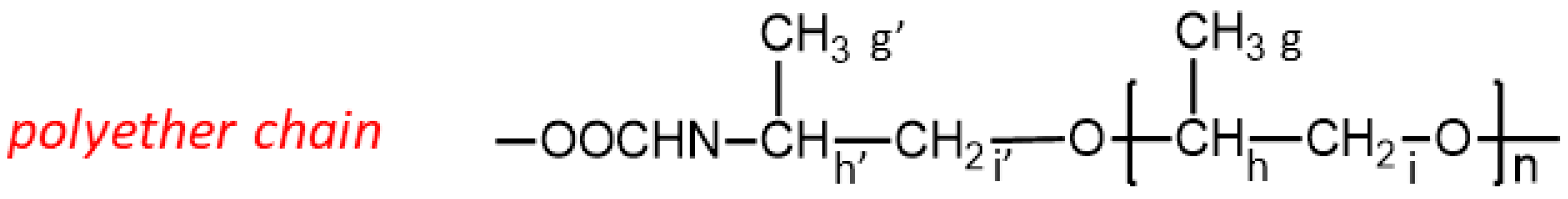3.1. Synthesis and Proprieties of Bio-Polyols
Renewable BPOs with several functionalization degrees were prepared from two different no-food vegetable oils, linseed and soybean oil, using a solvent-free method [
7], in which no purification or separation steps were required (
Scheme 1).
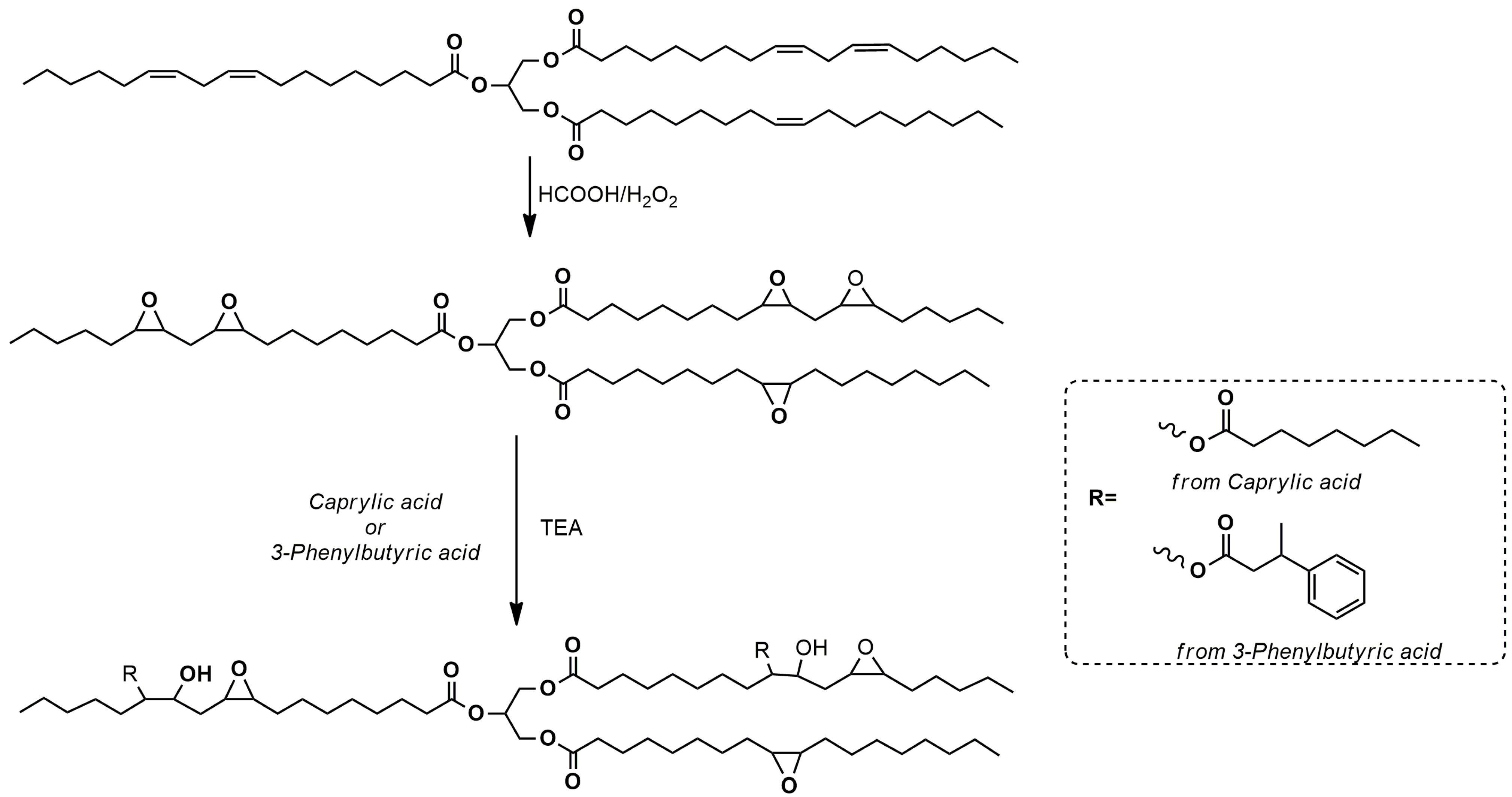
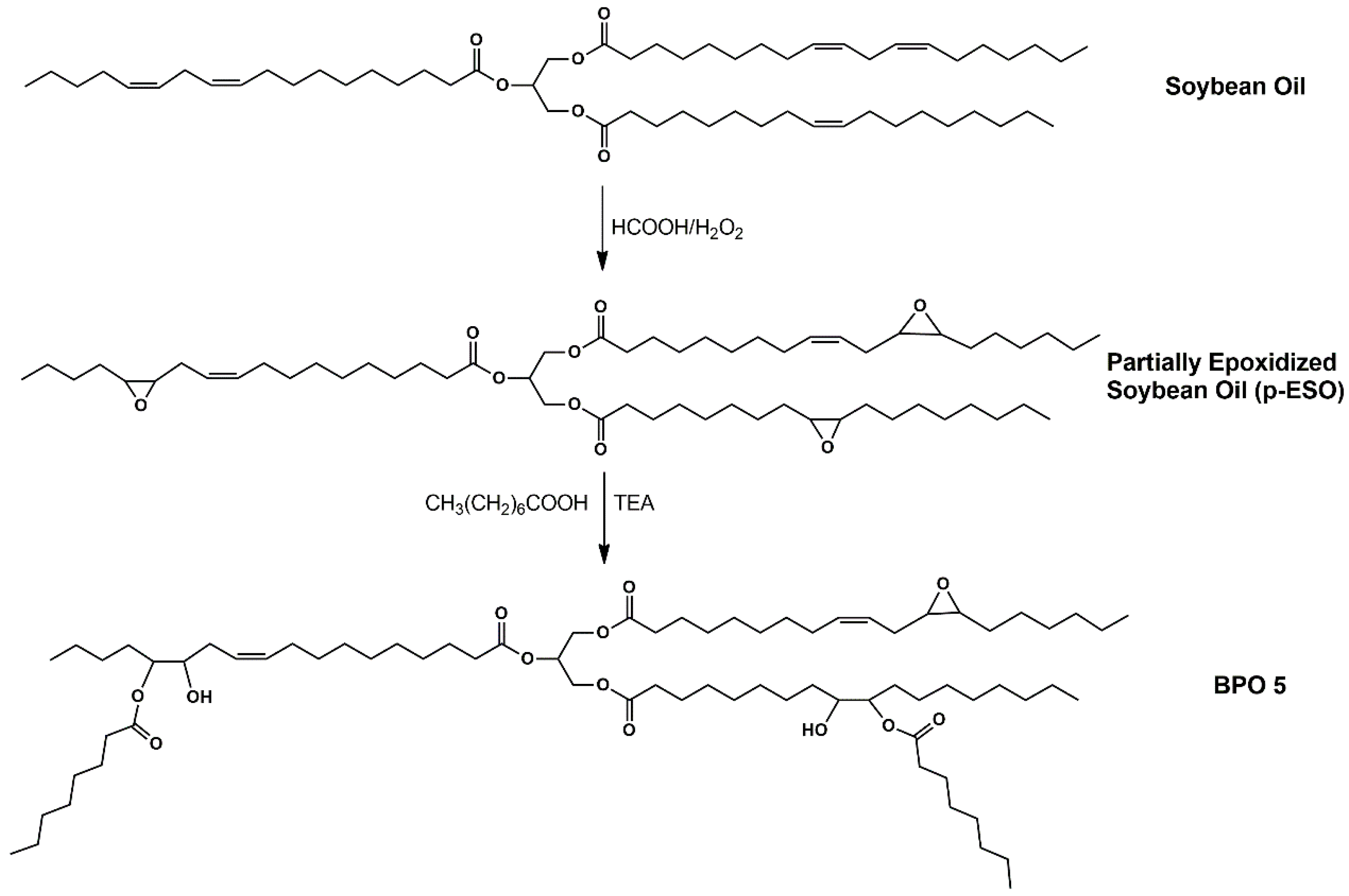
For BPOs from linseed oil, a commercial epoxidized linseed oil (ELO) was used, while for those from soybean oil, one partially and one completely epoxidized soybean oil (p-ESO and ESO, respectively) sample was prepared from a commercial soybean oil to evaluate the effect of the presence of residual double bonds on the final properties of the ensuing polyurethane foams. p-ESO and ESO were obtained from the reaction of soybean oil with formic acid and hydrogen peroxide overnight at 60 °C (
Scheme 1). Two oil/peracid ratios were used to tune the epoxidation degree (
Table 1).
In
Figure 1, the
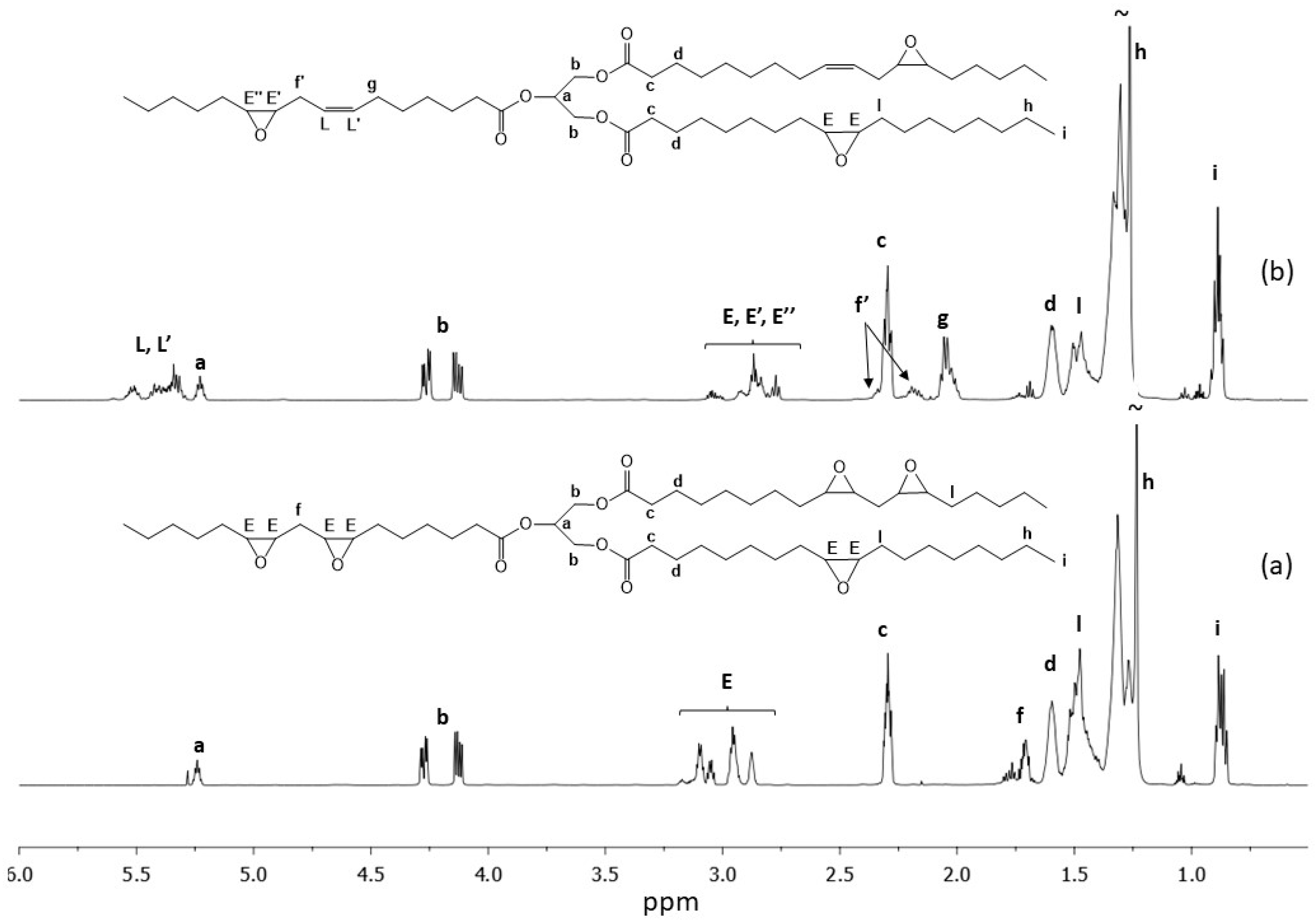
1H NMR spectra of partially and fully epoxidized soybean oils are reported.
For ESO (
Figure 1a), all the double bonds were converted into epoxy groups as confirmed by the absence of the protons related to olefinic
L,
L′ proton atoms in the region from 5.48 to 5.24 ppm [
29], and by the presence of resonances in the regions from 2.82 to 2.97 ppm and 3.00 to 3.17 ppm, related to protons of epoxy groups (E). The
1H NMR spectrum of p-ESO (
Figure 1b) shows the simultaneous presence of resonances belonging to epoxy groups (protons
E,
E′ and
E″) at 3.00–2.87 ppm and of resonances related to double bond in the region spanning from 5.48 to 5.24 ppm, (protons
L,
L′). Moreover, there is the appearance of two signals from 2.15 to 2.25 ppm and at 2.36, overlapped with the resonance at 2.34 (protons
c), due to methylene protons between an epoxy and an olefinic group (protons
f′). The presence of a resonance at 2.08 ppm ascribed to
g protons, due to allylic methylenes, further confirms the partial epoxidization.
1H NMR analysis assessed an estimated epoxy group per triglyceride of 2.8, 5.1 and 6.4 for p-ESO, ESO, and epoxidized linseed oil (ELO), respectively, (
Figure S1).
For the synthesis of the bio-based polyols, caprylic and 3-phenylbutyric acid, as the organic acids, respectively, were used to analyze the effect of an aliphatic short linear chain or the presence of an aromatic moiety on PU formulation and properties.
It is worth noting that the hydroxyl functionality, viscosity, residual double bonds, or epoxide in the bio-polyols backbone are key issues for the synthesis of PU foams. With a complete conversion of the epoxide group per triglyceride, a maximum total number of OH groups was estimated around 5–6. However, with these high functionalities, rigid foams were expected [
21,
30,
31]. Flexible foams require low crosslink density which can be obtained by using polyols with either low OH functionalities (in the range between 2 and 3) or molecular weights up to 1500 g/mol. Thus, polyols with those features were prepared and are reported in
Table 2. Highest viscosity values were observed for both bio-polyols from 3-phenylbutyric acid (
Table 2) due to the presence of the aromatic ring that increases the rigidity of the side-chain and the friction resistance [
32].
When caprylic acid is grafted on the epoxidized oil backbone, the viscosity dramatically decreases and as expected, the polyol from p-ESO and caprylic acid (BPO 5 in
Table 1) showed the lowest viscosity due to its structural properties (
Scheme 2). No bio-polyol was prepared by reacting p-ESO and 3-phenyl-butyrric acid since, as already mentioned, the presence of the aromatic moieties greatly increases the viscosity [
32].
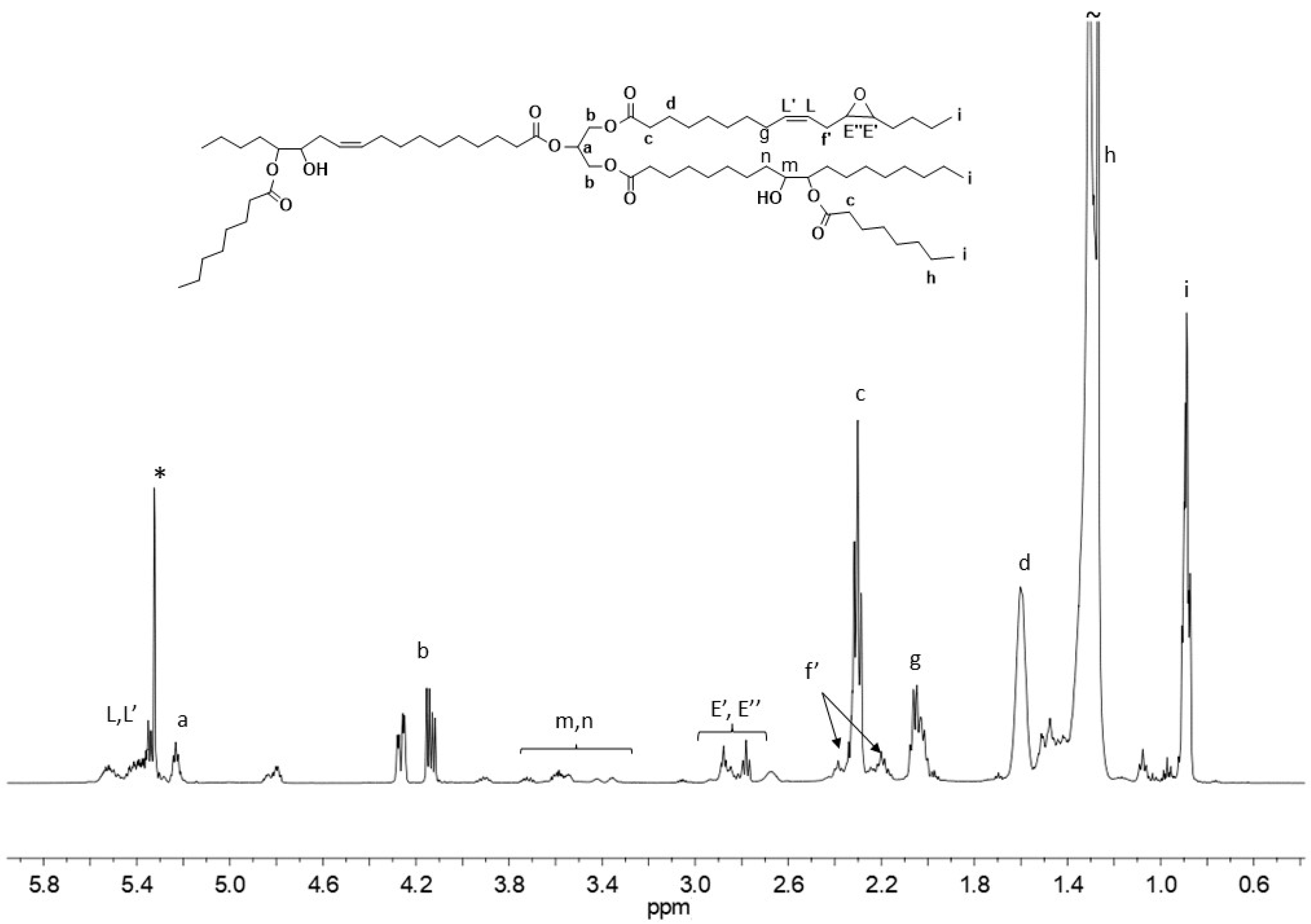
The
1H NMR spectrum of bio-polyol from p-ESO confirmed the simultaneous presence of resonances related to the double bond in the region spanning from 5.48 to 5.24 ppm, (proton
L), between 2.82 and 3.17 related to protons
E (epoxide group) and between 3.20 and 3.80 ppm due to methylene and methine groups in the beta and alpha positions to the hydroxyl groups (
Figure 2) [
33,
34]. A residual double bond functionality of 2 was determined for this bio-polyol.
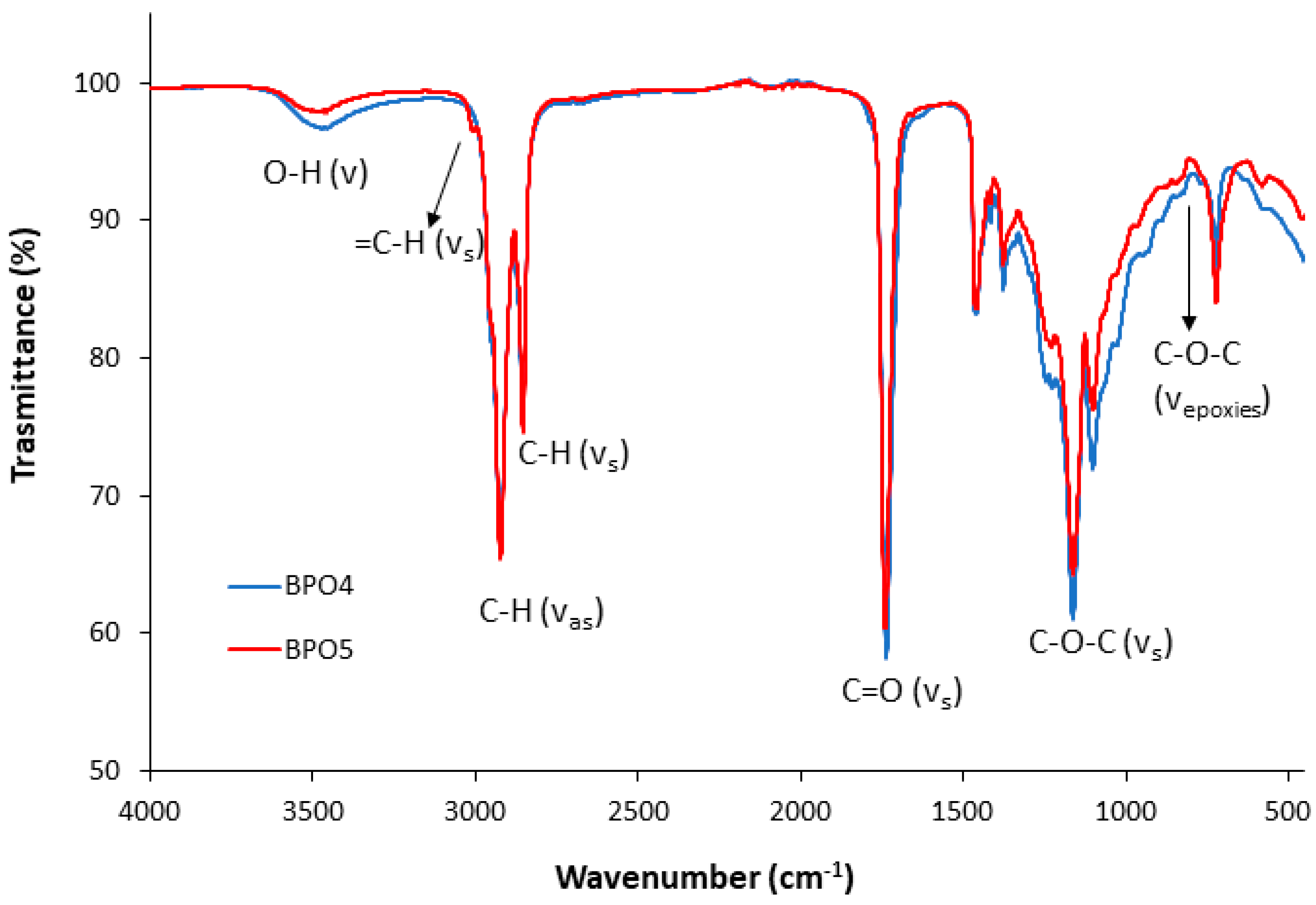
The FTIR spectra of the bio-polyols from p-ESO or ESO and caprylic acid are presented in
Figure 3. They both show between 3600 and 3200 cm
−1 the broad absorption peak characteristic for stretching vibrations of OH groups; at 1732 cm
−1, the C=O stretching vibration of esters and at ~2924 and 2854 cm
−1 the –CH
2 symmetric and asymmetric stretching [
35].
The oxirane ring vibration peak at 824 cm
−1 of epoxidized vegetable oils had a weak presence, according to NMR analysis. Moreover, the polyol from partially epoxidized oil showed the presence of a small absorption peak characteristic for C-H stretching ascribed to residual double bond around 2990 cm
−1 [
36].
The FT-IR spectra of BPOs from the aromatic acid (BPO 1 and 2), not reported, displayed the typical absorption peaks of monosubstituted aromatic groups from 3-phenyl butyric acid [
7].
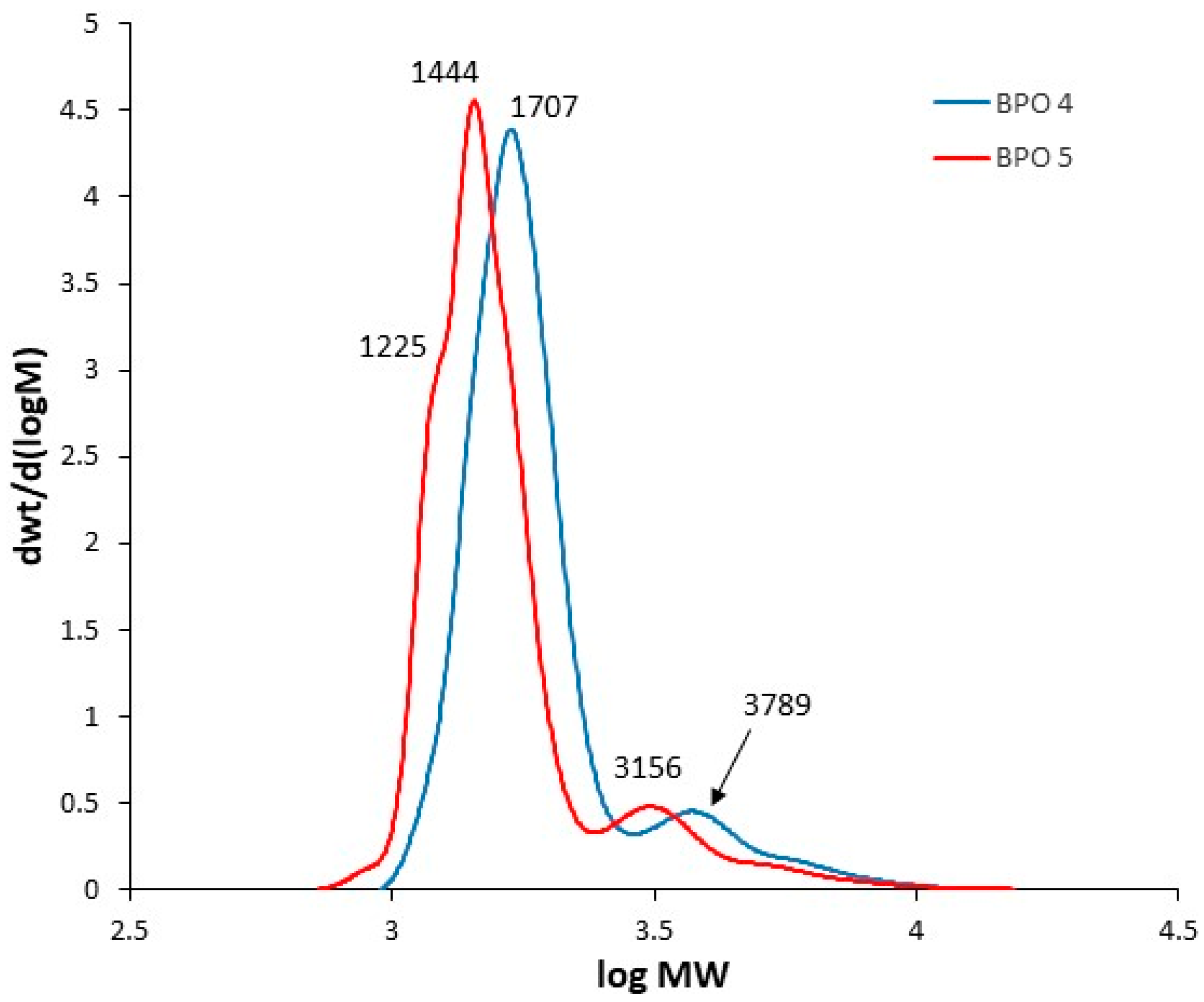
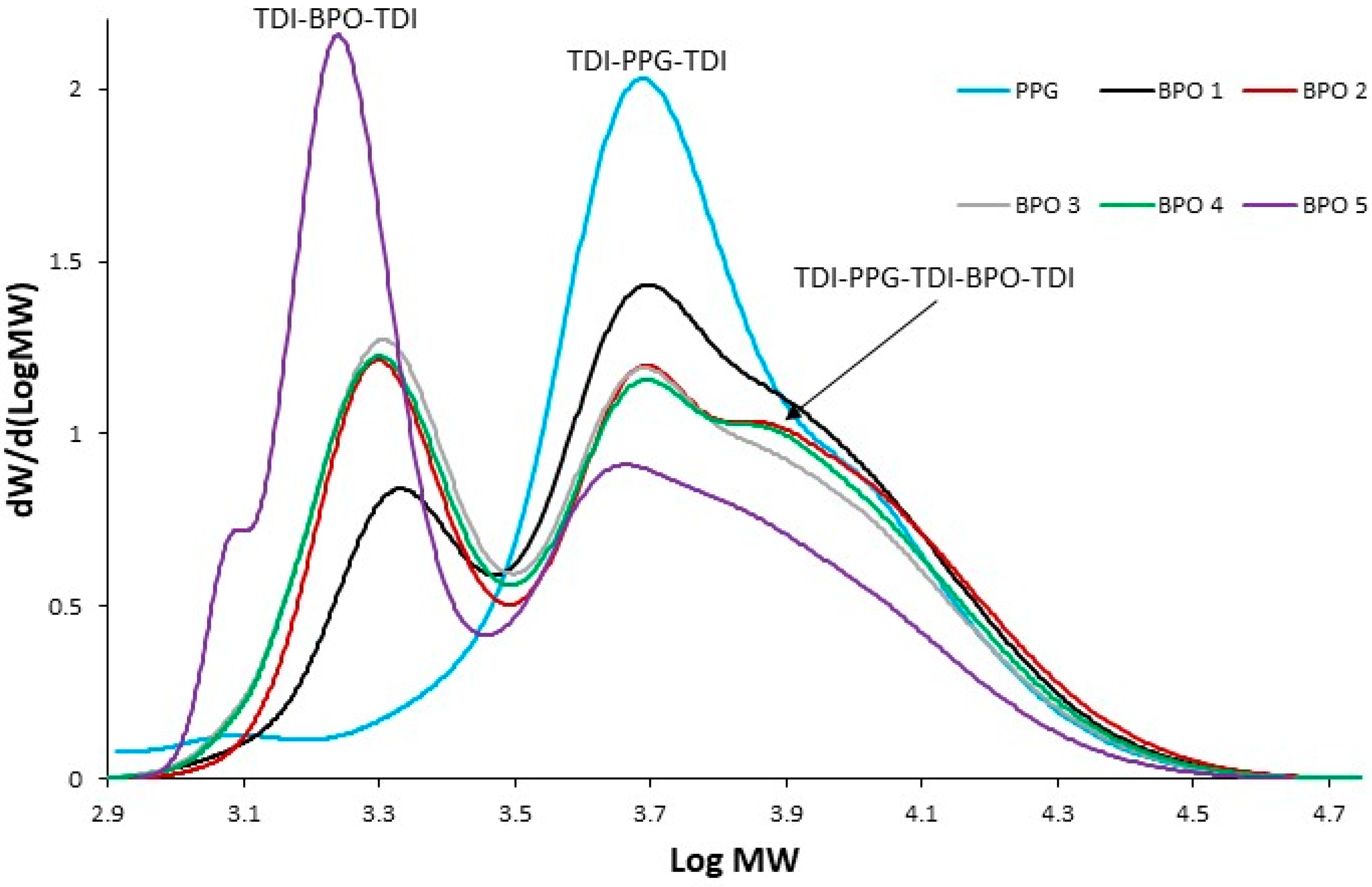
The SEC curves as a function of molecular weight (MW) for the bio-polyols from p-ESO or ESO and CA (
Figure 4) showed two fractions, the main one at lower molecular weight whose molecular weight depends on the functionalization degree of the bio-polyol and a second one at a higher molecular weight ranging from 3100 to 3800 g/mol, corresponding to the oligomeric fraction with an ester functionalization degree of between two and three per oil molecule.
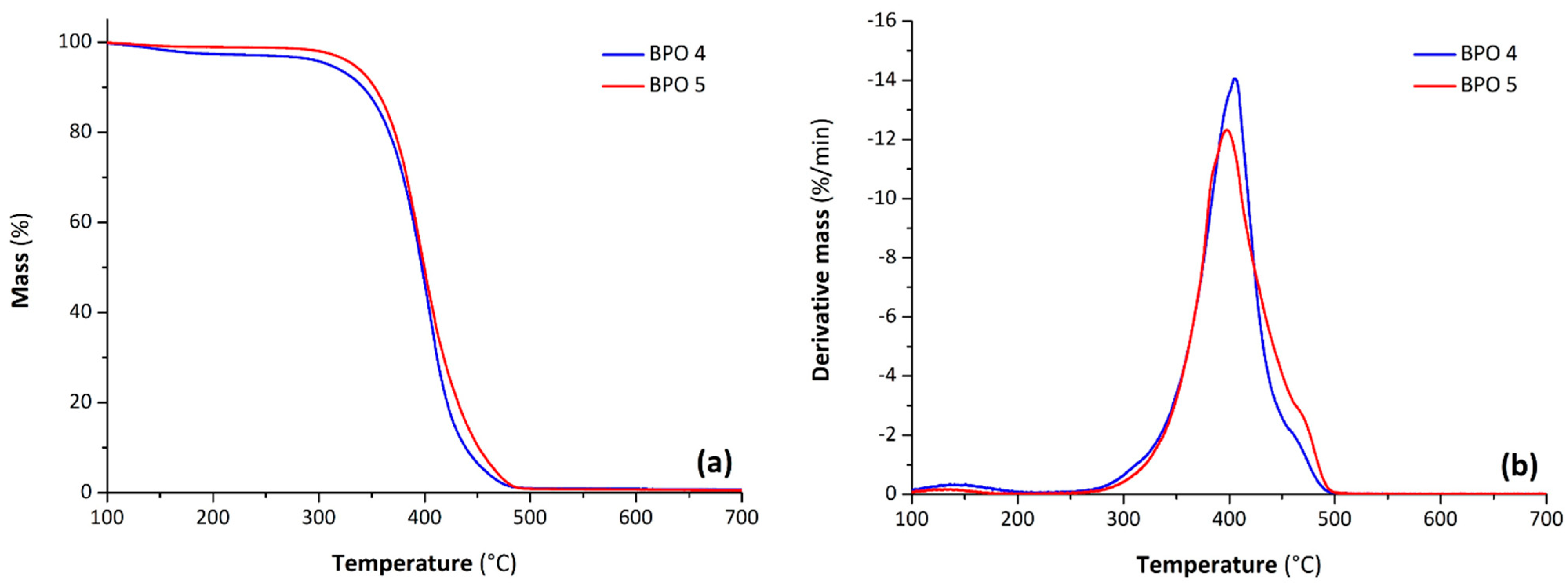
The polyols were characterized by TGA: the thermograms of bio-polyols from partially and completely epoxidized soybean oils are depicted in
Figure 5a,b, respectively. The thermal analysis proved that the use of p-ESO in the preparation of polyol does not produce significant differences from the bio-polyol from ESO. These samples, synthesized from CA, showed good thermal stability under nitrogen, as highlighted by a temperature of degradation at 5% mass loss of 310 °C for BPO 4 and 332 °C for BPO 5. Both samples exhibited a first degradation event with a mass loss of 2–4% ascribed to the presence of a small amount of unreacted acid at low temperature (see faint DTG peak centered at about 140 °C). The principal degradation stage of the bio-polyols from ESO and p-ESO occurred between 300 and 500 °C with a corresponding DTG peak at about 400 °C. Moreover, both the polyols presented a residual mass at 700 °C in nitrogen lower than 1%.
3.2. Prepolymer Synthesis
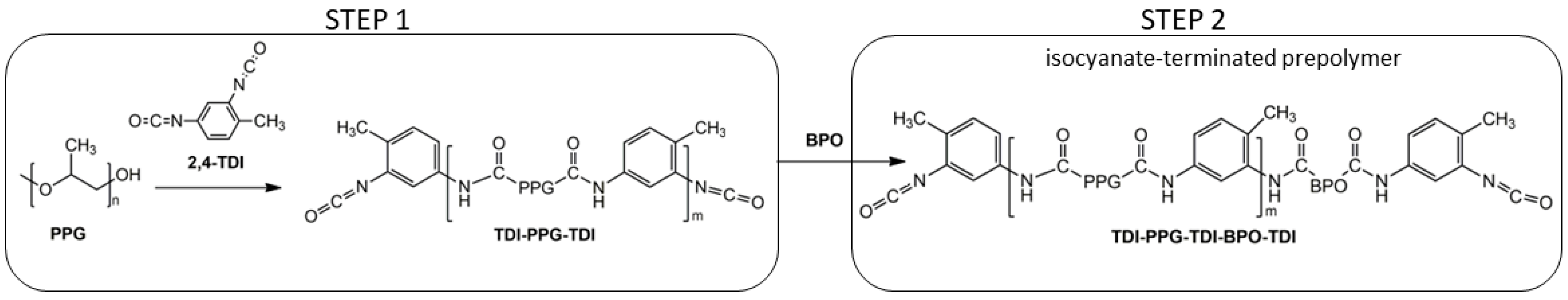
For the synthesis of polyurethane foams, a PU quasi-prepolymer was synthesized by a two-step reaction (
Scheme 3) in the presence of an excess of TDI (total index 200). The one-shot approach, by-passing prepolymer synthesis, was excluded since preliminary tests showed that it leads to easy cracking and crumbling foams. Moreover, a prepolymer leads to better compatibility between additional polyurethane formulation components, since the reacted systems provide lower surface tension to other chemicals within the solubility parameter range.
The first step (
Scheme 3) involves the reaction between the polypropylene glycol (PPG) and a large excess of diisocyanate, giving isocyanate-terminated intermediates [TDI-(PPG-TDI)
m]. For short reaction times, the majority of the prepolymer consists of a single adduct, TDI-PPG-TDI, with a molecular weight equal to the molecular weight of PPG plus the molecular weight of two diisocyanate units capping the polyol, while with an increased reaction time, longer oligomers (i.e., sequences of alternating TDI and PPG) can be observed.
In the second step, BPO reacts with the PPG-TDI adduct for 30 min at 40 °C, giving an isocyanate-terminated quasi-prepolymer. This step is more complex than the first one, because it involves the bio-polyols that are reactants with secondary −OH groups and with functionality ≥ 2. The number of branches of the quasi-prepolymer is strictly related to the functionality of the raw materials used to prepare the isocyanate-terminated prepolymer.
At the end of the second step, the products should be end-capped polyols (TDI-BPO-TDI) with a molecular weight corresponding to the molecular weight of bio-polyol plus twice the molecular weight of the diisocyanate, and species with higher molecular weights, which consist of isocyanate-terminated prepolymer TDI-(PPG-TDI)m-BPO-TDI with m corresponding to that obtained in the first step. In our conditions, the prepolymer should be TDI-PPG-TDI-BPO-TDI.
To have an insight into the synthesis of the prepolymers, the final products of each of the two steps were stirred with methanol for 30 min to cap any unreacted isocyanate groups and afterwards characterized by SEC and
1H NMR. In
Figure 6, SEC chromatograms of prepolymers from all bio-polyols are depicted and compared to that from PPG, obtained in the first step. Different products can be observed: (i) end-capped polyols, TDI-BPO-TDI, with lower molecular weight, (ii) end-capped PPG, TDI-PPG-TDI, molecular weight about 5000 g/mol, and (iii) species with higher molecular weight, corresponding to the TDI-PPG-TDI-BPO-TDI alternating structure, with linear or branched structures (e.g., for bio-polyols with functionality > 2).
It is worth noting, no significant differences were observed concerning the reactivity of the different bio-polyols with diisocyanate from SEC analysis. Moreover, BPO 5 (low OH functionality = 2), the vegetable oil lacking −OH groups in some portions of the backbone due to the presence of unreacted double bonds, resulted in being partially unreacted as shown in the SEC chromatogram of (see also
Figure 4).
Preferably, the number of branches of the isocyanate prepolymer and the crosslink density, i.e., the number of attachments between chains of polymers, should be kept low to control the final viscosity of the PU intermediate.
A prepolymer from bio-polyols and TDI in the absence of PPG was prepared for comparison to better understand how the reaction proceeds. It is worth noting that, in the absence of PPG, the reaction medium, made of bio-polyol and TDI in the presence of dibutyltin dilaurate as catalyst, reached the crosslinking point after 20 min at 40 °C, giving a semi-solid product. According to the Flory–Stockmayer theory, the higher the functionality of the monomers, the lower the conversion at the gel point, and consequently, the lower the gel time will become [
37,
38].
From all these considerations, it is possible to control the reaction between bio-polyols and TDI, (
Scheme 3), by introducing PPG, with linear long chains. It is reasonable to suppose that the end-capped PPG decreases the overall viscosity of the system, also partially reacting with the bio-polyol leading to linear copolymers limiting the growth of hyper-branched chains in the prepolymer; that is, the uncontrolled reaction between multiple end-capped BPO units.
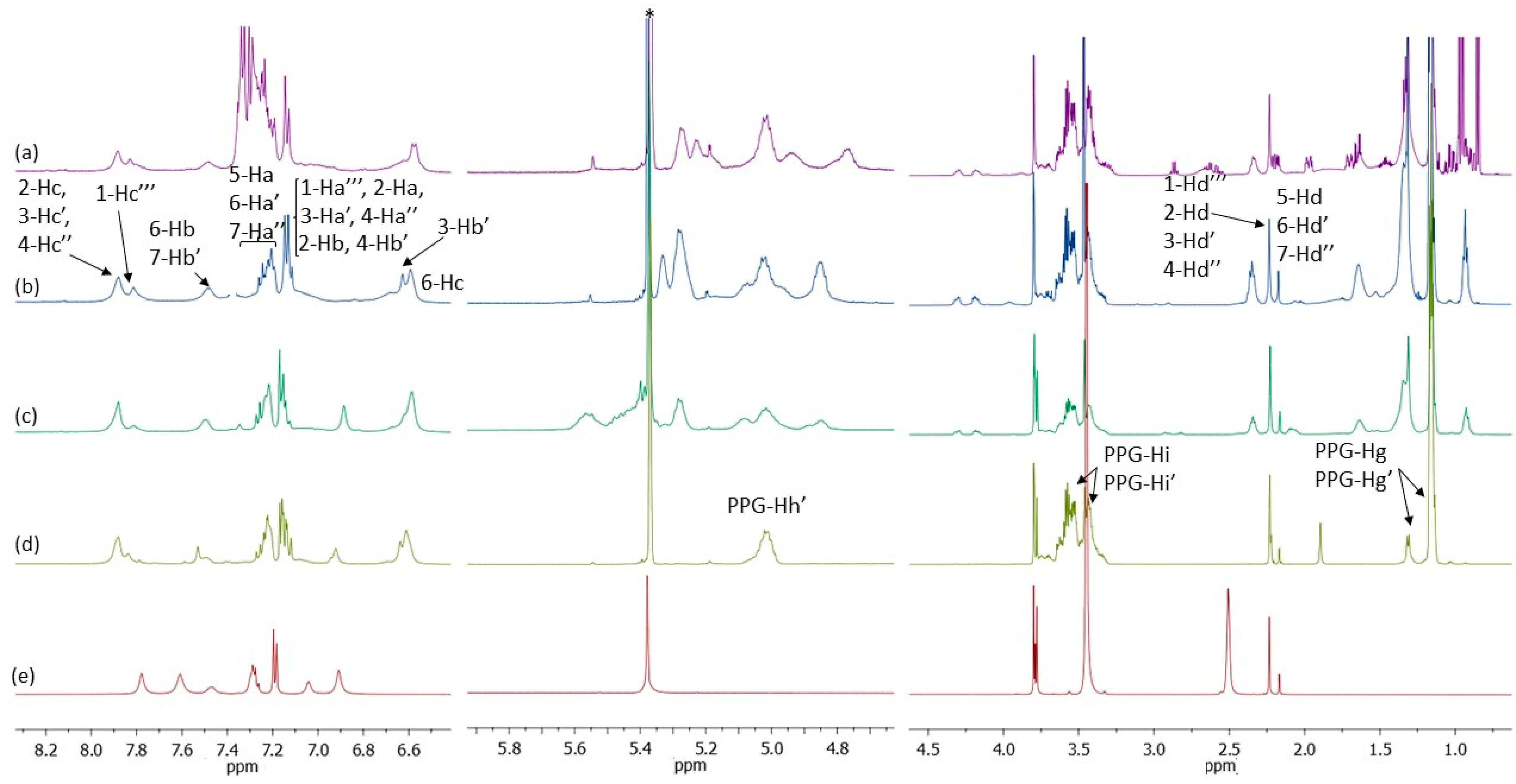
The
1H-NMR spectra (
Figure 7) of the prepolymers, quenched by methanol, validate the successful reaction between polyols (PPG and bio-polyols) and TDI. The complete assignment of the resonances ascribed to the structure coming from TDI (
Scheme 4) and PPG (
Scheme 5) have been reported; where no assignments are indicated, the corresponding resonances can be attributed to the BPOs. Moreover, the
1H NMR spectra of PPG-prepolymer and TDI are reported as reference (
Figure 7d,e).
The
1H-NMR spectra were analyzed and compared to those reported by Pegoraro et al. for prepolymers prepared from toluene 2,4-diisocyanate and toluene 2,6-diisocyanate using polypropylene glycol and acetone-d
6 as solvent [
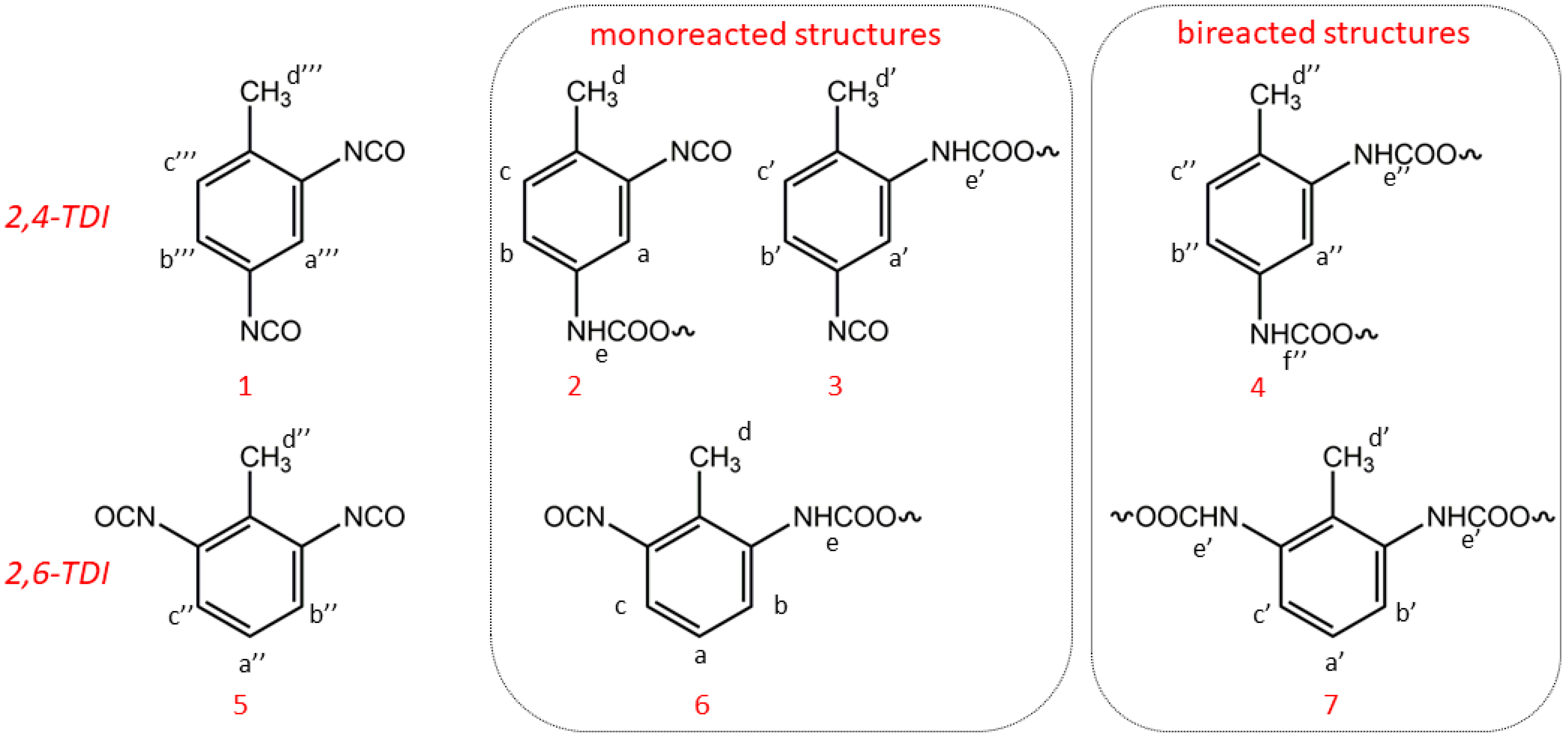
11]. Indeed, the complexity of the spectra acquired from samples elicited using commercial TDI where two positional isomers, toluene 2,4-diisocyanate (2,4-TDI) and toluene 2,6-diisocyanate (2,6-TDI) are present, usually in the ratio 80:20, is well known. Three structures with 2,4-TDI and two with 2,6-TDI, as reported in
Scheme 4, are expected by reacting with TDI. When 2,4-TDI is used, species can be formed (1) by reaction of the more reactive NCO in the para position to the methyl group, (2) from the less reactive NCO in ortho position to the methyl group, and (3) from a biurethane structure. With 2,6-TDI, due to the symmetry of the system, both monoreacted or bireacted structures can be expected.
Thus, the more reactive para-NCO group preferentially reacts with the OH groups of PPG or bio-polyol to form an adduct that is terminated with the less reactive ortho-NCO groups if the reaction is carried with an excess of NCO groups. The 1H NMR of prepolymers should show that a compound terminated with the less reactive ortho-NCO groups represented the main form of the urethanes in the polyol-TDI.
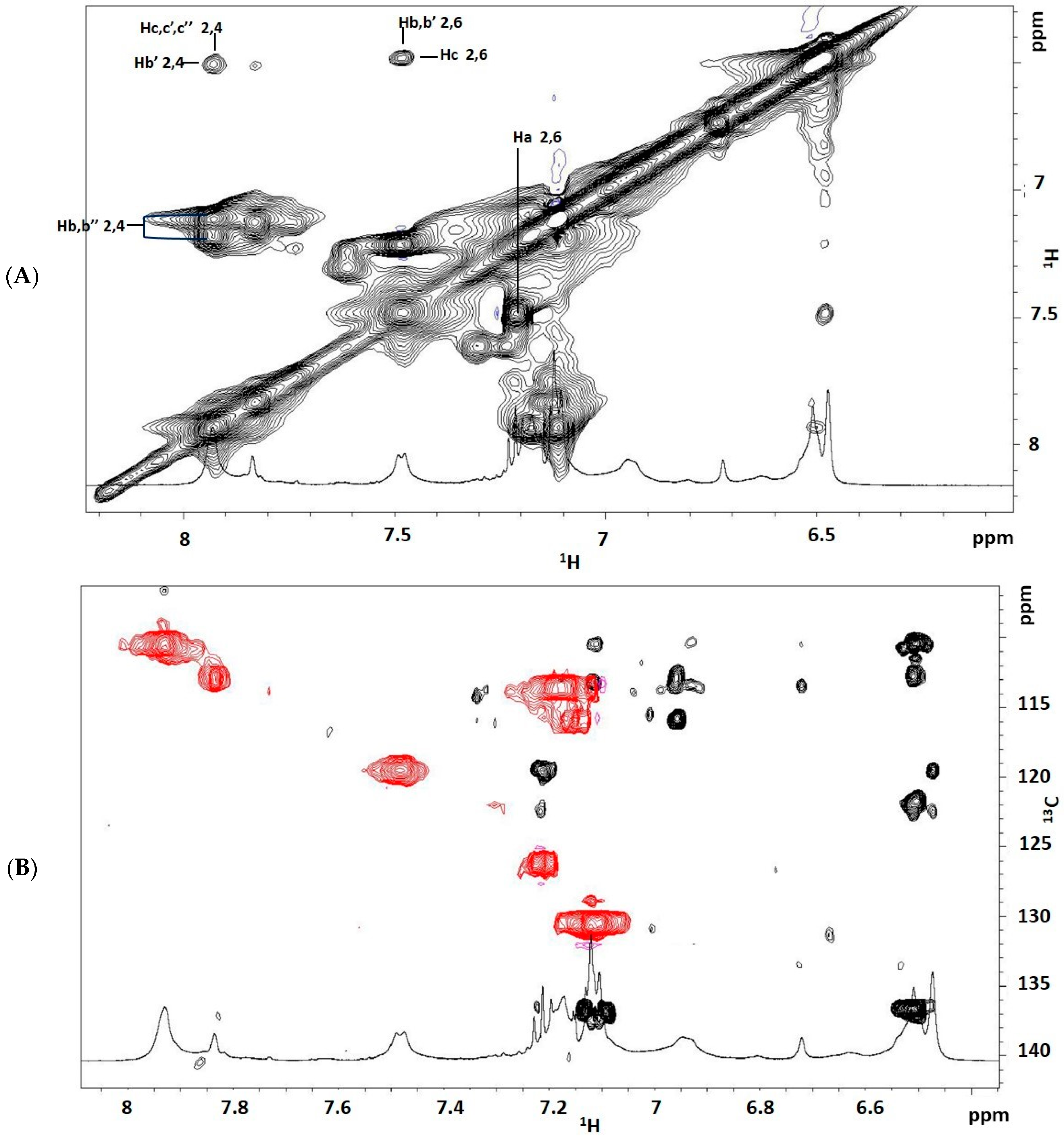
It is evident that the use of the two different polyols makes the NMR spectrum pattern more complex due to all the different expected chemical structures. The spectra on the left refer to the region spanning from 6.0 to 8.4 ppm, corresponding to those resonances arising entirely from aromatic protons of TDI and, for the prepolymer from BPO 2, at 7.2 ppm from proton atoms coming from 3-phenyl butyric acid. Resonances are labelled according to
Scheme 4 and
Scheme 5, where the number refers to the structure and the letter to the proton (i.e., protons 6-Ha are methyl protons of structure 6 of monoreacted structure from 2,6-TDI).
The complex group of signals in the 4.7–5.4 ppm range is due to the methine protons involved in the urethane linkages in bio-polyols confirming the reaction between polyol and TDI and the formation of urethane linkages. In detail, by analyzing spectrum (b) from PPG, and according to the literature [
11], it is evident that the signal at 5.02 ppm can be safely assigned to the PPG methine protons
h’ involved in the urethane linkages (
Scheme 5). Resonance centered at 5.27 ppm corresponds to methine proton of the glycerol structure of bio-polyols.
Looking at the spectra on the right, the two multiples centered at 4.29 and 4.18 ppm, absent in spectrum (b), are related to methylene protons of the glycerol structure of polyols while resonances in the 3.2–3.5 ppm range are assigned according to the literature to the methine h belonging to the internal units and to the methylene protons i and i′ of PPG, as well as to bio-polyols for comparison with the spectra of the starting bio-polyols.
Resonances at 2.10 and 2.22 ppm were assigned to methyl protons in MeOH-capped TDI, while those centered at 2.50 ppm to TDI methyl protons.
The characteristic resonances of the methyl proton atoms in the polyol are located at 0.8 ppm for bio-polyols and in the region spanning from 1.14 to 1.30 ppm for PPG; in detail, these two resonances are due to the methyl protons belonging to the internal portion of the polyether chain (more abundant) and to the terminal units involved in the urethane linkages, respectively.
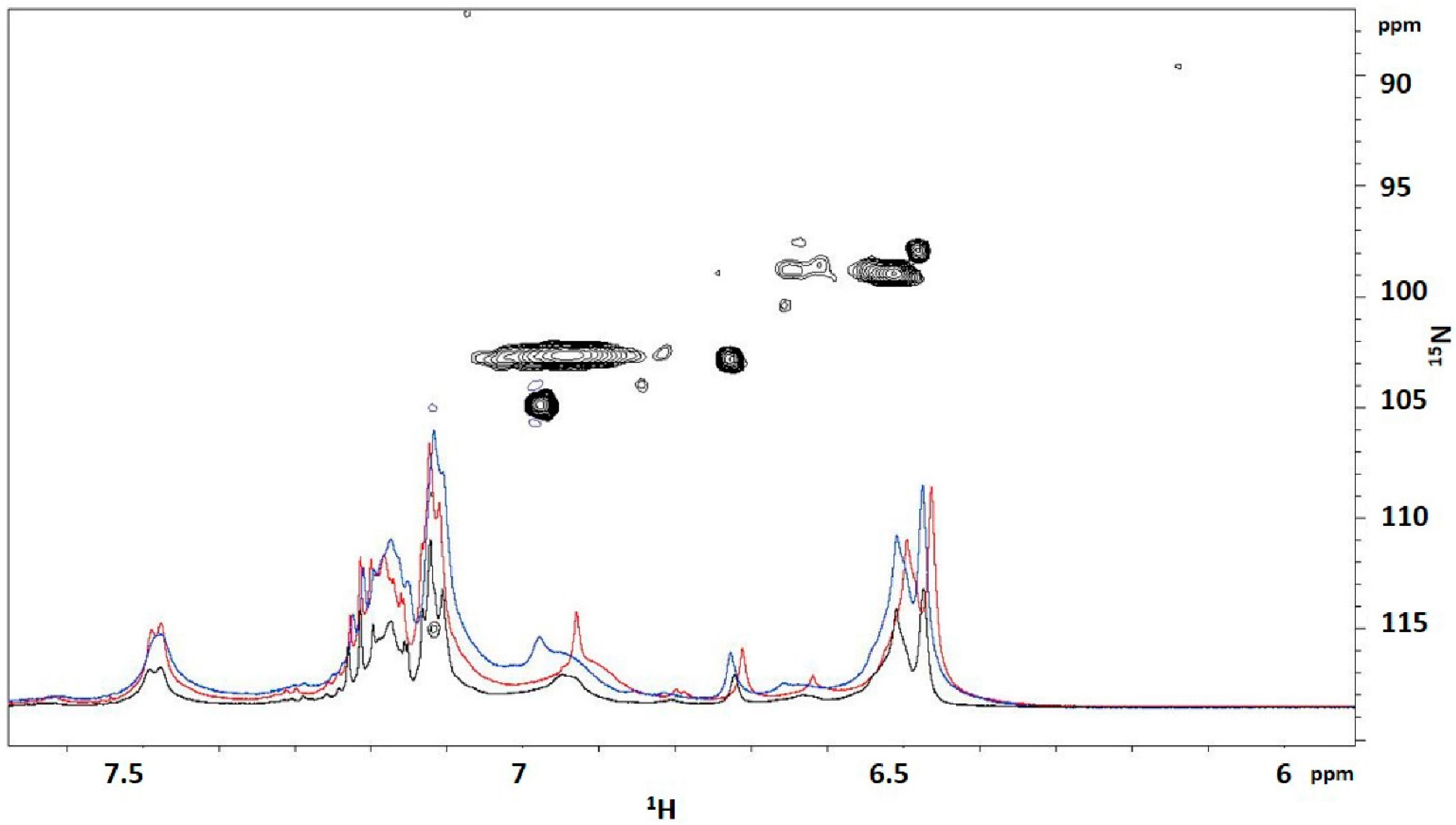
To confirm the presence of the urethane bonds, 1D experiments were carried out at two different temperatures, 25 and 35 °C, allowing the identification of NH resonances. By increasing the temperature or by diluting the sample, NH resonances experience an upfield shift because of the hydrogen bonds progressive breaking, as observed in the 1D overlapping in
Figure 8. To definitely assign urethane resonances, the
15N-HSQC spectra of the prepolymer from BPO 4 were acquired in natural abundance at 25 °C (
Figure 8). In this spectrum, the expected NH resonances (structures 2, 3, 4, 6 and 7 of
Scheme 4) were clearly visible between 6.4 and 7.1 ppm. The upfield shift urethane resonances appeared in a different spectral range, within the region around 8.5, as reported in the literature [
11]. This is due to the absence of H bonds with the solvent since spectra are acquired in CD
2Cl
2. Further experiments are necessary to univocally assign these resonances.
13C HMBC,
13C-HSQC, clean-TOCSY and COSY spectra were acquired to assign the aromatic protons of the prepolymers obtained from toluene 2,4-diisocyanate and toluene 2,6-diisocyanate from now on indicated as reported in
Scheme 4. In
Figure 9, the TOCSY aromatic region is reported which enabled the proton assignment (
Figure 9A), and the overlapping of
13C HMBC and
13C-HSQC (
Figure 9B) which enabled the carbon assignment.
In
Table 3, all the assigned aromatic resonances of the most abundant species are reported.
3.3. PU Foam Development and Formulation Optimization
The formulations of bio-based polyurethane foams are reported in
Table 4 and labelled with the number of the corresponding bio-polyol from
Table 2. For each formulation, a ratio PPG:bio-polyol equal to 1:1 was chosen; a chemical blowing agent (distilled water) was used, and its content kept constant at 2.6 parts per hundred parts (pphp). As blowing catalyst, 1,4-diazabicyclo [2.2.2]octane (DABCO) was tested for all the formulations and a constant amount of dibutyltin dilaurate, DBT, (0.1 pphp) was fixed. Glycerol, 6 wt/wt %, was used to enhance the bio-polyol miscibility with water and the overall reactivity and properties. TDI-1W was prepared from BPO 1 with a double content of water. TDI-3.2P was obtained doubling the amount of BPO 3 (PPG/bio-polyol ratio 1/2), thus achieving the largest content of sustainable material (47 wt %) with respect to other bio-based foams with an average amount of 25 wt %. For the sake of comparison, TDI-ref and TDI-refW were obtained exclusively from PPG in the absence of bio-polyols; moreover, for TDI-refW, a double amount of water was added.
After the curing reaction of all formulations, the absorption of −OH at 3510 cm
−1 and −NCO stretching vibration band at 2260 cm
−1 were not present in the FTIR spectra (see
Figures S2–S6) of all foams listed in
Table 4, thus suggesting that isocyanate and hydroxyl groups are completely reacted in the reaction conditions.
All the developed prepolymers showed a good miscibility with the other components of the formulation. The high viscosity of bio-polyol 1 (470.8 Pa·s, see
Table 2) did not affect the preparation of the foams, without hindering the miscibility and compatibility with PPG. However, the TDI-2 foam was characterized by some yellow-colored striations, probably due to poor homogeneity and miscibility of components. Thus, a too rigid (85 kPa 50% compression deflection value) and quite inhomogeneous foam with high density was produced. A less relevant problem connected with the miscibility of components was also in TDI-4.
The existence of a sharply defined foaming start time, rising time and gel point is a peculiar characteristic of such polyurethane systems. The cream time and the free rise time were recorded during the foaming process and are listed in
Table 5.
Summarizing, the rising was very fast for all foams (up to 45 s). TDI-1 and TDI-1W foams showed the best reactivity (25 and 18 s, respectively) probably due to the peculiar structure of the bio-polyol rich in aromatic moieties. These results seem to suggest that a better compatibility with the isocyanate can be achieved with this BPO. Moreover, in TDI-1W formulation, where double the amount of water was added as blowing agent, the exothermicity of the reaction was well controlled and, despite the short cream time, thorough mixing was achieved.
As observed in our previous study [
7], the relatively high viscosity of the bio-polyols facilitates the formation of the framework of the urethane group in the gel reaction stage between the bio-polyols and isocyanate, resulting in a dense structure. The apparent density of the foams was in the range of 40–90 kg/m
3.
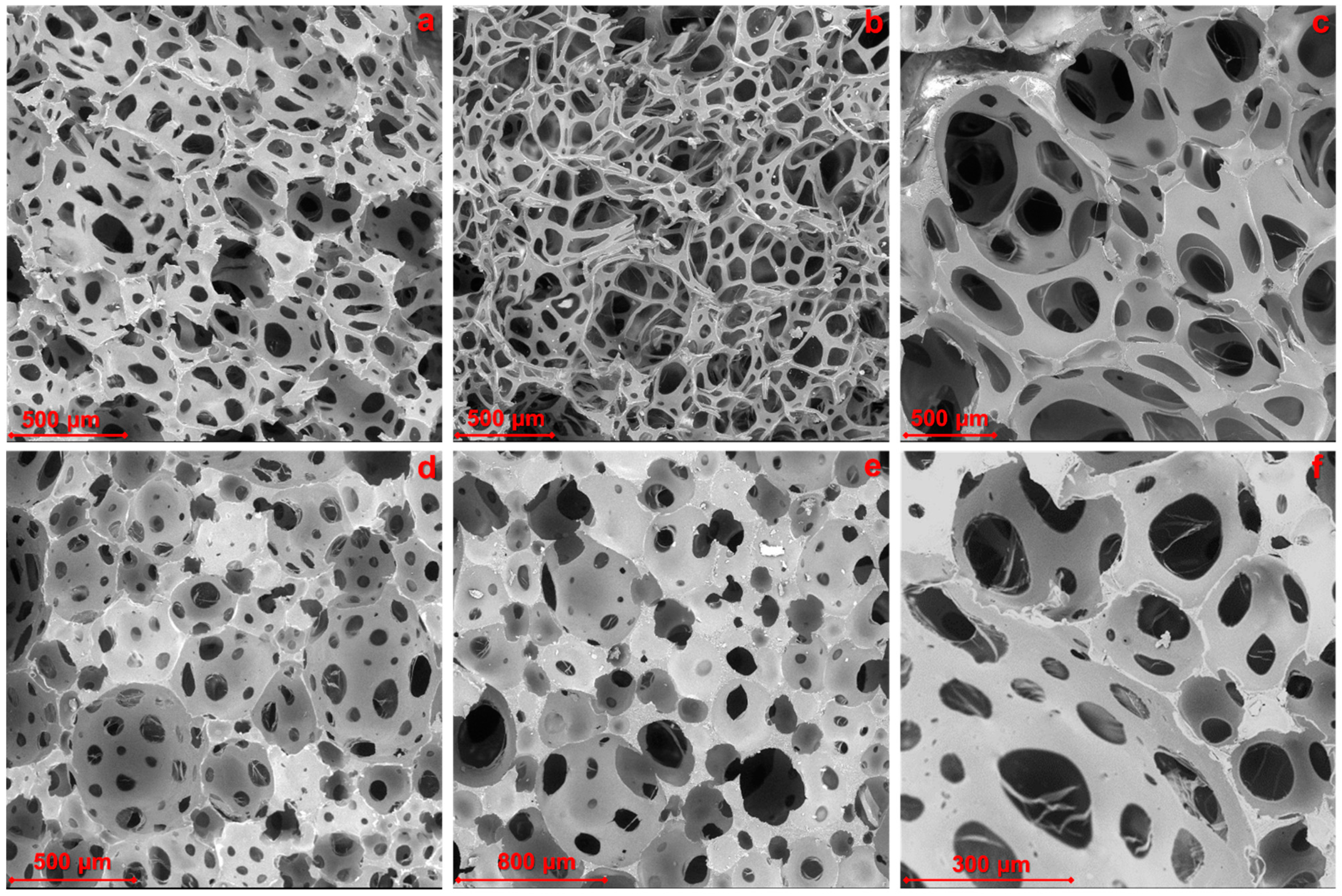
The cells’ cross-section size and surface were determined based on SEM images. The average pore sizes with standard deviations for the different systems are reported in
Table 6. The foam cells had an almost regular shape, indicating the quasi-isotropic growth of the bubble during the foaming process. In the case of samples with double the amount of water, the shape of the cells was less regular. This result may be attributed to high water content inducing a fast-blowing reaction with the isocyanate molecules. The pore size of foams was relatively uniform and presented a variability from 23 to 46% for the samples TDI-5 and TDI-ref, respectively. Recently, the authors reported on the preparation and characterization of formulations with some of the selected bio-polyols and Tolonate
TM, a partially bio-based aliphatic isocyanate [
7]. By comparing the dimensions of the cavities and cells from TDI with those obtained from Tolonate
TM, it was evident that smaller cells, as well as thinner struts formed between interconnecting pores, could be observed in TDI-based formulations. It is probably due to the higher reactivity of the aromatic isocyanate leading to fast crosslinking of the structure.
Figure 10,
Figure S7 and
Figure S8 show micrographs of the cross-section surface of selected foams.
The addition of bio-polyols based on ELO, regardless of the substituent groups, decreased the pore size; in particular, the foam TDI-3, based on ELO-CA, exhibited the lowest average pore size (54 µm). The presence of a double amount of the same bio-polyol in the sample TDI-3.2P resulted in a more than doubled pore size compared to TDI-3 (see
Table 5). For TDI-5 from a partially epoxidized bio-polyol, a larger size of pores could be observed, indicating that the epoxidation degree of the bio-polyol has a great effect on the strut thickness.
The cell morphology of TDI-1W foam obtained by increasing the water content resulted in a very well-developed cavity structure. The average pore size increased and the bulk density decreased with increasing water content. This phenomenon was equally evident in the reference foams (TDI-ref and TDI-refW).
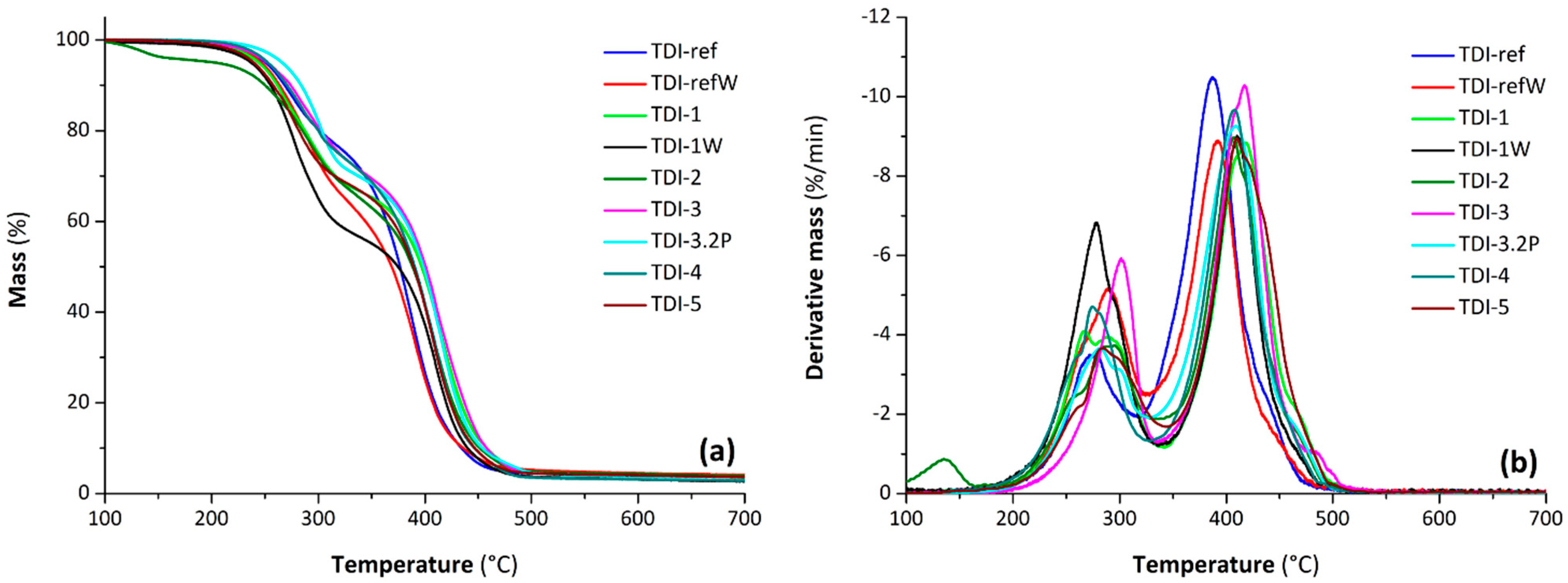
The TGA and DTG curves of the PU foams are shown in
Figure 11.
Generally, the thermograms of all samples presented a two-stage thermal decomposition under an inert atmosphere (
Figure 11a): (i) the first mass-loss stage, recorded in the range from 200 to 320 °C with a mass loss between 20 and 40% (depending on the sample), is related to the dissociation of the urethane bonds [
39]; (ii) the second stage occurred in the range from 320 to 500 °C and is attributed to the decomposition of polyol/bio-polyol components [
40]. It is worth noting that at the second decomposition stage, the reference foam degrades faster than the foams obtained from bio-polyols, as reported in our previous work for the foams based on Tolonate
TM and the same polyol/bio-polyols [
6]; in detail, the mass residue at 400 °C for the reference foams was close to 26%, while for the bio-based foams, it lay between 37 and 52%. TGA experimental data including the temperature at which the initial 5% mass loss occurs (
T5%) and the temperatures of maximum rate of mass loss (
Tmax,1 and
Tmax,2 related to the first and second decomposition stage, respectively) determined by main DTG peaks are summarized in
Table 6.
Regarding the T5%, the values ranged from 237 to 266 °C, except for TDI-2 where the partial presence of unreacted acid led to a mass loss highlighted by a DTG peak centered at 135 °C. The foams prepared with a double amount of water (TDI-1W and TDI-refW) exhibited a thermal stability comparable with the corresponding foams (TDI-1 and TDI-ref, respectively). The presence of a double content of bio-polyol in TDI-3.2P slightly reduced the characteristic degradation temperatures compared to TDI-3; the addition of the partially epoxidized bio-polyol in TDI-5 improved the thermal stability with respect to TDI-4.
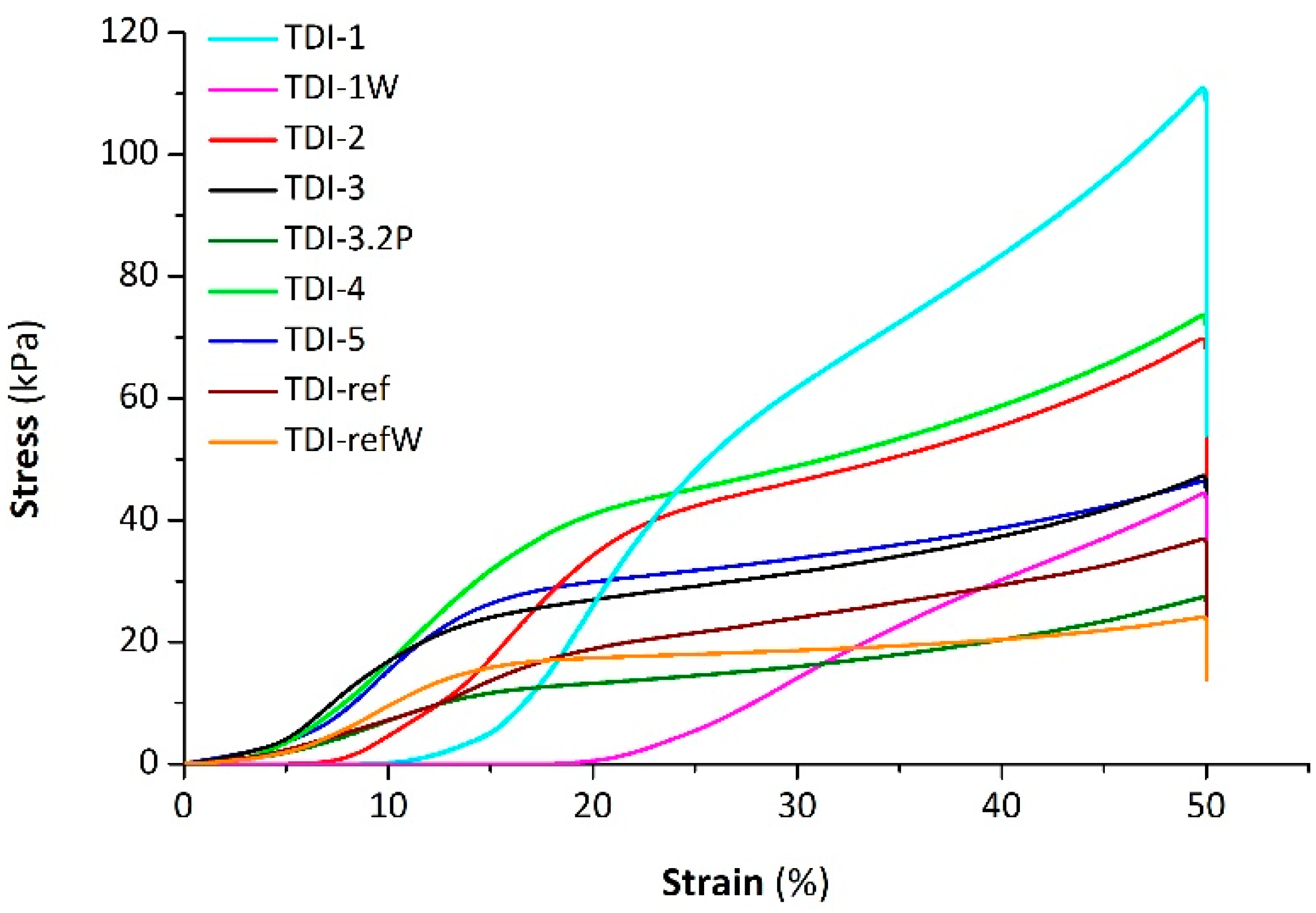
The compressive stress–strain curves of the investigated samples in
Figure 12 show the typical behavior of polyurethane foams: initial linear part, depending on the elastic deformation of the structure, followed by yield and constant enhancement of stress due to partial densification of cells as strain increases.
The mean values of Young’s modulus (
E) and the compression deflection value (
CDV), calculated as the ratio between the final load and the cross-section area of the sample at 50% of strain, are listed in
Table 6. The compressive mechanical properties of the foams prepared with bio-polyols were higher than those of the reference foam. In particular, the presence of polyol based on ELO and 3PBA in TDI-1 caused a greater increase (respectively, 280 and 150%) in the Young’s modulus and compressive deflection value in comparison to the reference foam. This result is strongly correlated to the difference in terms of cavity size between TDI-ref and bio-based foam. Indeed, Gibson et al. demonstrated that the compression strength of ductile cellular materials is inversely proportional to their cell size [
41,
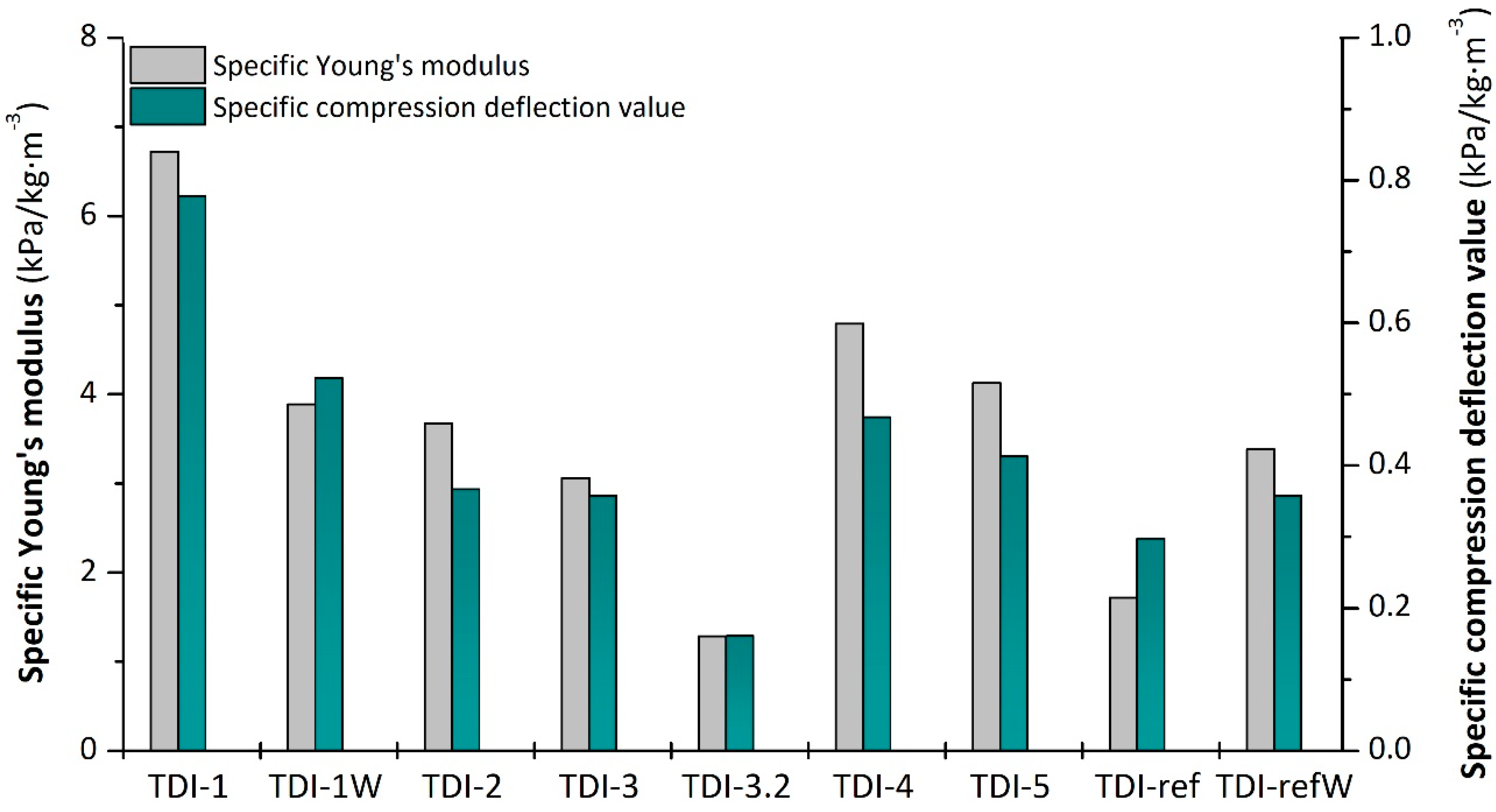
42]. Analogously, the bio-based foam containing the double content of water, i.e., TDI-1W, exhibited an enhancement of compressive properties compared to the respective reference foam (TDI-refW). Moreover, the compression properties of the foams obtained adding partially or totally ESO and CA, respectively, TDI-5 and TDI-4, were higher for the latter foam because of smaller pore size and higher density. As regards TDI-3.2P, mixing a double amount of bio-polyol caused an enhancement in pore size and a 50% decrease in modulus and compressive strength with respect to TDI-3, despite a similar density. The investigated foams differ in apparent density; thus their mechanical properties can be better compared by normalizing with respect to apparent density. The specific Young’s moduli and specific compression deflection values of all the analyzed samples are depicted in
Figure 13 and confirm the trends so far observed.
In general, the bio-based foams showed superior mechanical properties than the reference foams. This interesting result confirmed the possibility of replacing polyurethane foams from fossil resources with bio-based foams endowed with improved mechanical performance.