňák, D.; Khunová, V.; Vojtová, L. Effect of Halloysite Nanotube Structure on Physical, Chemical, Structural and Biological Properties of Elastic Polycaprolactone/Gelatin Nanofibers for Wound Healing Applications. Mater. Sci. Eng. C 2018, 91, 94–102. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural Halloysite Nano-Clay Electrolyte for Advanced All-Solid-State Lithium-Sulfur Batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Zhu, X.; Shchukin, D. Crystallohydrate Loaded Halloysite Nanocontainers for Thermal Energy Storage. Adv. Eng. Mater. 2018, 20, 1800618. [Google Scholar] [CrossRef]
- Ma, W.; Wu, H.; Higaki, Y.; Takahara, A. Halloysite Nanotubes: Green Nanomaterial for Functional Organic-Inorganic Nanohybrids. Chem. Rec. 2018, 18, 986–999. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A Review of Poly(Lactic Acid)-Based Materials for Antimicrobial Packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of Bioactive Functional Poly(Lactic Acid)/Curcumin Composite Film for Food Packaging Application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.; Kenny, J. Multifunctional Nanostructured PLA Materials for Packaging and Tissue Engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Rhim, J.; Lee, S.; Kim, Y. Pervaporation Separation of Water–Ethanol Mixtures Using Metal-Ion-Exchanged Poly(Vinyl Alcohol) (PVA)/Sulfosuccinic Acid (SSA) Membranes. J. Appl. Polym. Sci. 2002, 85, 1867–1873. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly(Lactic Acid) Fibers, Yarns and Fabrics: Manufacturing, Properties and Applications. Text. Res. J. 2021, 91, 1641–1669. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhu, P.; Wu, Y.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun Conductive Polyaniline–Polylactic Acid Composite Nanofibers as Counter Electrodes for Rigid and Flexible Dye-Sensitized Solar Cells. RSC Adv. 2012, 2, 652–657. [Google Scholar] [CrossRef]
- Culebras, M.; Geaney, H.; Beaucamp, A.; Upadhyaya, P.; Dalton, E.; Ryan, K.M.; Collins, M.N. Bio-derived Carbon Nanofibres from Lignin as High-Performance Li-Ion Anode Materials. ChemSusChem 2019, 12, 4516–4521. [Google Scholar] [CrossRef]
- Bediako, E.G.; Nyankson, E.; Dodoo-Arhin, D.; Agyei-Tuffour, B.; Łukowiec, D.; Tomiczek, B.; Yaya, A.; Efavi, J.K. Modified Halloysite Nanoclay as a Vehicle for Sustained Drug Delivery. Heliyon 2018, 4, 689. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, B.; Wang, A. Halloysite Nanotubes Template-Induced Fabrication of Carbon/Manganese Dioxide Hybrid Nanotubes for Supercapacitors. Ionics 2015, 21, 2329–2336. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and Biological Assessment of Silver Nanoparticles Immobilized Halloysite Nanotubes-Based Resin Composite for Dental Applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Kalahal, P.B.; Banapurmath, N.R.; Ayachit, N.H. Fabrication and assessment of poly(lactic acid)-poly(4-styrene sulfonate) flexible membranes as electrodes for supercapacitors. J. Energy Storage 2023, 72, 108513. [Google Scholar] [CrossRef]
- Tham, W.L.; Poh, B.T.; Ishak, Z.A.M.; Chow, W.S. Water Absorption Kinetics and Hygrothermal Aging of Poly(Lactic Acid) Containing Halloysite Nanoclay and Maleated Rubber. J. Polym. Environ. 2015, 23, 242–250. [Google Scholar] [CrossRef]
- Lim, K.; Chow, W.S.; Pung, S.Y. Accelerated Weathering and UV Protection-Ability of Poly(Lactic Acid) Nanocomposites Containing Zinc Oxide Treated Halloysite Nanotube. J. Polym. Environ. 2019, 27, 1746–1759. [Google Scholar] [CrossRef]
- Govindaraj, D.; Rajan, M. Investigation of Minerals Substituted Hydroxyapatite Based Nanocomposite Coated Titanium Implant for Bone Tissue Engineering Applications. Orient. J. Chem. 2021, 37, 1139–1145. [Google Scholar] [CrossRef]
- Dong, Y.; Haroosh, H.J. Processing-Structure-Property Relationship for Electrospun Poly Lactic Acid (PLA)/Halloysite Nanotube (HNT) Composite Mats. In Proceedings of the 9th Asian-Australasian Conference on Composite Materials (ACCM-9), Suzhou, China, 15–17 October 2014. [Google Scholar]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hassan, H.A.; Woei, C.B. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(Lactic Acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef] [PubMed]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Characterization of Polylactic Acid/Halloysite Nanotubes Bionanocomposite Films for Food Packaging. Food Packag. Shelf Life 2020, 23, 100450. [Google Scholar] [CrossRef]
- Rusling, J.F.; Suib, S.L. Characterizing Materials with Cyclic Voltammetry. Adv. Mater. 1994, 6, 922–930. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Kalahal, P.B.; Banapurmath, N.R. Insight into the Performance of Valve-Regulated Lead-Acid Battery Using Sodium Salt of Poly(4-Styrene Sulfonic Acid-Co-Maleic Acid)-Poly(Vinyl Alcohol) Gel Electrolyte. J. Energy Storage 2023, 72, 108261. [Google Scholar] [CrossRef]
- Taberna, P.; Portet, C.; Simon, P. Electrode Surface Treatment and Electrochemical Impedance Spectroscopy Study on Carbon/Carbon Supercapacitors. Appl. Phys. A Mater. Sci. Process. 2006, 82, 639–646. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Banapurmath, N.R.; Ayachit, N.H. Graphene-Doped Hydrogels Promoting Ionic Conductivity in Gel-Valve-Regulated Lead Acid Batteries. In Langmuir; ACS Publications: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Kalahal, P.B.; Banapurmath, N.R.; Angadi, A.R. Insight into the Performance of VRLA Battery Using PVA-TEOS Hybrid Gel Electrolytes with Titania Nanoparticles. J. Energy Storage 2023, 72, 108572. [Google Scholar] [CrossRef]
- Barros, K.S.; Martí-Calatayud, M.C.; Scarazzato, T.; Bernardes, A.M.; Espinosa, D.C.R.; Pérez-Herranz, V. Investigation of Ion-Exchange Membranes by Means of Chronopotentiometry: A Comprehensive Review on This Highly Informative and Multipurpose Technique. Adv. Colloid Interface Sci. 2021, 293, 102439. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, G.; Wan, T.; Chen, A.; Peng, Z.; Zhang, H. Polyaniline Growing on Polylactic Acid Substrate towards Flexible and Biodegradable Supercapacitors. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2022, 39, 193–202. [Google Scholar] [CrossRef]
- Ramadass, K.; Sathish, C.I.; MariaRuban, S.; Kothandam, G.; Joseph, S.; Singh, G.; Kim, S.; Cha, W.; Karakoti, A.S.; Belperio, T.; et al. Carbon Nanoflakes and Nanotubes from Halloysite Nanoclays and Their Superior Performance in CO2 Capture and Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 11922–11933. [Google Scholar] [CrossRef] [PubMed]
- Jekal, S.; Kim, M.-S.; Kim, D.-H.; Noh, J.; Kim, H.-Y.; Kim, J.; Yi, H.; Oh, W.-C.; Yoon, C.-M. Fabrication of Flexible All-Solid-State Asymmetric Supercapacitor Device via Full Recycling of Heated Tobacco Waste Assisted by PLA Gelation Template Method. Gels 2023, 9, 97. [Google Scholar] [CrossRef]



| Films | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| F-0 | 24 ± 1.2 | 1988 ± 3.2 | 2.9 ± 0.5 |
| F-1 | 27 ± 2.3 | 4072 ± 2.1 | 2.2 ± 0.7 |
| F-2 | 30 ± 1.9 | 8267 ± 3.4 | 1.5 ± 1.1 |
| F-3 | 28 ± 2.3 | 7071 ± 3.7 | 1.8 ± 1.2 |
| F-4 | 25 ± 2.4 | 5373 ± 1.9 | 2.5 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikkatti, B.S.; Sajjan, A.M.; Banapurmath, N.R.; Bhutto, J.K.; Verma, R.; Yunus Khan, T.M. Fabrication of Flexible Films for Supercapacitors Using Halloysite Nano-Clay Incorporated Poly(lactic acid). Polymers 2023, 15, 4587. https://doi.org/10.3390/polym15234587
Chikkatti BS, Sajjan AM, Banapurmath NR, Bhutto JK, Verma R, Yunus Khan TM. Fabrication of Flexible Films for Supercapacitors Using Halloysite Nano-Clay Incorporated Poly(lactic acid). Polymers. 2023; 15(23):4587. https://doi.org/10.3390/polym15234587
Chicago/Turabian StyleChikkatti, Bipin S., Ashok M. Sajjan, Nagaraj R. Banapurmath, Javed Khan Bhutto, Rajesh Verma, and T. M. Yunus Khan. 2023. "Fabrication of Flexible Films for Supercapacitors Using Halloysite Nano-Clay Incorporated Poly(lactic acid)" Polymers 15, no. 23: 4587. https://doi.org/10.3390/polym15234587
APA StyleChikkatti, B. S., Sajjan, A. M., Banapurmath, N. R., Bhutto, J. K., Verma, R., & Yunus Khan, T. M. (2023). Fabrication of Flexible Films for Supercapacitors Using Halloysite Nano-Clay Incorporated Poly(lactic acid). Polymers, 15(23), 4587. https://doi.org/10.3390/polym15234587









