Impact of Incorporating Free Calcium and Magnesium on the Heat Stability of a Dairy- and Soy-Protein-Containing Model Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Model Milk
2.3. Conductivity
2.4. Zeta Potential
2.5. Rheological Analysis
2.6. Particle Size Distribution Measurement
2.7. Dispersion Stability Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Interaction between Added Divalent Cations and Proteins before Heat-Treatment
3.2. Rheological Properties of the Heat-Treated Protein Solutions with Added Divalent Cations
3.3. Particle Size Analyses
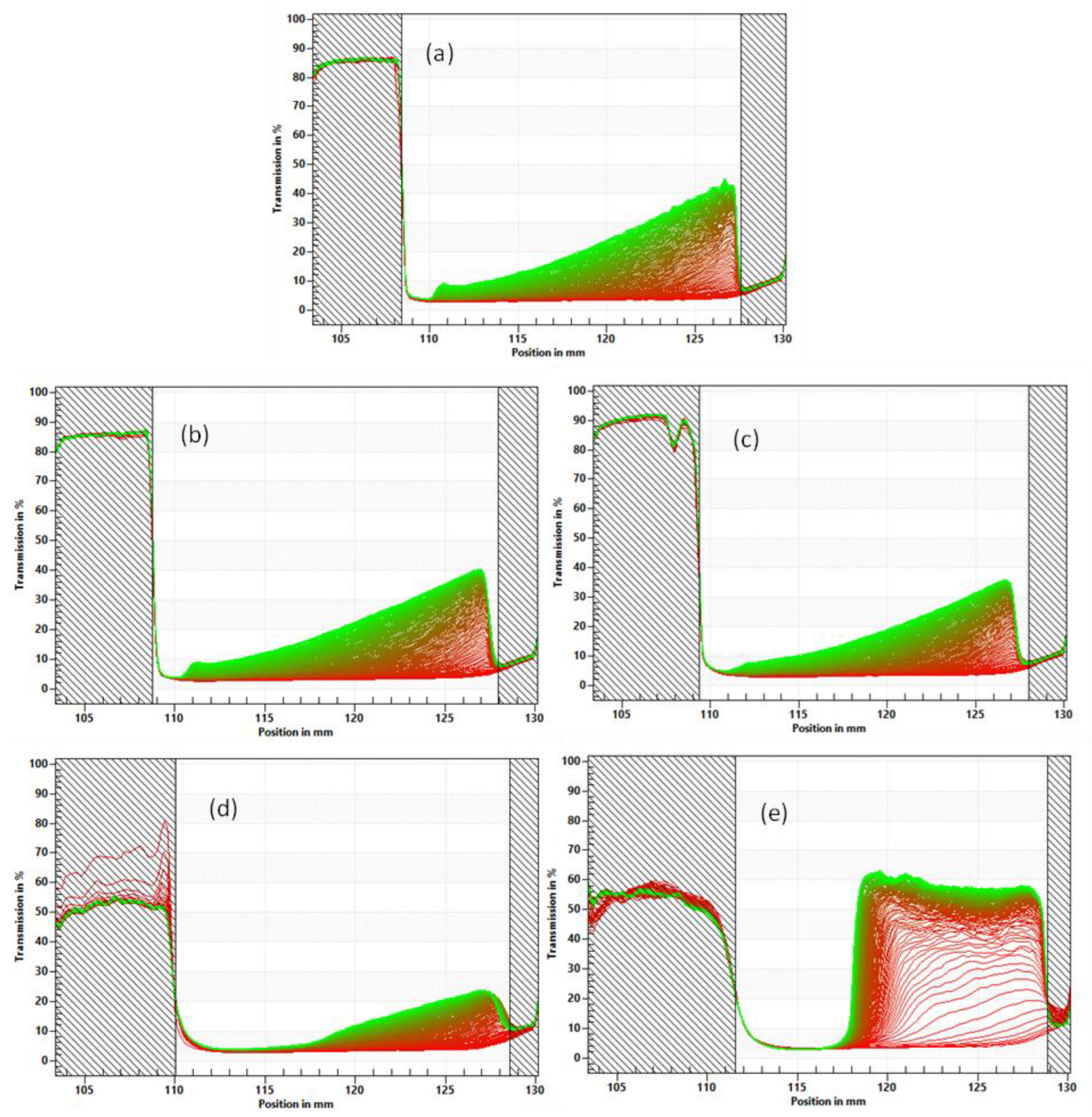
3.4. Emulsion Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Arora, R.G. Consumer buying behavior towards immunity booster products during COVID-19 pandemic: A study of systematic literature review. EPRA Int. J. Environ. Econ. Commer. Educ. Manag. (ECEM) 2023, 10, 6–16. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Interaction of dietary calcium and protein in bone health in humans. J. Nutr. 2003, 133, 852S–854S. [Google Scholar] [CrossRef]
- Hamada, A.M. Vitamins, omega-3, magnesium, manganese, and thyme can boost our immunity and protect against COVID-19. Eur. J. Biol. Res. 2020, 10, 271–295. [Google Scholar]
- Oh, H.E.; Deeth, H.C. Magnesium in milk. Int. Dairy J. 2017, 71, 89–97. [Google Scholar] [CrossRef]
- Liu, C.; Teng, Z.; Lu, Q.Y.; Zhao, R.Y.; Yang, X.Q.; Tang, C.H.; Liao, J.M. Aggregation kinetics and ζ-potential of soy protein during fractionation. Food Res. Int. 2011, 44, 1392–1400. [Google Scholar] [CrossRef]
- Glantz, M.; Månsson, H.L.; Stålhammar, H.; Paulsson, M. Effect of polymorphisms in the leptin, leptin receptor and acyl-CoA: Diacylglycerol acyltransferase 1 (DGAT1) genes and genetic polymorphism of milk proteins on bovine milk composition. J. Dairy Res. 2012, 79, 110–118. [Google Scholar] [CrossRef]
- Jeurnink, T.J.; De Kruif, K.G. Calcium concentration in milk in relation to heat stability and fouling. Neth. Milk Dairy J. 1995, 49, 151. [Google Scholar]
- Mohammad-Beigi, H.; Wijaya, W.; Madsen, M.; Hayashi, Y.; Li, R.; Rovers, T.A.M.; Jæger, C.T.; Buell, A.K.; Hougaard, A.B.; Kirkensgaard, J.J.K.; et al. Association of caseins with β-lactoglobulin influenced by temperature and calcium ions: A multi-parameter analysis. Food Hydrocoll. 2023, 137, 108373. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Restuccia, C.; Deeth, H.C. Effect of calcium on the physical properties of stirred probiotic yogurt. J. Dairy Sci. 2008, 91, 4164–4175. [Google Scholar] [CrossRef]
- Omoarukhe, E.D.; On-nom, N.; Grandison, A.S.; Lewis, M.J. Effects of different calcium salts on properties of milk related to heat stability. Int. J. Dairy Technol. 2010, 63, 504–511. [Google Scholar] [CrossRef]
- Singh, G.; Arora, S.; Sharma, G.S.; Sindhu, J.S.; Kansal, V.K.; Sangwan, R.B. Heat stability and calcium bioavailability of calcium-fortified milk. LWT-Food Sci. Technol. 2007, 40, 625–631. [Google Scholar] [CrossRef]
- Meza, B.E.; Zorrilla, S.E.; Olivares, M.L. Rheological methods to analyse the thermal aggregation of calcium enriched milks. Int. Dairy J. 2019, 97, 25–30. [Google Scholar] [CrossRef]
- Vyas, H.K.; Tong, P.S. Impact of source and level of calcium fortification on the heat stability of reconstituted skim milk powder. J. Dairy Sci. 2004, 87, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Tsioulpas, A.; Lewis, M.J.; Grandison, A.S. Effect of minerals on casein micelle stability of cows’ milk. J. Dairy Res. 2007, 74, 167–173. [Google Scholar] [CrossRef]
- Chen, B.Y.; Grandison, A.S.; Lewis, M.J. Comparison of heat stability of goat milk subjected to ultra-high temperature and in-container sterilization. J. Dairy Sci. 2012, 95, 1057–1063. [Google Scholar] [CrossRef]
- Philippe, M.; Le Graët, Y.; Gaucheron, F. The effects of different cations on the physicochemical characteristics of casein micelles. Food Chem. 2005, 90, 673–683. [Google Scholar] [CrossRef]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Lopez, F.; Cuomo, F.; Nostro, P.L.; Ceglie, A. Effects of solvent and alkaline earth metals on the heat-induced precipitation process of sodium caseinate. Food Chem. 2013, 136, 266–272. [Google Scholar] [CrossRef]
- Anderson, J.W.; Smith, B.M.; Washnock, C.S. Cardiovascular and renal benefits of dry bean and soybean intake. Am. J. Clin. Nutr. 1999, 70, 464S–474S. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.K.; Chakraborty, R.; Choudhuri, U.R. Effect of blending of soy milk with cow milk on sensory, textural and nutritional qualities of chhana analogue. J. Food Sci. Technol. 2002, 39, 702–704. [Google Scholar]
- Mujoo, R.; Trinh, D.T.; Ng, P.K.W. Characterization of storage proteins in different soybean varieties and their relationship to tofu yield and texture. Food Chem. 2003, 82, 265–273. [Google Scholar] [CrossRef]
- Riblett, A.L.; Herald, T.J.; Schmidt, K.A.; Tilley, K.A. Characterization of β-conglycinin and glycinin soy protein fractions from four selected soybean genotypes. J. Agric. Food Chem. 2001, 49, 4983–4989. [Google Scholar] [CrossRef]
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. [Google Scholar] [CrossRef]
- Anema, S.G.; Klostermeyer, H. ζ-Potentials of casein micelles from reconstituted skim milk heated at 120 °C. Int. Dairy J. 1996, 6, 673–687. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Phillips, J.A. Dietary guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- Gao, R.; Van Halsema, F.E.D.; Temminghoff, E.J.M.; Van Leeuwen, H.P.; Van Valenberg, H.J.F.; Eisner, M.D.; Giesbers, M.; Van Boekel, M.A.J.S. Modelling Ion Composition in Simulated Milk Ultrafiltrate (SMUF). I: Influence of Calcium Phosphate Precipitation. Food Chem. 2010, 122, 700–709. [Google Scholar] [CrossRef]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.; Sloth, K.H.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef]
- Crowley, S.V.; Megemont, M.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Heat stability of reconstituted milk protein concentrate powders. Int. Dairy J. 2014, 37, 104–110. [Google Scholar] [CrossRef]
- Tercinier, L. Study of the Interactions Between Milk Proteins and Hydroxyapatite Particles. Doctoral Thesis, Massey University: Riddet Institute, Palmerston North, New Zealand, 2016. [Google Scholar]
- Wade, T.; Beattie, J.K. Electroacoustic Determination of Size and Zeta Potential of Fat Globules in Milk and Cream Emulsions. Colloids Surf. B Biointerfaces 1997, 10, 73–85. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Detecting and Measuring Free Gadolinium in Nanoparticles for MRI Imaging. Charact. Nanoparticles Intend. Drug Deliv. 2011, 697, 101–108. [Google Scholar]
- Isusi, G.I.S.; Weilandt, M.; Majollari, I.; Karbstein, H.P.; Van Der Schaaf, U.S. Emulsions Stabilised with Pectin-Based Microgels: Investigations into the Effect of PH and Ionic Strength on Emulsion Stability. Food Funct. 2021, 12, 7227–7238. [Google Scholar] [CrossRef]
- Arii, Y.; Takenaka, Y. Initiation of protein association in tofu formation by metal ions. Biosci. Biotechnol. Biochem. 2014, 78, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.T.; Murphy, K.M.; Drapala, K.P.; O’Callaghan, T.F.; Fenelon, M.A.; O’Mahony, J.A.; McCarthy, N.A. Effect of pH and heat treatment on viscosity and heat coagulation properties of milk protein concentrate. Int. Dairy J. 2018, 85, 219–224. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. The effect of protein concentration and heat treatment temperature on micellar casein–soy protein mixtures. Food Hydrocoll. 2011, 25, 1448–1460. [Google Scholar] [CrossRef]
- Renkema, J.M.; Lakemond, C.M.; de Jongh, H.H.; Gruppen, H.; van Vliet, T. The effect of pH on heat denaturation and gel forming properties of soy proteins. J. Biotechnol. 2000, 79, 223–230. [Google Scholar] [CrossRef]
- Iwabuchi, S.; Watanabe, H.; Yamauchi, F. Thermal denaturation of beta-conglycinin. Kinetic resolution of reaction mechanism. J. Agric. Food Chem. 1991, 39, 27–33. [Google Scholar] [CrossRef]
- Beliciu, C.M.; Moraru, C.I. Physico-chemical changes in heat treated micellar casein–soy protein mixtures. LWT-Food Sci. Technol. 2013, 54, 469–476. [Google Scholar] [CrossRef]
- Roesch, R.R.; Corredig, M. Heat-induced soy−whey proteins interactions: Formation of soluble and insoluble protein complexes. J. Agric. Food Chem. 2005, 53, 3476–3482. [Google Scholar] [CrossRef]
- Singh, G.; Muthukumarappan, K. Influence of calcium fortification on sensory, physical and rheological characteristics of fruit yogurt. LWT-Food Sci. Technol. 2008, 41, 1145–1152. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P.; Gebhardt, R.; Roth, S.V.; Metwalli, E.; Doster, W. Effect of calcium concentration on the structure of casein micelles in thin films. Biophys. J. 2007, 93, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Noguchi, H. Interaction of 11S fraction of soybean protein with calcium ion. Agric. Biol. Chem. 1977, 41, 1575–1580. [Google Scholar]
- Scilingo, A.A.; Añon, M.C. Characterization of soybean protein isolates. The effect of calcium presence. J. Am. Oil Chem. Soc. 2004, 81, 63–69. [Google Scholar] [CrossRef]
- Nagano, T. Contribution of disulfide bonding to viscoelastic properties and microstructures of 11S globulin gels from soybeans: Magnesium chloride-induced gels. Food Sci. Technol. Res. 2013, 19, 51–57. [Google Scholar] [CrossRef][Green Version]
- Ye, A.; Singh, H. Influence of calcium chloride addition on the properties of emulsions stabilized by whey protein concentrate. Food Hydrocoll. 2000, 14, 337–346. [Google Scholar] [CrossRef]
- Keowmaneechai, E.; McClements, D.J. Effect of CaCl2 and KCl on physiochemical properties of model nutritional beverages based on whey protein stabilized oil-in-water emulsions. J. Food Sci. 2002, 67, 665–671. [Google Scholar] [CrossRef]
- Boulet, M.; Britten, M.; Lamarche, F. Aggregation of Some Food Proteins in Aqueous Dispersions: Effects of Concentration, PH and Ionic Strength. Food Hydrocoll. 2000, 14, 135–144. [Google Scholar] [CrossRef]
- Amagliani, L.; Schmitt, C. Globular Plant Protein Aggregates for Stabilization of Food Foams and Emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- Puchana-Rosero, M.J.; Adebayo, M.A.; Lima, E.C.; Machado, F.M.; Thue, P.S.; Vaghetti, J.C.; Umpierres, C.M.; Gutterres, M. Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 105–115. [Google Scholar] [CrossRef]
- Shimoni, G.; Levi, C.S.; Tal, S.L.; Lesmes, U. Emulsions stabilization by lactoferrin nano-particles under in vitro digestion conditions. Food Hydrocoll. 2003, 33, 264–272. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018, 229, 12–20. [Google Scholar] [CrossRef]
- Petzold, G.; Goltzsche, C.; Mende, M.; Schwarz, S.; Jaeger, W. Monitoring the stability of nanosized silica dispersions in presence of polycations by a novel centrifugal sedimentation method. J. Appl. Polym. Sci. 2009, 114, 696–704. [Google Scholar] [CrossRef]
- Kori, A.H.; Mahesar, S.A.; Sherazi, S.T.H.; Khatri, U.A.; Laghari, Z.H.; Panhwar, T. Effect of Process Parameters on Emulsion Stability and Droplet Size of Pomegranate Oil-in-Water. Grasas Y Aceites 2021, 72, e410. [Google Scholar] [CrossRef]




| Mineral Dose Level | pH | Conductivity of Milk (µS/cm) | Ζeta Potential (mV) | |
|---|---|---|---|---|
| No addition | Zero | 6.93 ± 0.03 e | 1072 ± 29 a | −39.5 ± 2.6 a |
| CaCl2 addition | 2 mM Ca | 6.86 ± 0.03 d | 1185 ± 33 b | −14.6 ± 0.3 cd |
| 3.5 mM Ca | 6.81 ± 0.05 b | 1267 ± 23 c | −12.3 ± 0.7 de | |
| 5 mM Ca | 6.76 ± 0.05 ab | 1349 ± 15 d | −9.9 ± 1.7 efg | |
| 6.5 mM Ca | 6.73 ± 0.06 a | 1450 ± 19 e | −8.9 ± 0.9 g | |
| MgCl2 addition | 2 mM Mg | 6.86 ± 0.03 d | 1186 ± 10 b | −17.24 ± 1.1 b |
| 3.5 mM Mg | 6.84 ± 0.02 c | 1229 ± 19 bc | −16.20 ± 1.6 bc | |
| 5 mM Mg | 6.78 ± 0.01 ab | 1365 ± 25 d | −11.60 ± 0.3 ef | |
| 6.5 mM Mg | 6.75 ± 0.02 ab | 1465 ± 21 e | −9.25 ± 0.0 fg |
| Mineral Dose Level | Starting Viscosity (mPa.s) | Minimum Viscosity (mPa.s) | Tc * (°C) | Ending Viscosity (mPa.s) | |
|---|---|---|---|---|---|
| No addition | Zero | 11.53 ± 0.15 a | 2.36 ± 0.03 a | 78.99 ± 0.68 f | 5.74 ± 1.26 a |
| CaCl2 addition | 2 mM Ca | 13.43 ± 2.49 b | 2.51 ± 0.01 a | 79.59 ± 0.19 f | 9.71 ± 1.44 a |
| 3.5 mM Ca | 11.27 ± 0.13 a | 2.91 ± 0.08 b | 74.98 ± 0.34 e | 9.01 ± 0.29 a | |
| 5 mM Ca | 11.11 ± 0.68 a | 3.71 ± 0.06 d | 66.18 ± 1.79 c | 31.86 ± 3.87 c | |
| 6.5 mM Ca | 11.55 ± 0.21 a | 4.88 ± 0.21 f | 54.38 ± 0.56 a | 49.47 ± 5.74 e | |
| MgCl2 addition | 2 mM Mg | 10.76 ± 0.26 a | 2.54 ± 0.01 a | 79.37 ± 0.24 f | 6.43 ± 0.54 a |
| 3.5 mM Mg | 10.66 ± 0.24 a | 2.80 ± 0.08 b | 74.00 ± 1.90 e | 8.25 ± 0.16 a | |
| 5 mM Mg | 10.81 ± 0.24 a | 3.35 ± 0.18 c | 70.08 ± 2.59 d | 16.00 ± 1.23 b | |
| 6.5 mM Mg | 10.93 ± 0.19 a | 3.96 ± 0.09 e | 60.45 ± 1.84 b | 41.82 ± 5.20 d |
| Mineral Dose Level | Instability Index | Sedimentation Height (mm) | |
|---|---|---|---|
| No addition | Zero | 0.174 ± 0.007 d | 2.57 ± 0.15 c |
| CaCl2 addition | 2.0 mM Ca | 0.162 ± 0.010 cd * | 2.53 ± 0.29 c |
| 3.5 mM Ca | 0.132 ± 0.012 b | 2.50 ± 0.17 c | |
| 5.0 mM Ca | 0.078 ± 0.010 a | 1.57 ± 0.21 b | |
| 6.5 mM Ca | 0.366 ± 0.010 f | 1.15 ± 0.13 a | |
| MgCl2 addition | 2.0 mM Mg | 0.156 ± 0.004 c | 2.57 ± 0.06 c |
| 3.5 mM Mg | 0.132 ± 0.003 b | 2.50 ± 0.10 c | |
| 5.0 mM Mg | 0.065 ± 0.004 a | 2.40 ± 0.10 c | |
| 6.5 mM Mg | 0.206 ± 0.014 e | 0.93 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tan, K.W.J.; Chiang, P.L.; Wong, W.X.; Chen, W.; Lin, Q. Impact of Incorporating Free Calcium and Magnesium on the Heat Stability of a Dairy- and Soy-Protein-Containing Model Emulsion. Polymers 2023, 15, 4424. https://doi.org/10.3390/polym15224424
Wang W, Tan KWJ, Chiang PL, Wong WX, Chen W, Lin Q. Impact of Incorporating Free Calcium and Magnesium on the Heat Stability of a Dairy- and Soy-Protein-Containing Model Emulsion. Polymers. 2023; 15(22):4424. https://doi.org/10.3390/polym15224424
Chicago/Turabian StyleWang, Wei, Kevin Wei Jie Tan, Poh Leong Chiang, Wai Xin Wong, Wenpu Chen, and Qi Lin. 2023. "Impact of Incorporating Free Calcium and Magnesium on the Heat Stability of a Dairy- and Soy-Protein-Containing Model Emulsion" Polymers 15, no. 22: 4424. https://doi.org/10.3390/polym15224424
APA StyleWang, W., Tan, K. W. J., Chiang, P. L., Wong, W. X., Chen, W., & Lin, Q. (2023). Impact of Incorporating Free Calcium and Magnesium on the Heat Stability of a Dairy- and Soy-Protein-Containing Model Emulsion. Polymers, 15(22), 4424. https://doi.org/10.3390/polym15224424





