Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges
Abstract
:1. Introduction
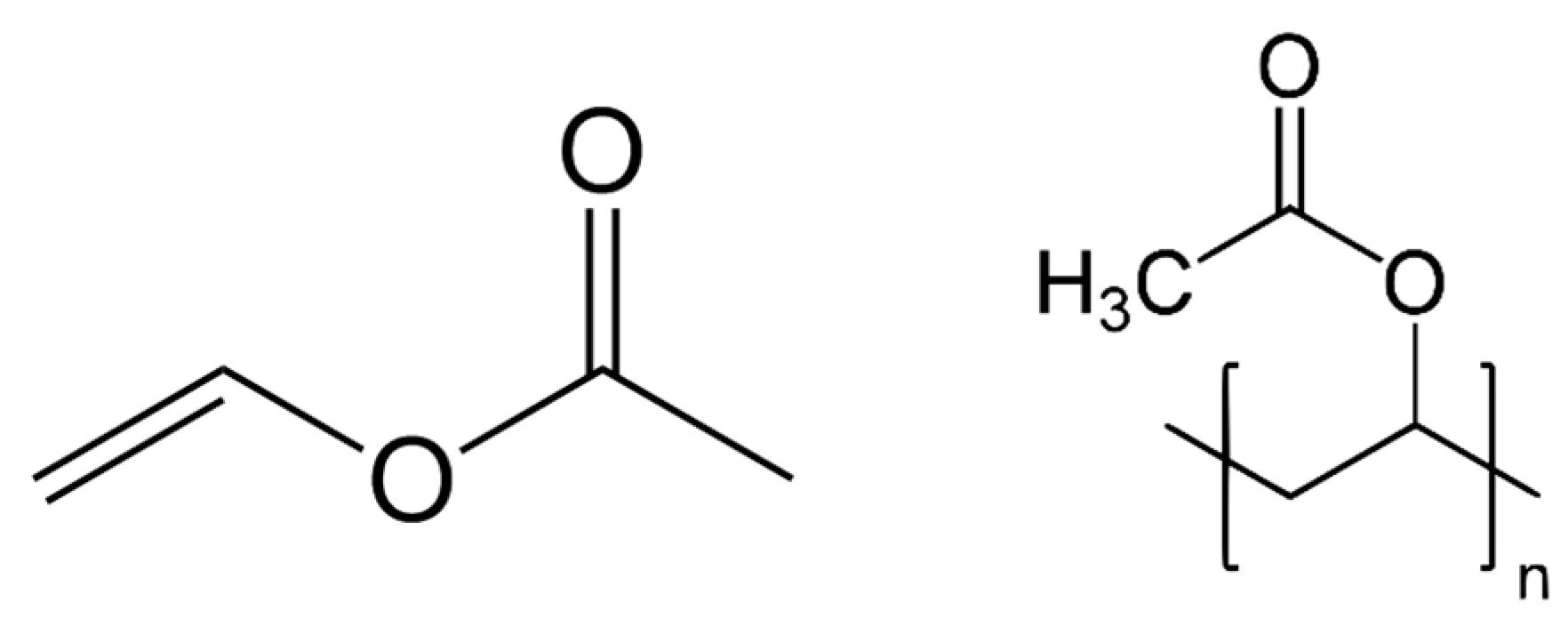
2. History and Formulation
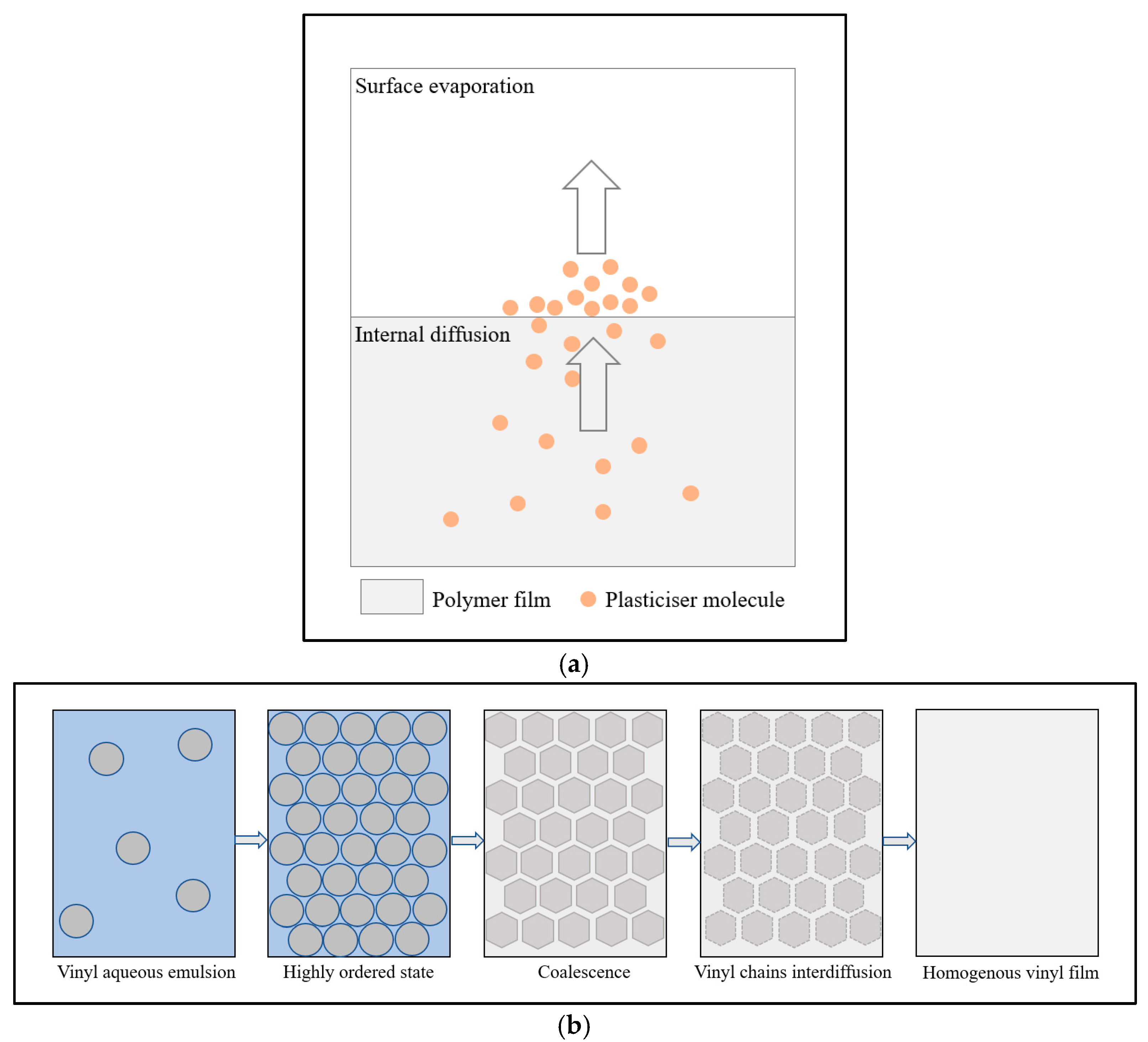
3. Physical Properties
4. Additives
| Additive | Role in the Emulsion Paints | Chemical Compounds Added to the Emulsion Paints [5,9,51,86,87,88,89,90,91] |
|---|---|---|
| Antifoam agent | Prevents the development of air bubbles during paint handling | Mineral and silicone oils |
| Octanol | ||
| Polydimethylsiloxane | ||
| Biocide | Prevents biological contamination of the paints during storage | Tin oxide |
| Zinc oxide | ||
| Mercury-based compounds | ||
| Acrylamide | ||
| 2-n-octyl-4-isothiazolin-3-one | ||
| Coalescing agent | Improves the coalescing process between polymer molecules during the drying phase | Ester alcohols |
| Benzoate esters | ||
| Glycols | ||
| Glycol ethers | ||
| N-methyl-2-pyrrolidone | ||
| Fillers | Reduces the cost of paint production and improves the handling of the paint | Calcium carbonate |
| Hydrated magnesium silicate | ||
| Freeze–thaw agent | Prevents the freezing of the paints when exposed to cold temperatures | Ethylene glycol |
| Propylene glycol | ||
| pH buffer | Modifies paint pH to make it optimal for all the paint components (optimal between pH 8 and 10) | Ammonia |
| Pigment dispersant | Improves the dispersion of the solid pigment particles | Oligophosphates (calcium and potassium salts) |
| Polyacrylic acids (sodium and ammonium salts) | ||
| Plasticisers | Improves the flexibility of the homopolymer | External |
| Dibutyl phthalate | ||
| Diethyl phthalate | ||
| Isobutyl phthalate | ||
| Bis (2-Ethylhexyl) phthalate | ||
| Dipropylene glycol dibenzoate | ||
| Diethylene glycol dibenzoate | ||
| Triphenyl phosphate | ||
| Internal | ||
| Vinyl versatates | ||
| N-butyl acrylate | ||
| 2-ethyl hexyl acrylate | ||
| Protective colloids | Improves the polymer solubility and sterically stabilises emulsion paints | Hydroxyethyl cellulose |
| Methyl cellulose | ||
| Poly(vinyl alcohol) | ||
| Surfactants | Disperses polymer and pigment molecules in water and electrostatically stabilises emulsion paint | Ethoxylated alkyl alcohols and phenols |
| Alkyl sulphonates and sulphates | ||
| Ethoxylated sulphonates and sulphates | ||
| Phosphates | ||
| Thickener | Increases the paint viscosity (thicker paint) and improves the paint’s workability | Hydroxyethyl cellulose |
| Methylcellulose | ||
| Hydrophobically modified carboxymethylcellulose | ||
| Hydrophobically modified ethoxylated urethane | ||
| Polysaccharides (xanthan and guar gums) | ||
| Wetting agent | Reduces the surface tension around the pigment particles and increases their wettability | Alkyl phenol ethoxylates |
| Acetylenic diols | ||
| Alkyl aryl sulfonates | ||
| Sulfosuccinates |
5. Ageing Behaviour and Material Degradation
5.1. Hydrolysis
5.2. Photochemical and Oxidative Degradation
5.3. Thermal Degradation
6. Analytical Methods for the Characterisation of Poly(Vinyl Acetate) Materials
7. Conservation Issues and Cleaning Effects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| DBP | dibutyl phthalate |
| DEP | diethyl phthalate |
| DEHP | bis(2-ethylhexyl) phthalate |
| DIBP | diisobutyl phthalate |
| DLS | dynamic light scattering |
| FTIR | Fourier transform infrared spectroscopy |
| LIF | laser-induced fluorescence |
| Mw | molecular weight |
| NMR | nuclear magnetic resonance |
| PEO | poly(ethene oxide) |
| PVAc | poly(vinyl acetate) |
| PVOH | poly(vinyl alcohol) |
| PyGCMS | pyrolysis–gas chromatography–mass spectrometry |
| SEC | size exclusion chromatography |
| SEM | scanning electron microscopy |
| SEM-EDX | scanning electron microscopy energy dispersive X-ray spectroscopy |
| Tg | glass transition temperature |
| TPP | triphenyl phosphate |
| UV | ultraviolet |
| UV–Vis | Ultraviolet–visible spectroscopy |
| VeoVa | vinyl versatate esters |
References
- Crook, J.; Learner, T. The Impact of Modern Paints, 1st ed.; Tate Gallery Publishing: London, UK, 2000. [Google Scholar]
- Learner, T. Modern paints: Uncovering the choices. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 3–16. [Google Scholar]
- Learner, T. A review of synthetic binding media in twentieth-century paints. Conservator 2000, 24, 96–103. [Google Scholar] [CrossRef]
- Izzo, F.C.; van den Berg, K.J.; van Keulen, H.; Ferriani, B.; Zendri, E. Modern Oil Paints—Formulations, Organic Additives and Degradation: Some Case Studies. In Issues in Contemporary Oil Paint; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., de Tagle, A., Heydenreich, G., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 75–104. [Google Scholar]
- Learner, T. Analysis of Modern Paints, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Burnstock, A.; van den Berg, K.J. Twentieth Century Oil Paint. The Interface Between Science and Conservation and the Challenges for Modern Oil Paint Research. In Issues in Contemporary Oil Paint; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., de Tagle, A., Heydenreich, G., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–19. [Google Scholar]
- Hillary, S.; Campbell, K.; Carlisle, M.; Khanjian, H.; Learner, T.; Schilling, M. The early use of synthetic emulsion paints by New Zealand artists. AICCM Bull. 2013, 34, 44–56. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Ávila, M.J.; Melo, M.J.; Ramos, A.M. Early aqueous dispersion paints: Portuguese artists’ use of polyvinyl acetate, 1960s–1990s. Stud. Conserv. 2013, 58, 211–225. [Google Scholar] [CrossRef]
- Pereira, A.I.M.L. The Perfect Paint in Modern Art Conservation: A Comparative Study of 21st Century Vinyl Emulsions. Ph.D. Thesis, NOVA School of Science and Technology, Lisbon, Portugal, 2015. [Google Scholar]
- Mancini, D.; Percot, A.; Bellot-Gurlet, L.; Colomban, P.; Carnazza, P. On-site contactless surface analysis of modern paintings from Galleria Nazionale (Rome) by reflectance FTIR and Raman spectroscopies. Talanta 2021, 227, 122159. [Google Scholar] [CrossRef] [PubMed]
- Spizzichino, V.; Angelini, F.; Caneve, L.; Colao, F.; Corrias, R.; Ruggiero, L. In situ study of modern synthetic materials and pigments in contemporary paintings by laser-induced fluorescence scanning. Stud. Conserv. 2015, 60, S178–S184. [Google Scholar] [CrossRef]
- Schmid, A. Cleaning of Matte White Polyvinyl Acetate Paint with Nanorestore Gels®. In Proceedings of the AIC/SPNHC Joint Virtual Annual Meeting, Virtual, 10 May–24 June 2021. [Google Scholar]
- Ferreira, J.L.A. Liaisons Dangereuses, Conservation of Modern and Contemporary art: A Study of the Synthetic Binding Media in Portugal. Ph.D. Thesis, NOVA School of Science and Technology, Lisbon, Portugal, 2011. [Google Scholar]
- Croll, S. Overview of Developments in the Paint Industry since 1930. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 17–29. [Google Scholar]
- Dredge, P.; Carter, A.; Osmond, G. Sidney Nolan: The Artist’s Materials; The Getty Conservation Institute: Los Angeles, CA, USA, 2020. [Google Scholar]
- Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Poly(vinyl acetate) paints in works of art: A photochemical approach: Part I. Polym. Degrad. Stab. 2010, 95, 453–461. [Google Scholar] [CrossRef]
- Carter, A.; Osmond, G.; Ormsby, B. Ian Fairweather and water-based emulsion house paints in Australia 1950–64. AICCM Bull. 2014, 34, 34–43. [Google Scholar] [CrossRef]
- Viana, C.D.R. Are All Vinyl Paints the Same? Masters Thesis, NOVA School of Science and Technology, Lisbon, Portugal, 2022. [Google Scholar]
- ‘Peter Grimes’s Apprentice‘, Sir Sidney Nolan. 1977. Available online: https://www.tate.org.uk/art/artworks/nolan-peter-grimess-apprentice-t03560 (accessed on 26 October 2023).
- Stols-Witlox, M.; Ormsby, B.; Gottsegen, M. Grounds, 1400–1900. In The Conservation of Easel Paintings, 1st ed.; Routledge: New York, NY, USA, 2012; pp. 161–185. [Google Scholar]
- Riley, B.; Moorhouse, P. Bridget Riley; Tate Publishing: London, UK, 2003; Volume 1st. [Google Scholar]
- Doménech-Carboó, M.T.; Silva, M.F.; Aura-Castro, E.; Doménech-Carboó, A.; Fuster-López, L.; Gimeno-Adelantado, J.V.; Kröner, S.U.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; et al. Multitechnique Approach to Evaluate Cleaning Treatments for Acrylic and Polyvinyl Acetate Paints. In Proceedings of the Cleaning 2010 Congress. New Insights into the Cleaning of Paintings, 1st ed.; Smithsonian Institution: Washington, DC, USA, 2010; pp. 125–134. [Google Scholar]
- Gettens, R.J.; Stout, G.L. Painting Materials: A Short Encyclopaedia; Dover Publications: New York, NY, USA, 1966. [Google Scholar]
- Klatte, F. Verfahren zur Herstellung Technisch Wertvoller Produkte aus Organischen Vinylestern. 1912. [Google Scholar]
- Blaikie, K.G.; Crozier, R.N. Polymerization of Vinyl Acetate. Ind. Eng. Chem. 1936, 28, 1155–1159. [Google Scholar] [CrossRef]
- Cuthbertson, A.C.; Gee, G.; Rideal, E.K. On the polymerization of vinyl acetate. Proc. R. Soc. Lond. A 1939, 170, 300–322. [Google Scholar] [CrossRef]
- Berber, H. Emulsion Polymerization: Effects of Polymerization Variables on the Properties of Vinyl Acetate Based Emulsion Polymers. In Polymer Science; Ylmaz, F., Ed.; InTech: London, UK, 2013; pp. 35–72. [Google Scholar]
- Gettens, R.J. Polymerized Vinyl Acetate and Related Compounds in the Restoration of Objects of Art. Tech. Stud. Field Fine Arts 1935, 4, 15–27. [Google Scholar]
- The History of Flashe. Available online: https://www.lefrancbourgeois.com/row/the-history-of-flashe/ (accessed on 15 September 2023).
- Standeven, H.A.L. Emulsion paints based on synthetic resins. In House Paints, 1900–1960—History and Use; Getty Conservation Institute: Los Angeles, CA, USA, 2011; pp. 85–114. [Google Scholar]
- Carter, A.; Osmond, G.; Ormsby, B. Characterisation of three early Australian emulsion house paints using FTIR and py-GC/MS. In ICOM-CC 17th Triennial Conference: Modern Materials and Contemporary Art; ICOM Committee for Conservation: Melbourne, VC, USA, 2014. [Google Scholar]
- Ferreira, J.L.; Melo, M.J.; Ramos, A.M.; Avila, M.J. “Eternity is in love with the productions of time”: Joaquim Rodrigo’s classical palette in a vinyl synthetic medium. In Modern Paints Uncovered: Proceedings from the Modern apints Uncovered Symposium, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 43–52. [Google Scholar]
- Golden Artist Colors, Inc. PVA Conservation Paints. Available online: https://goldenpaints.com/products/custom-products/pva-conservation-paints (accessed on 25 September 2023).
- Alderson, S.; Baade, B.; Deghetaldi, K. PVA Retouching Colors: A Brief History and Introduction to Golden’s Newly Formulated PVA Conservation Colors. In Postprints RECH 4; Academy of Arts, University of Split: Split, Croatia, 2017; pp. 80–87. [Google Scholar]
- Slinckx, M.; Scholten, H.P.H. Veova-9/(meth)acrylates, a New Class of Emulsion Copolymers. Surf. Coat. Int. 1994, 77, 107. [Google Scholar]
- Learner, T. The Characterisation of Acrylic Painting Materials and Implications for Their Use, Conservation and Stability. Ph.D. Thesis, University of London, London, UK, 1997. [Google Scholar]
- Horie, C.V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings, 2nd ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Chelazzi, D.; Chevalier, A.; Pizzorusso, G.; Giorgi, R.; Menu, M.; Baglioni, P. Characterization and degradation of poly(vinyl acetate)-based adhesives for canvas paintings. Polym. Degrad. Stab. 2014, 107, 314–320. [Google Scholar] [CrossRef]
- Down, J.L.; MacDonald, M.A.; Tetreault, J.; Williams, R.S. Adhesive Testing at the Canadian Conservation Institute: An Evaluation of Selected Poly(Vinyl Acetate) and Acrylic Adhesives. Stud. Conserv. 1996, 41, 19–44. [Google Scholar]
- Firmery, G. Les dispersions de PVAC pour le collage des panneaux peints fragilisés: Réversibilité du collage de joints endommagés. CeROArt 2014, EGG 4. [Google Scholar] [CrossRef]
- Cove, S. Retouching with a PVA Resin Medium. In Mixing and Matching—Aproaches to Retouching Paintings, 1st ed.; Ellison, R., Smithen, P., Turnbull, R., Eds.; Archetype Publications: London, UK, 2010; pp. 74–86. [Google Scholar]
- Kaboorani, A.; Riedl, B. Improving performance of polyvinyl acetate (PVA) as a binder for wood by combination with melamine based adhesives. Int. J. Adhes. Adhes. 2011, 31, 605–611. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B. Mechanical performance of polyvinyl acetate (PVA)-based biocomposites. In Biocomposites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 347–364. [Google Scholar]
- Zgueb, R.; Brichni, A.; Yacoubi, N. Improvement of the thermal properties of Sorel cements by polyvinyl acetate: Consequences on physical and mechanical properties. Energy Build. 2018, 169, 1–8. [Google Scholar] [CrossRef]
- Voskanyan, P.S. Use of Polyvinyl Acetate Plastics in Medicine. Int. Polym. Sci. Technol. 2008, 35, 21–25. [Google Scholar] [CrossRef]
- Angelova, L.V.; Terech, P.; Natali, I.; Dei, L.; Carretti, E.; Weiss, R.G. Cosolvent Gel-like Materials from Partially Hydrolyzed Poly(vinyl acetate)s and Borax. Langmuir 2011, 27, 11671–11682. [Google Scholar] [CrossRef]
- Natali, I.; Carretti, E.; Angelova, L.; Baglioni, P.; Weiss, R.; Dei, L. Structural and Mechanical Properties of “Peelable” Organoaqueous Dispersions with Partially Hydrolyzed Poly(vinyl acetate)-Borate Networks: Applications to Cleaning Painted Surfaces. Langmuir ACS J. Surf. Colloids 2011, 27, 13226–13235. [Google Scholar] [CrossRef]
- Angelova, L.V.; Berrie, B.H.; de Ghetaldi, K.; Kerr, A.; Weiss, R.G. Partially hydrolyzed poly(vinyl acetate)-borax-based gel-like materials for conservation of art: Characterization and applications. Stud. Conserv. 2014, 60, 227. [Google Scholar] [CrossRef]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of polyvinyl alcohol-borax/agarose (PVA-B/AG) double network hydrogel utilized for the cleaning of works of art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]
- Ricciotti, L.; Occhicone, A.; Manzi, S.; Saccani, A.; Ferone, C.; Tarallo, O.; Roviello, G. Sustainable Materials Based on Geopolymer–Polyvinyl Acetate Composites for Art and Design Applications. Polymers 2022, 14, 5461. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F. Analytical Study of Accelerated Light Ageing and Cleaning Effects on Acrylic and PVAc Dispersion Paints Used in Modern and Contemporary Art. Ph.D. Thesis, Universidad Politecnica de Valencia, Valencia, Spain, 2011. [Google Scholar]
- Singh, R.P.; Heldman, D.R. Chapter 15—Packaging Concepts. In Introduction to Food Engineering, 5th ed.; Singh, R.P., Heldman, D.R., Eds.; Food Science and Technology; Academic Press: Cambridge, MA, USA, 2014; pp. 767–791. [Google Scholar]
- De Sá, M.H.; Eaton, P.; Ferreira, J.L.; Melo, M.J.; Ramos, A.M. Ageing of vinyl emulsion paints-an atomic force microscopy study. Surf. Interface Anal. 2011, 43, 1160–1164. [Google Scholar] [CrossRef]
- Toja, F.; Saviello, D.; Nevin, A.; Comelli, D.; Lazzari, M.; Levi, M.; Toniolo, L. The degradation of poly(vinyl acetate) as a material for design objects: A multi-analytical study of the effect of dibutyl phthalate plasticizer. Part 1. Polym. Degrad. Stab. 2012, 97, 2441–2448. [Google Scholar] [CrossRef]
- Shashoua, Y. Conservation of Plastics, 1st ed.; Routledge: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- King, R.; Grau-Bové, J.; Curran, K. Plasticiser loss in heritage collections: Its prevalence, cause, effect, and methods for analysis. Herit. Sci. 2020, 8, 123. [Google Scholar] [CrossRef]
- Chiantore, O.; Scalarone, D. The Macro- and Microassessment of Physical and Aging Properties in Modern Paints. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 96–104. [Google Scholar]
- Silva, M.F.; Doménech-Carbó, M.T.; Osete-Cortina, L. Characterization of additives of PVAc and acrylic waterborne dispersions and paints by analytical pyrolysis–GC–MS and pyrolysis–silylation–GC–MS. J. Anal. Appl. Pyrolysis 2015, 113, 606–620. [Google Scholar] [CrossRef]
- Hayes, J.; Golden, M.; Smith, G.D. From Formulation to Finished Product: Causes and Potential Cures for Conservation Concerns in Acrylic Emulsion Paints. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; Getty Conservation Institute: Los Angeles, CA, USA, 2007; pp. 58–65. [Google Scholar]
- Husbands, M.J.; Standen, C.J.; Hayward, G. (Eds.) A Manual for Resins for Surface Coatings. 3; SITA-Technology: London, UK, 1987. [Google Scholar]
- Silva, M.F.; Doménech-Carbó, M.T.; Fuster-López, L.; Mecklenburg, M.F.; Martin-Rey, S. Identification of additives in poly(vinylacetate) artist’s paints using PY-GC-MS. Anal. Bioanal. Chem. 2010, 397, 357–367. [Google Scholar] [CrossRef]
- Zumbühl, S.; Attanasio, F.; Scherrer, N.C.; Muller, W.; Fenners, N.; Caseri, W. Solvent action on dispersion paint systems and the influence on the morphology–changes and destruction of the latex microstructure. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; Getty Publications: Los Angeles, CA, USA, 2006; pp. 257–268. [Google Scholar]
- Brown, G.L. Formation of films from polymer dispersions. J. Polym. Sci. 1956, 22, 423–434. [Google Scholar] [CrossRef]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef]
- GAC Inc. Technical Notes on Drying. Just Paint. Published 1 March 1996. Available online: https://justpaint.org/technical-notes-on-drying/ (accessed on 1 August 2023).
- Townsend, M. Investigating the Drying Process of Acrylic Color and Gel Medium. Just Paint. Published 1 September 2012. Available online: https://justpaint.org/investigating-the-drying-process-of-acrylic-color-and-gel-medium/ (accessed on 1 August 2023).
- Tumosa, C.S.; Mecklenburg, M.F. Weight Changes in Acrylic Emulsion Paints and the Implications for Accelerated Ageing. WAAC Newsl. 2003, 3, 12–14. [Google Scholar]
- Etemad, S.G.; Etesami, N.; Bagheri, R.; Thibault, J. Drying of latex films of poly(vinylacetate). Dry. Technol. 2002, 20, 1843–1854. [Google Scholar] [CrossRef]
- Keddie, J.L.; Routh, A.F. Drying of latex films. In Fundamentals of Latex Film Formation: Processes and Properties; Springer Laboratory Manuals in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Kiil, S. Drying of latex films and coatings: Reconsidering the fundamental mechanisms. Prog. Org. Coat. 2006, 57, 236–250. [Google Scholar] [CrossRef]
- Schmidt, M.; Krieger, S.; Johannsmann, D. Film formation of latex dispersions observed with evanescent dynamic light scattering. In Optical Methods and Physics of Colloidal Dispersions; Palberg, T., Ballauff, M., Eds.; Steinkopff: Darmstadt, Germany, 1997; pp. 191–193. [Google Scholar] [CrossRef]
- Makan, A.C.; Spallek, M.J.; du Toit, M.; Klein, T.; Pasch, H. Advanced analysis of polymer emulsions: Particle size and particle size distribution by field-flow fractionation and dynamic light scattering. J. Chromatogr. A 2016, 1442, 94–106. [Google Scholar] [CrossRef]
- Kato, H.; Nakamura, A.; Takahashi, K.; Kinugasa, S. Accurate Size and Size-Distribution Determination of Polystyrene Latex Nanoparticles in Aqueous Medium Using Dynamic Light Scattering and Asymmetrical Flow Field Flow Fractionation with Multi-Angle Light Scattering. Nanomaterials 2012, 2, 15–30. [Google Scholar] [CrossRef]
- Carro, S.; Herrera-Ordonez, J.; Castillo-Tejas, J. On the evolution of the rate of polymerization, number and size distribution of particles in styrene emulsion polymerization above CMC. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3152–3160. [Google Scholar] [CrossRef]
- Holthoff, H.; Borkovec, M.; Schurtenberger, P. Determination of light-scattering form factors of latex particle dimers with simultaneous static and dynamic light scattering in an aggregating suspension. Phys. Rev. E 1997, 56, 6945–6953. [Google Scholar] [CrossRef]
- Feng, H.; Verstappen, N.A.L.; Kuehne, A.J.C.; Sprakel, J. Well-defined temperature-sensitive surfactants for controlled emulsion coalescence. Polym. Chem. 2013, 4, 1842–1847. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- de Bruyn, H. The Emulsion Polymerization of Vinyl Acetate. Ph.D. Thesis, University of Sydney, Sydney, Australia, 1999. [Google Scholar]
- Lovell, P.A.; Schork, F.J. Fundamentals of Emulsion Polymerization. Biomacromolecules 2020, 21, 4396–4441. [Google Scholar] [CrossRef]
- Mills, J.S.; White, R. The Organic Chemistry of Museum Objects, 2nd ed.; Butterworth-Heinemann series in conservation and museology; Routledge: New York, NY, USA, 2015. [Google Scholar]
- Gentekos, D.T.; Sifri, R.J.; Fors, B.P. Controlling polymer properties through the shape of the molecular-weight distribution. Nat. Rev. Mater. 2019, 4, 761–774. [Google Scholar] [CrossRef]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, M.M.D.; Zendri, E.; Rosi, F.; Miliani, C. A Preliminary FTIR-based Exploration of the Surfactant Phase Separation Process in Contemporary Mural Paintings. e-Preserv. Sci. 2013, 10, 10–18. [Google Scholar]
- Doménech-Carbó, M.T.; Bitossi, G.; Osete-Cortina, L.; De La Cruz-Cañizares, J.; Yusá-Marco, D.J. Characterization of polyvinyl resins used as binding media in paintings by pyrolysis–silylation–gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Doménech-Carbó, M.T.; Fuster-Lopéz, L.; Martín-Rey, S.; Mecklenburg, M.F. Determination of the plasticizer content in poly(vinyl acetate) paint medium by pyrolysis–silylation–gas chromatography–mass spectrometry. J. Anal. Appl. Pyrolysis 2009, 85, 487–491. [Google Scholar] [CrossRef]
- Hellgren, A.-C.; Weissenborn, P.; Holmberg, K. Surfactants in water-borne paints. Prog. Org. Coat. 1999, 35, 79–87. [Google Scholar] [CrossRef]
- Martens, C.R. Waterborne Coatings: Emulsion and Water-Soluble Paints; Van Nostrand Reinhold: New York, NY, USA, 1981. [Google Scholar]
- Flick, E.W. Handbook of Paint Raw Materials, 2nd ed.; Noyes Publications: Park Ridge, NJ, USA, 1989. [Google Scholar]
- Oldring, P.; Hayward, G. Resins for Surface Coatings; SITA-Technology: London, UK, 1987; Volume II. [Google Scholar]
- Jablonski, E.; Learner, T.; Hayes, J.; Golden, M. Conservation Concerns for Acrylic Emulsion Paints: A Literature Review. Tate Pap. 2003, 2, 1–14. [Google Scholar]
- França De Sá, S.; Viana, C.; Ferreira, J.L. Tracing Poly(Vinyl Acetate) Emulsions by Infrared and Raman Spectroscopies: Identification of Spectral Markers. Polymers 2021, 13, 3609. [Google Scholar] [CrossRef]
- Cappitelli, F.; Sorlini, C. Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage. Appl. Environ. Microbiol. 2008, 74, 564–569. [Google Scholar] [CrossRef]
- Cappitelli, F.; Zanardini, E.; Sorlini, C. The Biodeterioration of Synthetic Resins Used in Conservation. Macromol. Biosci. 2004, 4, 399–406. [Google Scholar] [CrossRef]
- Amann, M.; Minge, O. Biodegradability of Poly(vinyl acetate) and Related Polymers. Adv. Polym. Sci. 2012, 245, 137–172. [Google Scholar] [CrossRef]
- Aruldass, S.; Mathivanan, V.; Mohamed, A.R.; Tye, C.T. Factors affecting hydrolysis of polyvinyl acetate to polyvinyl alcohol. J. Environ. Chem. Eng. 2019, 7, 103238. [Google Scholar] [CrossRef]
- Wheeler, O.L.; Ernst, S.L.; Crozier, R.N. Molecular weight degradation of polyvinyl acetate on hydrolysis. J. Polym. Sci. 1952, 8, 409–423. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Bitossi, G.; de la Cruz-Cañizares, J.; Bolívar-Galiano, F.; López-Miras, M.d.M.; Romero-Noguera, J.; Martín-Sánchez, I. Microbial deterioration of Mowilith DMC 2, Mowilith DM5 and Conrayt poly(vinyl acetate) emulsions used as binding media of paintings by pyrolysis-silylation-gas chromatography–mass spectrometry. J. Anal. Appl. Pyrolysis 2009, 85, 480–486. [Google Scholar] [CrossRef]
- Bradford, E.B.; Vanderhoff, J.W. Additional studies of morphological changes in latex films. J. Macromol. Sci. Part B 1972, 6, 671–693. [Google Scholar] [CrossRef]
- Scott, N.D.; Bristol, J.E. Hydrolysis of Polymerized Vinyl Esters. Published online 23 December 1941. Available online: https://patents.google.com/patent/US2266996A/en (accessed on 20 October 2023).
- Marin, E.; Rojas, J.; Ciro, Y. A review of polyvinyl alcohol derivatives: Promising materials for pharmaceutical and biomedical applications. Afr. J. Pharm. Pharmacol. 2014, 8, 674–684. [Google Scholar]
- Clarke, J.T.; Blout, E.R. Nature of the carbonyl groups in polyvinyl alcohol. J. Polym. Sci. 1946, 1, 419–428. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Melo, M.J.; Ramos, A.M. A mural painting by Estrela Faria: A FTIR study of a vinyl synthetic medium. In Contribution of the Seventh Biennial Gathering of the Infrared and Raman User’s Group; Museum of Modern Art: New York, NY, USA, 2006; pp. 75–76. [Google Scholar]
- Melo, M.J.; Ferreira, J.L.; Ramos, A.M.; Avila, M. Vinyl paints in Portugese modern art (1960–1990): A FTIR study. In Contributions of the Sevents Biennial Gathering of the Infrared and Raman User’s Group; Museum of Modern Art: New York, NY, USA, 2006; pp. 57–60. [Google Scholar]
- Ormsby, B.; Learner, T. The effects of wet surface cleaning treatments on acrylic emulsion artists’ paints—A review of recent scientific research. Stud. Conserv. 2009, 54, 29–41. [Google Scholar] [CrossRef]
- Erlebacher, J.; Browne, E.; Tumosa, C.; Mecklenburg, M.F. The Effects of Temperature and Relative Humidity on the Rapidly Loaded Mechanical Properties of Artists’ Acrylic Paint. In Materials Issues in Art and Archaeology III; Materials Research Society: San Francisco, CA, USA, 1992; pp. 359–370. [Google Scholar]
- Erlebacher, J.D.; Mecklenburg, M.F.; Tumosa, C.S. The Mechanical Behavior of Artist’s Acrylic Paints with Changing Temperature and Relative Humidity. Polym. Prepr. 1992, 33, 646–647. [Google Scholar]
- Hagan, E.; Murray, A. Effects of Water Exposure on the Mechanical Properties of Early Artists’ Acrylic Paints. In MRS Proceedings; Materials Research Society: San Francisco, CA, USA, 2005; pp. 41–47. [Google Scholar]
- dePolo, G.; Walton, M.; Keune, K.; Shull, K.R. After the paint has dried: A review of testing techniques for studying the mechanical properties of artists’ paint. Herit. Sci. 2021, 9, 68. [Google Scholar] [CrossRef]
- Dillon, C.E.; Lagalante, A.F.; Wolbers, R.C. Acrylic emulsion paint films: The effect of solution pH, conductivity, and ionic strength on film swelling and surfactant removal. Stud. Conserv. 2014, 59, 52–62. [Google Scholar] [CrossRef]
- del Gaudio, I.; Hunter-Sellars, E.; Parkin, I.P.; Williams, D.; Da Ros, S.; Curran, K. Water sorption and diffusion in cellulose acetate: The effect of plasticisers. Carbohydr. Polym. 2021, 267, 118185. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Borsu, M.; Geuskens, G. Photolysis and radiolysis of polyvinyl acetate. Eur. Polym. J. 1970, 6, 959–963. [Google Scholar] [CrossRef]
- Geuskens, G.; Borsu, M.; David, C. Photolysis and radiolysis of polyvinylacetate—II. Eur. Polym. J. 1972, 8, 883–892. [Google Scholar] [CrossRef]
- Buchanan, K.J.; McGill, W.J. Photodegradation of poly(vinyl esters)—I. Formation and quantitative measurement of volatile products. Eur. Polym. J. 1980, 16, 309–312. [Google Scholar] [CrossRef]
- Buchanan, K.J.; McGill, W.J. Photodegradation of poly(vinyl esters)—II. Volatile product formation and changes in the absorption spectra and molecular mass distributions. Eur. Polym. J. 1980, 16, 313–318. [Google Scholar] [CrossRef]
- Buchanan, K.J.; McGill, W.J. Photodegradation of poly(vinyl esters)—III. Photolysis mechanism for both polymeric and low molecular mass esters. Eur. Polym. J. 1980, 16, 319–324. [Google Scholar] [CrossRef]
- Vaidergorin, E.Y.L.; Marcondes, M.E.R.; Toscano, V.G. Photodegradation of poly(vinyl acetate). Polym. Degrad. Stab. 1987, 18, 329–339. [Google Scholar] [CrossRef]
- Madras, G.; Chattopadhyay, S. Optimum temperature for oxidative degradation of poly(vinyl acetate) in solution. Chem. Eng. Sci. 2001, 56, 5085–5089. [Google Scholar] [CrossRef]
- Norrish, R.G.W.; Bamford, C.H. Photo-decomposition of aldehydes and ketones. Nature 1937, 140, 195–196. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. Photochemical degradation study of polyvinyl acetate paints used in artworks by Py–GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Melchiorre Di Crescenzo, M.; Zendri, E.; Sánchez-Pons, M.; Fuster-López, L.; Yusá-Marco, D.J. The use of waterborne paints in contemporary murals: Comparing the stability of vinyl, acrylic and styrene-acrylic formulations to outdoor weathering conditions. Polym. Degrad. Stab. 2014, 107, 285–293. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Anal. Bioanal. Chem. 2011, 399, 2921–2937. [Google Scholar] [CrossRef] [PubMed]
- Toja, F.; Saviello, D.; Nevin, A.; Comelli, D.; Lazzari, M.; Valentini, G.; Toniolo, L. The degradation of poly(vinyl acetate) as a material for design objects: A multi-analytical study of the Cocoon lamps. Part 2. Polym. Degrad. Stab. 2013, 98, 2215–2223. [Google Scholar] [CrossRef]
- Naude, K.M.; Styler, S.A.; Ormsby, B. Photochemical Degradation of Non-Ionic Paint Surfactants: Implications for Art Conservation. unpublished work.
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl acetate) measured by thermal analysis–Fourier transform infrared spectroscopy. Polymer 2002, 43, 2207–2211. [Google Scholar] [CrossRef]
- Grassie, N. The thermal degradation of polyvinyl acetate. Part 1. Products and reaction mechanism at low temperatures. Trans. Faraday Soc. 1952, 48, 379–387. [Google Scholar] [CrossRef]
- Grassie, N. The thermal degradation of polyvinyl acetate. Part 2. Determination of the rate constants of the primary processes involved in the elimination of acetic acid. Trans. Faraday Soc. 1953, 49, 835–842. [Google Scholar] [CrossRef]
- Servotte, A.; Desreux, V. Thermal degradation of some vinyl polymers. I. Poly(vinyl acetate). J. Polym. Sci. Part C Polym. Symp. 1968, 22, 367–376. [Google Scholar] [CrossRef]
- Bataille, P.; Van, B.T. Mechanism of thermal degradation of poly(vinyl acetate). J. Therm. Anal. 1975, 8, 141–153. [Google Scholar] [CrossRef]
- Ballistreri, A.; Foti, S.; Montaudo, G.; Scamporrino, E. Evolution of aromatic compounds in Tthe thermal decomposition of vinyl polymers. J. Polym. Sci. Part A-1 Polym. Chem. 1980, 18, 1147–1153. [Google Scholar] [CrossRef]
- Troitskii, B.B.; Razuvaev, G.A.; Khokhlova, L.V.; Bortnikov, G.N. On the mechanism of the thermal degradation of polyvinyl acetate. J. Polym. Sci. Polym. Symp. 1973, 42, 1363–1375. [Google Scholar] [CrossRef]
- Rimez, B.; Rahier, H.; Van Assche, G.; Artoos, T.; Biesemans, M.; Van Mele, B. The thermal degradation of poly(vinyl acetate) and poly(ethylene-co-vinyl acetate), Part I: Experimental study of the degradation mechanism. Polym. Degrad. Stab. 2008, 93, 800–810. [Google Scholar] [CrossRef]
- Rimez, B.; Rahier, H.; Van Assche, G.; Artoos, T.; Van Mele, B. The thermal degradation of poly(vinyl acetate) and poly(ethylene-co-vinyl acetate), Part II: Modelling the degradation kinetics. Polym. Degrad. Stab. 2008, 93, 1222–1230. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Pinton, F.; Zendri, E. A preliminary study of the composition of commercial oil, acrylic and vinyl paints and their behaviour after accelerated ageing conditions. Conserv. Sci. Cult. Herit. 2014, 14, 353–369. [Google Scholar] [CrossRef]
- Pintus, V.; Viana, C.; Angelin, E.M.; De Sá, S.F.; Wienland, K.; Sterflinger, K.; Ferreira, J.L. Applicability of single-shot and double-shot Py-GC/MS for the detection of components in vinyl acetate-based emulsions used in modern-contemporary art. J. Anal. Appl. Pyrolysis 2022, 168, 105782. [Google Scholar] [CrossRef]
- Schossler, P.; Fortes, I.; Júnior, J.C.D.d.F.; Carazza, F.; Souza, L.A.C. Acrylic and Vinyl Resins Identification by Pyrolysis-Gas Chromatography/Mass Spectrometry: A Study of Cases in Modern Art Conservation. Anal. Lett. 2013, 46, 1869–1884. [Google Scholar] [CrossRef]
- Scalarone, D.; Chiantore, O. Py-GC/MS of Natural and Synthetic Resins. In Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 327–361. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Alderson, S.; Down, J.L.; Maines, C.A.; Williams, R.S.; Young, G.S. Potential substitutes for discontinued poly(vinyl acetate) resins used in conservation. J. Am. Inst. Conserv. 2019, 58, 158–179. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T.; Foster, G.; Druzik, J.; Schilling, M. Wet cleaning acrylic emulsion paint films: An evaluation of physical, chemical, and optical changes. In Modern Paints Uncovered: Proceedings from the Modern Paints Uncovered Symposium, 1st ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2006; pp. 189–199. [Google Scholar]
- Colombini, M.P.; Modugno, F. (Eds.) Organic Mass Spectrometry in Art and Archaeology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ropret, P.; Centeno, S.A.; Bukovec, P. Raman identification of yellow synthetic organic pigments in modern and contemporary paintings: Reference spectra and case studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 486–497. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Identification of synthetic organic pigments: The role of a comprehensive digital Raman spectral library. J. Raman Spectrosc. 2012, 43, 1536–1544. [Google Scholar] [CrossRef]
- Jovanović, V.; Erić, S.; Colomban, P.; Kremenović, A. Identification of Lithol Red Synthetic Organic Pigment Reveals the Cause of Paint Layer Degradation on the Lazar Vozarević Painting “Untitled” with Copper Plates. Heritage 2019, 2, 2612–2624. [Google Scholar] [CrossRef]
- Scherrer, N.C.; Stefan, Z.; Francoise, D.; Annette, F.; Renate, K. Synthetic organic pigments of the 20th and 21st century relevant to artist’s paints: Raman spectra reference collection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Edwards, H.G.M.; Moens, L. A Decade of Raman Spectroscopy in Art and Archaeology. Chem. Rev. 2007, 107, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, C.; Nunberg, S.; Alcala, S.; Dittmer, J. Nuclear magnetic resonance analysis for treatment decisions: The case of a white sculptural environment by Louise Nevelson. Microchem. J. 2018, 137, 480–484. [Google Scholar] [CrossRef]
- Souza, C.; Tavares, M.I. Nuclear magnetic resonance study of commercial poly(vinyl acetate). J. Appl. Polym. Sci. 1998, 70, 2457–2461. [Google Scholar] [CrossRef]
- Learner, T. The analysis of synthetic paints by pyrolysis-gas chromatography-mass spectrometry (PyGCMS). Stud. Conserv. 2001, 46, 225–241. [Google Scholar]
- Peris-Vicente, J.; Baumer, U.; Stege, H.; Lutzenberger, K.; Gimeno Adelantado, J.V. Characterization of Commercial Synthetic Resins by Pyrolysis-Gas Chromatography/Mass Spectrometry: Application to Modern Art and Conservation. Anal. Chem. 2009, 81, 3180–3187. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T.; Schilling, M.; Druzik, J.; Khanjian, H.; Carson, D.; Foster, G.; Sloan, M. The Effects of Surface Cleaning on Acrylic Emulsion Paintings: A Preliminary Investigation. Tate Papers No 6. Available online: https://www.tate.org.uk/research/tate-papers/06/effects-of-surface-cleaning-on-acrylic-emulsion-painting-preliminary-investigation (accessed on 6 July 2023).
- Lee, J.; Bonaduce, I.; Modugno, F.; La Nasa, J.; Ormsby, B.; van den Berg, K.J. Scientific investigation into the water sensitivity of twentieth century oil paints. Microchem. J. 2018, 138, 282–295. [Google Scholar] [CrossRef]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Papaspyropoulos, K.G.; Schreiner, M.; Kyriacou, G. A novel methodological approach for the assessment of surface cleaning of acrylic emulsion paints. Microchem. J. 2018, 141, 25–39. [Google Scholar] [CrossRef]
- Smithen, P. A history of the treatment of acrylic painting. In Modern Paints Uncovered, 1st ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2006; pp. 165–174. [Google Scholar]
- Roy, P. Problems of dirt accumulation and its removal from unvarnished paintings: A practical review. In Dirt and Pictures Separated, 1st ed.; Hackney, S., Townsend, J., Eastaugh, N., Eds.; Institute of Conservation and Tate Gallery: London, UK, 1990; pp. 3–6. [Google Scholar]
- Murray, A.; de Berenfeld, C.C.; Chang, S.Y.S.; Jablonski, E.; Klein, T.; Riggs, M.C.; Robertson, E.C.; Tse, W.M.A. The Condition and Cleaning of Acrylic Emulsion Paintings. MRS Online Proc. Libr. 2002, 712, 14. [Google Scholar] [CrossRef]
- Cardaba, I.; Poggi, G.; Baglioni, M.; Chelazzi, D.; Maguregui, I.; Giorgi, R. Assessment of aqueous cleaning of acrylic paints using innovative cryogels. Microchem. J. 2020, 152, 104311. [Google Scholar] [CrossRef]
- Angelova, L.V.; Ormsby, B.; Richardson, E. Diffusion of water from a range of conservation treatment gels into paint films studied by unilateral NMR: Part I: Acrylic emulsion paint. Microchem. J. 2016, 124, 311–320. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T. Artists’ acrylic emulsion paints: Materials, meaning and conservation treatment options. AICCM Bull. 2014, 34, 57–65. [Google Scholar] [CrossRef]
- Kampasakali, E.; Ormsby, B.; Cosentino, A.; Miliani, C.; Learner, T. A Preliminary Evaluation of the Surfaces of Acrylic Emulsion Paint Films and the Effects of Wet-Cleaning Treatment by Atomic Force Microscopy (AFM). Hastings Cent. Stud. 2011, 56, 216–230. [Google Scholar] [CrossRef]
- Chelazzi, D.; Fratini, E.; Giorgi, R.; Mastrangelo, R.; Rossi, M.; Baglioni, P. Gels for the Cleaning of Works of Art. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; pp. 291–314. [Google Scholar] [CrossRef]
- Angelova, L.V.; Ormsby, B.; Townsend, J.; Wolbers, R.; International Academic Projects, Tate Modern (Gallery) (Eds.) Gels in the Conservation of Art; Archetype Publications: London, UK, 2017. [Google Scholar]
- Baglioni, P.; Berti, D.; Bonini, M.; Carretti, E.; Dei, L.; Fratini, E.; Giorgi, R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv. Colloid Interface Sci. 2013, 205, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Stoveland, L.P.; Frøysaker, T.; Stols-Witlox, M.; Grøntoft, T.; Steindal, C.C.; Madden, O.; Ormsby, B. Evaluation of novel cleaning systems on mock-ups of unvarnished oil paint and chalk-glue ground within the Munch Aula Paintings Project. Herit. Sci. 2021, 9, 144. [Google Scholar] [CrossRef]
- Chung, J.Y.; Ormsby, B.; Lee, J.; Burnstock, A. An investigation of options for surface cleaning unvarnished water-sensitive oil paints based on recent developments for acrylic paints. In ICOM-CC 18th Triennial Conference Preprints; Bridgland, J., Ed.; ICOM Committee for Conservation: Copengahen, Denmark, 2017; pp. 1–13. [Google Scholar]
- Ormsby, B.; Kampasakali, E.; Learner, T. Surfactants and acrylic dispersion paints: Evaluating changes induced by wet surface cleaning treatments. In Cleaning 2010 Congress New Insights into the Cleaning of Paintings; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2013; pp. 159–164. [Google Scholar]
- Hagan, E.W.S.; Charalambides, M.N.; Young, C.T.; Learner, T.J.S.; Hackney, S. Tensile properties of latex paint films with TiO2 pigment. Mech. Time-Depend. Mater. 2009, 13, 149. [Google Scholar] [CrossRef]
- Meek, M.E.; Long, G. Di-n-Butyl Phthalate; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Sekizawa, J.; Dobson, S. Diethyl Phthalate; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Lundberg, P. Diethylhexyl Phthalate; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Diisobutyl Phthalate (DIBP). European Chemical Agency, 2014; pp. 1–43. Available online: https://echa.europa.eu/documents/10162/12934bbe-ad6f-4671-a931-1ef44f51cb10 (accessed on 22 September 2023).
- Diisobutyl Phthalate—The Chemical Company. Available online: https://thechemco.com/chemical/diisobutyl-phthalate/ (accessed on 22 September 2023).
- Programme International sur la Sécurité des Substances Chimiques (Ed.) Triphenyl Phosphate; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Polyethylene glycol [MAK Value Documentation, 1998]. In The MAK-Collection for Occupational Health and Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 248–270. [CrossRef]

| Material Aspect Investigated | Techniques Employed | Research Aims | References |
|---|---|---|---|
| Polymer, colourants, and additives identification | Fourier transform infrared spectroscopy (FTIR) | Chemical characterisation of the vinyl binder, colourants, and additives Monitor photooxidative and thermal degradation of vinyl polymer Monitor polymer or additive extraction during cleaning treatments | Ferreira et al. [8,16,32,102] Pereira et al. [9] Mancini et al. [10] Carter et al. [17,31] Viana [18] Doménech-Carbó et al. [22,122] Alderson et al. [34,139] Silva et al. [51] Toja et al. [54,123] Zümbuhl et al. [62] De Sá et al. [91] Wei et al. [119] Melchiorre Di Crescenzo et al. [120] Pintus et al. [121] Holland et al. [125] Izzo et al. [134] Ormsby et al. [140] |
| Pyrolysis–gas chromatography–mass spectrometry (PyGCMS) | Chemical characterisation of vinyl binder, colourants, and additives (plasticisers, surfactants) | Ferreira et al. [8] Pereira et al. [9] Carter et al. [17,31] Viana [18] Doménech-Carbó et al. [22] Silva et al. [51,58,61,85] Doménech-Carbó et al. [84,97,122] Wei et al. [119] Pintus et al. [121,135] Toja et al. [123] Izzo et al. [134] Schossler et al. [136] Ormsby et al. [140] Learner [149] Peris-Vicente et al. [150] | |
| Nuclear magnetic resonance (NMR) | Monitor structural changes in polymer backbone during thermal and photooxidative ageing | Toja et al. [54] | |
| Chemical characterisation of the poly(vinyl acetate) restoration layer | Kehlet et al. [147] | ||
| Structural characterisation of commercial poly(vinyl acetate) products | De Souza and Tavares [148] | ||
| Raman spectroscopy | Chemical characterisation of poly(vinyl acetate) paints (pigments, extenders) | Ferreira et al. [8] | |
| Chemical characterisation of poly(vinyl acetate) paintings (organic and inorganic pigments) | Mancini et al. [10] | ||
| Chemical characterisation of poly(vinyl acetate) paintings (organic and inorganic pigments) | Spizzichino et al. [11] | ||
| Chemical characterisation of poly(vinyl acetate) resins after light ageing | Viana [18] | ||
| Chemical characterisation of poly(vinyl acetate) homopolymer, and plasticised and non-plasticised resins (polymer and plasticisers) | De Sá et al. [91] | ||
| Ultraviolet–visible (UV–Vis) spectroscopy | The reflectance spectrum of the light-aged poly(vinyl acetate) paint films | Ferreira et al. [16] | |
| The reflectance spectrum of the light-aged poly(vinyl acetate) paint films | Doménech-Carbó et al. [122] | ||
| Fluorescence spectroscopy | Fluorescence emission of poly(vinyl acetate) films after thermal-oxidative and photooxidative ageing | Toja et al. [54] | |
| Laser-induced fluorescence (LIF) | Chemical characterisation of poly(vinyl acetate) paintings (vinyl binder and pigments) | Spizzichino et al. [11] | |
| Scanning electron microscopy energy dispersive X-ray spectroscopy (SEM-EDX) | Elemental analysis of the poly(vinyl acetate) paint films | Melchiorre Di Crescenzo et al. [120] | |
| Elemental analysis of the poly(vinyl acetate) paint films | Doménech-Carbó et al. [122] | ||
| Physical properties | Mechanical (tensile) testing | The effect of different cleaning strategies (immersion, swabbing, gels, and microemulsions) on the tensile properties of the paint films | Doménech-Carbó et al. [22] |
| Mechanical testing of the poly(vinyl acetate) paint films after light ageing (λ = 340 nm) | Alderson et al. [34] | ||
| Mechanical testing of the daylight- and UV light-aged poly(vinyl acetate) paint films, and the effect of cleaning | Silva [51] | ||
| Mechanical testing of the poly(vinyl acetate) paint films and its dependence on the paints’ additives | Silva et al. [61] | ||
| Mechanical testing of the poly(vinyl acetate) paint films and the effect of cleaning treatments | Zumbühl et al. [62] | ||
| Mechanical testing of the daylight- and UV light-aged poly(vinyl acetate) paint films | Doménech-Carbó et al. [122] | ||
| Thermal analysis (thermogravimetric analysis, differential scanning calorimetry, or dynamic mechanical analysis) | Monitor glass transition temperature during photooxidative (λ ≥ 290 nm) and thermal-oxidative ageing (60 ± 2 °C) of plasticised and unplasticized poly(vinyl acetate) films | Toja et al. [54] | |
| Monitor mass loss during thermal degradation of poly(vinyl acetate) films | Holland and Hay [125] | ||
| Dynamic light scattering (DLS) | Determination of particle size and its influence on the cross-linking of polymer chains | Ferreira et al. [13,16] | |
| Size exclusion chromatography (SEC) | Determination of Mw of poly(vinyl acetate) paints and its change during ageing | Ferreira et al. [13,16] | |
| Determination of Mw of poly(vinyl acetate) resins and its change during ageing | Viana et al. [18] | ||
| Gel permeation chromatography | Determination of Mw of poly(vinyl acetate) resins | Alderson et al. [34] | |
| Surface analysis | Atomic force microscopy (AFM) | Surface analysis of poly(vinyl acetate) resins and paints after light ageing and cleaning treatments | Pereira [9] |
| Surface analysis of poly(vinyl acetate) paints before and after cleaning treatments | Doménech-Carbó et al. [22] | ||
| Surface changes of pure poly(vinyl acetate), resin, and white paint after light ageing (λ ≥ 300 nm) | De Sá et al. [53] | ||
| Surface changes of the poly(vinyl acetate) paint films after daylight and UV light ageing | Doménech-Carbó et al. [122] | ||
| Scanning electron microscopy (SEM) | Surface morphology of the poly(vinyl acetate) paint films exposed to daylight and UV light ageing and after cleaning treatments | Doménech-Carbó et al. [22,122] | |
| Surface morphology of the poly(vinyl acetate) paint films during immersion and solvent cleaning | Silva [51] | ||
| Surface morphology of the poly(vinyl acetate) paint films during immersion and solvent cleaning | Zumbühl et al. [62] | ||
| Surface morphology of poly(vinyl acetate) paint films after light ageing (295 < λ < 370 nm) | Melchiorre Di Crescenzo et al. [120] |
| Additives | Solubility in Water at 25 °C (g/L) | Solubility in Other Solvents |
|---|---|---|
Dibutyl phthalate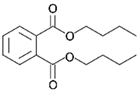 | 0.01 [168,169] | Soluble in alcohol, benzene, and ether [168] |
Diethyl phthalate | 1 [170] | Soluble in alcohol, acetone, benzene, ketones, ethers, esters, aliphatic solvents, and aromatic hydrocarbons [170] |
Bis(2-ethylhexyl) phthalate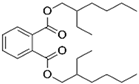 | 0.0003–0.0004 [171] | Miscible with most common organic solvents [171] |
Diisobutyl phthalate | 0.02 [169,172] | Soluble in ethanol, ether, and benzene [173] |
Triphenyl phosphate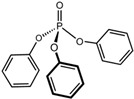 | 0.0019 [174] | Soluble in acetone, benzene, chloroform, and ether; moderately soluble in ethanol [174] |
Poly(ethylene oxide) | ~550 (depends on polymer Mw) [175] | Soluble in aliphatic ketones, alcohols, chloroform, glycol ethers, esters, and aromatic hydrocarbons; insoluble in ether and most aliphatic hydrocarbons [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, M.; Ormsby, B. Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges. Polymers 2023, 15, 4348. https://doi.org/10.3390/polym15224348
Novak M, Ormsby B. Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges. Polymers. 2023; 15(22):4348. https://doi.org/10.3390/polym15224348
Chicago/Turabian StyleNovak, Morana, and Bronwyn Ormsby. 2023. "Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges" Polymers 15, no. 22: 4348. https://doi.org/10.3390/polym15224348
APA StyleNovak, M., & Ormsby, B. (2023). Poly(Vinyl Acetate) Paints: A Literature Review of Material Properties, Ageing Characteristics, and Conservation Challenges. Polymers, 15(22), 4348. https://doi.org/10.3390/polym15224348




