Characterization of Moringa oleifera Seed Oil for the Development of a Biopackage Applied to Maintain the Quality of Turkey Ham
Abstract
:1. Introduction
2. Materials and Methods
2.1. M. oleifera Seed Oil Extraction
2.2. Physico-Chemical Characterization of the MOS
Antioxidant Capacity and Total Phenols in MOS
2.3. Fatty Acid Profile of MOS
2.4. MOS Susceptibility to Oxidative Stress
2.5. Development of a Biopackaging with MOS
2.6. Opacity and Filters of Biopackaging
2.7. Water Vapor Permeability (PVA) of Biopackaging
2.8. Protective Capacity of Biopackaging on the Useful Life of Turkey Ham
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Composition of M. oleifera Seed Oil
3.2. MOS Fatty Acid Profile
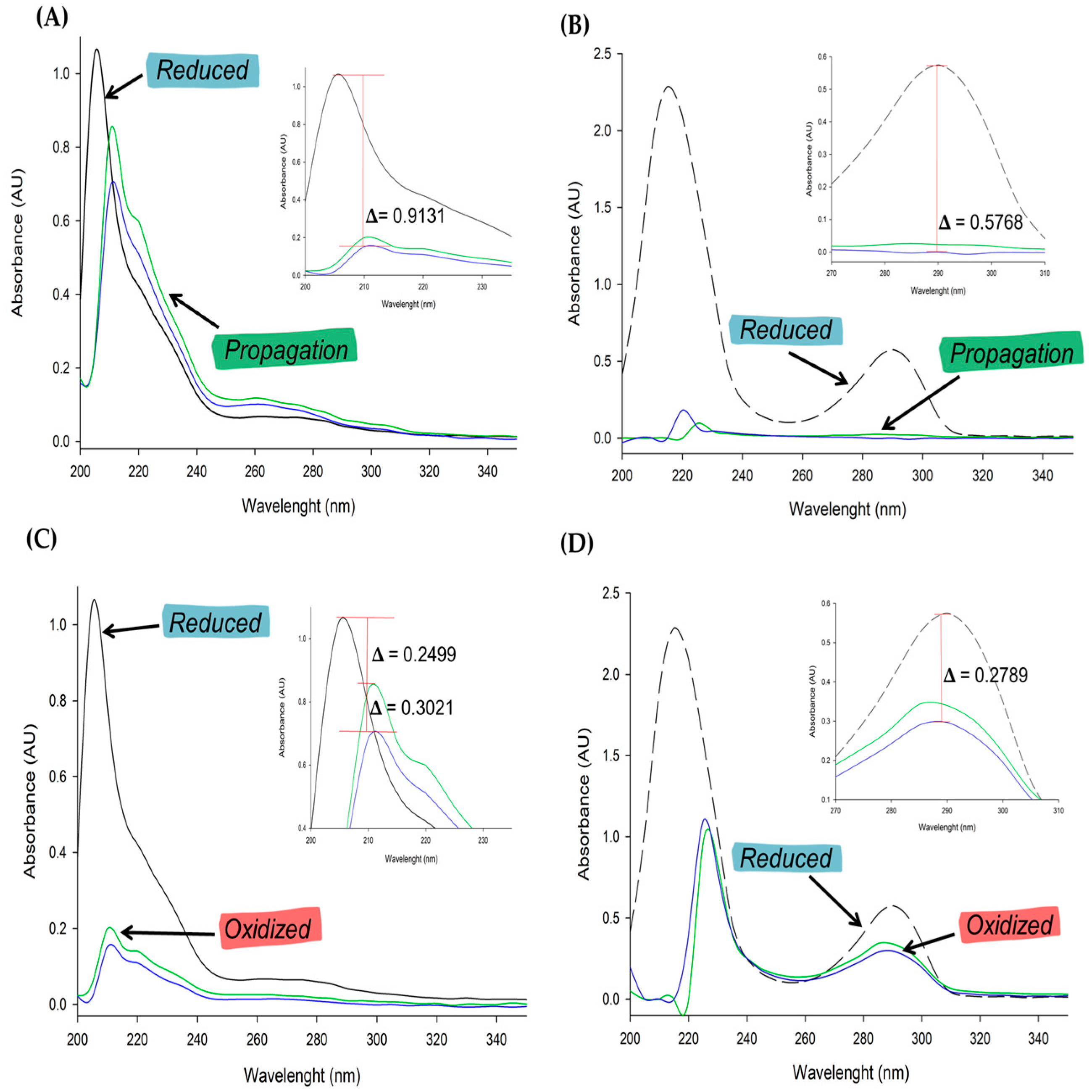
3.3. Susceptibility of MOS to Oxidative Stress
3.4. Opacity and Filters of the Biopackaging
3.5. Water Vapor Permeability of Biopackaging
3.6. Protective Capacity of Biopackaging on the Useful Life of Turkey Ham
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Bajer, D.; Janczak, K.; Bajer, K. Novel Starch/Chitosan/Aloe Vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef]
- Magni, S.; Bonasoro, F.; Della Torre, C.; Parenti, C.C.; Maggioni, D.; Binelli, A. Plastics and biodegradable plastics: Ecotoxicity comparison between polyvinylchloride and Mater-Bi® micro-debris in a freshwater biological model. Sci. Total Environ. 2020, 720, 137602. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in Chitosan as a Primary Biopolymer for Functional Films and Coatings Manufacture for Food and Natural Products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Han, B.; Chen, P.; Guo, J.; Yu, H.; Zhong, S.; Li, D.; Jiang, B. A Novel Intelligent Indicator Film: Preparation, Characterization, and Application. Molecules 2023, 28, 3384. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage bacteria and meat quality. In Meat Quality Analysis; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. [Google Scholar]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; Li, J.; Chen, L.; Ma, Y. Protective Effects of Four Natural Antioxidants on Hydroxyl-Radical-Induced Lipid and Protein Oxidation in Yak Meat. Foods 2022, 11, 3062. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: A promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Ezz El-Din Ibrahim, M.; Alqurashi, R.M.; Alfaraj, F.Y. Antioxidant Activity of Moringa oleifera and Olive Olea europaea L. Leaf Powders and Extracts on Quality and Oxidation Stability of Chicken Burgers. Antioxidants 2022, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Dinesha, B.L.; Nidoni, U.; Ramachandra, C.T.; Naik, N.; Sankalpa, K.B. Effect of extraction methods on physicochemical, nutritional, antinutritional, antioxidant and antimicrobial activity of Moringa (Moringa oleifera Lam.) seed kernel oil. J. Appl. Nat. Sci. 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Anzano, A.; de Falco, B.; Ammar, M.; Ricciardelli, A.; Grauso, L.; Sabbah, M.; Lanzotti, V. Chemical analysis and antimicrobial activity of Moringa oleifera lam. Leaves and Seeds. Molecules 2022, 27, 8920. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, K.; Lee, W.J.; Reaney, M.T.; Zhang, N.; Wang, Y. Recent progress in the thermal treatment of oilseeds and oil oxidative stability: A review. Fundam. Res. 2021, 1, 767–784. [Google Scholar] [CrossRef]
- Aviara, N.A.; Musa, W.B.; Owolarafe, O.K.; Ogunsina, B.S.; Oluwole, F.A. Effect of processing conditions on oil point pressure of Moringa oleifera seed. J. Food Sci. Technol. 2015, 52, 4499–4506. [Google Scholar] [CrossRef]
- Ceriani, R.; Paiva, F.R.; Goncalves, C.B.; Batista, E.A.; Meirelles, A.J. Densities and viscosities of vegetable oils of nutritional value. J. Chem. Eng. Data 2008, 53, 1846–1853. [Google Scholar] [CrossRef]
- Khaneghah, M.; Shoeibi, S.; Ameri, M. Effects of storage conditions and PET packaging on quality of edible oils in Iran. Adv. Environ. Biol. 2012, 694–702. [Google Scholar]
- Ogbunugafor, H.A.; Eneh, F.U.; Ozumba, A.N.; Igwo-Ezikpe, M.N.; Okpuzor, J.; Igwilo, I.O. Physico-chemical and antioxidant properties of Moringa oleifera seed oil. Pak. J. Nutr. 2011, 10, 409–414. [Google Scholar] [CrossRef]
- Thimmappa, S.P.; Sudhir, A.; Rai, K.M.L.; Deepakumari, H.N.; Bhatt, M. Potassium Iodate: A New Versatile Reagent to Determine the Iodine Value of Edible Oils. Lett. Appl. NanoBioScience 2021, 11, 3537–3541. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; B’chir, F.; Bourgou, S.; Achour, N.B.; Jlassi, C.; Chalh, A. Composition and characterization of cold pressed Moringa oleifera seed oil. J. Oleo Sci. 2022, 71, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Kayanan, B.U.R.; Sagum, R.S. Microwave and ultrasound pretreatment of Moringa oleifera Lam. Seeds: Effects on oil expression, oil quality, and bioactive component. J. Oleo Sci. 2021, 70, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Borello, E.; Domenici, V. Determination of pigments in virgin and extra-virgin olive oils: A comparison between two near UV-vis spectroscopic techniques. Foods 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Frangu, A.; Ashrafi, A.M.; Sýs, M.; Arbneshi, T.; Metelka, R.; Adam, V.; Richtera, L. Determination of trolox equivalent antioxidant capacity in berries using amperometric tyrosinase biosensor based on multi-walled carbon nanotubes. Appl. Sci. 2020, 10, 2497. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Kanavouras, A. Alterations of PET material physical properties during storage of olive oil. Food Packag. Shelf Life 2019, 21, 100336. [Google Scholar] [CrossRef]
- ASTM International. Available online: https://img.antpedia.com/standard/files/pdfs_ora/20200926/ASTM%20E96-2016.pdf (accessed on 15 January 2023).
- Sierra, F.R.G.; Jiménez-Sánchez, A. Elaboración de películas biodegradables con policaprolactona y almidóncelulosa de la cáscara de plátano verde (Musa paradisíaca). Aliment. Cienc. Ing. 2021, 28, 19–33. [Google Scholar] [CrossRef]
- Hunt, M.C.; King, A.; Barbut, S.; Clause, J.; Cornforth, D.; Hanson, D.; Mohan, A. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; Volume 61820, pp. 1–135. [Google Scholar]
- Farhoosh, R.; Moosai, S.M.R.; Sharif, A. Investigation on frying oils quality in terms of color index, refractive index and viscosity during frying process. J. Food Sci. Technol. 2008, 5, 13–19. [Google Scholar]
- Li, Y.; Lee, E.K. Negative tunneling magnetoresistance in spin filtering magnetic junctions with spin-orbit coupling. In Proceedings of the 6th International Conference on Natural Computation, Yantai, China, 10–12 August 2010; pp. 4088–4092. [Google Scholar] [CrossRef]
- Pan, J.; Shen, H.; You, J.; Luo, Y. Changes in physiochemical properties of myofibrillar protein from silver carp (Hypophthalmichthys mollitrix) during heat treatment. J. Food Biochem. 2011, 35, 939–952. [Google Scholar] [CrossRef]
- Ekwu, F.C.; Nwagu, A. Effect of processing on the quality of cashew nut oils. J. Sci. Agric. Food Technol. Environ. 2004, 4, 105–110. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practice of the American Oil Chemist Society, 5th ed.; AOAC Press: Champaign, IL, USA, 1993. [Google Scholar]
- Asuquo, J.E.; Anusiem, A.C.I.; Etim, E.E. Extraction and characterization of rubber seed oil. Int. J. Mod. Chem. 2012, 1, 109–115. [Google Scholar]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005, 93, 253–263. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Thangamani, A.; Karthishwaran, K.; Cheruth, A.J. Essential oil from the seeds of Moringa peregrina: Chemical composition and antioxidant potential. S. Afr. J. Bot. 2020, 129, 100–105. [Google Scholar] [CrossRef]
- Sanyal, A.; Decocq, G. Adaptive evolution of seed oil content in angiosperms: Accounting for the global patterns of seed oils. BMC Evol. Biol. 2016, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283. Available online: http://www.jstor.org/stable/4254186 (accessed on 23 November 2022). [CrossRef]
- Dahot, M.U.; Memon, A.R. Nutritive significance of oil extracted from Moringa oleifera seeds. J. Pharmacol. (Univ. Karachi) 1985, 20, 75–79. [Google Scholar]
- Ferrao, A.M.B.; Ferrao, J.E. Fatty acids of the oil of Moringueiro (Moringa oleifera [M. pterygosperma]). Agron. Angolana 1970, 30, 3–16. [Google Scholar]
- Geng, L.; Liu, K.; Zhang, H. Lipid oxidation in foods and its implications on proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef]
- Barthel, G.; Grosch, W. Peroxide value determination—Comparison of some methods. J. Am. Oil Chem. Soc. 1974, 51, 540–544. [Google Scholar] [CrossRef]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L. A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit. Contam. Part A 2020, 37, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Cacique, A.P.; Barbosa, É.S.; de Pinho, G.P.; Silvério, F.O. Miniaturized Methodologies for Determining the Total Phenol and Flavonoid Concentrations and the Antioxidant Activity. Food Anal. Methods 2021, 14, 1110–1120. [Google Scholar] [CrossRef]
- Gonçalves, T.R.; Rosa, L.N.; Gonçalves, R.P. Monitoring the Oxidative Stability of Monovarietal Extra Virgin Olive Oils by UV–Vis Spectroscopy and MCR–ALS. Food Anal Methods 2018, 11, 1936–1943. [Google Scholar] [CrossRef]
- Lapčíková, B.; Valenta, T.; Lapčík, L.; Fuksová, M. Thermal aging of edible oils: Spectrophotometric study. Potravin. Slovak J. Food Sci. 2018, 12, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Fito, M.; de la Torre, R.; Covas, M.I. Olive oil and oxidative stress. Mol. Nutr. Food Res. 2007, 51, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Bekbölet, M. Light Effects on Food. J. Food Prot. 1990, 53, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Puyol, S.; Benítez, J.J.; Heredia-Guerrero, J.A. Transparency of polymeric food packaging materials. Food Res. Int. 2022, 161, 111792. [Google Scholar] [CrossRef]
- Majling, J.; Šimon, P.; Khunová, V. Optical transmittance thermal analysis of the poly (ethylene terephthalate) foils. J. Therm. Anal. Calorim. 2002, 67, 201–206. [Google Scholar] [CrossRef]
- Hülsmann, P.; Wallner, G.M. Permeation of water vapour through polyethylene terephthalate (PET) films for back-sheets of photovoltaic modules. Polym. Test. 2017, 58, 153–158. [Google Scholar] [CrossRef]
- Cazón, P.; Morales-Sanchez, E.; Velazquez, G.; Vázquez, M. Measurement of the water vapor permeability of chitosan films: A laboratory experiment on food packaging materials. J. Chem. Educ. 2022, 99, 2403–2408. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Vidaurre, E.C.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Pu Acurio Rocafuerte, R.M.; Cabezas Rodríguez, H.K. Elaboración de Biopelículas a Partir de Residuos de Tallos de Flores. Bachelor’s Thesis, Facultad de Ingeniería Química, Universidad de Guayaquil, Guayaquil, Ecuador, 2022. [Google Scholar]
- Morrissey, P.A.; Sheehy, P.J.A.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid stability in meat and meat products. Meat Sci. 1998, 49, S73–S86. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Cava, R. Oxidative and colour changes in meat from three lines of free-range reared Iberian pigs slaughtered at 90 kg live weight and from industrial pig during refrigerated storage. Meat Sci. 2003, 65, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Balev, D.; Vulkova, T.; Dragoev, S.; Zlatanov, M.; Bahtchevanska, S. A comparative study on the effect of some antioxidants on the lipid and pigment oxidation in dry-fermented sausages. Int. J. Food Sci. Technol. 2005, 40, 977–983. [Google Scholar] [CrossRef]
- Haile, D.M.; De Smet, S.; Claeys, E.; Vossen, E. Effect of light, packaging condition and dark storage durations on colour and lipid oxidative stability of cooked ham. J. Food Sci. Technol. 2013, 50, 239–247. [Google Scholar] [CrossRef]
- Tomasevic, I.; Tomovic, V.; Milovanovic, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Djekic, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Acidity | 0.71 mg KOH/g |
| Peroxide | 1.74 meq O2/kg |
| Saponification | 184.06 ± 0.427 mg KOH g−1 |
| Iodine | 30.96 ± 0.707 I2 100 g−1 |
| Viscosity | 51.5 MPa·s (20 °C) |
| L* | 96.143 ± 5.889 |
| a* | −6.993 ± 1.810 |
| b* | 22.953 ± 1.716 |
| DPPH | 17.30 ± 0.636% |
| ABTS | 11.89 ± 0.784% |
| F-C | 8.58 ± 0.235 mg trolox g−1 |
| Fatty Acids | Percentage |
|---|---|
| Palmitic (C 16:0) | 5.45 |
| Palmitoleic (C 16:1) | 1.64 |
| Estearic (C 18:0 C) | 7.03 |
| Oleic (C 18:1 w-9) | 71.7 |
| Linoleic (C 18:2 w-6) | 0.54 |
| Araquidic (C 20:0) | 4.21 |
| Eicosene (C 20:1 w-9) | 1.67 |
| Behenic (C 22:0) | 6.93 |
| Lignoceric (C 24:0) | 0.84 |
| SAFA | 24.46 |
| MUFA | 75.01 |
| PUFA | 0.54 |
| Time | Control | Biopackage | Synthetic Package (PT) |
|---|---|---|---|
| Four | 74.16 ± 0.86% a | 74.16 ± 0.86% a | 74.16 ± 0.86% a |
| Seven | 71.36 ± 1.05% a | 72.52 ± 0.31% a | 72. 96 ± 1.52% a |
| Twenty-two | 27.45 ± 1.94% b | 68.47 ± 0.21% b | 70.02 ± 1.14% ab |
| Time | Control | Biopackage | Synthetic Package (PT) |
|---|---|---|---|
| Four | 1.9583 ± 0.2574 N a | 2.2555 ± 0.1995 N a | 2.1755 ± 0.1218 N a |
| Seven | 6.8641 ± 0.9726 N b | 4.1623 ± 0.129 N b | 4.5923 ± 0.096 N b |
| Twenty-two | 122.603 ± 0.9211 N c | 4.0761 ± 0.3344 N b | 4.0179 ± 0.1717 N b |
| Control | Biopackage | Synthetic Package (PT) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | L* | a* | b* | ∆E | L* | a* | b* | ∆E | L* | a* | b* | ∆E |
| Four | 74.54 ± 2.13 | 7.34 ± 1.96 | −8.01 ± 1.53 | 73.8± 0.25a | 5.34 ± 1.33 a | −5.01 ± 1.87 a | 75.6± 0.44 a | 6.77 ± 1.45 a | −7.66 ± 0.90 a | |||
| Seven | 91.11 ± 2.14 b | −2.2 ± 0.92 b | 3.525 ± 0.94 b | 79.3 ± 2.96 b | 3.53 ± 0.59 b | −2.84 ± 2.72 b | 76.8 ± 1.09 a | 5.53 ± 0.11 b | −4.66 ± 1.41 b | |||
| Twenty-two | 91.37± 1.81 b | −3.35 ± 1.66 ab | 2.14 ± 0.86 ab | 22.37 ± 1.03 b | 81.7± 1.34 b | 4.69 ± 0.83 b | −5.28 ± 2.84 ab | 8.1 ± 0.34 b | 79.1± 1.40 b | 4.43 ± 1.11 b | −3.03 ± 0.71 ab | 7.37± 1.04 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cueto Covarrubias, L.A.; Valdez Solana, M.A.; Avitia Domínguez, C.; Téllez Valencia, A.; Meza Velázquez, J.A.; Sierra Campos, E. Characterization of Moringa oleifera Seed Oil for the Development of a Biopackage Applied to Maintain the Quality of Turkey Ham. Polymers 2024, 16, 132. https://doi.org/10.3390/polym16010132
Cueto Covarrubias LA, Valdez Solana MA, Avitia Domínguez C, Téllez Valencia A, Meza Velázquez JA, Sierra Campos E. Characterization of Moringa oleifera Seed Oil for the Development of a Biopackage Applied to Maintain the Quality of Turkey Ham. Polymers. 2024; 16(1):132. https://doi.org/10.3390/polym16010132
Chicago/Turabian StyleCueto Covarrubias, Lesly Adamari, Mónica Andrea Valdez Solana, Claudia Avitia Domínguez, Alfredo Téllez Valencia, Jorge Armando Meza Velázquez, and Erick Sierra Campos. 2024. "Characterization of Moringa oleifera Seed Oil for the Development of a Biopackage Applied to Maintain the Quality of Turkey Ham" Polymers 16, no. 1: 132. https://doi.org/10.3390/polym16010132
APA StyleCueto Covarrubias, L. A., Valdez Solana, M. A., Avitia Domínguez, C., Téllez Valencia, A., Meza Velázquez, J. A., & Sierra Campos, E. (2024). Characterization of Moringa oleifera Seed Oil for the Development of a Biopackage Applied to Maintain the Quality of Turkey Ham. Polymers, 16(1), 132. https://doi.org/10.3390/polym16010132





