Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Characterisation of Films
2.3.1. Final Concentration of Phenolic Acids in the Films
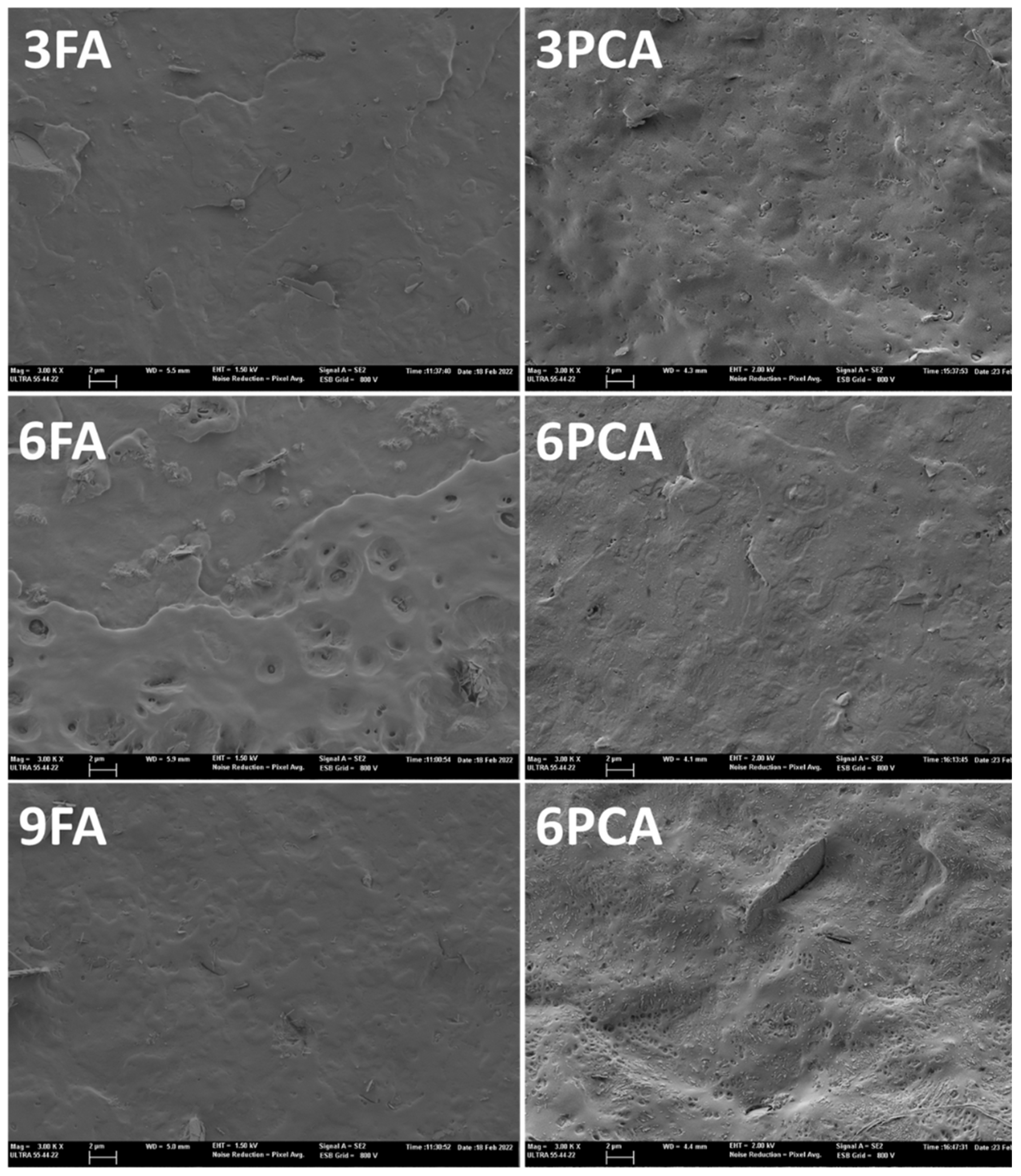
2.3.2. Microstructural Analyses
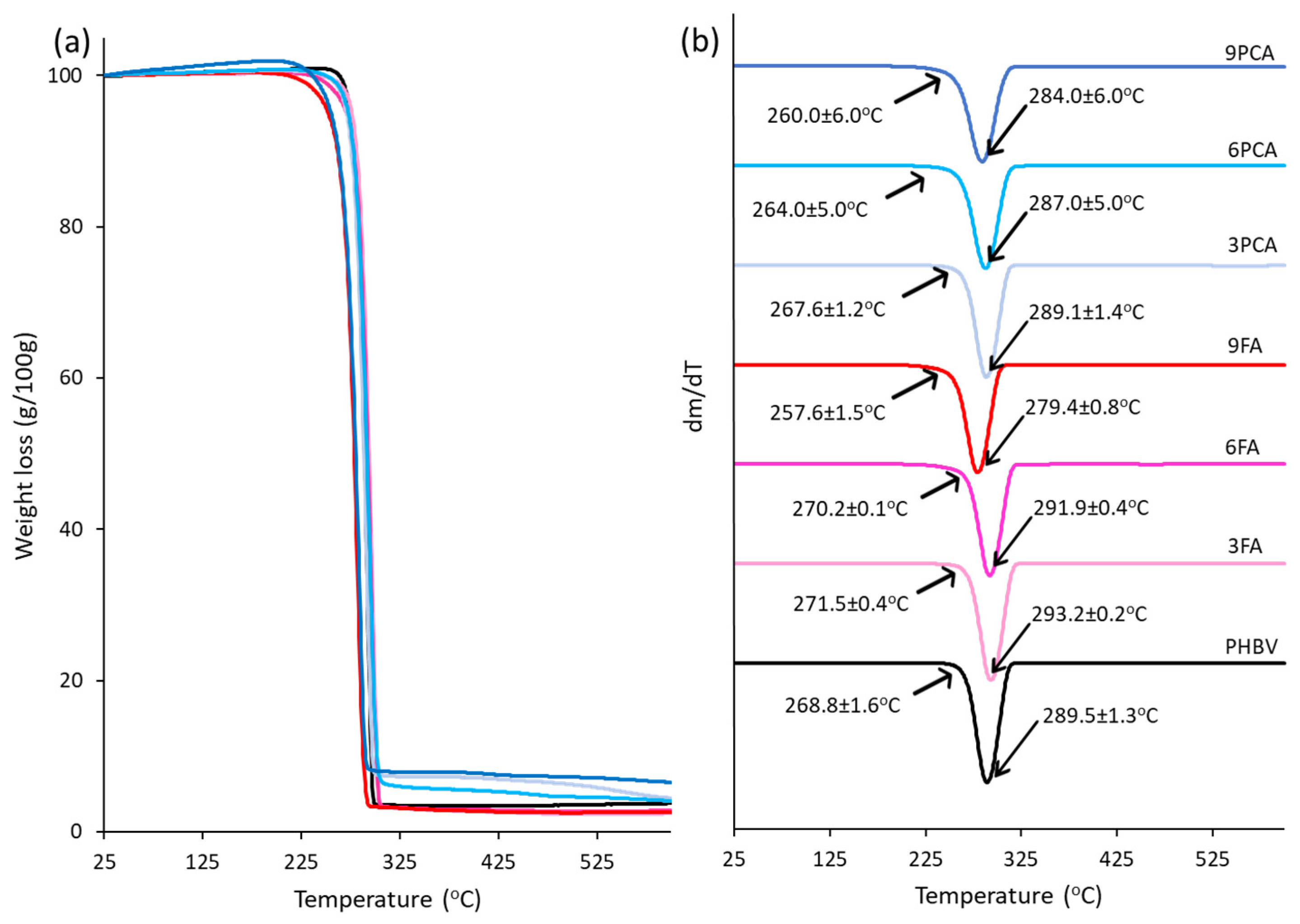
2.3.3. Thermal Analyses
2.3.4. X-ray Diffraction (XRD)
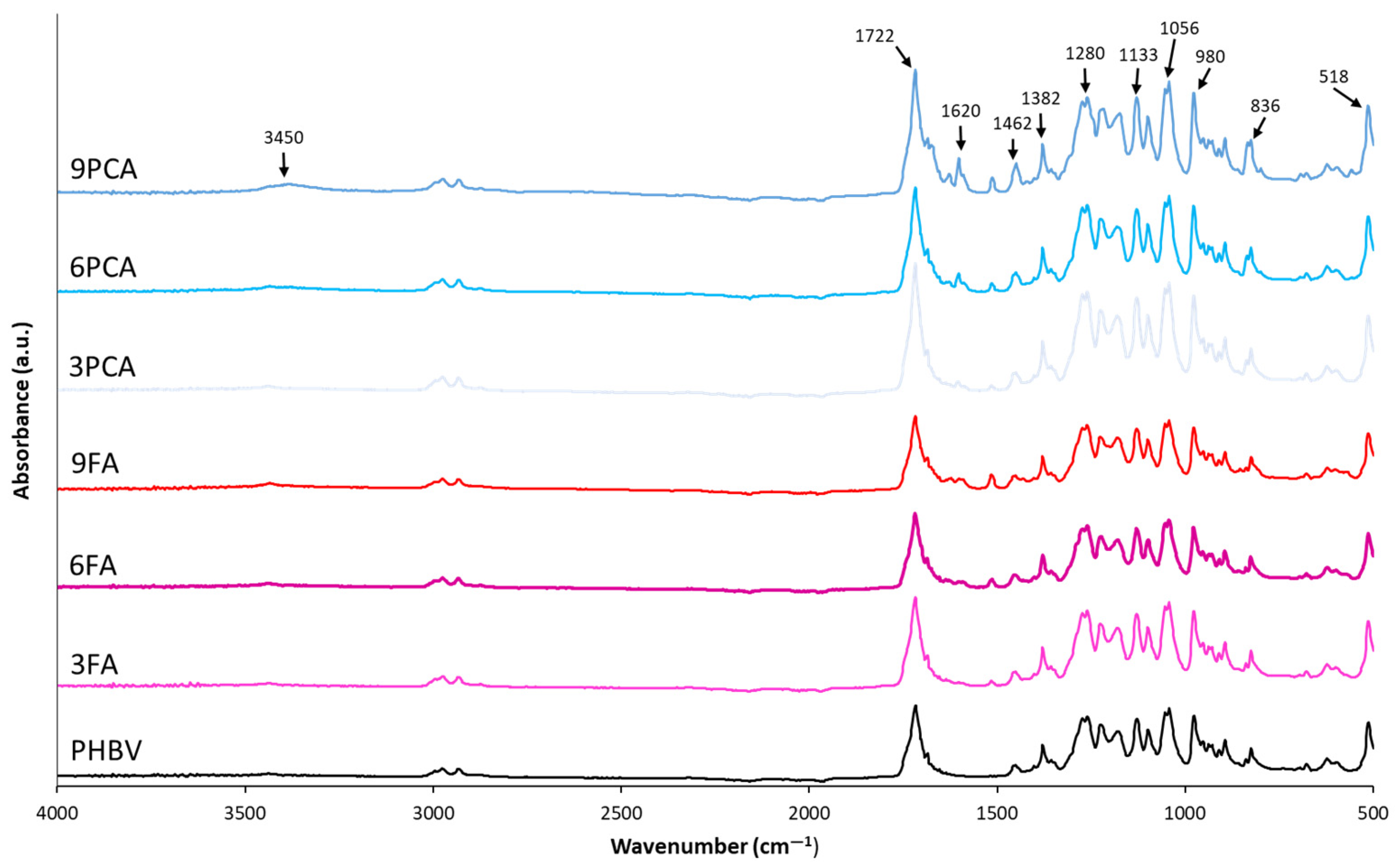
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.6. Tensile Properties
2.3.7. Barrier Properties
2.3.8. Optical Properties
2.4. Migration Analyses in Different Food Simulants
2.5. Statistical Analysis
3. Results and Discussion
3.1. Structural and Molecular Properties of the Films
3.2. Thermal Behaviour and Crystallinity of the Films
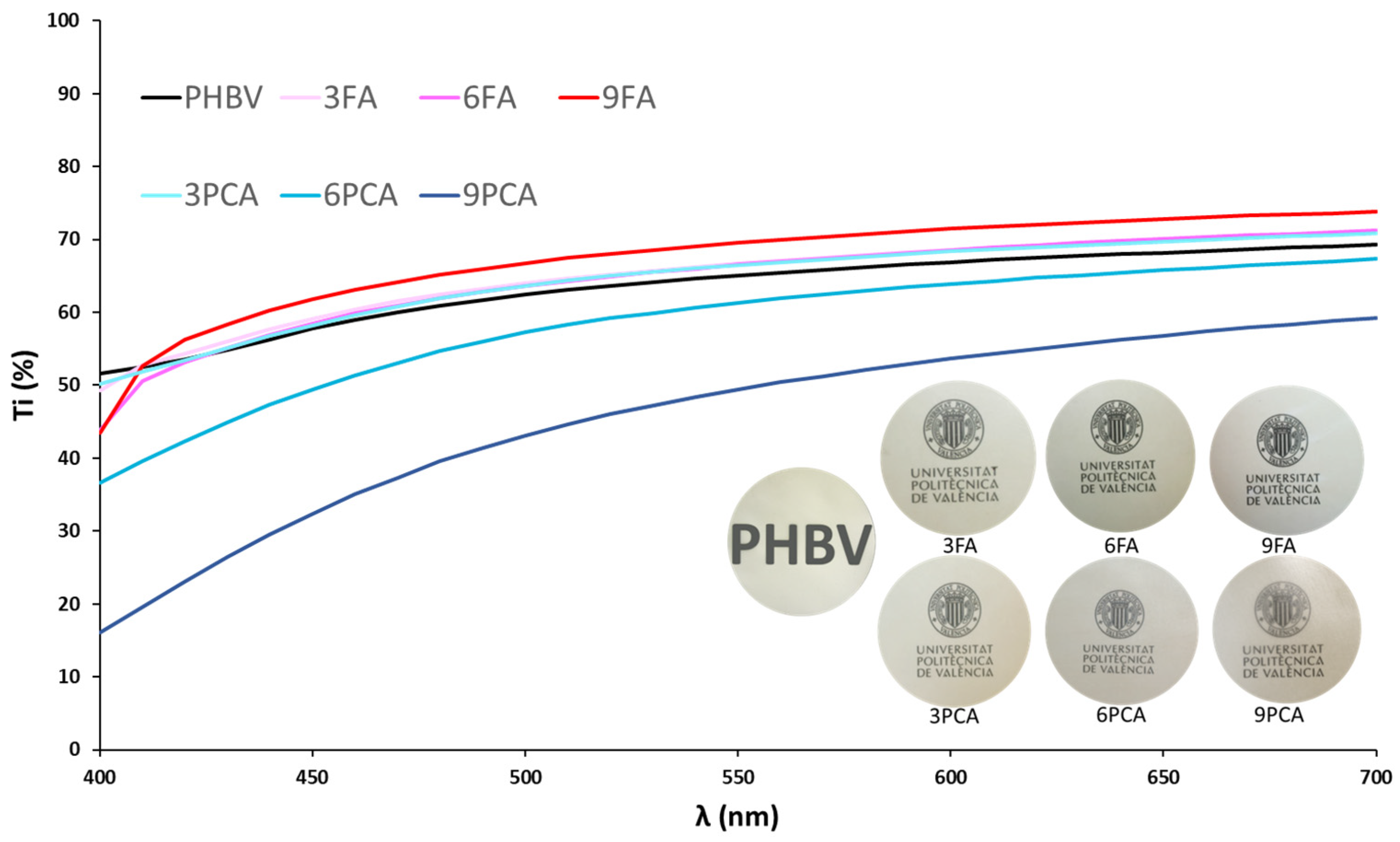
3.3. Mechanical, Barrier and Optical Properties of the Films
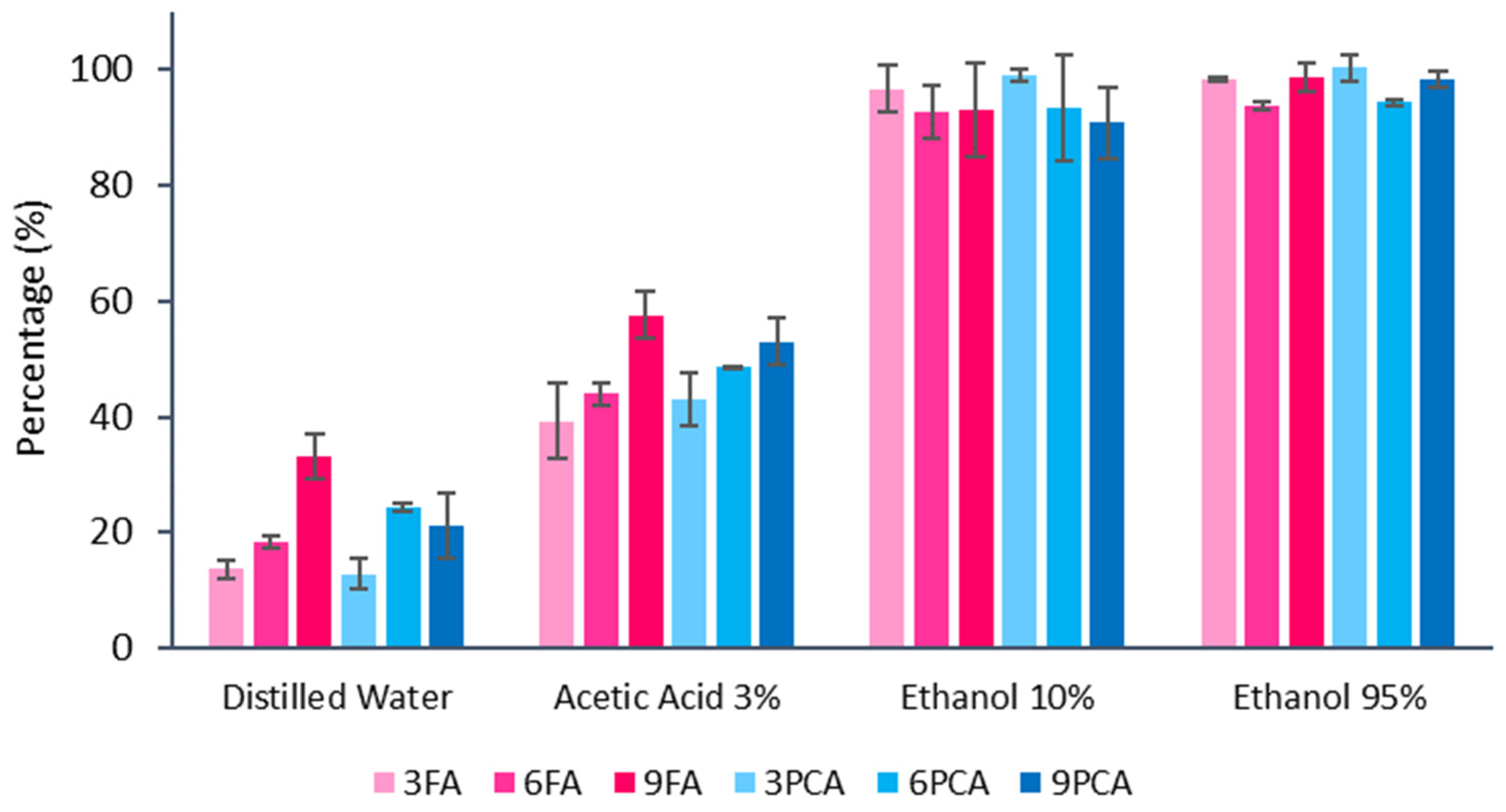
3.4. Migration in Food Simulants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ryberg, M.W.; Hauschild, M.Z.; Wang, F.; Averous-Monnery, S.; Laurent, A. Global Environmental Losses of Plastics across Their Value Chains. Resour. Conserv. Recycl. 2019, 151, 104459. [Google Scholar] [CrossRef]
- Phelan, A.; Meissner, K.; Humphrey, J.; Ross, H. Plastic Pollution and Packaging: Corporate Commitments and Actions from the Food and Beverage Sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Rillig, M.C.; Kim, S.W.; Kim, T.-Y.; Waldman, W.R. The Global Plastic Toxicity Debt. Environ. Sci. Technol. 2021, 55, 2717–2719. [Google Scholar] [CrossRef]
- Kaur, L.; Khajuria, R.; Parihar, L.; Dimpal Singh, G. Polyhydroxyalkanoates: Biosynthesis to commercial production—A review. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1098–1106. [Google Scholar] [CrossRef]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and Functional Properties of Commercial Polyhydroxyalkanoates: A Comprehensive and Comparative Study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Biobased and Biodegradable Polymers in a Circular Economy Context: Understanding Quercetin and Gallic Acid Impacts on PHBV Thermal Properties. Polym. Degrad. Stab. 2022, 201, 109975. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Chatellard, L.; Gontard, N.; Aouf, C. Effect of Quercetin and Gallic Acid on the Microbial Degradation of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Materials. J. Polym. Environ. 2023, 31, 1478–1488. [Google Scholar] [CrossRef]
- Müller, K. Active packaging concepts-are they able to reduce food waste? In Proceedings of the 5th International Workshop Cold Chain Management, Bonn, Germany, 10–11 June 2013. [Google Scholar]
- Latos-Brozio, M.; Masek, A. The Application of (+)-Catechin and Polydatin as Functional Additives for Biodegradable Polyesters. Int. J. Mol. Sci. 2020, 21, 414. [Google Scholar] [CrossRef]
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending Poly(3-Hydroxybutyrate) with Tannic Acid: Influence of a Polyphenolic Natural Additive on the Rheological and Thermal Behavior. Eur. Polym. J. 2015, 63, 123–131. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Biodegradable Active Materials Containing Phenolic Acids for Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3910–3930. [Google Scholar] [CrossRef]
- Gijsman, P. Polymer Stabilization. In Handbook of Environmental Degradation of Materials, 2nd ed.; William Andrew Publishing: Oxford, UK, 2012; pp. 673–714. [Google Scholar]
- Requena, R.; Vargas, M.; Chiralt, A. Release Kinetics of Carvacrol and Eugenol from Poly(Hydroxybutyrate-Co-Hydroxyvalerate) (PHBV) Films for Food Packaging Applications. Eur. Polym. J. 2017, 92, 185–193. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and Carvacrol Migration from PHBV Films and Antibacterial Action in Different Food Matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.; Díez García, A.; López, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-Polyester Bilayer Films with Phenolic Acids for Pork Meat Preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Biodegradable Polyester Materials Containing Gallates. Polymers 2020, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L. Comparative Study of PHBV/TBP and PHBV/BPA Blends. Polym. Int. 2004, 53, 903–910. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L.; Xin, J.H. Modified Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Using Hydrogen Bonding Monomers. Polymer 2004, 45, 6275–6284. [Google Scholar] [CrossRef]
- Xiang, H.X.; Chen, S.H.; Cheng, Y.H.; Zhou, Z.; Zhu, M.F. Structural Characteristics and Enhanced Mechanical and Thermal Properties of Full Biodegradable Tea Polyphenol/Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Composite Films. Express Polym. Lett. 2013, 7, 778–786. [Google Scholar] [CrossRef]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A Review of the Current Evidence of Fruit Phenolic Compounds as Potential Antimicrobials against Pathogenic Bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Pernin, A.; Bosc, V.; Maillard, M.-N.; Dubois-Brissonnet, F. Ferulic Acid and Eugenol Have Different Abilities to Maintain Their Inhibitory Activity Against Listeria Monocytogenes in Emulsified Systems. Front. Microbiol. 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Dędek, K.; Rosicka-Kaczmarek, J.; Nebesny, E.; Kowalska, G. Characteristics and Biological Properties of Ferulic Acid. Biotechnol. Food Sci. 2019, 83, 71–85. [Google Scholar]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus Mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Miyague, L.; Macedo, R.E.F.; Meca, G.; Holley, R.A.; Luciano, F.B. Combination of Phenolic Acids and Essential Oils against Listeria Monocytogenes. LWT—Food Sci. Technol. 2015, 64, 333–336. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial Activity of Ferulic Acid against Cronobacter Sakazakii and Possible Mechanism of Action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Takahashi, H.; Kashimura, M.; Koiso, H.; Kuda, T.; Kimura, B. Use of Ferulic Acid as a Novel Candidate of Growth Inhibiting Agent against Listeria Monocytogenes in Ready-to-Eat Food. Food Control 2013, 33, 244–248. [Google Scholar] [CrossRef]
- Boz, H. P-Coumaric Acid in Cereals: Presence, Antioxidant and Antimicrobial Effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of Active Phenolic Acids on Properties of PLA-PHBV Blend Films. Food Packag. Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Yang, F.; Li, Z.; Qiu, Z. Miscibility and Crystallization Behavior of Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Phenolic Blends. J. Appl. Polym. Sci. 2011, 123, 2781–2786. [Google Scholar] [CrossRef]
- Otero-Pazos, P.; Rodríguez-Bernaldo De Quirósquirós, A.; Sendón, R.S.; Benito-Peñ, E.; Gonzaíez-Vallejo, V.; Cruz Moreno-Bondi, M.; Angulo, I.; Paseiro-Losada, P. Active Food Packaging Based on Molecularly Imprinted Polymers: Study of the Release Kinetics of Ferulic Acid. J. Agric. Food Chem. 2014, 62, 11215–11221. [Google Scholar] [CrossRef] [PubMed]
- Moll, E.; González-Martínez, C.; Chiralt, A. Release and Antibacterial Action of Phenolic Acids Incorporated into PHBV Films. Food Packag. Shelf Life 2023, 38, 101112. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Physicochemical and Antimicrobial Properties of Cassava Starch Films with Ferulic or Cinnamic Acid. LWT 2021, 144, 111242. [Google Scholar] [CrossRef]
- Miguel, O.; Egiburu, J.L.; Iruin, J.J. Blends of Bacterial Poly(3-Hydroxybutyrate) with Synthetic Poly(3-Hydroxybutyrate) and Poly(Epichlorohydrin): Transport Properties of Carbon Dioxide and Water Vapour. Polymer 2001, 42, 953–962. [Google Scholar] [CrossRef]
- American Society for Testing Materials D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: Philadelphia, PA, USA, 2002; Volume 14.
- American Society for Testing Materials D3985-05; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: Philadelphia, PA, USA, 2010; pp. 1–7. [CrossRef]
- American Society for Testing Materials and Annual Book of ASTM Standards E 96/E 96M; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: Philadelphia, PA, USA, 2009. Available online: www.astm.org (accessed on 30 September 2023).
- Cano, A.; Jiménez, A.; Cháfer, M.; Gónzalez, C.; Chiralt, A. Effect of Amylose:Amylopectin Ratio and Rice Bran Addition on Starch Films Properties. Carbohydr. Polym. 2014, 111, 543–555. [Google Scholar] [CrossRef]
- Hutchings, J.B. Food and Colour Appearance, Chapman and Hall Food Science Book, 2nd ed.; Springer: Gaithersburg, MD, USA, 1999. [Google Scholar]
- UNE-EN 1186-1; Materiales y Artículos en Contacto con Productos Alimenticios. Plásticos. Parte 1: Guía para la Elección de Condiciones y Métodos de Ensayo para la Migración Global. Asociación Española de Normalización y Certificación: Madrid, Spain, 2002; pp. 1–52.
- UNE-EN 1186-2; Materiales y Artículos en Contacto con Productos Alimenticios. Plásticos. Parte 2: Métodos de Ensayo para la Migración Global en Aceites Vegetales. Asociación Española de Normalización: Madrid, Spain, 2022; pp. 1–45.
- UNE-EN 1186-3; Materiales y Artículos en Contacto con Productos Alimenticios. Plásticos. Parte 3: Métodos de Ensayo para la Migración Global en Simulantes Evaporables. Asociación Española de Normalización: Madrid, Spain, 2022; pp. 1–29.
- Ordoñez, R.; Atarés, L.; Chiralt, A. Effect of Ferulic and Cinnamic Acids on the Functional and Antimicrobial Properties in Thermo-Processed PLA Films. Food Packag. Shelf Life 2022, 33, 100882. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Alves, R.S.; Brandão, P.; Campos, L.M.A.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solid-Liquid Phase Equilibrium of Trans-Cinnamic Acid, p-Coumaric Acid and Ferulic Acid in Water and Organic Solvents: Experimental and Modelling Studies. Fluid. Phase Equilib. 2020, 521, 112747. [Google Scholar] [CrossRef]
- Kaniuk, Ł.; Ferraris, S.; Spriano, S.; Luxbacher, T.; Krysiak, Z.; Berniak, K.; Zaszczynska, A.; Marzec, M.M.; Bernasik, A.; Sajkiewicz, P.; et al. Time-Dependent Effects on Physicochemical and Surface Properties of PHBV Fibers and Films in Relation to Their Interactions with Fibroblasts. Appl. Surf. Sci. 2021, 545, 148983. [Google Scholar] [CrossRef]
- Kim, G.-M.; Michler, G.H.; Henning, S.; Radusch, H.-J.; Wutzler, A. Thermal and Spectroscopic Characterization of Microbial Poly(3-Hydroxybutyrate) Submicrometer Fibers Prepared by Electrospinning. J. Appl. Polym. Sci. 2007, 103, 1860–1867. [Google Scholar] [CrossRef]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable Resource Based Biocomposites from Natural Fiber and Polyhydroxybutyrate-Co-Valerate (PHBV) Bioplastic. Compos. Part. A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Bai, J.; Dai, J.; Li, G. Electrospun Composites of PHBV/Pearl Powder for Bone Repairing. Prog. Nat. Sci. Mater. Int. 2015, 25, 327–333. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Martins-Franchetti, S.M.; Chinaglia, D.L. Biodegradation of the Films of PP, PHBV and Its Blend in Soil. J. Polym. Environ. 2009, 17, 280–285. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of Different Molecular Weights of Chitosan by P-Coumaric Acid: Preparation, Characterization and Effect of Molecular Weight on Its Water Solubility and Antioxidant Property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Ramorobi, L.M.; Matowane, G.R.; Mashele, S.S.; Bonnet, S.L.; Noreljaleel, A.E.M.; Swain, S.S.; Makhafola, T.J.; Chukwuma, C.I. Bioactive Synergism between Zinc Mineral and P-coumaric Acid: A Multi-mode Glycemic Control and Antioxidative Study. J. Food Biochem. 2022, 46, e14360. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Characterisation of Inclusion Complex of Trans-Ferulic Acid and Hydroxypropyl-β-Cyclodextrin. Food Chem. 2011, 124, 1069–1075. [Google Scholar] [CrossRef]
- Wang, S.; Kong, L.; Zhao, Y.; Tan, L.; Zhang, J.; Du, Z.; Zhang, H. Lipophilization and Molecular Encapsulation of P-Coumaric Acid by Amylose Inclusion Complex. Food Hydrocoll. 2019, 93, 270–275. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, Characterization and Antioxidant Property of Water-Soluble Ferulic Acid Grafted Chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the Thermoformability of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Acid Lactic) Blends Compatibilized with Diisocyanates. Polym. Test. 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Chikh, A.; Benhamida, A.; Kaci, M.; Pillin, I.; Bruzaud, S. Synergistic Effect of Compatibilizer and Sepiolite on the Morphology of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Butylene Succinate) Blends. Polym. Test. 2016, 53, 19–28. [Google Scholar] [CrossRef]
- Feijoo, P.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Torres-Giner, S.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Development and Characterization of Fully Renewable and Biodegradable Polyhydroxyalkanoate Blends with Improved Thermoformability. Polymers 2022, 14, 2527. [Google Scholar] [CrossRef]
- Furushima, Y.; Schick, C.; Toda, A. Crystallization, Recrystallization, and Melting of Polymer Crystals on Heating and Cooling Examined with Fast Scanning Calorimetry. Polym. Cryst. 2018, 1, e10005. [Google Scholar] [CrossRef]
- Sato, H.; Nakamura, M.; Padermshoke, A.; Yamaguchi, H.; Terauchi, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Thermal Behavior and Molecular Interaction of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) Studied by Wide-Angle X-Ray Diffraction. Macromolecules 2004, 37, 3763–3769. [Google Scholar] [CrossRef]
- Liu, W.J.; Yang, H.L.; Wang, Z.; Dong, L.S.; Liu, J.J. Effect of Nucleating Agents on the Crystallization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). J. Appl. Polym. Sci. 2002, 86, 2145–2152. [Google Scholar] [CrossRef]
- Buzarovska, A.; Bogoeva-Gaceva, G.; Grozdanov, A.A.; Avella, A.M.; Gentile, A.G.; Errico, A.M. Crystallization Behavior of Poly(Hydroxybytyrate-Co-Valerate) in Model and Bulk PHBV/Kenaf Fiber Composites. J. Mater. Sci. 2007, 42, 6501–6509. [Google Scholar] [CrossRef]
- Eraslan, K.; Aversa, C.; Nofar, M.; Barletta, M.; Gisario, A.; Salehiyan, R.; Goksu, Y.A. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBH): Synthesis, Properties, and Applications—A Review. Eur. Polym. J. 2022, 167, 111044. [Google Scholar] [CrossRef]
- Naphade, R.; Jog, J. Electrospinning of PHBV/ZnO Membranes: Structure and Properties. Fibers Polym. 2012, 13, 692–697. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, C.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Enhanced Crystallinity and Antibacterial of PHBV Scaffolds Incorporated with Zinc Oxide. Hindawi J. Nanomater. 2020, 2020, 6014816. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. The Effects of Process Engineering on the Performance of PLA and PHBV Blends. Macromol. Mater. Eng. 2011, 296, 719–728. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen Scavengers for Food Packaging Applications: A Review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Effect of Plasticizers on Thermal and Physical Properties of Compression-Moulded Poly[(3-Hydroxybutyrate)-Co-(3-Hydroxyvalerate)] Films. Polym. Test. 2016, 56, 45–53. [Google Scholar] [CrossRef]
- Official Journal of the European Union and European Food Safety Authority, “Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food,” May 2009, Brussels, Belgium. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:135:0003:0011:EN:PDF (accessed on 30 September 2023).
- Official Journal of the European Union and European Food Safety Authority; “Commission Regulation (EC) No 1935/2004 of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food”, October 2004, Brussels, Belgium. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R1935 (accessed on 30 September 2023).
- Angellier-Coussy, H.; Kemmer, D.; Gontard, N.; Peyron, S. Physical–Chemical and Structural Stability of PHBV/Wheat Straw Fibers Based Biocomposites under Food Contact Conditions. J. Appl. Polym. Sci. 2020, 137, 49231. [Google Scholar] [CrossRef]
- Chea, V.; Angellier-Coussy, H.; Peyron, S.; Kemmer, D.; Gontard, N. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Films for Food Packaging: Physical-Chemical and Structural Stability under Food Contact Conditions. J. Appl. Polym. Sci. 2016, 133, 41850. [Google Scholar] [CrossRef]
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the Food Contact Suitability of Aged Bio-Nanocomposite Materials Dedicated to Food Packaging Applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of Cellulose and Lignin on Disintegration, Antimicrobial and Antioxidant Properties of PLA Active Films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Sandoval, G.; Quintana, P.G.; Baldessari, A.; Ballesteros, A.O.; Plou, F.J. Lipase-Catalyzed Preparation of Mono- and Diesters of Ferulic Acid. Biocatal. Biotransform. 2015, 33, 89–97. [Google Scholar] [CrossRef]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Siddiqui, N.A. Solubility and Thermodynamics of Ferulic Acid in Different Neat Solvents: Measurement, Correlation and Molecular Interactions. J. Mol. Liq. 2017, 236, 144–150. [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Li, P.; Yang, B.; Wang, F.; Ding, L.; Wang, B. Measurement and Correlation of the Solubility of P-Coumaric Acid in Nine Pure and Water + Ethanol Mixed Solvents at Temperatures from 293.15 to 333.15 K. J. Chem. Eng. Data 2016, 61, 3457–3465. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of Synthetic Phenolic Antioxidants from Extruded Poly Lactic Acid (PLA) Film. Food Control 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent Solvent Induced Crystallization and Hydrolytic Degradation of PLA by Water-Ethanol Solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef]
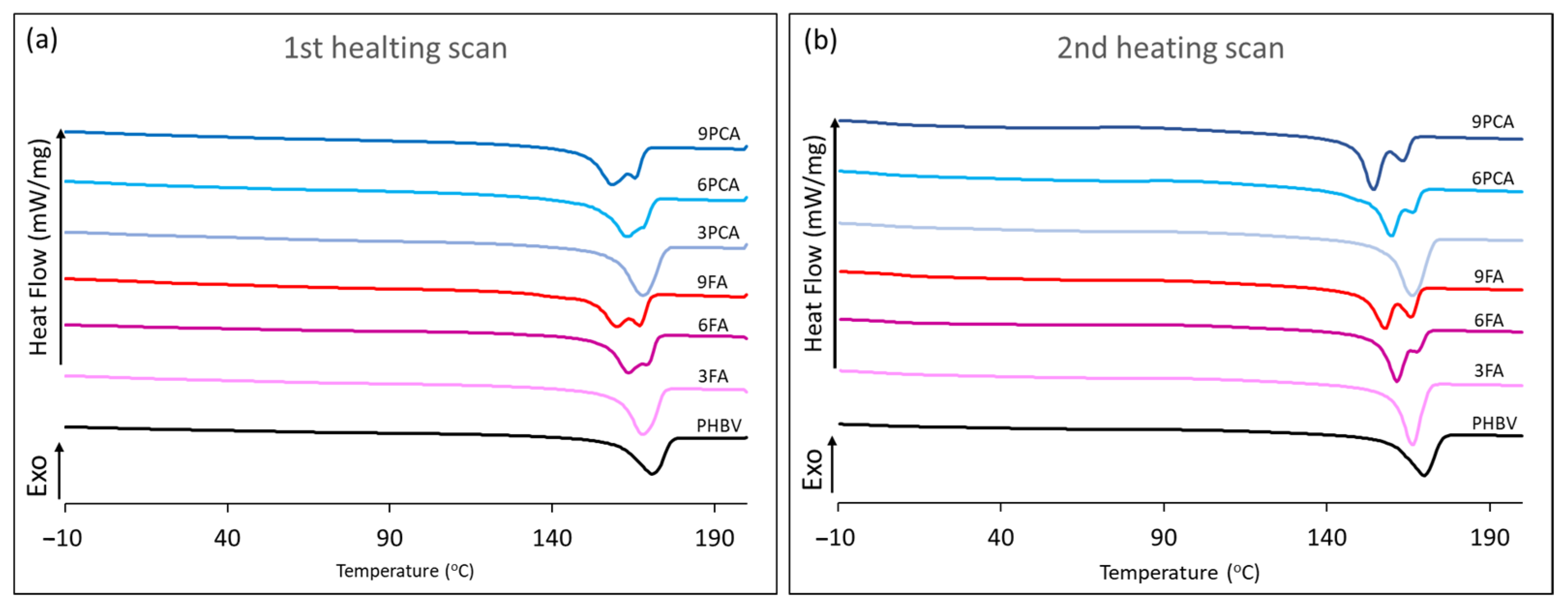


| First Heating | Second Heating | |||||
|---|---|---|---|---|---|---|
| Film | Tg (°C) | Tm1 (°C) | ∆Hm1 (J/g PHBV) | ꭓc (%) | Tm2 (°C) | ∆Hm2 (J/g PHBV) |
| PHBV | 7.2 ± 0.0 c | 171.3 ± 1.0 a | 85.1 ± 3.0 c | 64.4 ± 0.2 a | 170.2 ± 0.4 a | 88.0 ± 6.0 bc |
| 3FA | 7.5 ± 0.2 c | 167.8 ± 0.3 b | 76.0 ± 2.0 a | 57.8 ± 1.5 c | 166.3 ± 0.7 b | 71.1 ± 1.2 a |
| 6FA | 9.0 ± 0.2 b | 163.6 ± 0.9 c | 75.9 ± 0.2 ab | 57.5 ± 0.1 bc | 161.6 ± 0.5 c | 69.7 ± 0.3 a |
| 9FA | 15.0 ± 0.2 a | 163.7 ± 4.9 c | 75.5 ± 0.5 a | 57.2 ± 0.4 c | 157.8 ± 0.0 e | 70.7 ± 0.1 a |
| 3PCA | 7.1 ± 0.7 c | 167.9 ± 0.4 b | 88.0 ± 9.0 c | 66.5 ± 7.5 a | 166.2 ± 1.2 b | 92.0 ± 6.0 c |
| 6PCA | 5.3 ± 0.5 d | 163.1 ± 0.3 c | 83.7 ± 0.4 bc | 63.4 ± 0.4 ab | 159.8 ± 0.7 d | 87.4 ± 0.0 bc |
| 9PCA | 7.0 ± 0.0 c | 158.8 ± 0.7 d | 84.7 ± 1.3 c | 64.1 ± 1.0 a | 154.4 ± 0.7 f | 82.6 ± 0.1 b |
| PHBV | 3FA | 6FA | 9FA | 3PCA | 6PCA | 9PCA | |
|---|---|---|---|---|---|---|---|
| t (µm) | 160 ± 16 a | 152 ± 24 ab | 152 ± 16 bc | 137 ± 15 de | 144 ± 12 bcd | 143 ± 23 cd | 131 ± 12 e |
|
WVP × 1012 (g/msPa) | 5.4 ± 0.4 a | 4.2 ± 0.7 bcd | 4.5 ± 0.4 bc | 3.4 ± 0.1 e | 3.8 ± 0.3 cde | 4.7 ± 0.2 ab | 3.6 ± 0.3 de |
|
OP × 1013 (cm3/msPa) | 3.70 ± 0.20 a | 3.00 ± 0.20 b | 3.00 ± 0.04 b | 2.78 ± 0.14 b | 2.39 ± 0.03 c | 2.70 ± 0.20 b | 1.26 ± 0.06 d |
| EM (MPa) | 1520 ± 90 a | 1480 ± 50 a | 1280 ± 40 b | 1060 ± 90 c | 1480 ± 70 a | 1260 ± 70 b | 1490 ± 50 a |
| TS (MPa) | 37 ± 2 a | 37 ± 2 a | 33 ± 3 b | 31 ± 4 b | 38 ± 2 a | 38 ± 2 a | 28 ± 7 c |
| E% | 3.6 ± 0.4 c | 4.6 ± 0.7 b | 4.7 ± 0.8 b | 4.2 ± 0.7 b | 4.2 ± 0.5 b | 5.6 ± 0.7 a | 2.4 ± 1.0 d |
| L* | 83.9 ± 0.4 b | 81.7 ± 0.5 d | 80.8 ± 0.3 e | 81.0 ± 0.5 e | 82.0 ± 0.2 cd | 82.1 ± 0.4 b | 85.1 ± 0.5 a |
| Cab* | 13.5 ± 0.3 e | 14.0 ± 0.4 d | 14.7 ± 0.5 c | 15.9 ± 0.5 ab | 15.5 ± 0.4 b | 16.0 ± 0.7 a | 11.8 ± 0.5 f |
| hab* | 89.8 ± 0.5 a | 87.9 ± 0.4 c | 86.6 ± 0.5 e | 86.3 ± 0.7 e | 89.7 ± 0.4 ab | 89.3 ± 0.4 b | 87.4 ± 0.8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moll, E.; Chiralt, A. Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging. Polymers 2023, 15, 4222. https://doi.org/10.3390/polym15214222
Moll E, Chiralt A. Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging. Polymers. 2023; 15(21):4222. https://doi.org/10.3390/polym15214222
Chicago/Turabian StyleMoll, Eva, and Amparo Chiralt. 2023. "Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging" Polymers 15, no. 21: 4222. https://doi.org/10.3390/polym15214222
APA StyleMoll, E., & Chiralt, A. (2023). Polyhydroxybutyrate-co-hydroxyvalerate (PHBV) with Phenolic Acids for Active Food Packaging. Polymers, 15(21), 4222. https://doi.org/10.3390/polym15214222







