Electrical, Thermal, and Structural Characterization of Plant-Based 3D Printed Gel Polymer Electrolytes for Future Electrochemical Applications
Abstract
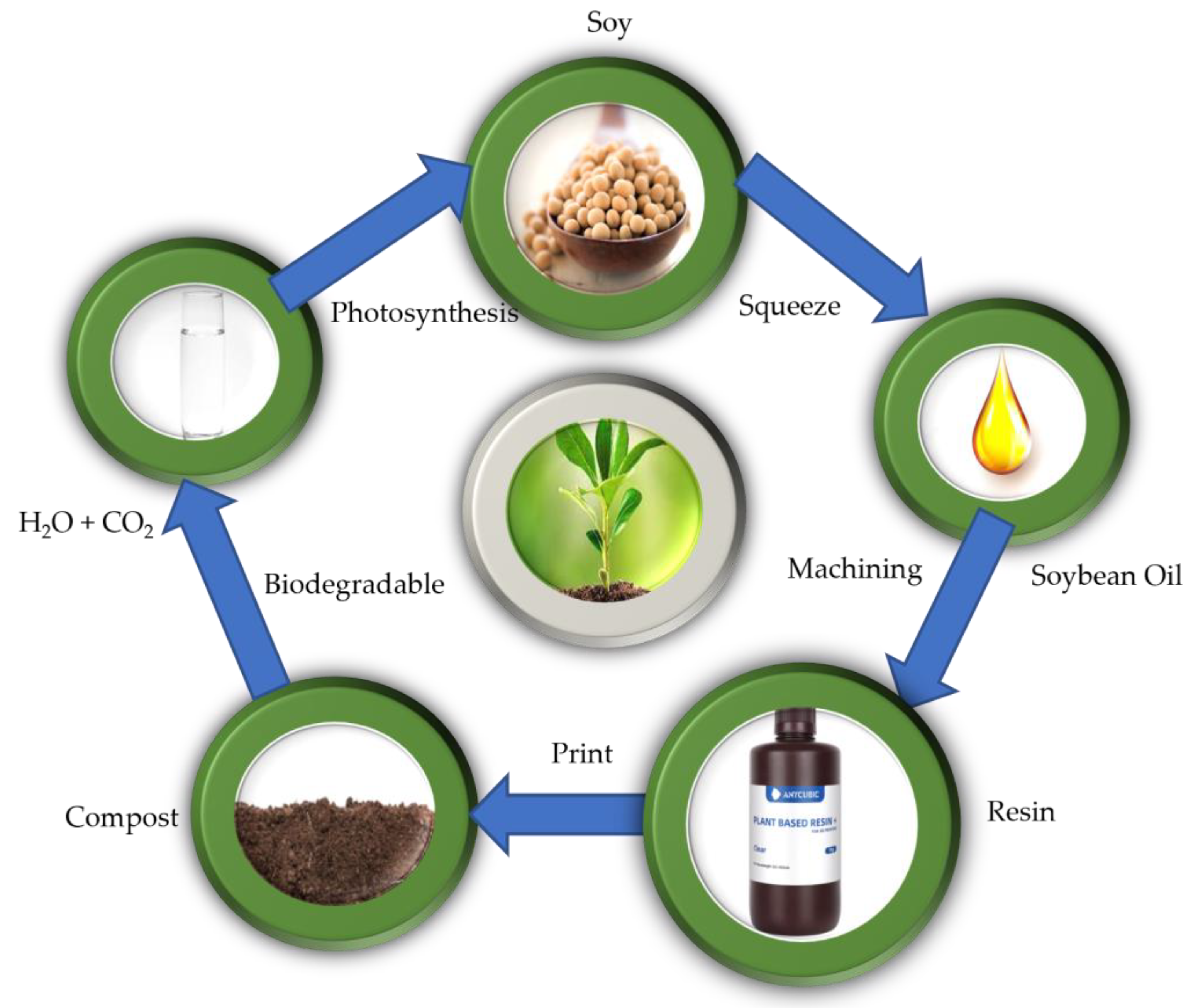
1. Introduction
2. Materials and Methods
2.1. Materials
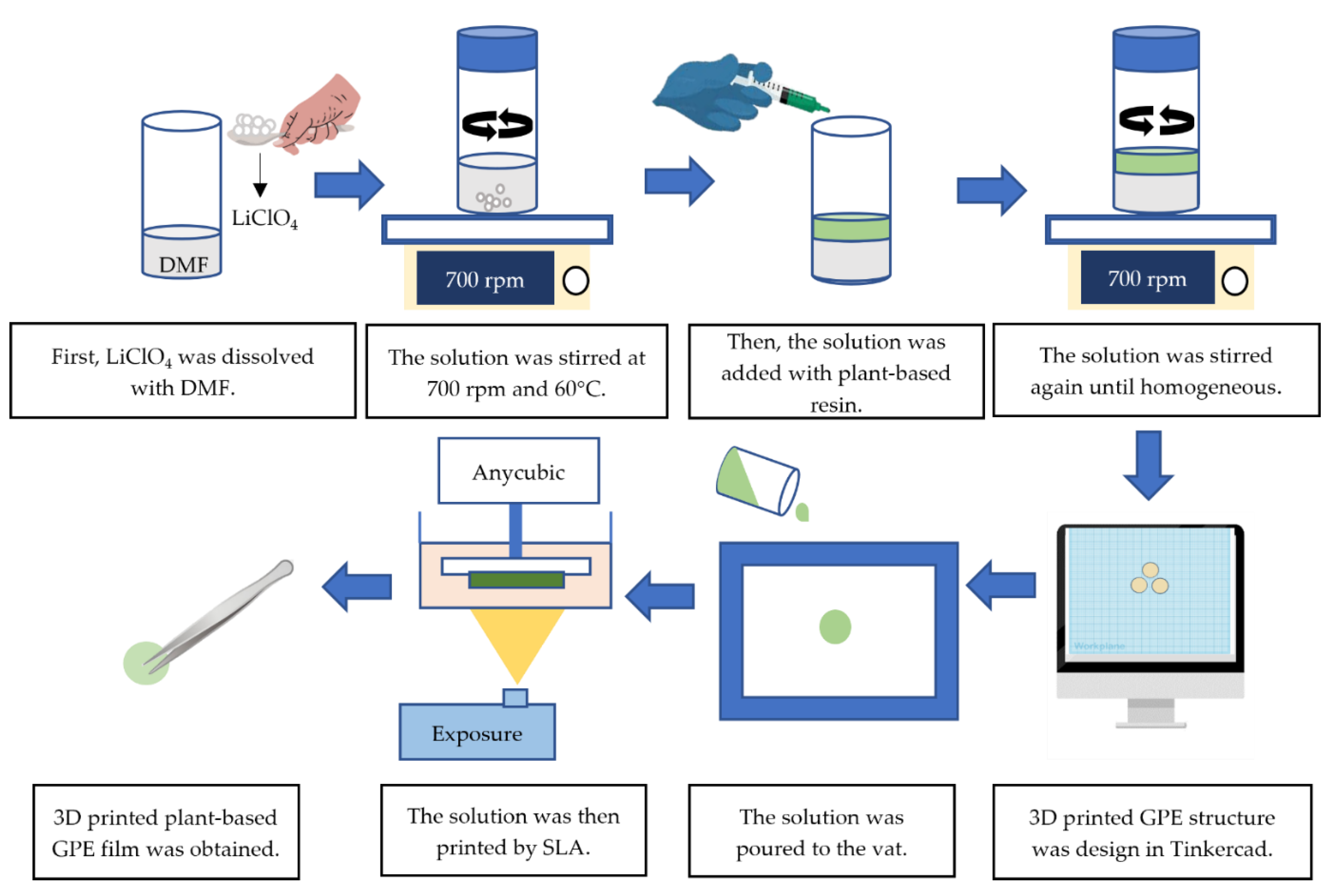
2.2. Preparation of 3D Printing Plant-Based Gel Polymer Electrolyte by Stereolithography
2.3. Characterizations
3. Results and Discussion
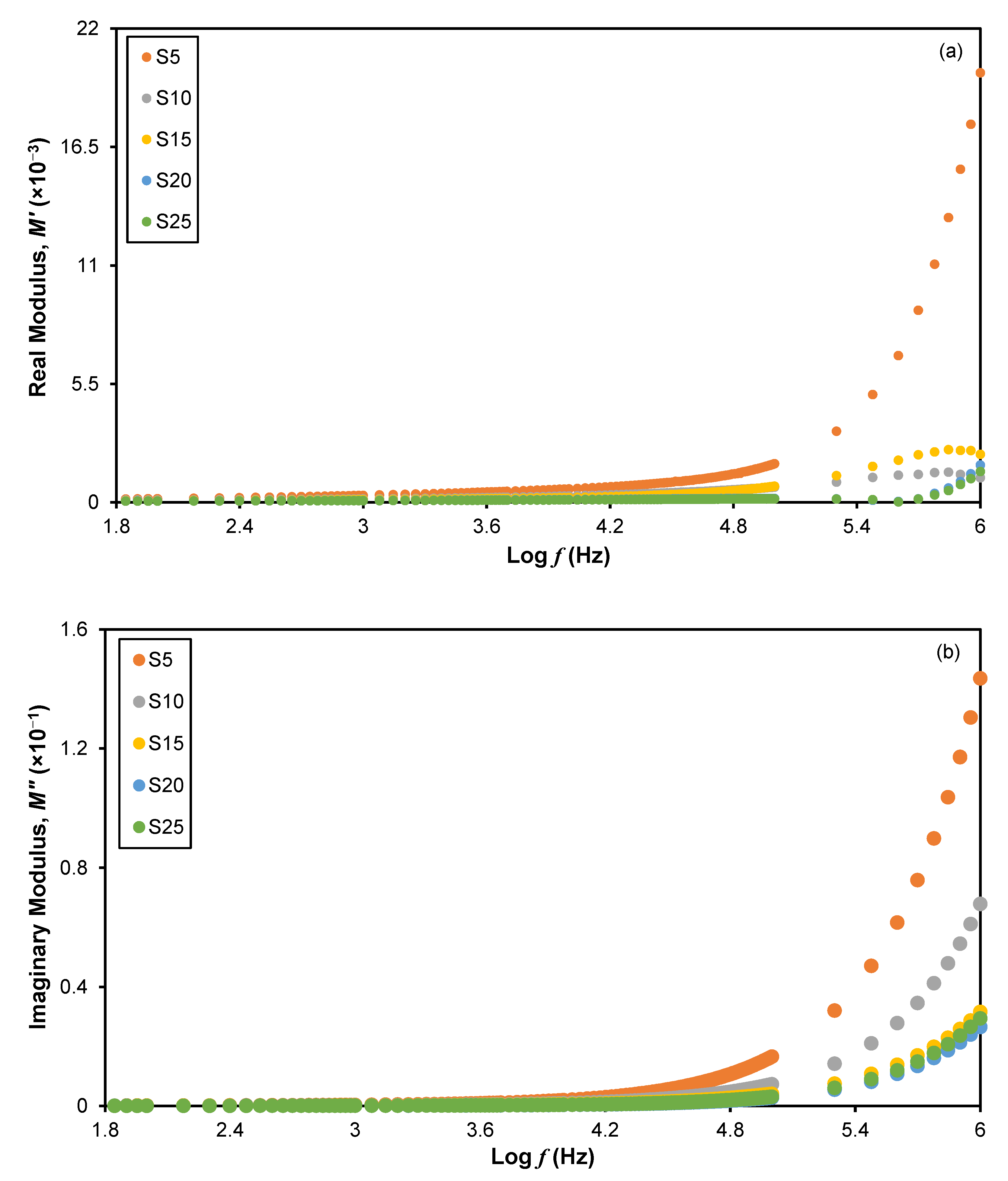
3.1. Electrochemical Impedance Spectroscopy (EIS)
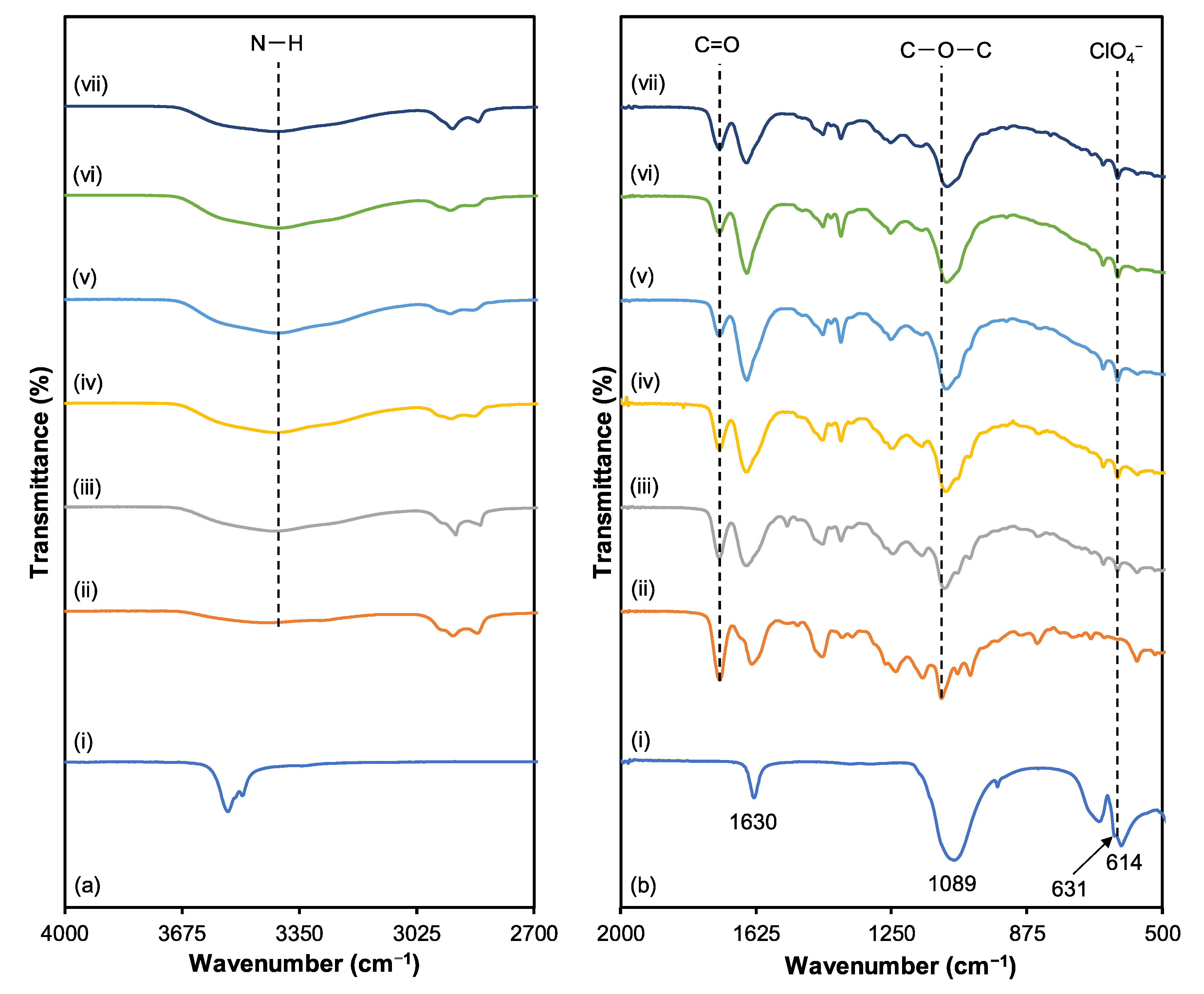
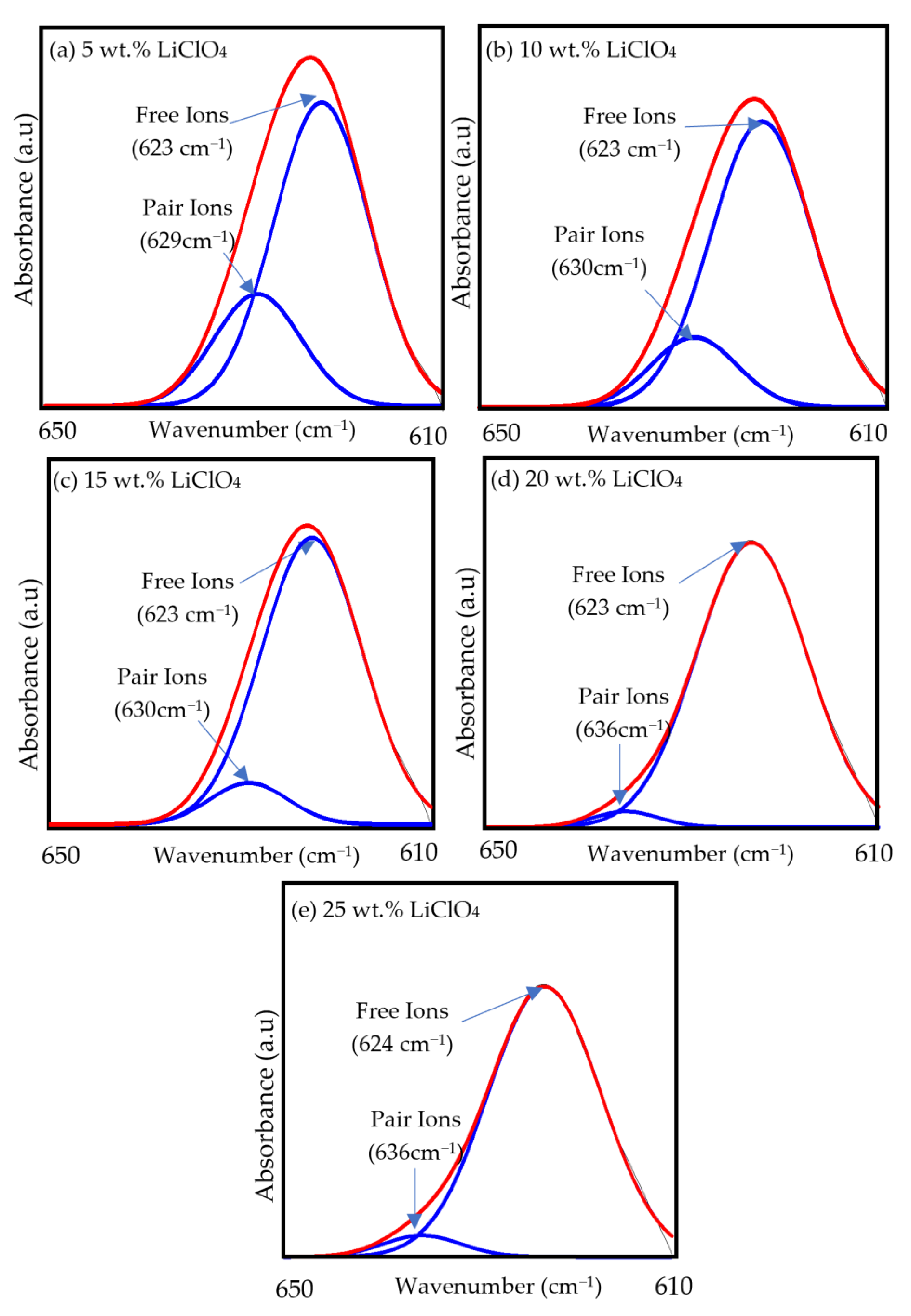
3.2. Fourier Transform Infrared (FTIR)
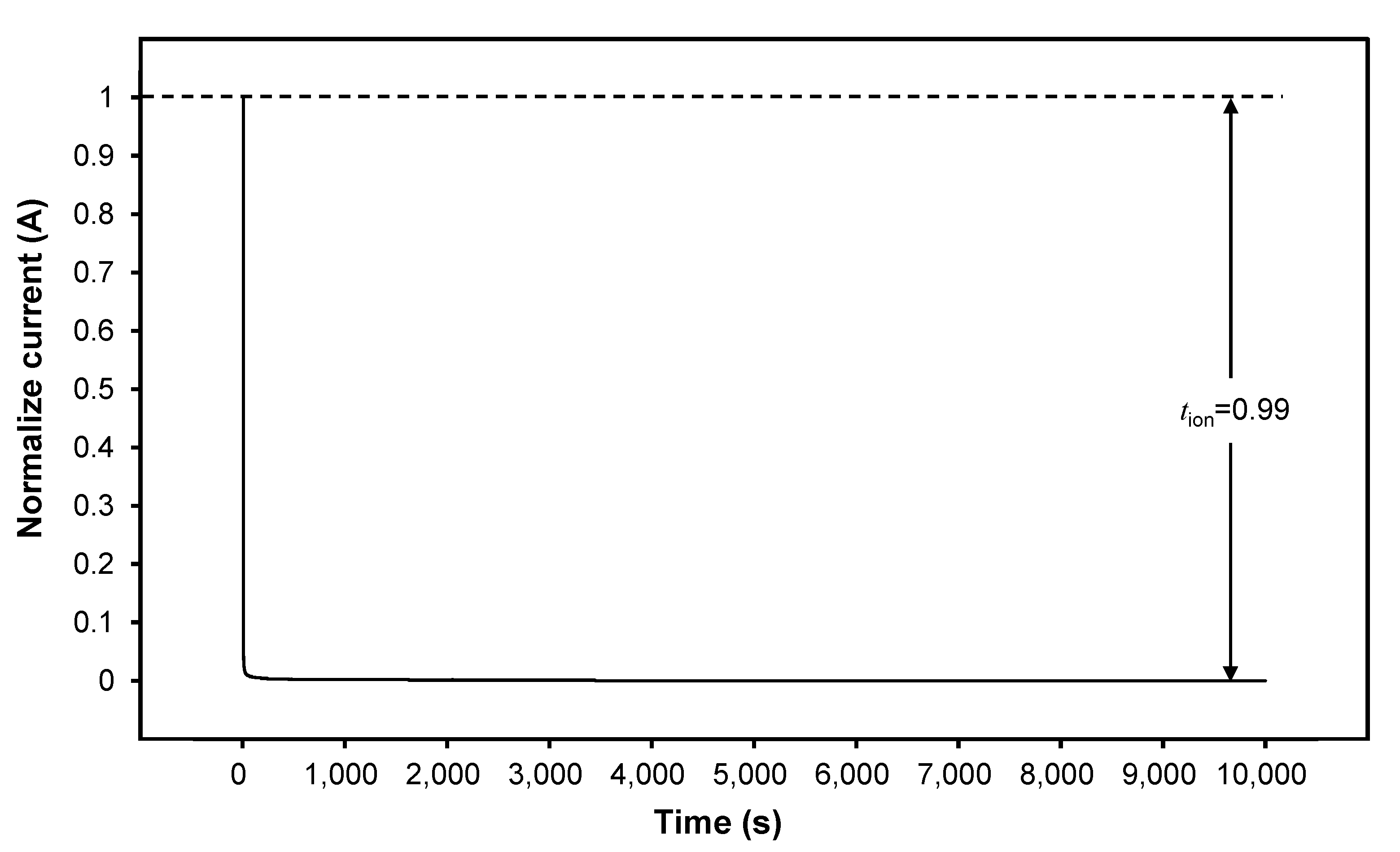
3.3. Transference Number Measurement (TNM)
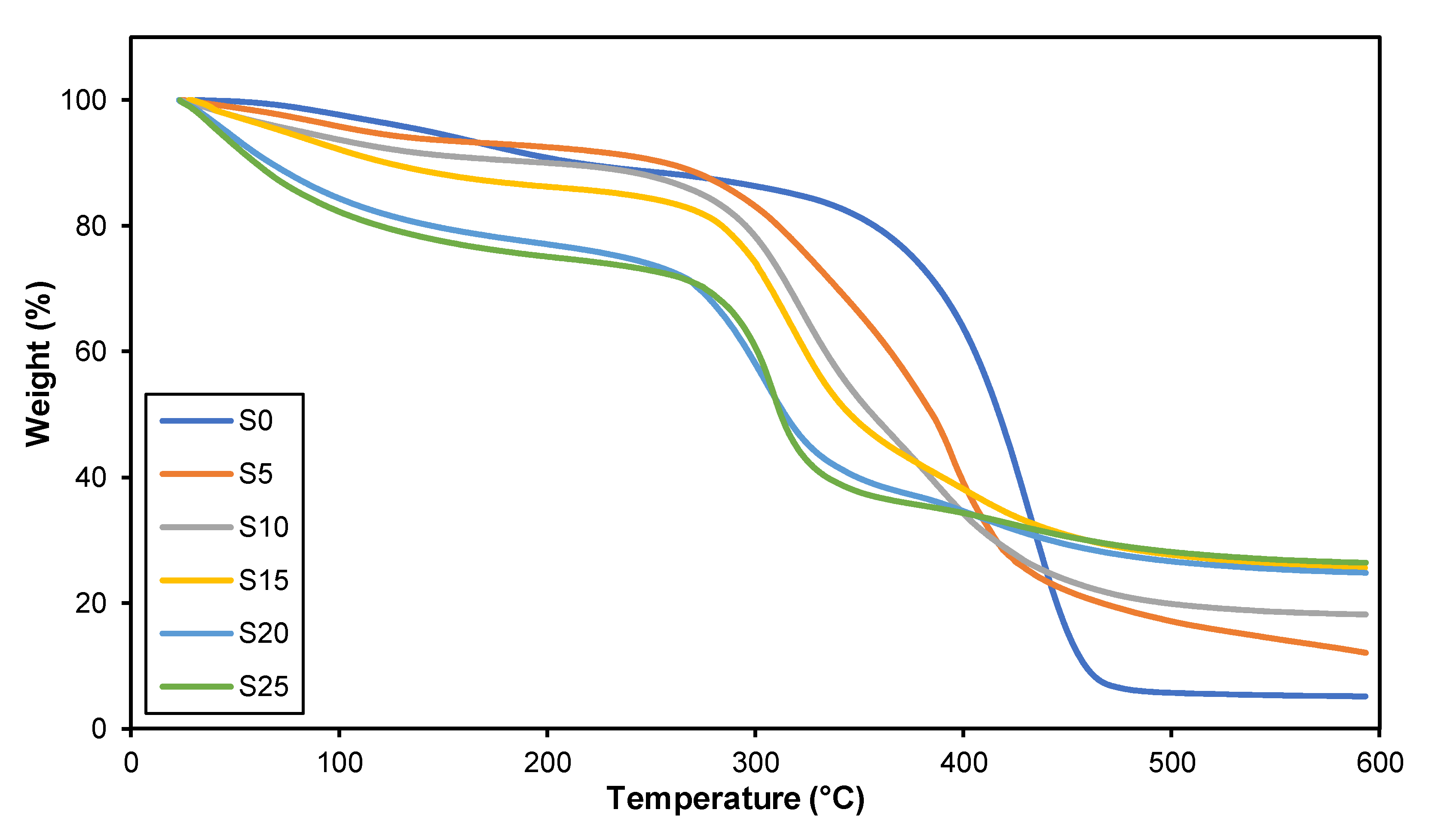
3.4. Thermal Gravimetric Analysis (TGA)
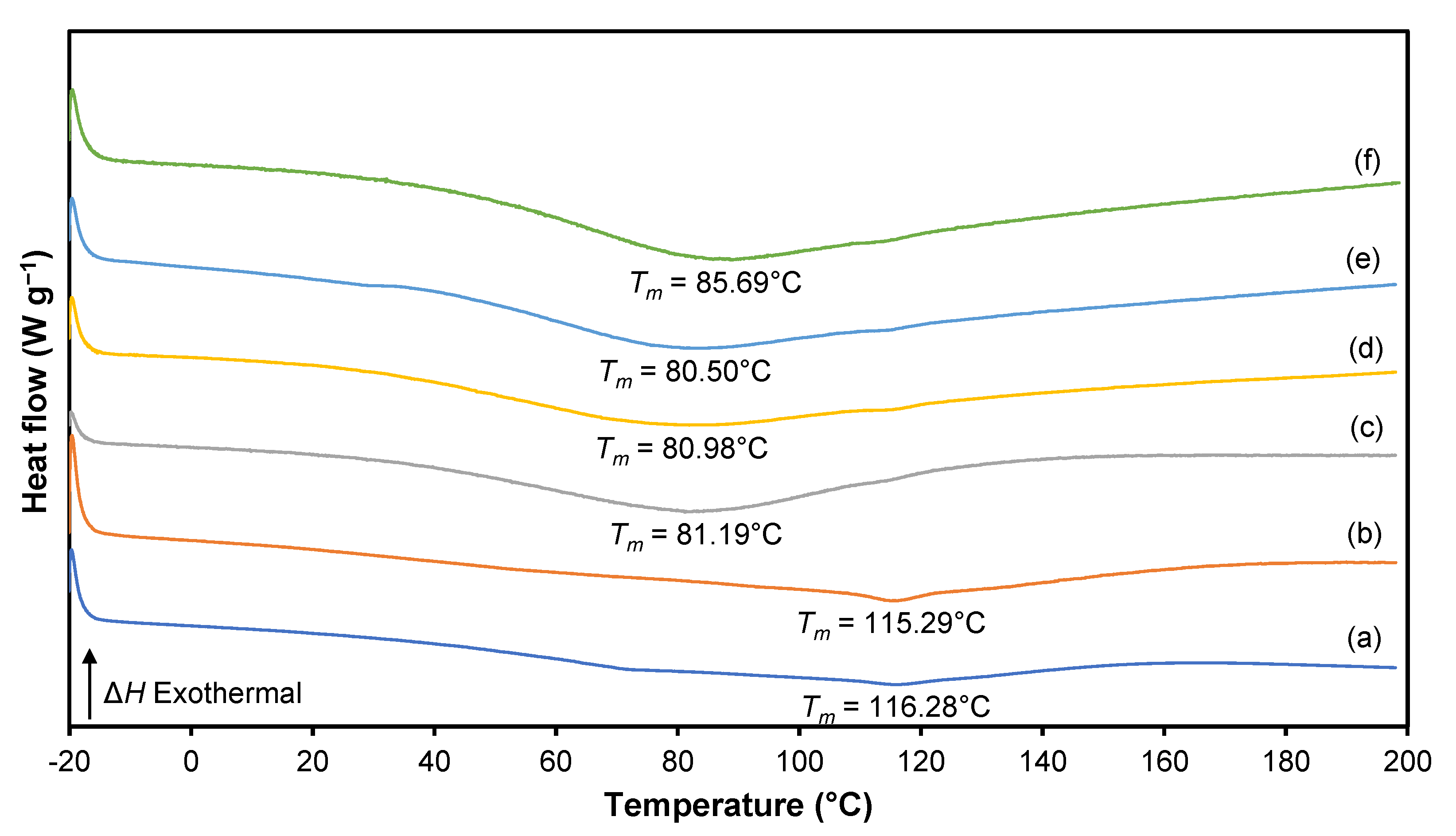
3.5. Differential Scanning Calorimetry (DSC)
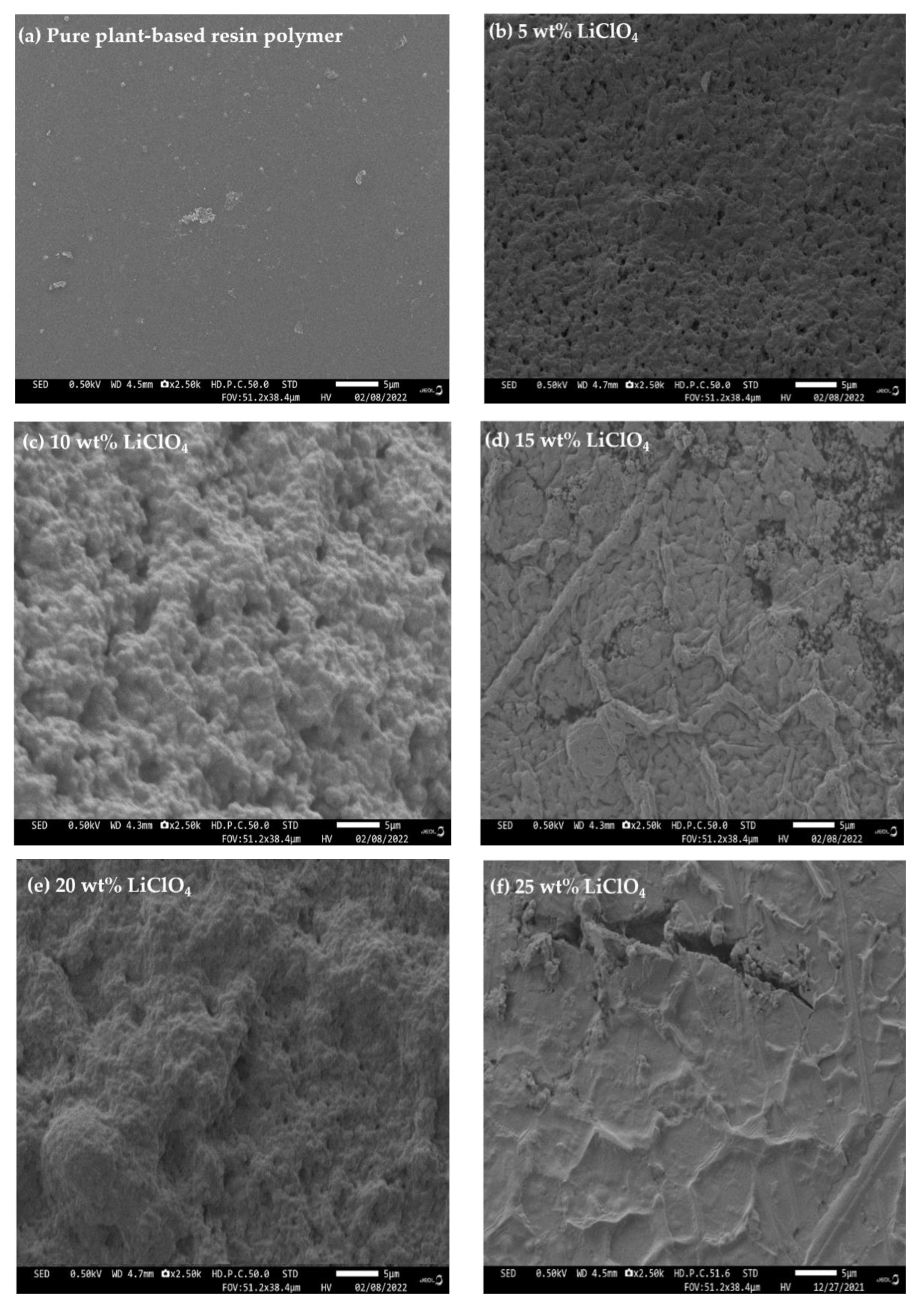
3.6. Field Emission Scanning Electron Microscope (FESEM)
3.7. Pure Plant-Based Polymer Biodegradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koohi-Fayegh, S.; Rosen, M.A. A Review of Energy Storage Types, Applications and Recent Developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the Challenge of Developing Advanced Technologies for Electrochemical Energy Storage and Conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Pramanik, P.K.D.; Sinhababu, N.; Mukherjee, B.; Padmanaban, S.; Maity, A.; Upadhyaya, B.K.; Holm-Nielsen, J.B.; Choudhury, P. Power Consumption Analysis, Measurement, Management, and Issues: A State-of-the-Art Review of Smartphone Battery and Energy Usage. IEEE Access 2019, 7, 182113–182172. [Google Scholar] [CrossRef]
- Latham, R.; Linford, R.; Schlindwein, W. Biomedical Applications of Batteries. Solid State Ion. 2004, 172, 7–11. [Google Scholar] [CrossRef]
- Uktamaliyev, B.I.; Abdukarimov, A.A.; Mamatkarimov, O.O.; Ergasheva, M. Ionic Conductivity and Dielectric Constant of a Solid Polymer Electrolyte Containing Salts Litf 2 and Mgtf 2. Converter 2021, 2021, 897–902. [Google Scholar]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A Conceptual Review on Polymer Electrolytes and Ion Transport Models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Nair, J.R.; Imholt, L.; Brunklaus, G.; Winter, M. Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges. Electrochem. Soc. Interface 2019, 28, 55–61. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent Advances in Gel Polymer Electrolyte for High-Performance Lithium Batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Mazuki, N.F.; Kufian, M.Z.; Nagao, Y.; Samsudin, A.S. Correlation Studies Between Structural and Ionic Transport Properties of Lithium-Ion Hybrid Gel Polymer Electrolytes Based PMMA-PLA. J. Polym. Environ. 2022, 30, 1864–1879. [Google Scholar] [CrossRef]
- Vondrák, J.; Sedlaříková, M.; Velická, J.; Klápště, B.; Novák, V.; Reiter, J. Gel Polymer Electrolytes Based on PMMA III. PMMA Gels Containing Cadmium. Electrochim. Acta 2003, 48, 1001–1004. [Google Scholar] [CrossRef]
- Le, H.T.T.; Ngo, D.T.; Kalubarme, R.S.; Cao, G.; Park, C.N.; Park, C.J. Composite Gel Polymer Electrolyte Based on Poly(Vinylidene Fluoride-Hexafluoropropylene) (PVDF-HFP) with Modified Aluminum-Doped Lithium Lanthanum Titanate (A-LLTO) for High-Performance Lithium Rechargeable Batteries. ACS Appl. Mater. Interfaces 2016, 8, 20710–20719. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Kuo, P.-L.; Hsieh, C.T.; Teng, H. Design of Poly(Acrylonitrile)-Based Gel Electrolytes for High-Performance Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 19360–19370. [Google Scholar] [CrossRef]
- Saroja, A.P.V.K.; Kumar, A.; Moharana, B.C.; Kamaraj, M.; Ramaprabhu, S. Design of Porous Calcium Phosphate Based Gel Polymer Electrolyte for Quasi-Solid State Sodium Ion Battery. J. Electroanal. Chem. 2020, 859, 113864. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer Electrolytes for Lithium Ion Batteries: A Critical Study. Ionics 2017, 23, 497–540. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, K.H.; Lee, Y.H.; Kim, K.G.; Jeong, Y.U.; Kim, S.Y.; Kim, H.D. Poly(Urethane Acrylate)-Based Gel Polymer Films for Mechanically Stable, Transparent, and Highly Conductive Polymer Electrolyte Applications. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Franceschi, E.; de Cezaro, A.; Ferreira, S.R.S.; Kunita, M.H.; Muniz, E.C.; Rubira, A.F.; Oliveira, J.V. Co-Precipitation of Beta-Carotene and Bio-Polymer Using Supercritical Carbon Dioxide as Antisolvent. Open Chem. Eng. J. 2014, 5, 11–20. [Google Scholar] [CrossRef][Green Version]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Imam, S.H.; Bilbao-Sainz, C.; Chiou, B.S.; Glenn, G.M.; Orts, W.J. Biobased Adhesives, Gums, Emulsions, and Binders: Current Trends and Future Prospects. J. Adhes. Sci. Technol. 2013, 27, 1972–1997. [Google Scholar] [CrossRef]
- Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Biak, D.R.A.; Tajau, R. Structural and Rheological Properties of Nonedible Vegetable Oil-Based Resin. Polymers 2021, 13, 2490. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, S.R.; Aung, M.M.; Ahmad, A.; Mansor, A.; TianKhoon, L. Preparation and Characterization of Jatropha Oil-Based Polyurethane as Non-Aqueous Solid Polymer Electrolyte for Electrochemical Devices. Electrochim. Acta 2016, 222, 293–302. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Guo, X.; Kong, B.; Zhang, M.; Qian, X.; Mi, S.; Sun, W. The Boom in 3D-Printed Sensor Technology. Sensors 2017, 17, 1166. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, N.S.; Mwasiagi, J.I.; Nkiwane, L.C.; Njuguna, D. Use of Regression to Study the Effect of Fabric Parameters on the Adhesion of 3D Printed PLA Polymer onto Woven Fabrics. Fash. Text. 2019, 6, 24. [Google Scholar] [CrossRef]
- Phillips, B.T.; Allder, J.; Bolan, G.; Nagle, R.S.; Redington, A.; Hellebrekers, T.; Borden, J.; Pawlenko, N.; Licht, S. Additive Manufacturing Aboard a Moving Vessel at Sea Using Passively Stabilized Stereolithography (SLA) 3D Printing. Addit. Manuf. 2020, 31, 100969. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; et al. 3D Printed Functional Nanomaterials for Electrochemical Energy Storage. Nano Today 2017, 15, 107–120. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Norjeli, M.F.; Tamchek, N.; Osman, Z.; Mohd Noor, I.S.; Kufian, M.Z.; Ghazali, M.I.B.M. Additive Manufacturing Polyurethane Acrylate via Stereolithography for 3D Structure Polymer Electrolyte Application. Gels 2022, 8, 589. [Google Scholar] [CrossRef]
- Jusoh Taib, E.R.; Abdullah, L.C.; Aung, M.M.; Basri, M.; Salleh, M.Z.; Saalah, S.; Mamat, S.; Chee, C.Y.; Wong, J.L. Physico-Chemical Characterisation of Epoxy Acrylate Resin from Jatropha Seed Oil Abstract Purpose. Pigment Resin Technol. 2017, 46, 1–14. [Google Scholar] [CrossRef]
- Tulcan, A.; Vasilescu, M.D.; Tulcan, L. Comparative Study of the Influence of Bio-Resin Color on the Dimension, Flatness and Straightness of the Part in the 3d Printing Process. Polymers 2021, 13, 1412. [Google Scholar] [CrossRef]
- Satzer, P.; Achleitner, L. 3D Printing: Economical and Supply Chain Independent Single-Use Plasticware for Cell Culture. New Biotechnol. 2022, 69, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Osman, Z.; Mohd Ghazali, M.I.; Othman, L.; Md Isa, K.B. AC Ionic Conductivity and DC Polarization Method of Lithium Ion Transport in PMMA-LiBF 4 Gel Polymer Electrolytes. Results Phys. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvasekarapandian, S.; Hirankumar, G.; Bhuvaneswari, M.S. Vibrational, Ac Impedance and Dielectric Spectroscopic Studies of Poly(Vinylacetate)-N,N-Dimethylformamide-LiClO4 Polymer Gel Electrolytes. J. Power Sources 2004, 134, 235–240. [Google Scholar] [CrossRef]
- Kasturi, P.R.; Ramasamy, H.; Meyrick, D.; Sung Lee, Y.; Kalai Selvan, R. Preparation of Starch-Based Porous Carbon Electrode and Biopolymer Electrolyte for All Solid-State Electric Double Layer Capacitor. J. Colloid Interface Sci. 2019, 554, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.L.; Ahmad, A.; Hassan, N.H.; Rana, U.A. [MG49-LiClO4]:[TiO2-SiO2] Polymer Electrolytes: In Situ Preparation and Characterization. Int. J. Polym. Sci. 2016, 2016, 9838067. [Google Scholar] [CrossRef]
- Ahmad, A.; Rahman, M.Y.A.; Ali, M.L.M.; Hashim, H.; Kalam, F.A. Solid Polymeric Electrolyte of PVC-ENR-LiClO4. Ionics 2007, 13, 67–70. [Google Scholar] [CrossRef]
- Deepa, M.; Sharma, N.; Agnihotry, S.A.; Chandra, R. FTIR Investigations on Ion-Ion Interactions in Liquid and Gel Polymeric Electrolytes: LiCF3SO3-PC-PMMA. J. Mater. Sci. 2002, 37, 1759–1765. [Google Scholar] [CrossRef]
- Aziz, S.B. Li+ Ion Conduction Mechanism in Poly (ε-Caprolactone)-Based Polymer Electrolyte. Iran. Polym. J. 2013, 22, 877–883. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A.; Ahmed, H.M. Effect of High Salt Concentration (HSC) on Structural, Morphological, and Electrical Characteristics of Chitosan Based Solid Polymer Electrolytes. Polymers 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Shukur, M.F.; Sonsudin, F.; Yahya, R.; Ahmad, Z.; Ithnin, R.; Kadir, M.F.Z. Electrical Properties of Starch Based Silver Ion Conducting Solid Biopolymer Electrolyte. Adv. Mater. Res. 2013, 701, 120–124. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Dennis, J.O.; Shukur, M.F.B.A.; Nasef, M.M.E.; Usman, F. Study on Dielectric Properties of Gel Polymer Electrolyte Based on PVA-K2CO3 Composites. Int. J. Electrochem. Sci. 2021, 16, 150296. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of Dielectric Relaxation and AC Conductivity Behavior of Plasticized Polymer Nanocomposite Electrolytes. Int. J. Electrochem. Sci. 2008, 3, 597–608. [Google Scholar] [CrossRef]
- Pawlicka, A.; Tavares, F.C.; Dörr, D.S.; Cholant, C.M.; Ely, F.; Santos, M.J.L.; Avellaneda, C.O. Dielectric Behavior and FTIR Studies of Xanthan Gum-Based Solid Polymer Electrolytes. Electrochim. Acta 2019, 305, 232–239. [Google Scholar] [CrossRef]
- Ramya, C.S.; Selvasekarapandian, S.; Hirankumar, G.; Savitha, T.; Angelo, P.C. Investigation on Dielectric Relaxations of PVP-NH4SCN Polymer Electrolyte. J. Non. Cryst. Solids 2008, 354, 1494–1502. [Google Scholar] [CrossRef]
- Santhosh, P.; Gopalan, A.; Vasudevan, T.; Lee, K.P. Evaluation of a Cross-Linked Polyurethane Acrylate as Polymer Electrolyte for Lithium Batteries. Mater. Res. Bull. 2006, 41, 1023–1037. [Google Scholar] [CrossRef]
- Bao, J.; Tao, C.; Yu, R.; Gao, M.; Huang, Y.; Chen, C. Solid Polymer Electrolyte Based on Waterborne Polyurethane for All-Solid-State Lithium Ion Batteries. J. Appl. Polym. Sci. 2017, 134, 45554. [Google Scholar] [CrossRef]
- Adam, N.I.; Hanibah, H.; Subban, R.H.Y.; Kassim, M.; Mobarak, N.N.; Ahmad, A.; Badri, K.H.; Su’ait, M.S. Palm-Based Cationic Polyurethane Membranes for Solid Polymer Electrolytes Application: A Physico-Chemical Characteristics Studies of Chain-Extended Cationic Polyurethane. Ind. Crops Prod. 2020, 155, 112757. [Google Scholar] [CrossRef]
- Mustapa, S.R.; Aung, M.M.; Rayung, M. Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane. Polymers 2021, 13, 132. [Google Scholar] [CrossRef]
- Whba, R.A.G.; TianKhoon, L.; Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Influence of Binary Lithium Salts on 49% Poly(Methyl Methacrylate) Grafted Natural Rubber Based Solid Polymer Electrolytes. Arab. J. Chem. 2020, 13, 3351–3361. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Characterization of Novel Castor Oil-Based Polyurethane Polymer Electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Sim, L.H.; Gan, S.N.; Chan, C.H.; Yahya, R. ATR-FTIR Studies on Ion Interaction of Lithium Perchlorate in Polyacrylate/Poly(Ethylene Oxide) Blends. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 287–292. [Google Scholar] [CrossRef]
- Firman, P.; Xu, M.; Eyring, E.M.; Petrucci, S. Molecular Relaxation Dynamics of Lithium Perchlorate in Acyclic Polyether Solvents. J. Phys. Chem. 1993, 97, 3606–3613. [Google Scholar] [CrossRef]
- Rajendran, S.; Mahendran, O.; Kannan, R. Ionic Conductivity Studies in Composite Solid Polymer Electrolytes Based on Methylmethacrylate. J. Phys. Chem. Solids 2002, 63, 303–307. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chuang, Y.C.; Su, J.H.; Yu, H.C.; Chen-Yang, Y.W. High Discharge Capacity Solid Composite Polymer Electrolyte Lithium Battery. J. Power Sources 2011, 196, 2802–2809. [Google Scholar] [CrossRef]
- Saikia, D.; Chen, Y.H.; Pan, Y.C.; Fang, J.; Tsai, L.D.; Fey, G.T.K.; Kao, H.M. A New Highly Conductive Organic-Inorganic Solid Polymer Electrolyte Based on a Di-Ureasil Matrix Doped with Lithium Perchlorate. J. Mater. Chem. 2011, 21, 10542–10551. [Google Scholar] [CrossRef]
- Kumar, D.; Hashmi, S.A. Ion Transport and Ion-Filler-Polymer Interaction in Poly(Methyl Methacrylate)-Based, Sodium Ion Conducting, Gel Polymer Electrolytes Dispersed with Silica Nanoparticles. J. Power Sources 2010, 195, 5101–5108. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High Electrochemical and Mechanical Performance of Zinc Conducting-Based Gel Polymer Electrolytes. Sci. Rep. 2021, 11, 13268. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Y.M.; Shukur, M.F.; Hamsan, M.H.; Jumbri, K.; Kadir, M.F.Z. Plasticized Solid Polymer Electrolyte Based on Natural Polymer Blend Incorporated with Lithium Perchlorate for Electrical Double-Layer Capacitor Fabrication. Ionics 2019, 25, 5473–5484. [Google Scholar] [CrossRef]
- Sheela, T.; Bhajantri, R.F.; Nambissan, P.M.G.; Ravindrachary, V.; Lobo, B.; Naik, J.; Rathod, S.G. Ionic Conductivity and Free Volume Related Microstructural Properties of LiClO4/PVA/NaAlg Polymer Composites: Positron Annihilation Spectroscopic Studies. J. Non-Cryst. Solids 2016, 454, 19–30. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Krishna Bhat, D. Lithium Salts Doped Biodegradable Gel Polymer Electrolytes for Supercapacitor Application. J. Mater. Environ. Sci. 2015, 6, 1218–1227. [Google Scholar]
- Liu, Q.; Yang, G.; Li, X.; Zhang, S.; Chen, R.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Polymer Electrolytes Based on Interactions between [Solvent-Li+] Complex and Solvent-Modified Polymer. Energy Storage Mater. 2022, 51, 443–452. [Google Scholar] [CrossRef]
- Liao, F.; Zeng, X.R.; Li, H.Q.; Lai, X.J.; Zhao, F.C. Synthesis and Properties of UV Curable Polyurethane Acrylates Based on Two Different Hydroxyethyl Acrylates. J. Cent. South Univ. Technol. 2012, 19, 911–917. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical Characterization of Corn Starch-LiOAc Electrolytes and Application in Electrochemical Double Layer Capacitor. Electrochim. Acta 2014, 136, 204–216. [Google Scholar] [CrossRef]









| Ingredients | Compositions (%) |
|---|---|
| Concentration of fatty acids, soya, epoxidized, Bu esters | 45 |
| Isooctyl acrylate (C11H20O2) | 30 |
| 2-((2,2-Bis(((1-oxoallyl)oxy)methyl)butoxy)methyl)-2-ethyl-1,3-propanediyl diacrylate | 15 |
| 2-hydroxy-1-(4-(4-(2-hydroxy-2-methylpropionyl)benzyl)phenyl)-2-methylpropan-1-one | 5 |
| Polychloro copper phthalocyanine | 5 |
| Major Characteristics | Regular Resin | Plant-Based Resin |
|---|---|---|
| Resin odor | Smelly | Slightly |
| Ingredient | Industrial chemical | Soy oil |
| Washing odor | Pungent | Detergent smell |
| Range of wavelength | 405 nm | 355–410 nm |
| Eco-friendly | Difficult to degrade | Biodegradable |
| Designation | LiClO4 Content (wt.%) | Plant-Based Resin (g) | DMF (g) | LiClO4 (g) |
|---|---|---|---|---|
| S0 | 0 | 2.00 | 2.00 | 0 |
| S5 | 5 | 2.00 | 2.00 | 0.21 |
| S10 | 10 | 2.00 | 2.00 | 0.44 |
| S15 | 15 | 2.00 | 2.00 | 0.71 |
| S20 | 20 | 2.00 | 2.00 | 1.00 |
| S25 | 25 | 2.00 | 2.00 | 1.33 |
| Anycubic Photon S Settings | Parameters |
|---|---|
| Layer height | 0.5 mm |
| Exposure time | 10 s |
| Off-time | 6.5 s |
| Exposure on 8 bottom layers | 70 s |
| Distance of Z-lift | 6 mm |
| Speed | 1 mm s−1 |
| Sample | Rb (Ω) | Ionic Conductivity, σ (S cm−1) |
|---|---|---|
| S0 | 7.82 × 105 | 2.27 × 10−8 |
| S5 | 5.18 × 101 | 3.42 × 10−4 |
| S10 | 2.09 × 101 | 8.56 × 10−4 |
| S15 | 9.07 | 1.85 × 10−3 |
| S20 | 8.86 | 3.05 × 10−3 |
| S25 | 9.95 | 2.41 × 10−3 |
| Functional Groups | Wavenumber (cm−1) | ||||||
|---|---|---|---|---|---|---|---|
| LiClO4 | S0 | S5 | S10 | S15 | S20 | S25 | |
| C=O | - | 1732 | 1731 | 1730 | 1729 | 1727 | 1727 |
| N−H | - | 3419 | 3421 | 3423 | 3426 | 3429 | 3431 |
| C−O−C | - | 1115 | 1110 | 1106 | 1101 | 1098 | 1093 |
| LiClO4 characteristics | 1630 | - | 1630 | 1630 | 1630 | 1630 | 1630 |
| ClO4 asymmetric vibration | 1089 | - | 1089 | 1089 | 1089 | 1089 | 1089 |
| LiClO4 ion pairs | 631 | - | 629 | 630 | 630 | 636 | 636 |
| ClO4− free ions | 614 | - | 623 | 623 | 623 | 623 | 624 |
| Sample | Percentage of Free Ions (%) | Percentage of Ion Pairs (%) |
|---|---|---|
| S5 | 74.84 | 25.16 |
| S10 | 82.86 | 17.14 |
| S15 | 89.85 | 10.15 |
| S20 | 96.80 | 3.20 |
| S25 | 94.75 | 5.25 |
| Sample | Tdmax1 (°C) (First Stage) | Tdmax2 (°C) (Second Stage) | Weight Change (%) | Residue (%) |
|---|---|---|---|---|
| S0 | - | 457.00 | 94.855 | 5.1450 |
| S5 | 337.00 | 423.00 | 87.924 | 12.076 |
| S10 | 286.99 | 397.63 | 81.846 | 18.154 |
| S15 | 285.17 | 373.62 | 74.391 | 25.609 |
| S20 | 273.24 | 350.31 | 75.194 | 24.806 |
| S25 | 289.45 | 329.78 | 73.602 | 26.398 |
| Sample | Tg (°C) | Tm (°C) |
|---|---|---|
| S0 | −18.54 | 116.28 |
| S5 | −18.06 | 115.29 |
| S10 | −18.87 | 81.19 |
| S15 | −18.94 | 80.98 |
| S20 | −19.58 | 80.50 |
| S25 | −18.60 | 85.69 |
| Days | Weight of Pure Plant-Based Polymer (g) | Weight Loss of Pure Plant-Based Polymer after Burial (%) |
|---|---|---|
| 1 | 0.1711 | - |
| 5 | 0.1690 | 1.23 |
| 10 | 0.1678 | 1.93 |
| 15 | 0.1652 | 3.45 |
| 20 | 0.1647 | 3.74 |
| 25 | 0.1636 | 4.38 |
| 30 | 0.1635 | 4.44 |
| 35 | 0.1630 | 4.73 |
| 40 | 0.1623 | 5.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamood, M.A.H.; Norjeli, M.F.; Abu Bakar, A.A.; Abdullah, S.M.; Tamchek, N.; Mohd Noor, I.S.; Sabeeh, A.H.; Alforidi, A.F.; Khawaji, I.H.; Mohd Ghazali, M.I. Electrical, Thermal, and Structural Characterization of Plant-Based 3D Printed Gel Polymer Electrolytes for Future Electrochemical Applications. Polymers 2023, 15, 4713. https://doi.org/10.3390/polym15244713
Mahamood MAH, Norjeli MF, Abu Bakar AA, Abdullah SM, Tamchek N, Mohd Noor IS, Sabeeh AH, Alforidi AF, Khawaji IH, Mohd Ghazali MI. Electrical, Thermal, and Structural Characterization of Plant-Based 3D Printed Gel Polymer Electrolytes for Future Electrochemical Applications. Polymers. 2023; 15(24):4713. https://doi.org/10.3390/polym15244713
Chicago/Turabian StyleMahamood, Muhammad Afiq Hazizi, Muhammad Faishal Norjeli, Ahmad Adnan Abu Bakar, Shahino Mah Abdullah, Nizam Tamchek, Ikhwan Syafiq Mohd Noor, Ala H. Sabeeh, Ahmad Fudy Alforidi, Ibrahim H. Khawaji, and Mohd Ifwat Mohd Ghazali. 2023. "Electrical, Thermal, and Structural Characterization of Plant-Based 3D Printed Gel Polymer Electrolytes for Future Electrochemical Applications" Polymers 15, no. 24: 4713. https://doi.org/10.3390/polym15244713
APA StyleMahamood, M. A. H., Norjeli, M. F., Abu Bakar, A. A., Abdullah, S. M., Tamchek, N., Mohd Noor, I. S., Sabeeh, A. H., Alforidi, A. F., Khawaji, I. H., & Mohd Ghazali, M. I. (2023). Electrical, Thermal, and Structural Characterization of Plant-Based 3D Printed Gel Polymer Electrolytes for Future Electrochemical Applications. Polymers, 15(24), 4713. https://doi.org/10.3390/polym15244713










