Towards the Understanding of the Aging Behavior of p-PVC in Close Contact with Minced Meat in the Artwork POEMETRIE by Dieter Roth
Abstract
:1. Introduction
1.1. “POEMETRIE” by Dieter Roth—Challenges in the Preservation of Modern Polymeric Materials Combined with Natural Organic Fats
1.2. p-PVC Properties and Issues
1.3. Relevance to Other POEMETRIE Copies
1.4. Aim of the Research
- Documentation and examination of the artwork—based on the localization and characterization of damage phenomena, material identification, and state of degradation.
- Selection and preparation of replicas—based on the material identification of the artwork.
- Artificial accelerated thermal aging studies—based on the prepared replicas with and without fillings to study material changes and interactions and on the difficulties in reproducing those specific phenomena.
2. Experimental Part
2.1. Documentation of the Artwork—Methods
2.1.1. Photographic Documentation in Visible (Vis) and Ultraviolet (UV) Induced Fluorescence Light
2.1.2. pH Measurements
2.1.3. Fourier Transform Infrared Spectroscopy in Attenuated Total Reflection (FTIR-ATR)
2.1.4. Pyrolysis—Gas Chromatography/Mass Spectrometry (Py-GC/MS)
2.2. Selection and Preparation of Replicas
2.2.1. Complexity in the p-PVC-Based Replica Materials
2.2.2. Selection of p-PVC Replicas
- Transparent Dia-slide cases (M67 ref.: 0111 01, PANODIA, Lyon, France) were purchased, containing bis(2-ethylhexyl) phthalate (DEHP) as a primary plasticizer and as repp material for the artwork’s bags. The Dia-slide sheets consist of two p-PVC foils, each with a layer thickness of 0.04 mm. The individual slots are separated by weld seams.
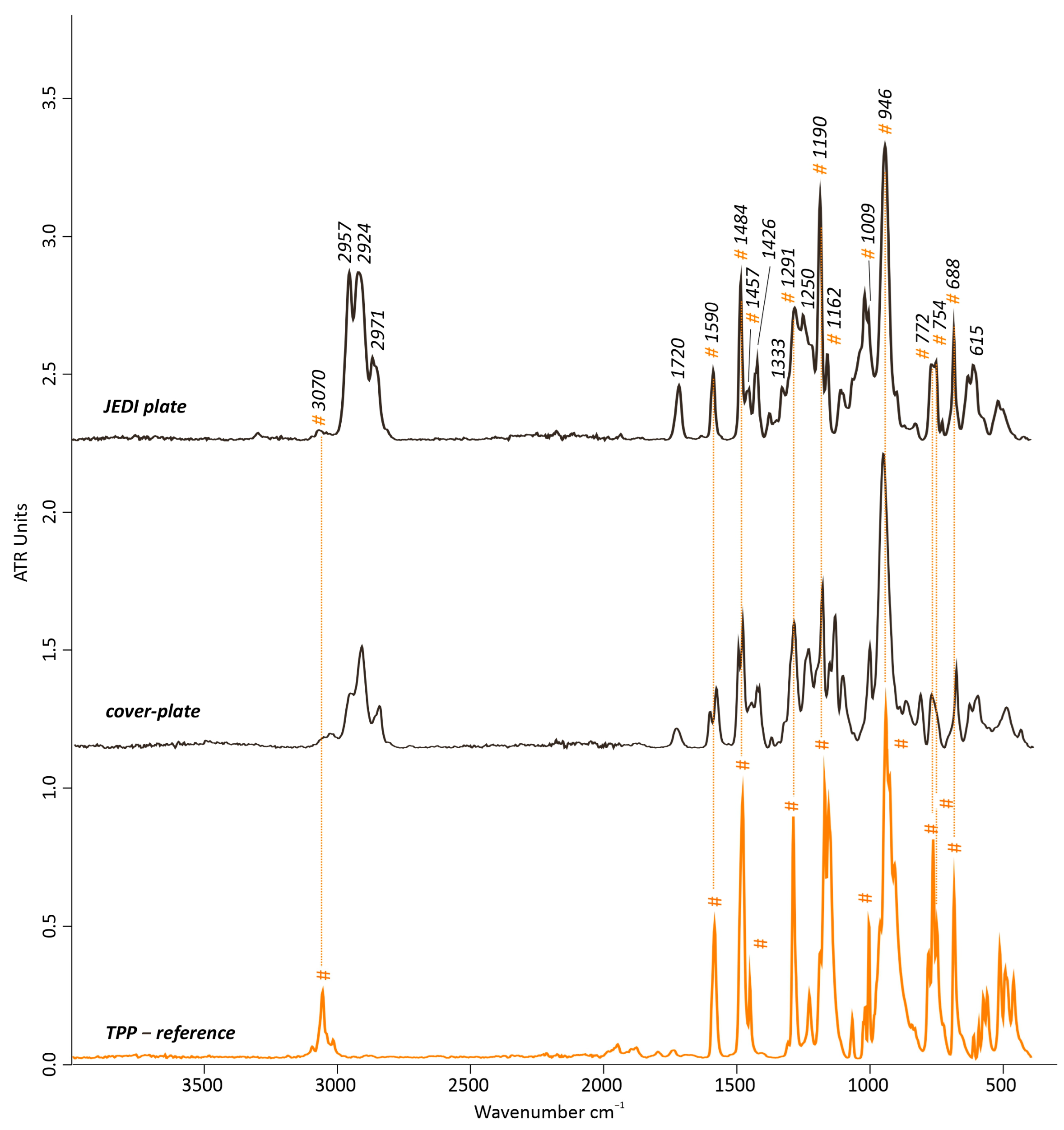
- No p-PVC containing TCP as plasticizer could be commercially found as replica material for the translucent yellowish covering plate. Because of the non-availability of tricresyl phosphate (TCP)-based p-PVC types of materials, a p-PVC containing triphenyl phosphate (TPP) (FB01//B2, JEDI Kunststoff GmbH, Eitorf, Germany) was instead selected. Possible differences between phthalate- and phosphate-based plasticizers were still studied. The JEDI plate has a thickness of 1 mm.
2.2.3. Filling
2.2.4. Methods
2.3. Artificially-Accelerated Thermal Aging
3. Results and Discussion
3.1. Documentation and Examination of the Artwork
3.1.1. UV/Vis-Imaging and pH Measurements
3.1.2. Material Identification by FTIR-ATR and Py-GC/MS
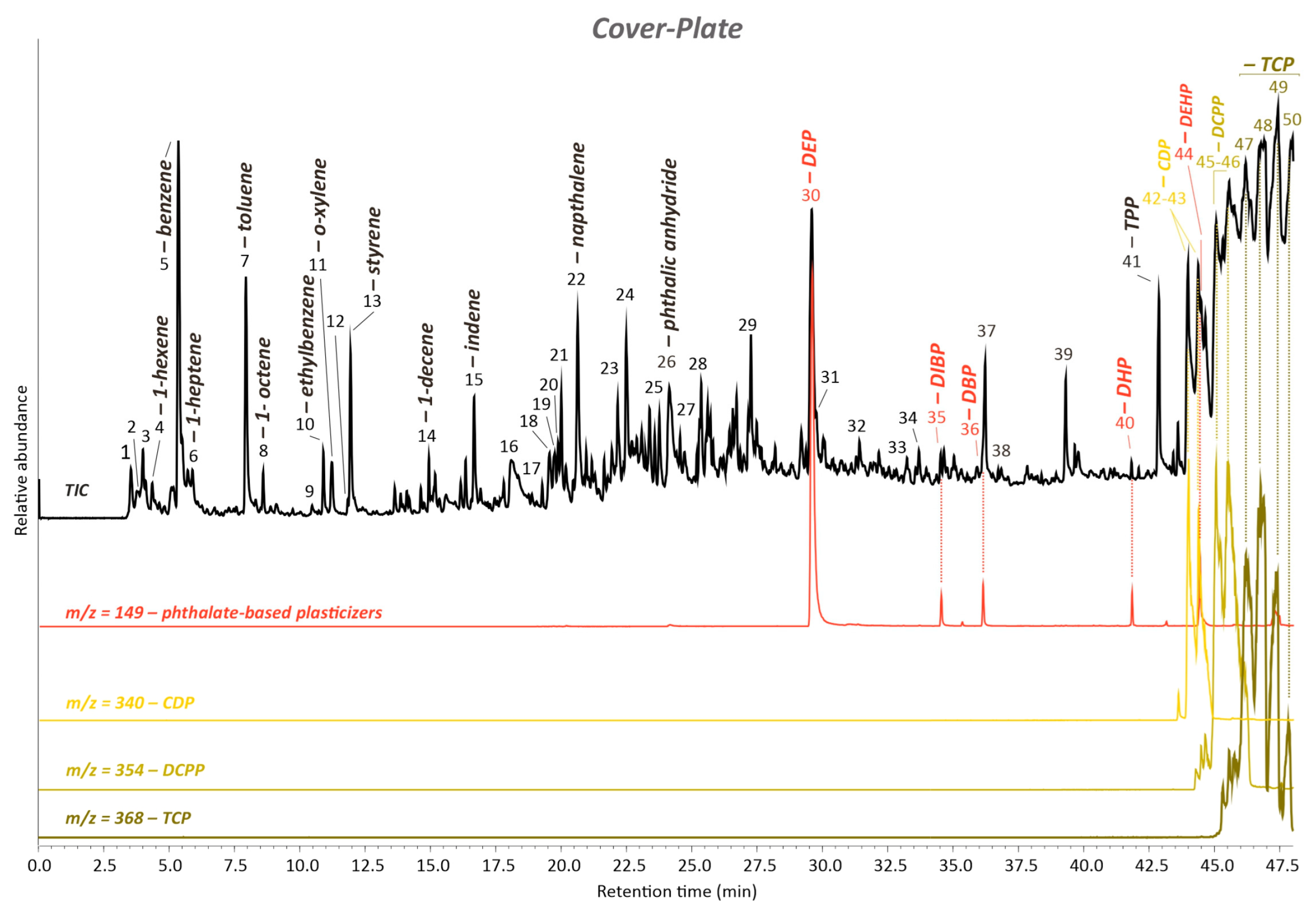
Artwork’s Cover Plate and Cover Plate’s Exudate
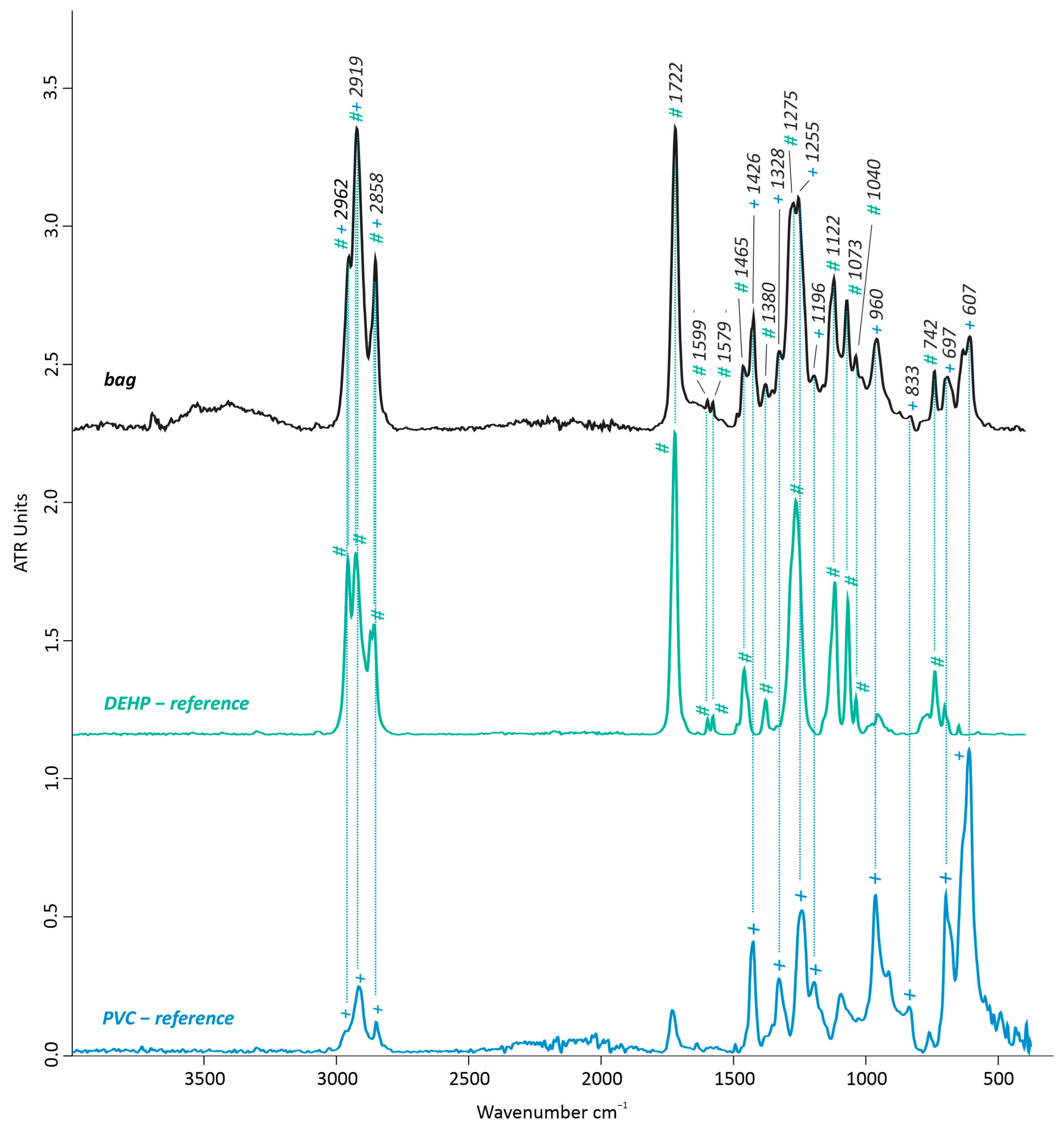
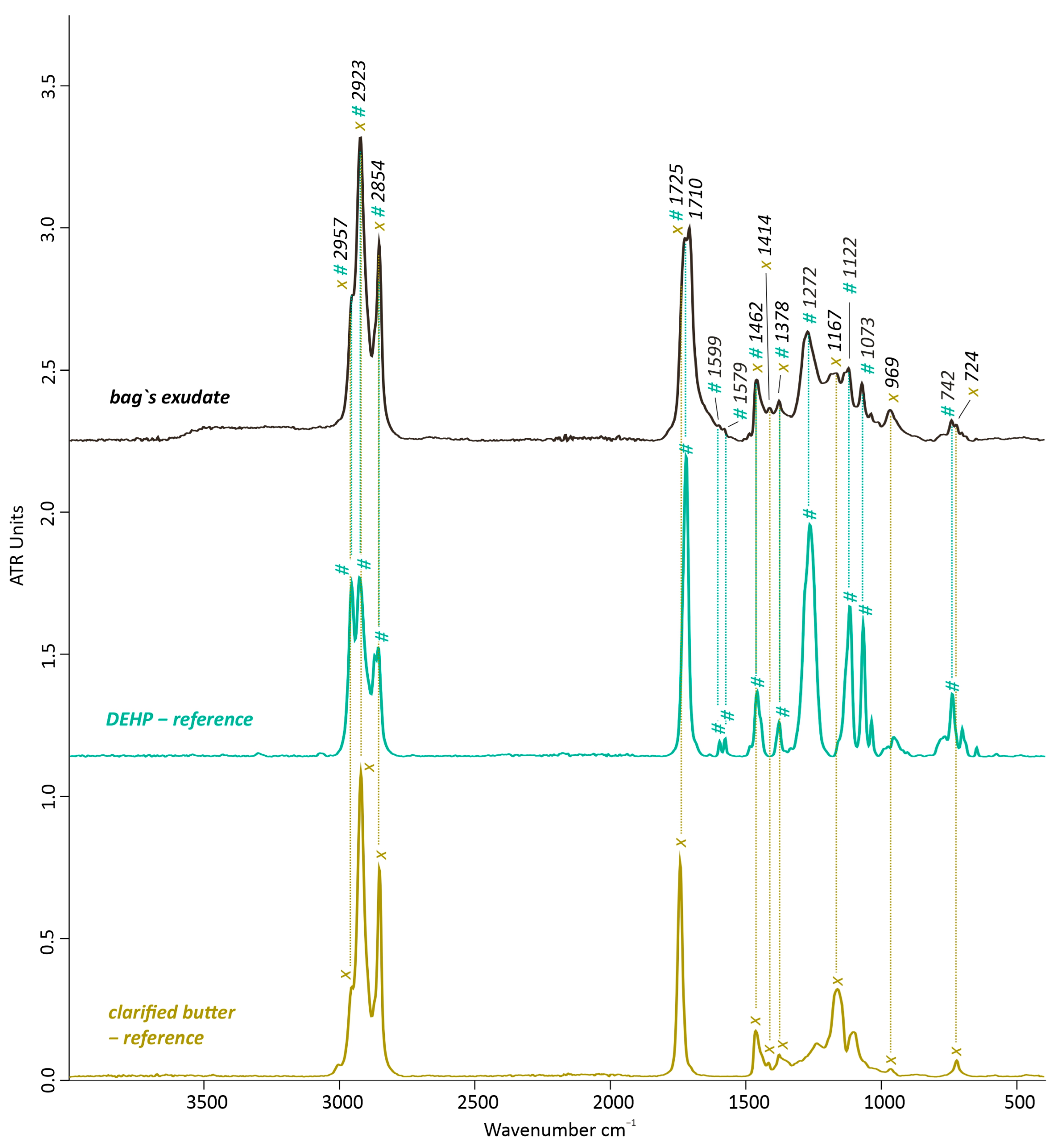
Artwork’s Bag and Bag’s Exudate
3.2. Replica Materials
3.2.1. JEDI Plate
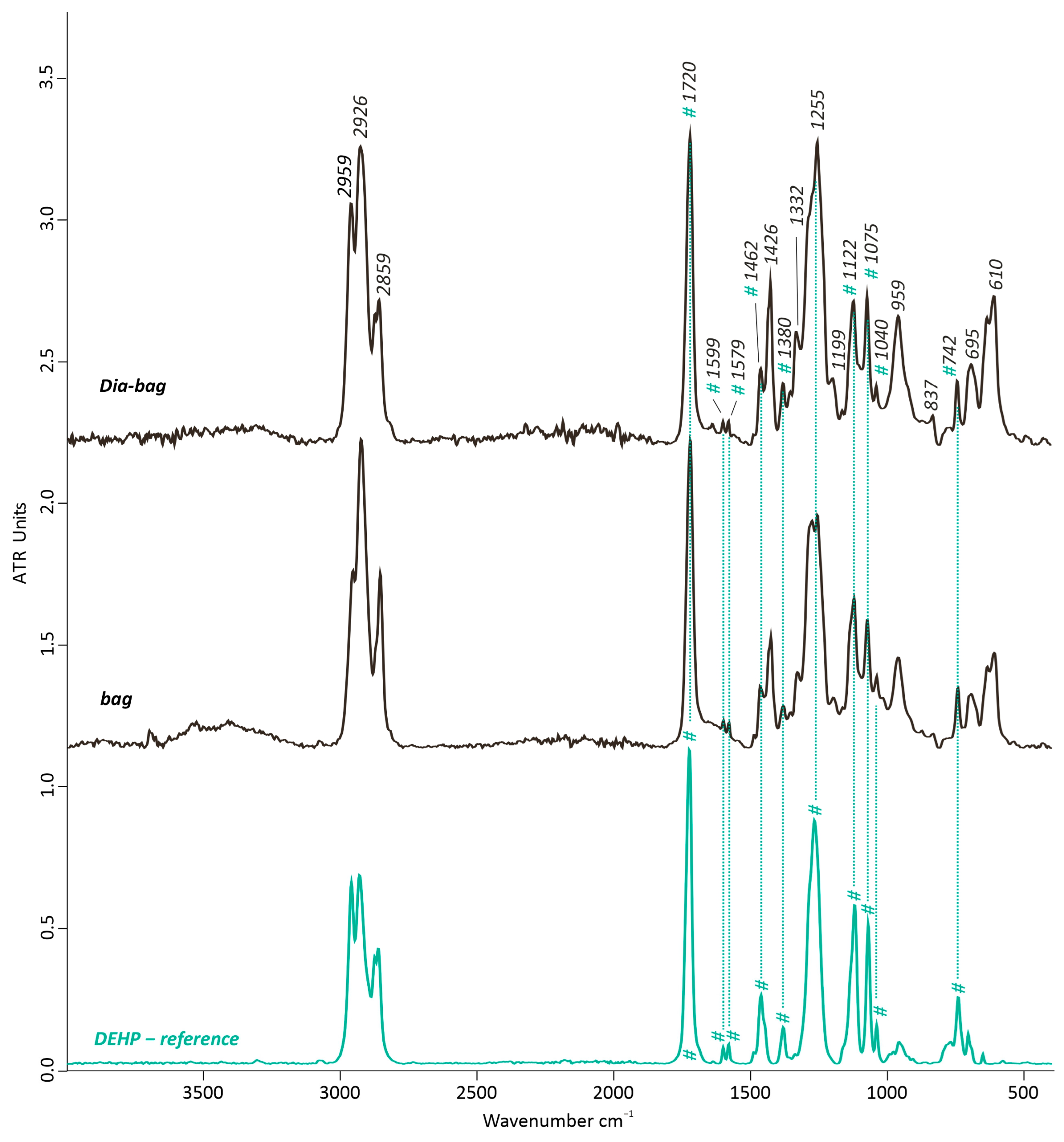
3.2.2. Dia-Bag
3.3. Artificial Thermal Aging Studies
3.3.1. Dia-Bag without Filling and JEDI Plate
3.3.2. Thermal Aging on Dia-Bags with Fillings
4. Conclusions
- −
- It was important to notice that the distribution of dust and particle agglomeration, as well as sticky areas, were found mostly in some specific areas of the artwork by UV/Vis imaging, which suggested a vertically aligned presentation or hanging of the object, which was not clear and known from the historical documentation. Also, while the yellowish fluorescence in the UV images points toward organic fatty compounds, the pH measurements helped to characterize the exudates as highly acidic.
- −
- Analytical investigations allowed the determination of the exact chemical composition of the p-PVC-based cover plate and bags, as well as the replicas selected for the thermal aging studies, additionally pointing out their differences in terms of plasticizer types. Despite the extensive agreement between the FTIR-ATR spectra of the samples from the artwork and the selected replica materials, Py-GC/MS showed clear differences in the material composition.
- −
- Very importantly, this research also demonstrated that despite several types of PVC-based materials that can be characterized by different plasticizer-type compositions, they still may present similar degradation pathways, such as migration of the plasticizers, a decrease in flexibility and an increase in brittleness, as well as an increase in yellowing as a color change. For instance, the p-PVC-based cover plate and bags of the artwork and the recent p-PVC-based replicas used for the thermal aging studies were all affected by those degradation pathways, regardless of their slightly different plasticizer compositions as determined by Py-GC/MS.
- −
- On the other hand, the well-known stickiness issue can be strictly influenced by the intrinsic characteristics of the p-PVC material formulation, such as plasticizer composition, but also by the environmental conditions. Indeed, the most recent p-PVC formulation, such as the one used for this study, was not affected by stickiness after artificially accelerated thermal aging. Instead, the new typology of p-PVC representing the replicas suffered from a migration of a detected ammine-wax type of lubricant, which for the first time was successfully identified as N,N’-ethylenebis(stearamide) (EBS) by FTIR-ATR and only after the thermal aging. These results open new aspects and degradation pathways of the most recent p-PVC formulation, which need to be considered not only in the field of modern and contemporary art but, for instance, in industrial applications and food packaging, and which provide an important source of information.
- −
- The determination of fatty organic components mostly on the back side of the thermally aged replica’s bags filled with minced meat and clarified butter points to the fact that they can follow different directions during exudation or migration, since fatty exudates were mostly found on the front side of the artwork. Factors such as air/oxygen exposure, temperature at the surface, plasticizers, and additive types, whether vertical or horizontal, should be considered. This can thus significantly suggest that special care should be considered while planning the exhibition of those artworks and in which position they should be hung and placed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobke, D. Melancholischer Nippes: Dieter Roths frühe Objekte und Materialbilder (1960–1975). Ph.D. Thesis, Universität Hamburg, Hamburg, Germany, 1997. Available online: https://ediss.sub.uni-hamburg.de/bitstream/ediss/163/1/Dissertation_gesamt.pdf (accessed on 26 November 2023).
- Shashoua, Y.R. Conservation of Plastics Materials Science Degradation Preservation. Butterworth-Heinemann. In Conservation of Plastics; Routledge: London, UK, 2008. [Google Scholar] [CrossRef]
- Shashoua, Y.R.R. Inhibition the Deterioration of Plasticized Poly (Vinyl Chloride)—A Museum Perspective. Ph.D. Thesis, The Technical University of Denmark, Lyngby, Denmark, 2001. Available online: http://www.cwaller.de/didaktik_teil4/shashoua_2001.pdf (accessed on 26 November 2023).
- Ryf, S. Weichmacherverlust Bei Objekten Aus Weich-PVC Am Beispiel Einiger Multiples Von Joseph Beuys, Semesterarbeit; Hochschule der Künste Bern: Bern, Switzerland, 2005. [Google Scholar]
- Waentig, F. Kunststoffe in Der Kunst: Eine Studie Unter Konservatorischen Gesichtspunkten; Petersberg: Imhof, Germany, 2004; pp. 239–248. [Google Scholar]
- Rijavec, T.; Strlič, M.; Cigić, I.K. Plastics in Heritage Collections: Poly(vinyl chloride) Degradation and Characterization. Acta Chim. Slov. 2020, 67, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, G.W.; Pongratz, S. Beständigkeit Von Kunststoffen—Bd. 1; Carl Hansen Verlag: Munich, Germany, 2007; pp. 362–363. [Google Scholar]
- Cruz, R.M.S.; Rico, B.P.M.; Vieira, M.C. 9—Food packaging and migration. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 281–301. [Google Scholar] [CrossRef]
- Ardıç, M.; Kahve, H.; Duran, A. Chemical Migration in Food Technology. Acad. J. Sci. 2015, 4, 163–166. Available online: https://www.researchgate.net/publication/290429224_CHEMICAL_MIGRATION_IN_FOOD_TECHNOLOGY (accessed on 26 November 2023).
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Fiigge, K. Migration of Components from Plastics-Packaging Materials into Packed Goods-Test Methods and Diffusion Models. Prog. Polym. Sci. 1980, 6, 187–252. [Google Scholar] [CrossRef]
- Rijavec, T.; Strlič, M.; Cigić, I.K. Damage function for poly(vinyl chloride) in heritage collections. Polym. Degrad. Stab. 2023, 211, 110329. [Google Scholar] [CrossRef]
- Klempová, S.; Oravec, M.; Vizárová, K. Analysis of thermally and UV–Vis aged plasticized PVC using UV–Vis, ATR-FTIR and Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 294, 122541. [Google Scholar] [CrossRef] [PubMed]
- Royaux, A.; Fabre-Francke, I.; Balcar, N.; Barabant, G.; Bollard, C.; Lavédrine, B.; Cantin, S. Aging of plasticized polyvinyl chloride in heritage collections: The impact of conditioning and cleaning treatments. Polym. Degrad. Stab. 2017, 137, 109–121. [Google Scholar] [CrossRef]
- Morales Muñoz, C.; Egsgaard, H.; Sanz-Landaluze, J.; Dietz, C. A model approach for finding cleaning solutions for plasticized poly(vinyl chloride) surfaces of collections objects. J. Am. Inst. Conserv. 2014, 53, 236–251. [Google Scholar] [CrossRef]
- Rijavec, T.; Ribar, D.; Markelj, J.; Strlič, M.; Cigić, I.K. Machine learning-assisted non-destructive plasticizer identification and quantification in historical PVC objects based on IR spectroscopy. Sci. Rep. 2022, 12, 5017. [Google Scholar] [CrossRef]
- Dutta, P.K.; Graf, K.R. Migration of Plasticizer in Vinyl Resins: An Infrared Spectroscopic Study. J. Appl. Polym. Sci. 1984, 29, 2247–2250. Available online: https://onlinelibrary.wiley.co (accessed on 26 November 2023).
- Petre, A.; Budrugeac, P.; Segal, E. Thermal Degradation of Polyvinyl Chloride. J. Therm. Anal. Calorim. 1999, 56, 1065–1070. Available online: https://www.academia.edu/6225326/Thermal_Degradation_of_Polyvinyl_Chloride?email_work_card=view-paper (accessed on 26 November 2023). [CrossRef]
- Jakubowicz, I.; Yarahmadi, N.; Gevert, T. Effects of accelerated and natural ageing on plasticized polyvinyl chloride (PVC). Polym. Degrad. Stab. 1999, 66, 415–421. [Google Scholar] [CrossRef]
- Ito, M.; Nagai, K. Analysis of degradation mechanism of plasticized PVC under artificial aging conditions. Polym. Degrad. Stab. 2007, 92, 260–270. [Google Scholar] [CrossRef]
- Braun, D.; Sonderhof, D. Assignment of UV-absorption maxima of degraded PVC. Polym. Bull. 1985, 14, 39–43. [Google Scholar] [CrossRef]
- Al-Dossary, A.K.; Gilbert, M.; Hitt, D. Evaluating PVC degradation using UV and Raman spectroscopies. Adv. Mater. Res. 2010, 83–86, 923–930. [Google Scholar] [CrossRef]
- Baruya, A.; Gerrard, D.L.; Maddams, W.F. Resonance Raman spectrum of degraded poly (vinyl chloride). 4. determination of conjugated polyene sequence lengths. Macromolecules 1983, 16, 578–580. [Google Scholar] [CrossRef]
- Kip, B.J.; van Aaken, S.M.; Meier, R.J.; Kenneth, P.J.W.; Gerrard, D.L. Considerations for Raman Spectroscopic Determination of Polyene Length Distribution in Degraded Poly(vinyl chloride). Macromolecules 1992, 25, 4290–4296. [Google Scholar] [CrossRef]
- Fumitaka, M.; Fujimaki, S.; Sakamoto, Y.; Kudo, Y.; Miyagawa, H. Screening of Phthalates in Polymer Materials by Pyrolysis-GC-MS. Anal. Sci. 2015, 31, 3–5. [Google Scholar] [CrossRef]
- Akoueson, F.; Chbib, C.; Monchy, S.; Paul-Pont, I.; Doyen, P.; Dehaut, A.; Duflos, G. Identification and quantification of plastic additives using pyrolysis-GC/MS: A review. Sci. Total Environ. 2021, 773, 145073. [Google Scholar] [CrossRef]
- Landesmuseum Westfalen-Lippe (LWL), Dieter Roth, POEMETRIE (1968). Available online: https://www.lwl.org/323sc-download/LWL/Kultur/stiftung-cremer/popups/roth-poetry/index.html (accessed on 16 September 2023).
- Zucker Art Books, New York, Dieter Roth, POEMETRIE (1968). Available online: https://www.zuckerartbooks.com/inc/scripts/image_pdf.php?file_id=20562&caption=Dieter%20Roth%2C%20%3Ci%3EPoemetry%2C%20Deluxe%20Edition%20No.4%20of%20the%20review%20%E2%80%98Poeting%E2%80%99%3C%2Fi%3E%2C%201968%3Cbr%3E33%2013%2F16%20x%2010%203%2F16%20x%201%2015%2F16%20in.%20%2886%20x%2026%20x%205%20cm%29%3Cbr%3E6071-BK%2C%20Price%20Upon%20Request (accessed on 16 September 2023).
- Hughes, A. Measurement of Surface PH of paper using Agarose Gel Plugs. In GELS in the Conservation of Art; Archetype Publications: London, UK, 2017; pp. 62–66. [Google Scholar]
- European Commission, Institute of Health and Consumer Protection. BIS (2-ETHYLHEXYL) PHTHALATE (DEHP), SUMMARY RISK ASSESSMENT REPORT. 2008. Available online: https://echa.europa.eu/documents/10162/060d4981-4dfb-4e40-8c69-6320c9debb01 (accessed on 26 November 2023).
- World Health Organisation, Environmental. Health Criteria 110 Tricresyl phosphate. 1990. Available online: https://apps.who.int/iris/bitstream/handle/10665/39784/9241571101-eng.pdf?sequence=1 (accessed on 26 November 2023).
- Betrand, P.A. Reactions of Tricresyl Phosphate with Bearing Materials. AEROSPACE REPORT NO. TR-97(8565)-1; The Aerospace Corporation: El Segundo, CA, USA, 1997; Available online: https://apps.dtic.mil/sti/pdfs/ADA327918.pdf (accessed on 26 November 2023).
- Coltro, L.; Pitta, J.; Madaleno, E. Performance evaluation of new plasticizers for stretch PVC films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef]
- Beltan, M.; Garcia, J.C.; Marcilla, A. Infrared Spectral Changes in PVC and Plasticized PVC during Gelation and Fusion. Eur. Polym. J. 1997, 33, 453–462. [Google Scholar] [CrossRef]
- Wampler, T.P. Applied Pyrolysis Handbook; CRC Press/Talor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Moldoveanu, S.C. Analytical Pyrolysis of Synthetic Organic Polymers; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Beriain, M.J.; Ibañez, F.C.; Beruete, E.; Gómez, I.; Beruete, M. Estimation of Fatty Acids in Intramuscular Fat of Beef by FT-MIR Spectroscopy. Foods 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Pintus, V.; Viana, C.; Angelin, E.M.; De Sá, S.F.; Wienland, K.; Sterflinger, K.; Ferreira, J.L. Applicability of single-shot and double-shot Py-GC/MS for the detection of components in vinyl acetate-based emulsions used in modern-contemporary art. J. Anal. Appl. Pyrolysis 2022, 168, 105782. [Google Scholar] [CrossRef]
- Schiller, M. PVC Additives—Performance, Chemistry, Developments, and Sustainability; Carl Hansen Verlag: Munich, Germany, 2015. [Google Scholar]
- Chow, W.S.; Tham, W.; Poh, B.; Ishak, Z. Mechanical and Thermal Oxidation Behavior of Poly(Lactic Acid)/Halloysite Nanotube Nanocomposites Containing N,N′-Ethylenebis(Stearamide) and SEBS-g-MA. J. Polym. Environ. 2018, 26, 2973–2982. [Google Scholar] [CrossRef]
- Huynh, M.D.; Trung, T.H.; Nguyen, D.; Giang, N. The melting rheology, mechanical properties, thermal stability and morphology of polylactic acid/ethylene bis stearamide modified gypsum composite. Vietnam. J. Chem. 2020, 58, 251–255. [Google Scholar] [CrossRef]
- Smith, B.C. Organic Nitrogen Compounds, VII: Amides—The Rest of the Story. 2020. Available online: https://www.spectroscopyonline.com/view/organic-nitrogen-compounds-vii-amides-rest-story (accessed on 28 September 2023).
- Bai, X.; Stein, B.K.; Smith, K..; Isaac, D.H. Effects of Reprocessing on Additives in ABS Plastics, Detected by Gas Chromatography/Mass Spectrometry. Prog. Rubber Plast. Recycl. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Wang, F.C.Y.; Buzanowski, W.C. Polymer additive analysis by pyrolysis–gas chromatography III. Lubricants. J. Chromatogr. A 2000, 891, 313–324. [Google Scholar] [CrossRef]









| Spot # | Material | Method | Results |
|---|---|---|---|
| I. | Plate (non-sticky) | FTIR-ATR | PVC + TCP |
| Py-GC/MS | PVC + TCP + TPP + CDP + DCPP + DEP + DIBP + DBP + DHP + DEHP + benzenepropanoyl bromide | ||
| I.i | pH | 4.9 | |
| I.ii | pH | 4.9 | |
| I.iii | Plate’s exudates | FTIR-ATR | TCP |
| I.iv | Plate (sticky area) | pH | 4.6 |
| II. | Bag (non-sticky) | FTIR-ATR | PVC + DEHP |
| Py-GC/MS | PVC + TPP + CDP + DEP + DHP + DEHP + DnOP + phthalic acid decyl hexyl ester | ||
| II.i | pH | 5.8 | |
| II.ii | pH | 5.1 | |
| III. | Bag’s exudates | FTIR-ATR | DEHP + fats |
| III.i | Bag (sticky area—directly on a droplet) | pH | 3.9 |
| III.ii | pH | 4.5 | |
| III.iii | pH | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gassmann, P.; Bohlmann, C.; Pintus, V. Towards the Understanding of the Aging Behavior of p-PVC in Close Contact with Minced Meat in the Artwork POEMETRIE by Dieter Roth. Polymers 2023, 15, 4558. https://doi.org/10.3390/polym15234558
Gassmann P, Bohlmann C, Pintus V. Towards the Understanding of the Aging Behavior of p-PVC in Close Contact with Minced Meat in the Artwork POEMETRIE by Dieter Roth. Polymers. 2023; 15(23):4558. https://doi.org/10.3390/polym15234558
Chicago/Turabian StyleGassmann, Paula, Carolin Bohlmann, and Valentina Pintus. 2023. "Towards the Understanding of the Aging Behavior of p-PVC in Close Contact with Minced Meat in the Artwork POEMETRIE by Dieter Roth" Polymers 15, no. 23: 4558. https://doi.org/10.3390/polym15234558
APA StyleGassmann, P., Bohlmann, C., & Pintus, V. (2023). Towards the Understanding of the Aging Behavior of p-PVC in Close Contact with Minced Meat in the Artwork POEMETRIE by Dieter Roth. Polymers, 15(23), 4558. https://doi.org/10.3390/polym15234558






