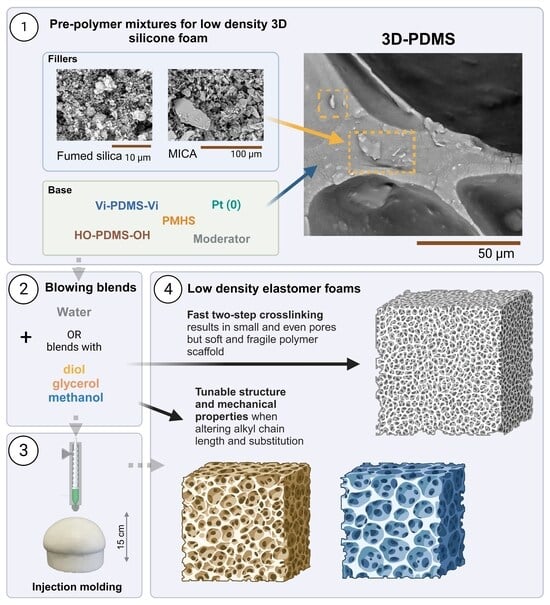
Enhanced Low-Density Silicone Foams Blown by Water–Hydroxyl Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polysiloxane Elastomer Foams
2.2. Determining the Physicomechanical Properties of the Prepared Foams
3. Results and Discussion
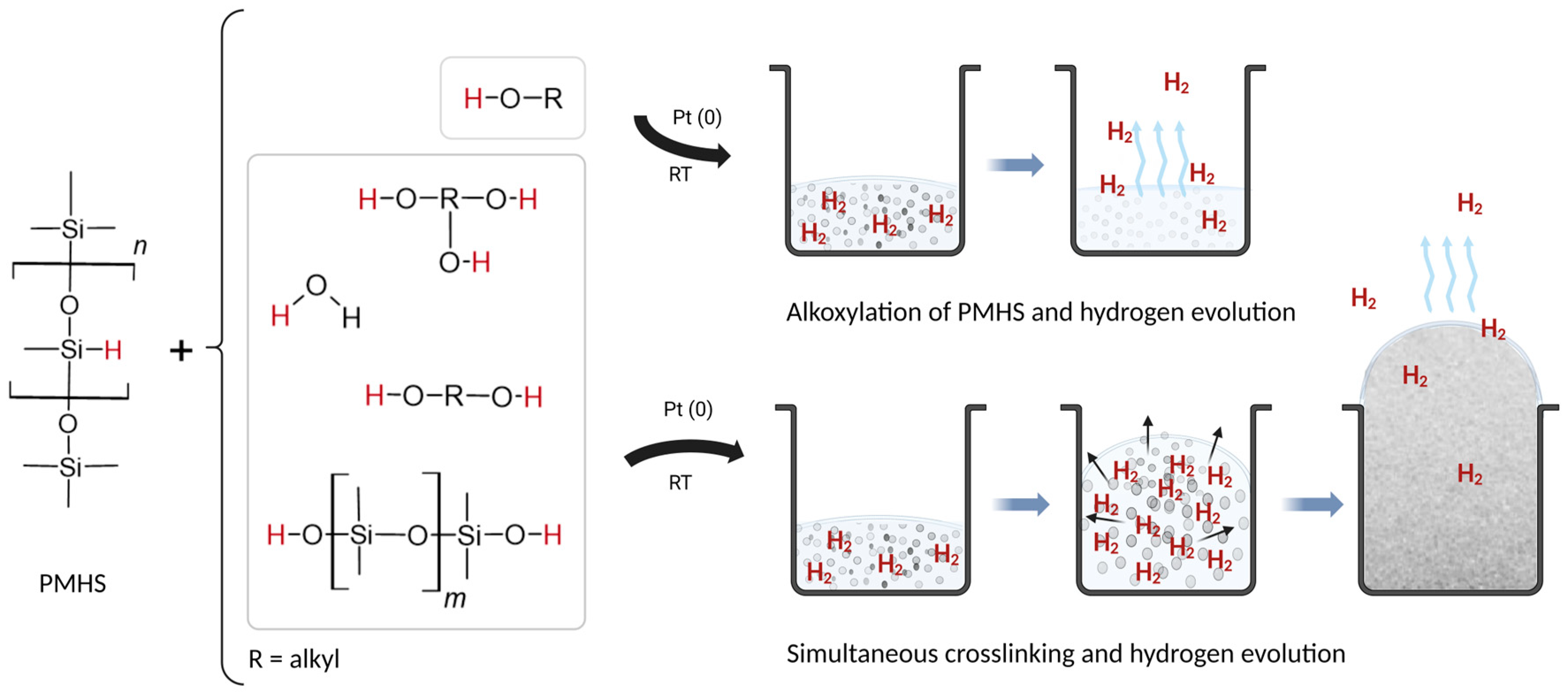
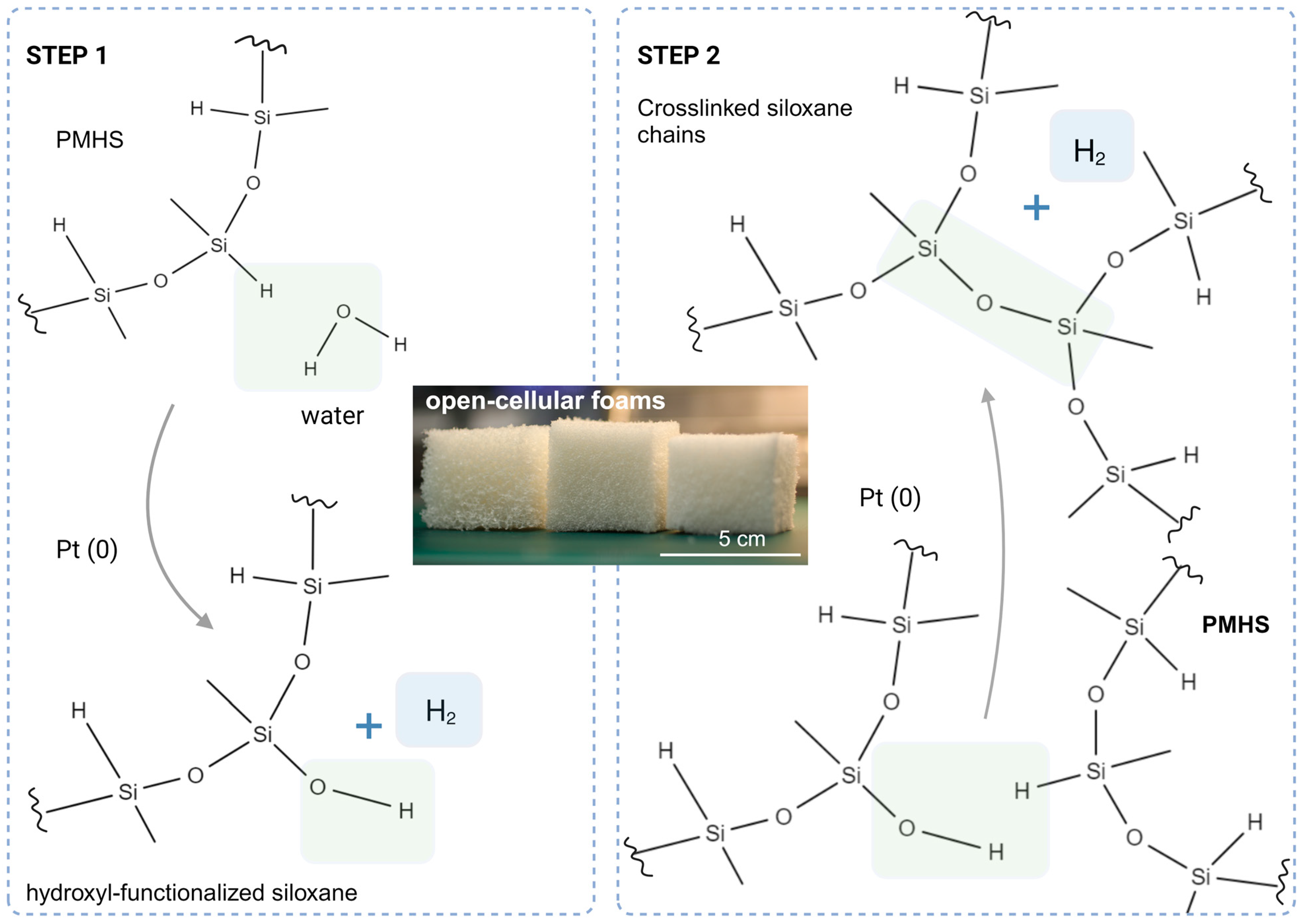
3.1. Reaction Mechanisms of SIF Expansion
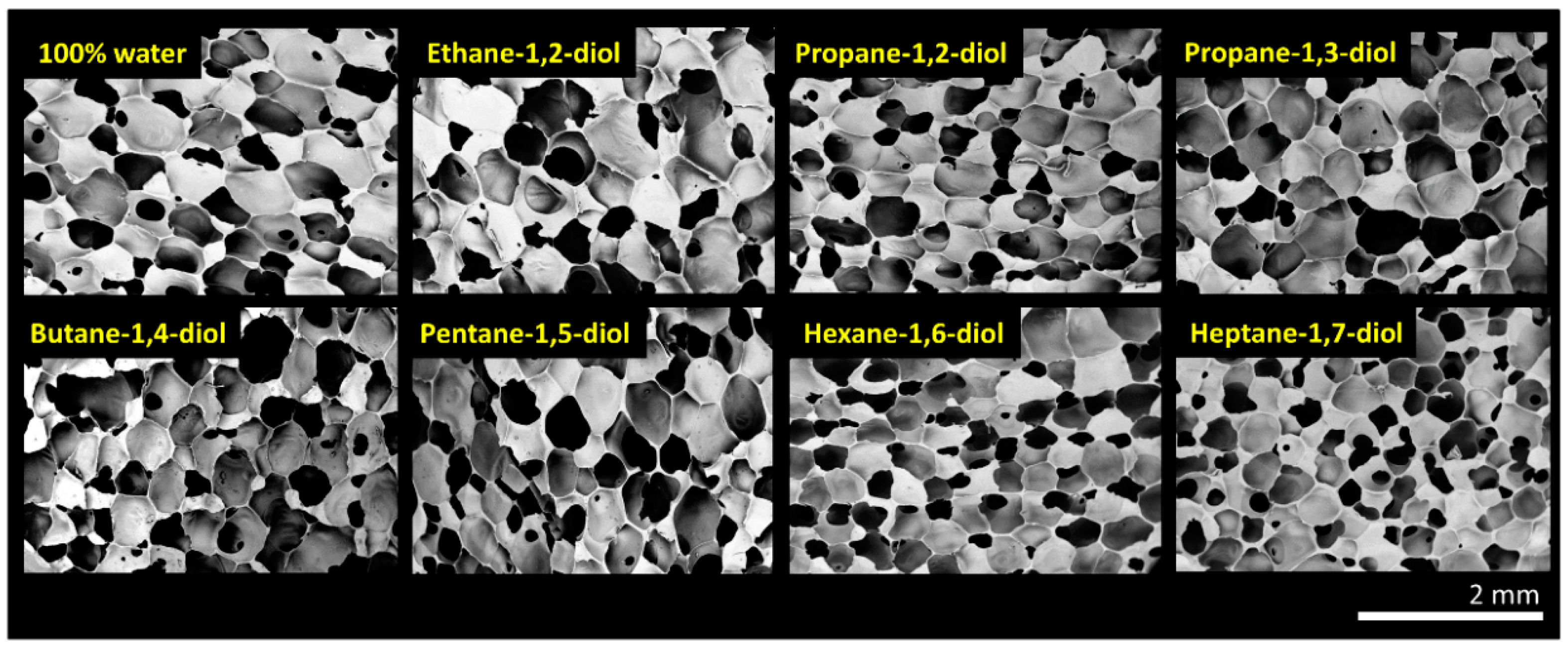
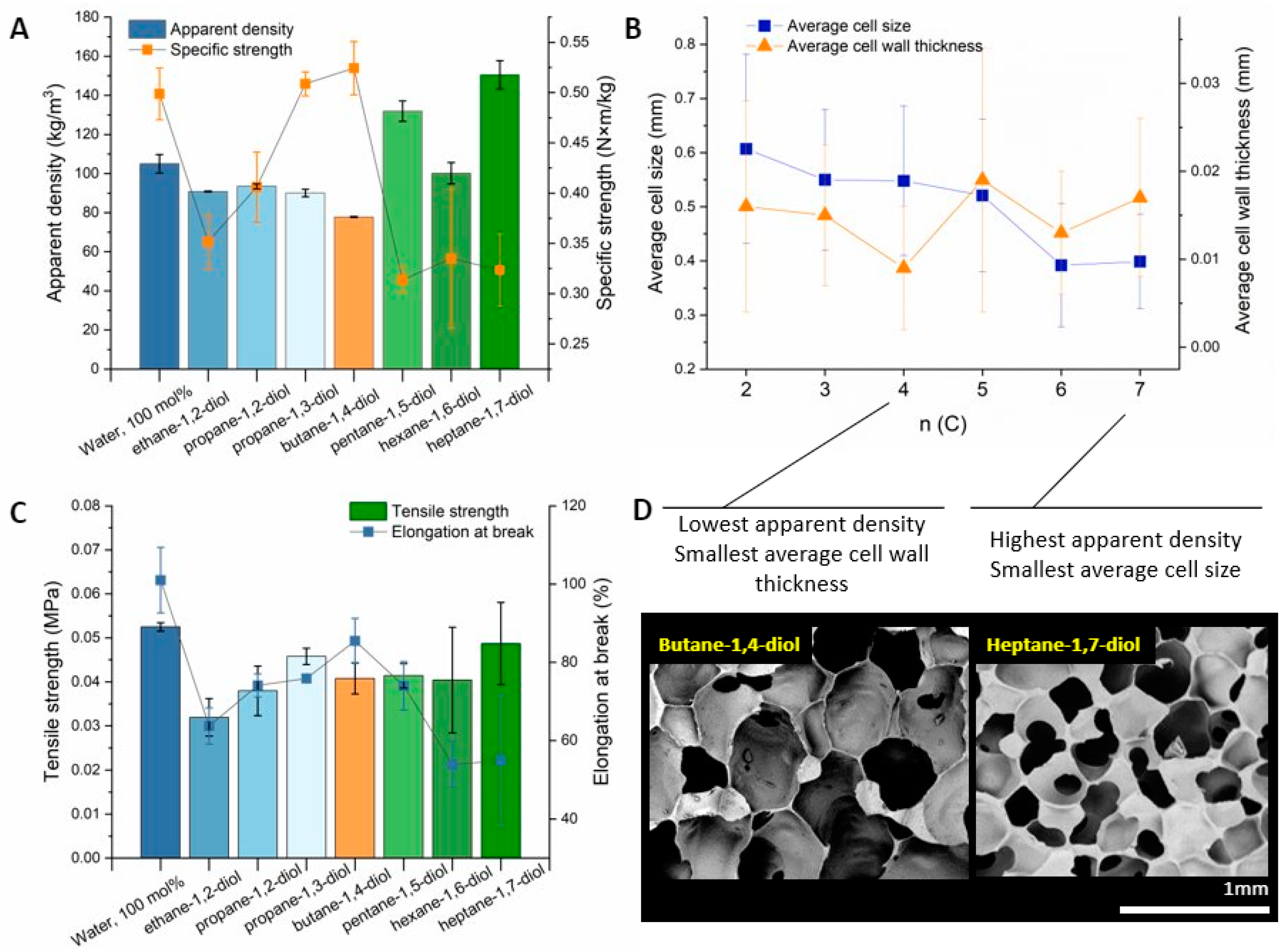
3.2. Tuning Structure and Mechanical Properties of Foams with Diols
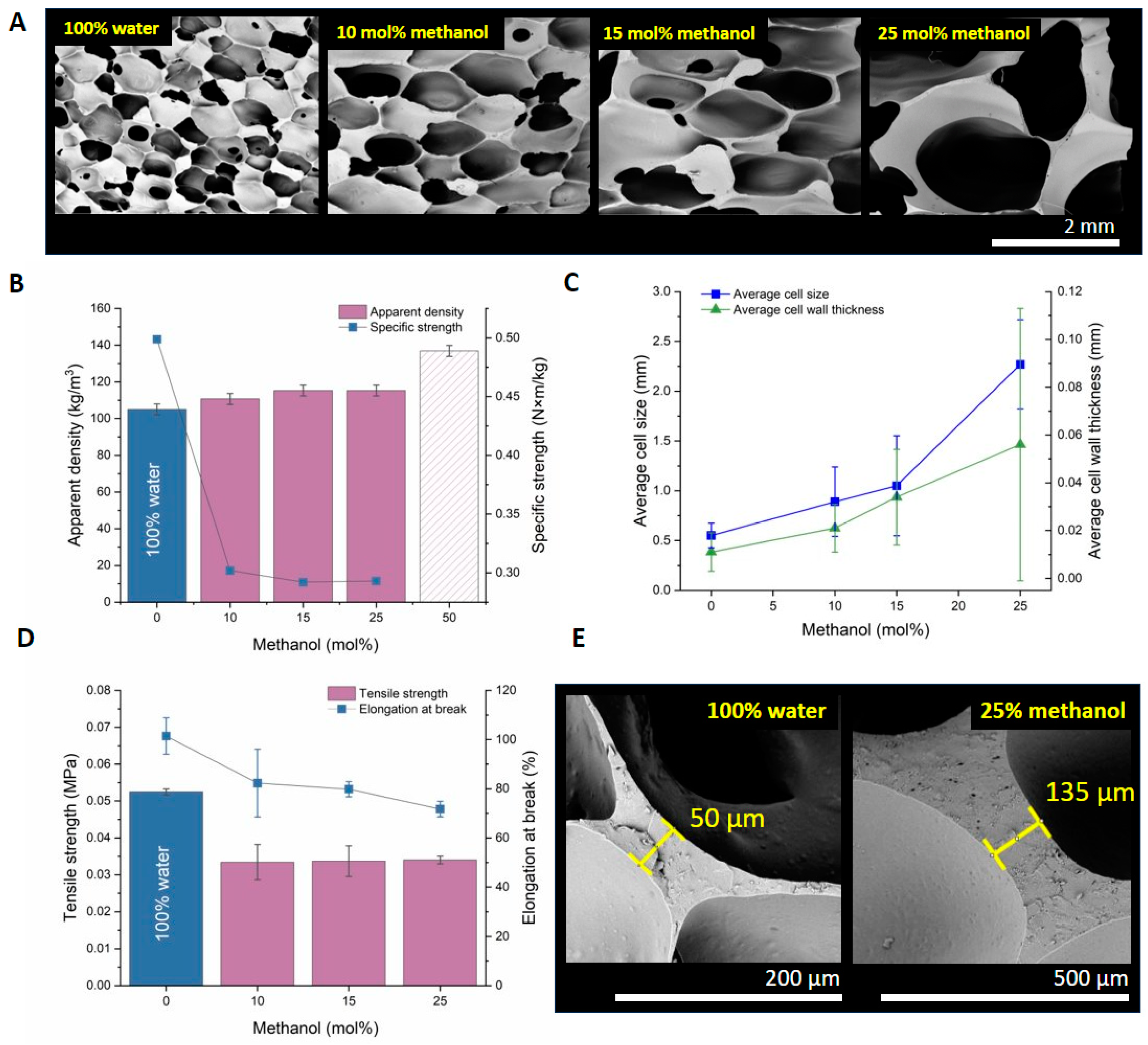
3.3. Tuning of Foam Morphology with Methanol
3.4. Optimizing Tensile Strength and Density with Diols
3.5. Comparison of Water and Water–Diol Blown Foams
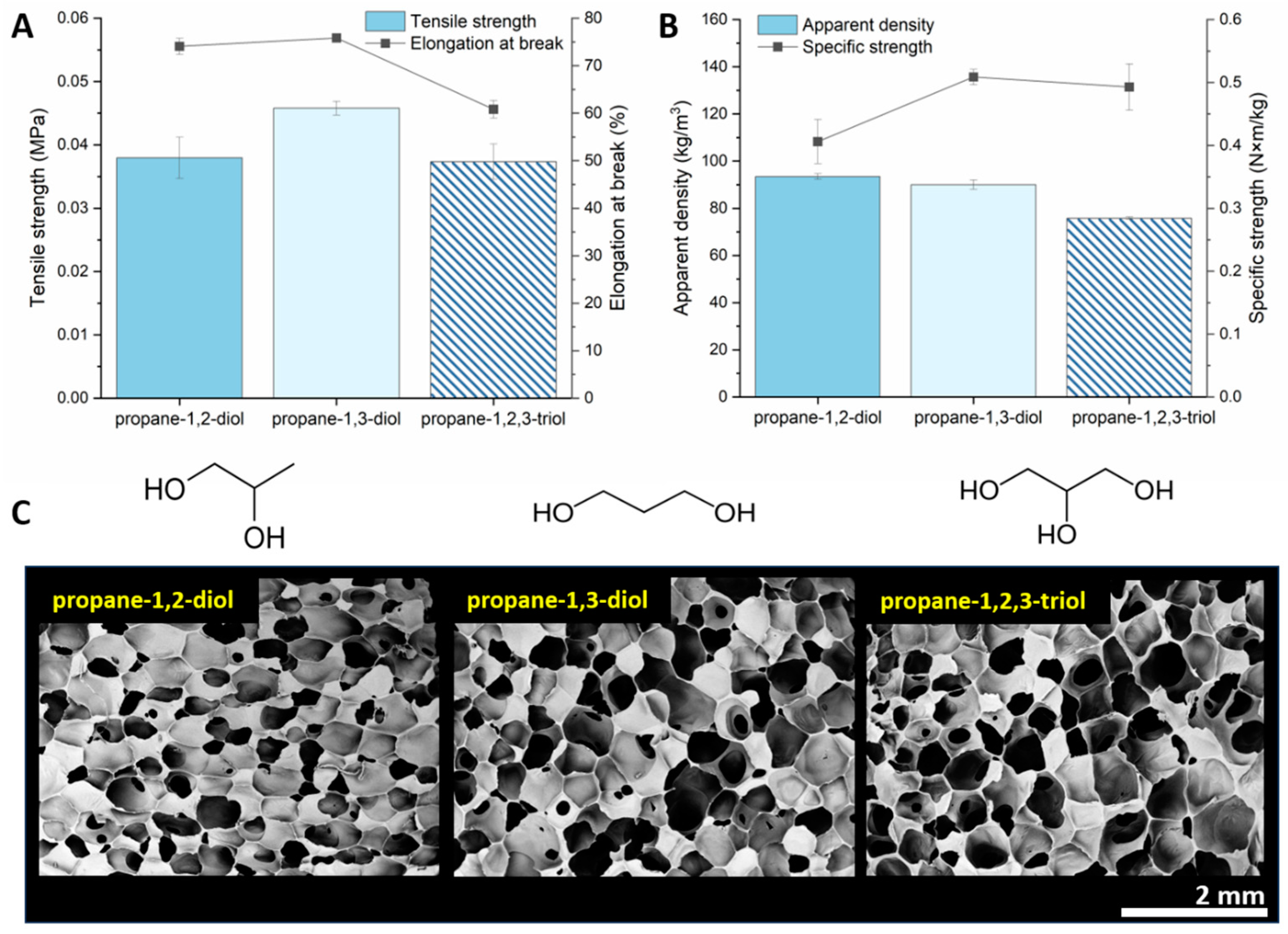
3.6. Effect of Functional Group Position
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Polymer System | Blowing Agent | Density, (kg‧m−3) | Elongation at Break (%), Eb | Tensile Strength, (kPa) | Average Cell Size, (mm) | Average Wall Thickness, (mm) | Comments | Curing | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Water | |||||||||
| Vi-PDMS/OH-PDMS/PMHS | water | 220 | - | - | 0.200–0.720 | 0.030–0.060 | surfactant for W/O emulsion | RT (several minutes) | [26] |
| Vi-PDMS/OH-PDMS/PMHS | water (0.2–1.8 wt%) | 214 | - | - | 0.510 | - | Inhibitor used | 15 min at RT + 2 h at 100 °C | [28] |
| Vi-PDMS/OH-PDMS/PMHS | water (1 wt%) | 105 | 101 | 52.5 | 0.6, SD = 0.2 | 0.011, SD = 0.008 | - | 3 min at RT | This work |
| PDMS/CFPS/D-66 | water (0.07 mL, 1.33%) | 250 | 42 | 35 | >1 | - | isocyanate + water reaction | RT (3–5+ min) | [27] |
| PDMS/CFPS/D-17 | water (0.07 mL, 1.33%) | 240 | - | - | >1 | - | isocyanate + water reaction | RT (3–5+ min) | [27] |
| PDMS/PMDI | water (0.07 mL, 1.12%), CO2 | 270 | - | - | >1, 0.4–1.0 | - | isocyanate + water reaction | RT (3–5+ min) | [27] |
| Elastosil LR 3003/50 | water (1–3 phr) | −52% | - | - | 0.005 (mm2) | n/a | water mixed with silica (8:1) | 140 °C/180 °C + 4 h/200 °C | [3] |
| Alcohols | |||||||||
| Vi-PDMS/H-PDMS/PMHS | ethanol (0 wt%) | 450 | 73 | 325.6 | 0.55 | 0.95 | 3 h at 80 °C | [29] | |
| Vi-PDMS/H-PDMS/PMHS | ethanol (1.5 wt%) | 200 | 31 | 52.8 | 1.6 | 1.4 | 3 h at 80 °C | [29] | |
| Vi-PDMS/OH-PDMS/PMHS | 1:9 methanol/water (1 wt%) | 111 | 84 | 41.8 | 0.9, SD = 0.4 | 0.021, SD = 0.010 | 3 min at RT | This work | |
| Vi-PDMS/OH-PDMS/PMHS | 1:9 butane-1,4-diol/water (1 wt%) | 78 | 86 | 40.8 | 0.6, SD = 0.2 | 0.009, SD = 0.007 | 3 min at RT | This work | |
| Other | |||||||||
| OH-PDMS/PMHS (Rhodorsil RTFoam 3240) | no additional blowing agent, H2 from reaction | 197 | - | - | 0.486 +/− 0.006 | - | RT | [4] | |
| VMQ/DBPMH/MHS | scCO2 | 109–548 | - | - | 0.073–0.291 | 0.019 | compounded rubber sheets + scCO2 | 50 °C to 80 °C | [42] |
| Combinative | |||||||||
| Sylgard 184 | glycerol (137 phr)/ethanol (1 wt%) | 282 | - | - | 1.37 a | - | 120 °C | [32] | |
| Sylgard 184 | glycerol (110 phr)/ethanol (1.1 wt%) | 300 | - | - | 1.63 a | - | 120 °C | [32] | |
| Vi-PDMS/OH-PDMS/PMHS | 10 mol% glycerol/water (1 wt%) | 76 | 61 | 37.3 | 0.632, SD = 0.141 | 0.016, SD = 0.010 | 3 min at RT | This work | |
| R-(OH)n | Diol, (mol%) | Water, (mol%) | Average Wall Thickness, (mm) | Average Cell Size, (mm) | Apparent Density, (kg‧m−3) (U = 3 kg‧m−3) |
|---|---|---|---|---|---|
| Water | 0 | 100 | 0.011, SD = 0.008 | 0.6, SD = 0.2 | 105 |
| Ethane-1,2-diol | 10 | 90 | 0.016, SD = 0.012 | 0.6, SD = 0.2 | 91 |
| Propane-1,2-diol | 10 | 90 | 0.013, SD = 0.008 | 0.6, SD = 0.2 | 94 |
| Propane-1,3-diol | 10 | 90 | 0.015, SD = 0.008 | 0.6, SD = 0.2 | 90 |
| Butane-1,4-diol | 10 | 90 | 0.009, SD = 0.007 | 0.6, SD = 0.2 | 78 |
| Pentane-1,5-diol | 10 | 90 | 0.019, SD = 0.015 | 0.5, SD = 0.2 | 132 |
| Hexane-1,6-diol | 10 | 90 | 0.013, SD = 0.007 | 0.4, SD = 0.2 | 100 |
| Heptane-1,7-diol | 10 | 90 | 0.017, SD = 0.009 | 0.4, SD = 0.1 | 151 |
| R-(OH)n | Alkanol, (mol%) | Water, (mol%) | Average Wall Thickness, (mm) | Average Cell Size, (mm) | Apparent Density, (kg‧m−3) (U = 3 kg‧m−3) |
|---|---|---|---|---|---|
| Methanol | 0 | 100 | 0.011, SD = 0.008 | 0.6, SD = 0.2 | 105 |
| 10 | 90 | 0.021, SD = 0.010 | 0.9, SD = 0.4 | 111 | |
| 15 | 85 | 0.034, SD = 0.020 | 1.1, SD = 0.5 | 115 | |
| 25 | 75 | 0.056, SD = 0.056 | 2.3, SD = 0.5 | 115 |
References
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Kwak, Y.; Kang, Y.; Park, W.; Jo, E.; Kim, J. Fabrication of fine-pored polydimethylsiloxane using an isopropyl alcohol and water mixture for adjustable mechanical, optical, and thermal properties. RSC Adv. 2021, 11, 18061–18067. [Google Scholar] [CrossRef]
- Marl, S.; Rüppel, A.; Hartung, M.; Klier, K.; Giesen, R.-U.; Heim, H.-P. Liquid Silicone Rubber Foams Made with Water as Blowing Agent. Adv. Eng. Mater. 2022, 24, 2100382. [Google Scholar] [CrossRef]
- Verdejo, R.; Saiz-Arroyo, C.; Carretero-Gonzalez, J.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Lopez-Manchado, M.A. Physical properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur. Polym. J. 2008, 44, 2790–2797. [Google Scholar] [CrossRef]
- Chen, B.; Qian, Z.; Song, G.; Zhuang, Z.; Sun, X.; Ma, S.; Liang, Y.; Ren, L.; Ren, L. Gas-permeable and stretchable on-skin electronics based on a gradient porous elastomer and self-assembled silver nanowires. Chem. Eng. J. 2023, 463, 142350. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Timusk, M.; Nigol, I.A.; Vlassov, S.; Oras, S.; Kangur, T.; Linarts, A.; Šutka, A. Low-density PDMS foams by controlled destabilization of thixotropic emulsions. J. Colloid Interface Sci. 2022, 626, 265–275. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Skov, A.L. How to tailor flexible silicone elastomers with mechanical integrity: A tutorial review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Bäumer, M.; Schowalter, M.; Birkenstock, J.; Wilhelm, M.; Grathwohl, G. Generation of Pt- and Pt/Zn-containing ceramers and their structuring as macro/microporous foams. Chem. Eng. J. 2014, 247, 205–215. [Google Scholar] [CrossRef]
- Obi, B.E. Foaming Processes. In Polymeric Foams Structure-Property-Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–188. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, L.; Wang, Y.; Xu, T.; Zhang, C. Thermal insulation and stability of polysiloxane foams containing hydroxyl-terminated polydimethylsiloxanes. RSC Adv. 2018, 8, 9901–9909. [Google Scholar] [CrossRef]
- Dezaki, M.L.; Bodaghi, M. Soft Magneto-Responsive Shape Memory Foam Composite Actuators. Macro Mater. Amp. Eng. 2022, 307, 2200490. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Wang, G.; Chen, J.; Guo, F.; Tang, W.; Wang, W.; Yan, Z.; Li, G. Gradient structure design of lightweight and flexible silicone rubber nanocomposite foam for efficient electromagnetic interference shielding. Chem. Eng. J. 2020, 390, 124589. [Google Scholar] [CrossRef]
- Ghahramani, P.; Moradi-Dastjerdi, R.; Behdinan, K.; Naguib, H.E. Mechanical characterization of multifunctional highly porous carbon nanotube-reinforced foams. Polym. Compos. 2023, 44, 2093–2101. [Google Scholar] [CrossRef]
- Gonzalez, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tiné, M.R. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, Y.; Yang, H.; Oh, J.H. Fabrication of hierarchically porous structured PDMS composites and their application as a flexible capacitive pressure sensor. Compos. Part B Eng. 2021, 211, 108607. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Cheng, S.; Liu, C.; Gao, Q.; Lian, W.; Yuan, Y.; Li, A.; Li, C.; Guan, S. Polydimethylsiloxane Composite Sponge Decorated with Graphene/Carbon Nanotube via Polydopamine for Multifunctional Applications. ACS Appl. Polym. Mater. 2023, 5, 6022–6033. [Google Scholar] [CrossRef]
- Abshirini, M.; Saha, M.C.; Cummings, L.; Robison, T. Synthesis and characterization of porous polydimethylsiloxane structures with adjustable porosity and pore morphology using emulsion templating technique. Polym. Eng. Sci. 2021, 61, 1943–1955. [Google Scholar] [CrossRef]
- Yan, J.; Cao, J.; Xue, L.; Feng, S.; Zhang, H.; Wang, D. Thiol Oxidative Coupling Synthesis of Silicone Foams for Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2, 1634–1643. [Google Scholar] [CrossRef]
- Grande, J.B.; Fawcett, A.S.; McLaughlin, A.J.; Gonzaga, F.; Bender, T.P.; Brook, M.A. Anhydrous formation of foamed silicone elastomers using the Piers–Rubinsztajn reaction. Polymer 2012, 53, 3135–3142. [Google Scholar] [CrossRef]
- Mabe, A.; Perez, L.P.; Wu, A.; Wilson, T. Effects of Varying Composition and Kinetics on the Microstructural and Mechanical Properties of Polysiloxane Foams; LLNL-TR-748401; Lawrence Livermore National Laboratory (LLNL): Livermore, CA, USA, 2018. [Google Scholar] [CrossRef]
- Coste, G.; Negrell, C.; Caillol, S. From gas release to foam synthesis, the second breath of blowing agents. Eur. Polym. J. 2020, 140, 110029. [Google Scholar] [CrossRef]
- Brook, M.A. New Control over Silicone Synthesis using SiH Chemistry: The Piers-Rubinsztajn Reaction. Chem. Eur. J. 2018, 24, 8458–8469. [Google Scholar] [CrossRef] [PubMed]
- Modic, F.J.; Striker, R.A. Low Viscosity Silicone Foam Compositions. EP0355380A2, 28 February 1990. [Google Scholar]
- Riesco, R.; Boyer, L.; Blosse, S.; Lefebvre, P.M.; Assemat, P.; Leichle, T.; Accardo, A.; Malaquin, L. Water-in-PDMS Emulsion Templating of Highly Interconnected Porous Architectures for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 28631–28640. [Google Scholar] [CrossRef]
- Guo, B.F.; Wang, P.H.; Cao, C.F.; Qu, Z.H.; Lv, L.Y.; Zhang, G.D.; Gong, L.-X.; Song, P.; Gao, J.-F.; Mai, Y.W.; et al. Restricted assembly of ultralow loading of graphene oxide for lightweight, mechanically flexible and flame retardant polydimethylsiloxane foam composites. Compos. Part B 2022, 247, 110290. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Preparation of flexible, self-extinguishing silicone foams. J. Appl. Polym. Sci. 2011, 119, 1696–1703. [Google Scholar] [CrossRef]
- Cao, C.F.; Wang, P.H.; Zhang, J.W.; Guo, K.Y.; Li, Y.; Xia, Q.Q.; Zhang, G.D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-step and green synthesis of lightweight, mechanically flexible and flame-retardant polydimethylsiloxane foam nanocomposites via surface-assembling ultralow content of graphene derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Tan, Y.; Yao, J.; Zhu, H.-P. Effects of ethanol content on the properties of silicone rubber foam. J. Polym. Eng. 2020, 40, 543–550. [Google Scholar] [CrossRef]
- Nakagawa, S.; Xia, J.; Yoshie, N. Quantifying the effects of cooperative hydrogen bonds between vicinal diols on polymer dynamics. Soft Matter 2022, 18, 1275–1286. [Google Scholar] [CrossRef]
- Mazurek, P.; Hvilsted, S.; Skov, A.L. Green silicone elastomer obtained from a counterintuitively stable mixture of glycerol and PDMS. Polymer 2016, 87, 1–7. [Google Scholar] [CrossRef]
- Mazurek, P.; Ekbrant, B.E.F.; Madsen, F.B.; Yu, L.; Skov, A.L. Glycerol-silicone foams—Tunable 3-phase elastomeric porous materials. Eur. Polym. J. 2019, 113, 107–114. [Google Scholar] [CrossRef]
- ISO 1798:2008; Flexible Cellular Polymeric Materials—Determination of Tensile Strength and Elongation at Break. ISO: Geneva, Switzerland, 2008.
- ASTM D 3574-17; Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams. ASTM: West Conshohocken, PA, USA, 2017.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Liao, M.; Schneider, A.F.; Laengert, S.E.; Gale, C.B.; Chen, Y.; Brook, M.A. Living synthesis of silicone polymers controlled by humidity. Eur. Polym. J. 2018, 107, 287–293. [Google Scholar] [CrossRef]
- Yang, K.; Cai, Z.; Jaiswal, A.; Tyagi, M.; Moore, J.S.; Zhang, Y. Dynamic Odd-Even Effect in Liquid n-Alkanes near Their Melting Points. Angew. Chem. Int. Ed. 2016, 55, 14090–14095. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, M.-C.D.; Blanc, D.; Chaumont, P.; Cassagnau, P. Study of the Coalescence Mechanisms during Silicone Foaming: Study of the Coalescence Mechanisms During Silicone Foaming. Macromol. Mater. Eng. 2014, 299, 336–343. [Google Scholar] [CrossRef]
- Pang, Y.; Cao, Y.; Zheng, W.; Park, C.B. A comprehensive review of cell structure variation and general rules for polymer microcellular foams. Chem. Eng. J. 2022, 430, 132662. [Google Scholar] [CrossRef]
- Rebane, I.; Mäeorg, U.; Johanson, U.; Ilisson, M.; Piirimägi, P.; Tamm, T. Kinetics of catalyzed dehydrocondensation of hydrogen functionalized siloxane. J. Appl. Polym. Sci 2022, 139, 52304. [Google Scholar] [CrossRef]
- Sato, T.; Buchner, R. The cooperative dynamics of the H-bond system in 2-propanol/water mixtures: Steric hindrance effects of nonpolar head group. J. Chem. Phys. 2003, 119, 10789–10800. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Luo, Y.; Liao, X.; Tang, W.; Yang, J.; Tian, C.; Li, G. Design of lightweight silicone rubber foam for outstanding deformation recoverability based on supercritical CO2 foaming technology. J. Mater. Sci. 2022, 57, 2292–2304. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebane, I.; Levin, K.J.; Mäeorg, U.; Johanson, U.; Piirimägi, P.; Tätte, T.; Tamm, T. Enhanced Low-Density Silicone Foams Blown by Water–Hydroxyl Blends. Polymers 2023, 15, 4425. https://doi.org/10.3390/polym15224425
Rebane I, Levin KJ, Mäeorg U, Johanson U, Piirimägi P, Tätte T, Tamm T. Enhanced Low-Density Silicone Foams Blown by Water–Hydroxyl Blends. Polymers. 2023; 15(22):4425. https://doi.org/10.3390/polym15224425
Chicago/Turabian StyleRebane, Ingrid, Karl Jakob Levin, Uno Mäeorg, Urmas Johanson, Peeter Piirimägi, Tauri Tätte, and Tarmo Tamm. 2023. "Enhanced Low-Density Silicone Foams Blown by Water–Hydroxyl Blends" Polymers 15, no. 22: 4425. https://doi.org/10.3390/polym15224425
APA StyleRebane, I., Levin, K. J., Mäeorg, U., Johanson, U., Piirimägi, P., Tätte, T., & Tamm, T. (2023). Enhanced Low-Density Silicone Foams Blown by Water–Hydroxyl Blends. Polymers, 15(22), 4425. https://doi.org/10.3390/polym15224425