Water Hyacinth Fiber as a Bio-Based Carbon Source for Intumescent Flame-Retardant Poly (Butylene Succinate) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of WHF/APP/PBS Composites
2.2.2. Determination of Flame Retardancy of WHF/APP/PBS Composites
2.2.3. Determination of Melt Flow Index of WHF/APP/PBS Composites
2.2.4. Determination of Thermal Stability of WHF/APP/PBS Composites
2.2.5. Determination of Combustion Behavior of WHF/APP/PBS Composites
2.2.6. Determination of Morphologies and Structure of Char Layer
2.2.7. Determination of Evolved Products during Thermal Decomposition of WHF/APP/PBS Composites
3. Results and Discussion
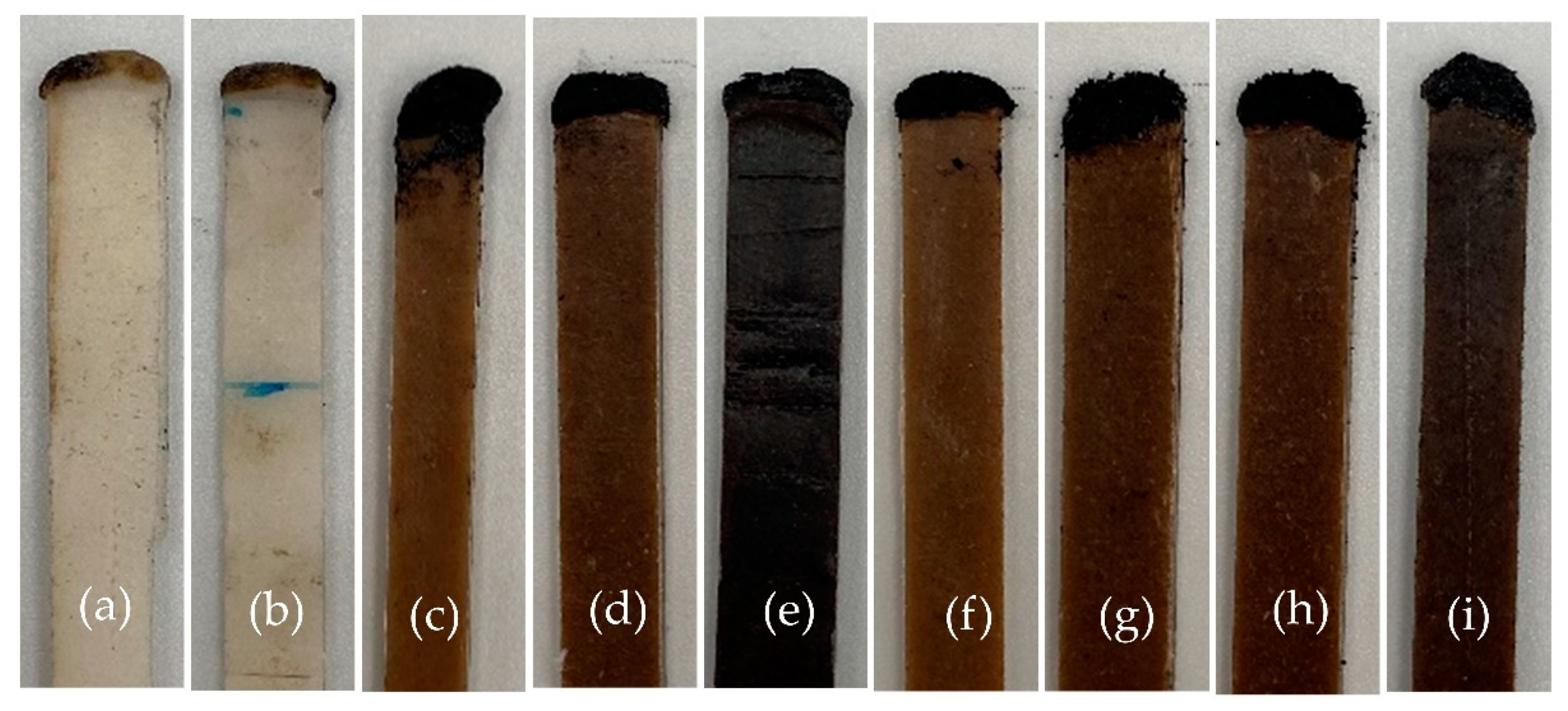
3.1. Flame Retardancy of WHF/APP/PBS Composites
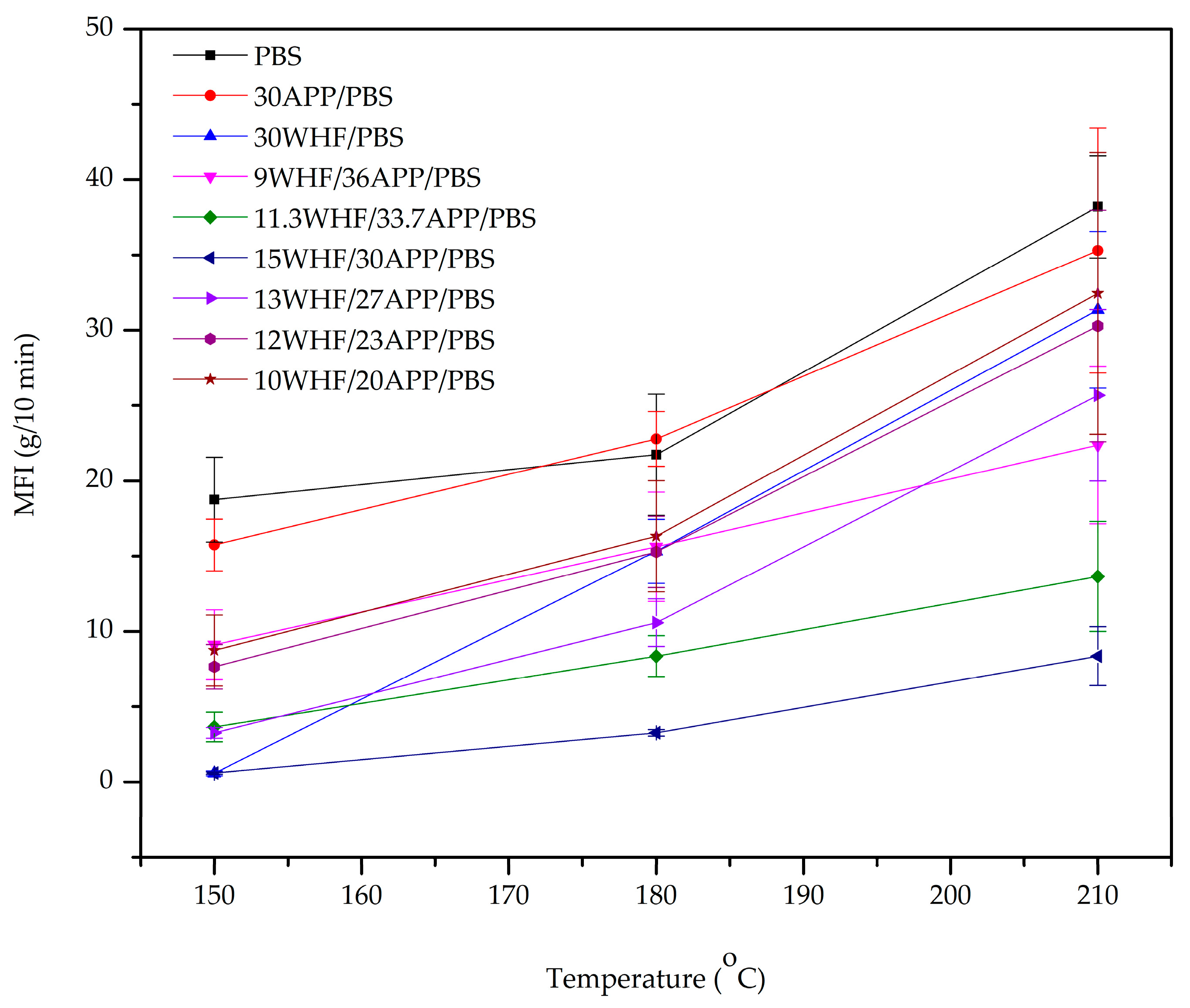
3.2. Melt Flow Index (MFI) of WHF/APP/PBS Composites
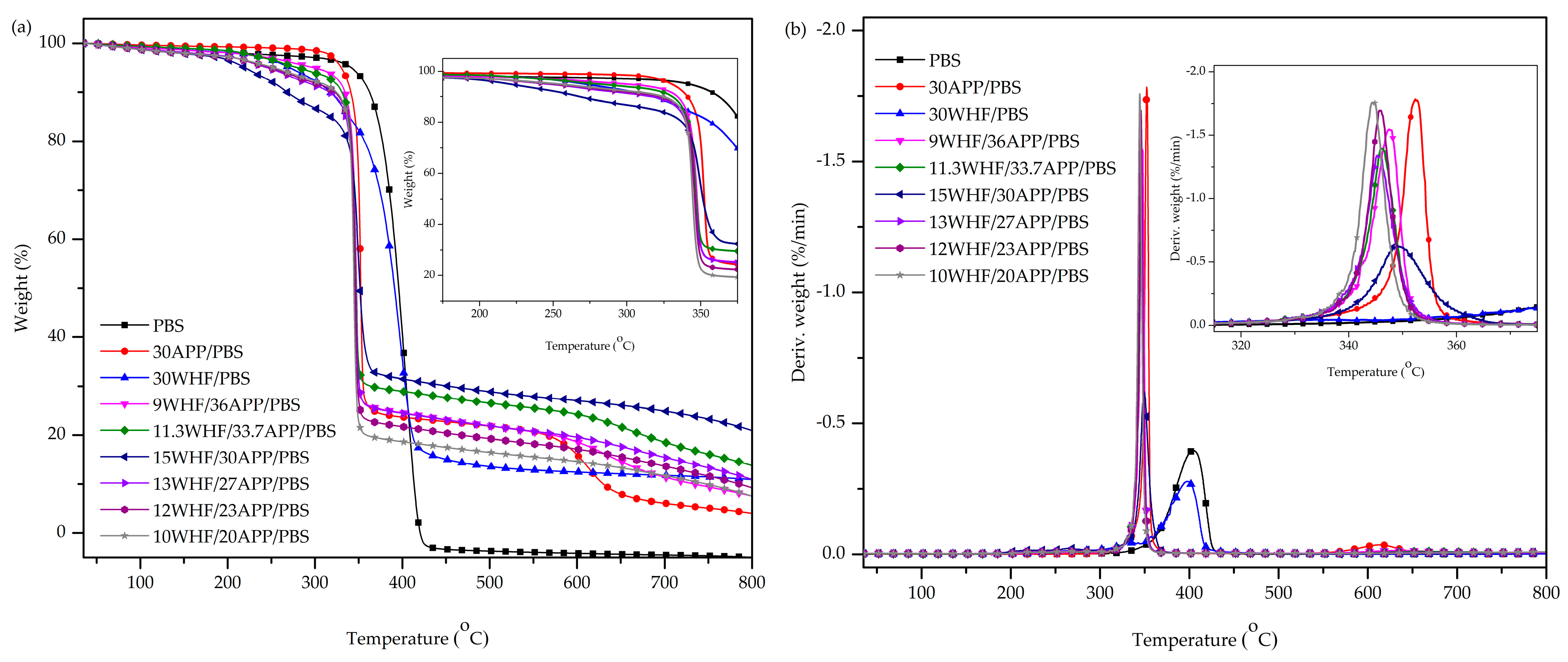
3.3. Thermal Stability of WHF/APP/PBS Composites
3.4. Combustion Behavior of WHF/APP/PBS Composites
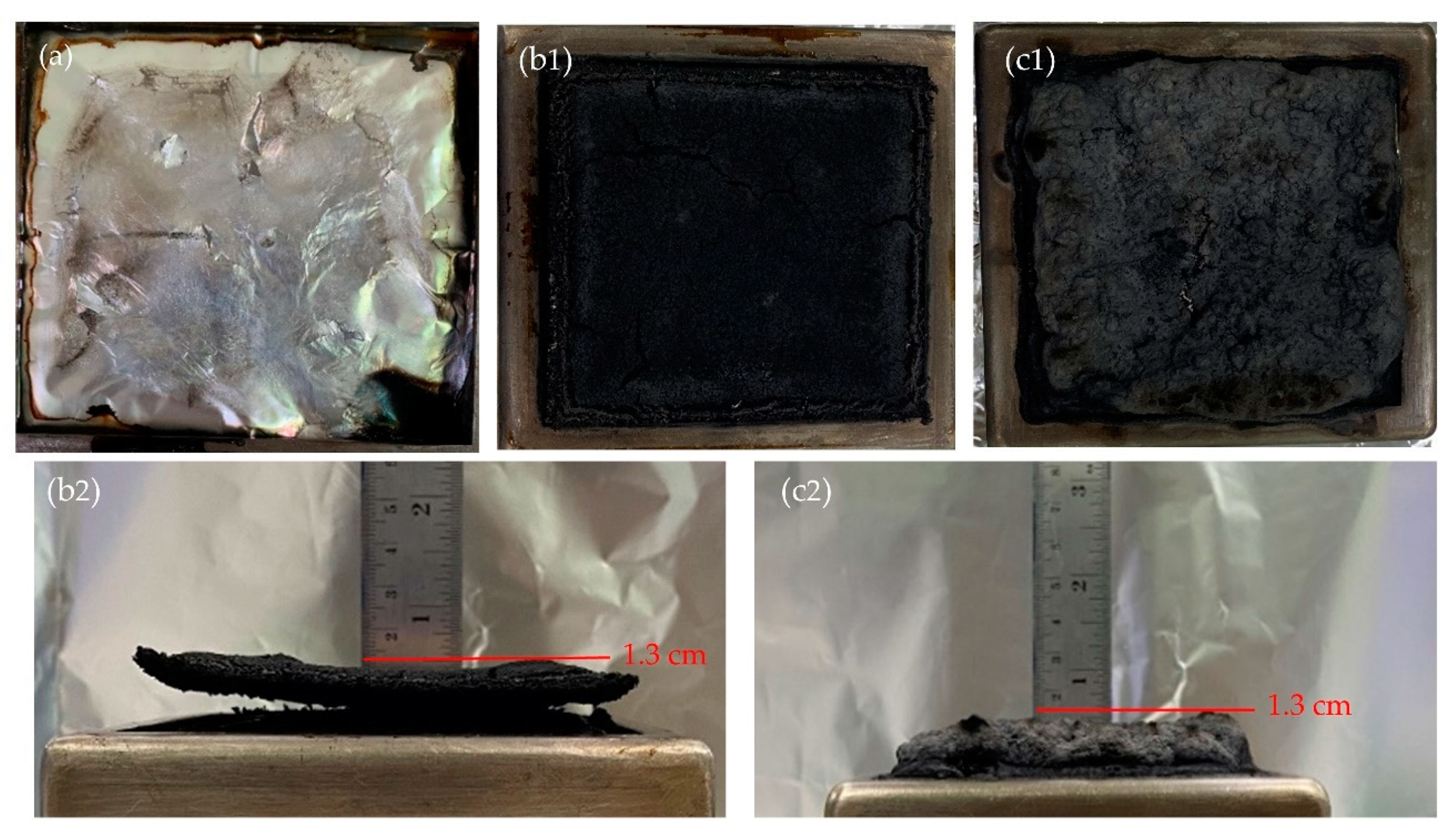
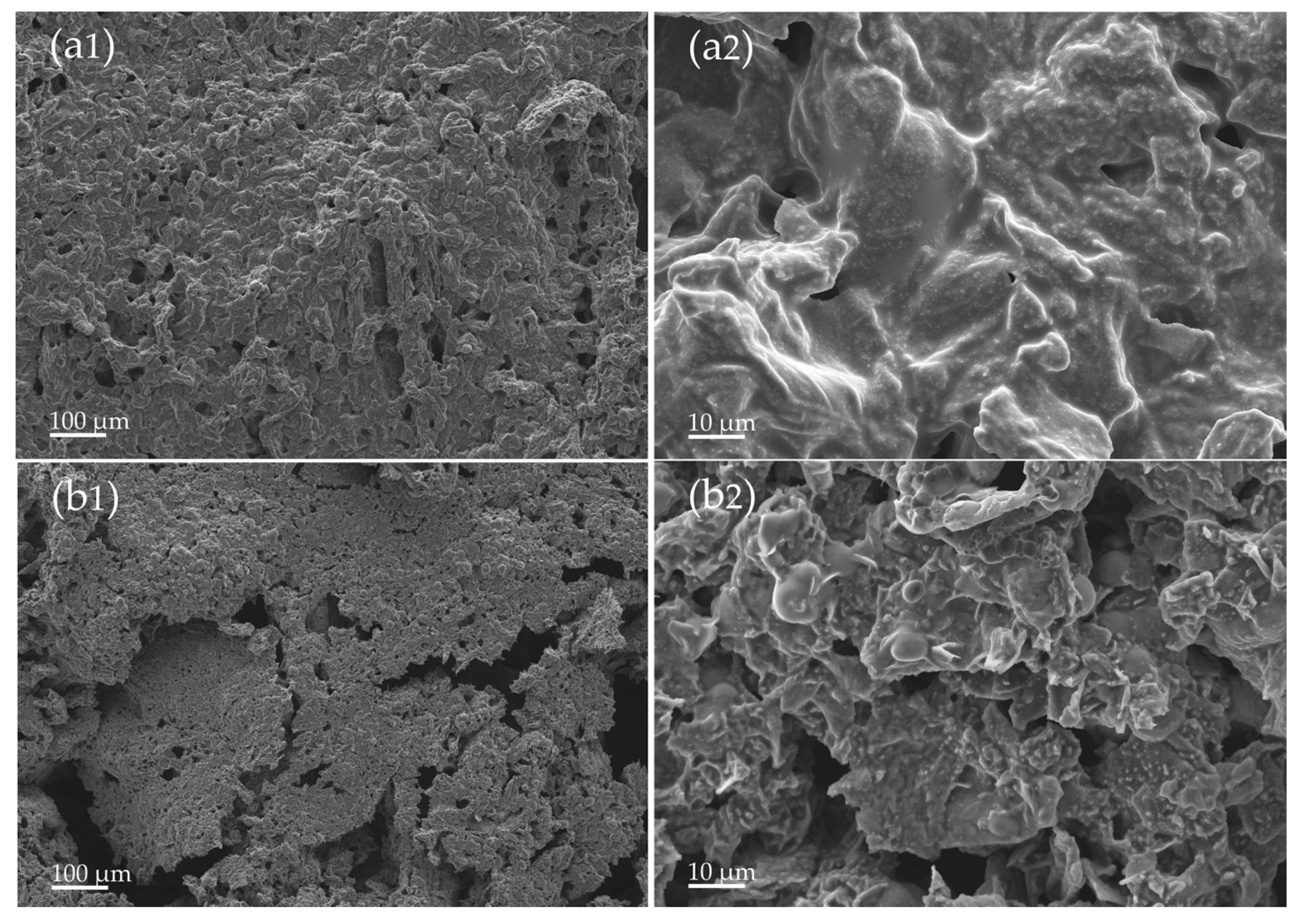
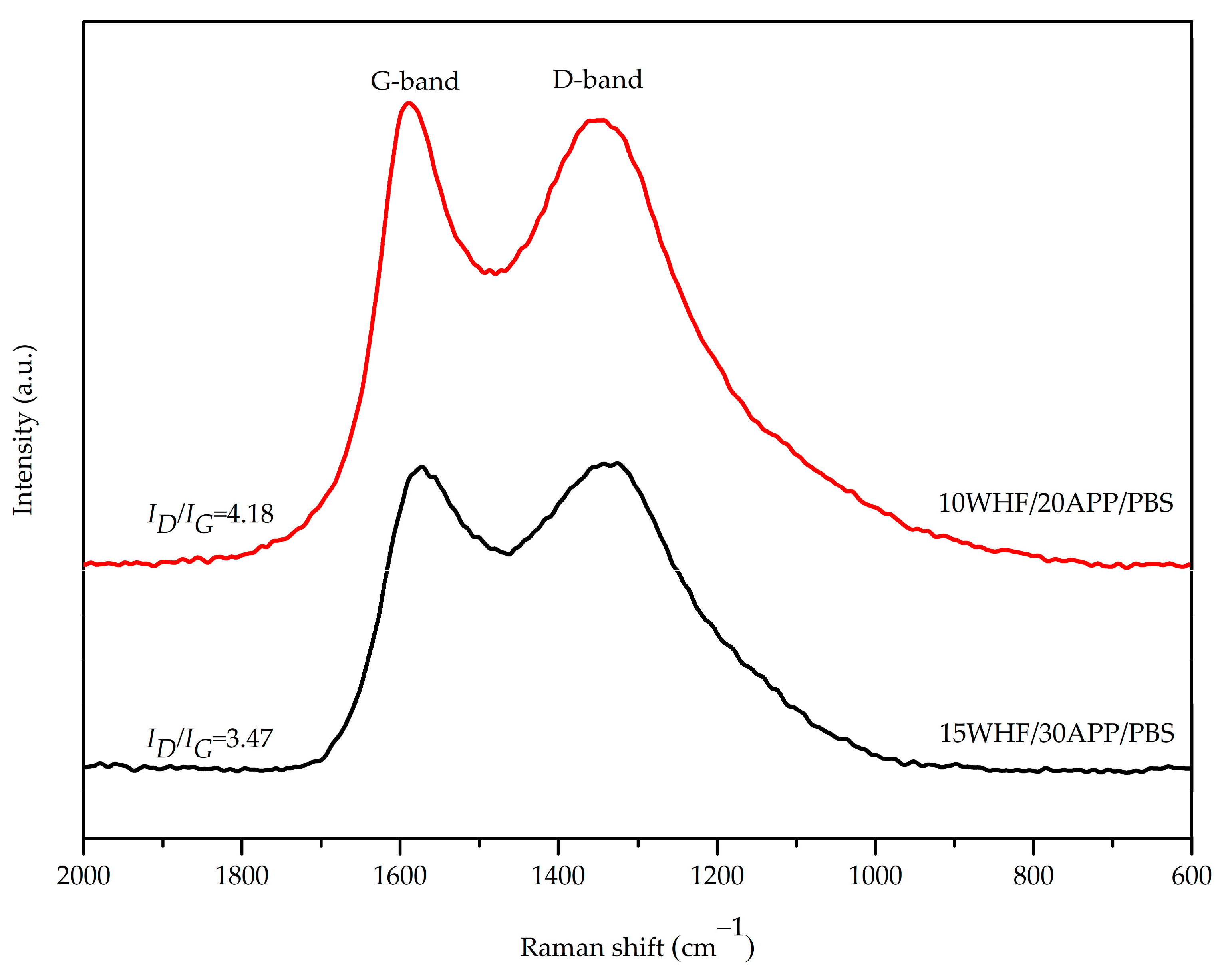
3.5. Morphologies and Structure of Char Residue
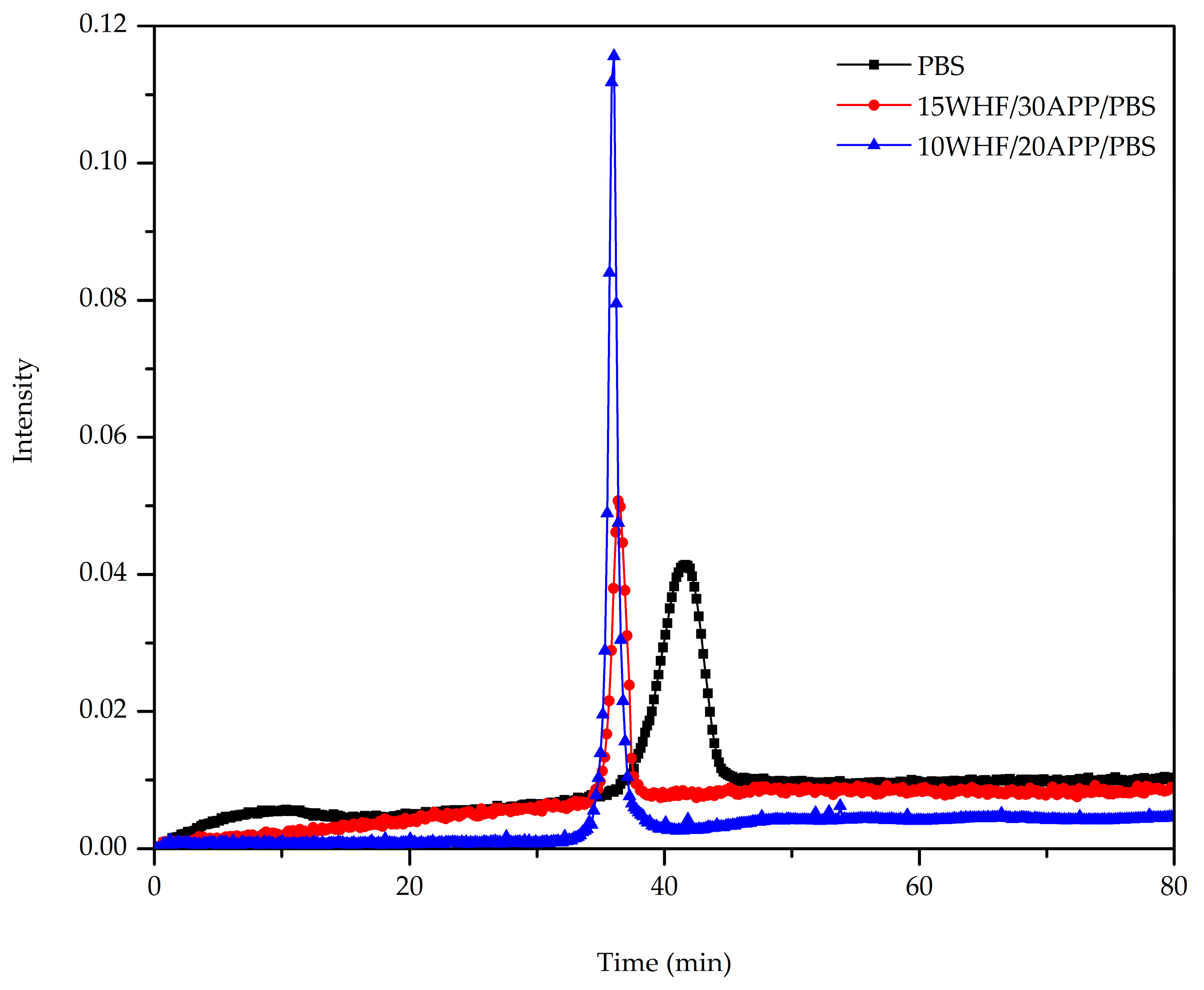
3.6. Evolved Products during Thermal Decomposition of WHF/APP/PBS Composites
3.7. Proposed Intumescent Flame-Retardant Mechanisms
3.8. Tensile Properties of PBS and PBS Composites
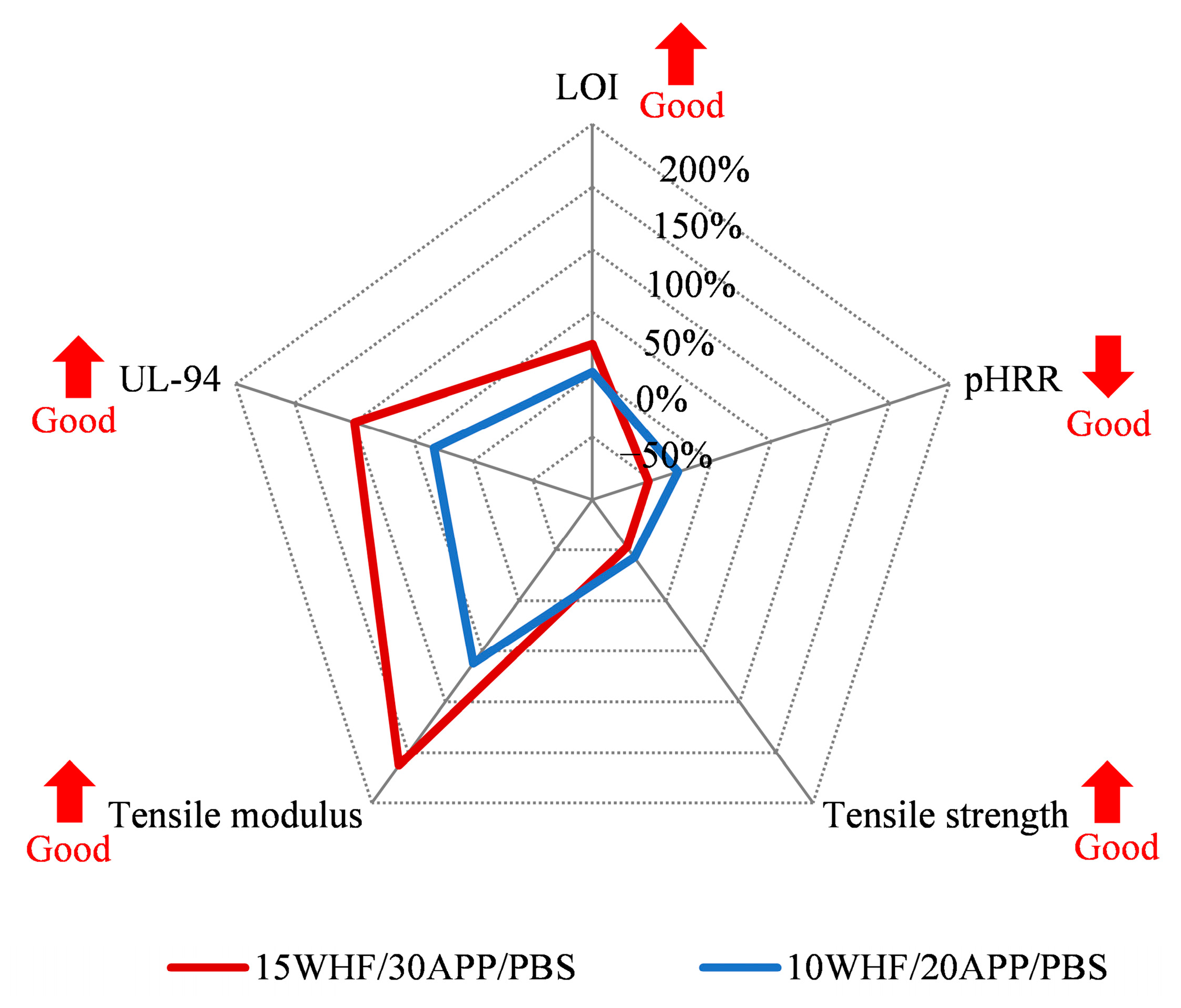
3.9. Comprehensive Performance of WHF/APP/PBS Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pongputthipat, W.; Ruksakulpiwat, Y.; Chumsamrong, P. Development of biodegradable biocomposite films from poly(lactic acid), natural rubber and rice straw. Polym. Bull. 2023, 80, 10289–10307. [Google Scholar] [CrossRef]
- Pavia, F.C.; Brucato, V.; Mistretta, M.C.; Botta, L.; La Mantia, F.P. A biodegradable, bio-based polymer for the production of tools for aquaculture: Processing, properties and biodegradation in sea water. Polymers 2023, 15, 927. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T. Processability and properties of aliphatic polyesters, ‘BIONELLE’, synthesized by polycondensation reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Suwanniroj, A.; Suppakarn, N. Influence of glycidyl methacrylate grafted poly(butylene succinate) (PBS-g-GMA) on flame retardancy and mechanical properties of water hyacinth fiber/ammonium polyphosphate/poly(butylene succinate) composites. J. Appl. Polym. Sci. 2022, 139, e53063. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Bourbigot, S. Recent developments in fire retardancy of polybutylene succinate. Polym. Degrad. Stab. 2021, 183, 109466. [Google Scholar] [CrossRef]
- Chen, H.; Wen, X.; Guan, Y.; Min, J.; Wen, Y.; Yang, H.; Chen, X.; Li, Y.; Yang, X.; Tang, T. Effect of particle size on the flame retardancy of poly(butylene succinate)/Mg(OH)2 composites. Fire Mater. 2016, 40, 1090–1096. [Google Scholar] [CrossRef]
- Chen, Q.; Wen, X.; Chen, H.; Qi, Y.; Gong, J.; Yang, H.; Li, Y.; Tang, T. Study of the effect of nanosized carbon black on flammability and mechanical properties of poly(butylene succinate). Polym. Adv. Technol. 2015, 26, 128–135. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, J.; Gui, Z.; Hu, Y.; Jiang, S. The influence of melamine phosphate modified MoS2 on the thermal and flammability of poly(butylene succinate) composites. Polym. Adv. Technol. 2016, 27, 1397–1400. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Motaung, T.E. The effect of expanded graphite/clay nanoparticles on thermal, rheological, and fire-retardant properties of poly(butylene succinate). Polym. Compos. 2021, 42, 6370–6382. [Google Scholar] [CrossRef]
- Dumazert, L.; Rasselet, D.; Pang, B.; Gallard, B.; Kennouche, S.; Lopez-Cuesta, J.M. Thermal stability and fire reaction of poly(butylene succinate) nanocomposites using natural clays and FR additives. Polym. Adv. Technol. 2018, 29, 69–83. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, Z.; Yang, W.; Luo, J.; Pan, B.; Lu, C. Enhancement of organoclay on thermal and flame retardant properties of polystyrene/magnesium hydroxide composite. Polym. Compos. 2016, 37, 746–755. [Google Scholar] [CrossRef]
- Yue, X.; Li, C.; Li, Y. Using colloidal lignin intercalated montmorillonite nanosheets as synergistic and reinforced agent for flame-retardant poly(butylene succinate) composites. Polym. Adv. Technol. 2021, 32, 2552–2565. [Google Scholar] [CrossRef]
- Kuan, C.F.; Kuan, H.C.; Ma, C.C.M.; Chen, C.H. Flame retardancy and nondripping properties of ammonium polyphosphate/poly(butylene succinate) composites enhanced by water crosslinking. J. Appl. Polym. Sci. 2006, 102, 2935–2945. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Dai, G.; Yuan, S.; Cai, F.; Li, B.; Hu, Y. Investigation on flame retardancy and thermal degradation of flame retardant poly(butylene succinate)/bamboo fiber biocomposites. J. Appl. Polym. Sci. 2012, 125, E485–E489. [Google Scholar] [CrossRef]
- Bourbigot, M.; Bras, L.M.; Duquense, S.; Rochery, M. Recent advances for intumescent polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Bourbigot, M.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413–1422. [Google Scholar] [CrossRef]
- Yue, X.; Li, Y.; Li, J.; Xu, Y. Improving fire behavior and smoke suppression of flame retardant PBS composites using lignin chelate as carbonization agent and catalyst. J. Appl. Polym. Sci. 2021, 138, 51199. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, S.; Li, H.; Sun, J.; Tang, W.F.; Zhao, L.; Gu, X. Preparation of intumescent flame retardant poly(butylene succinate) using urea intercalated kaolinite as synergistic agent. Fibers Polym. 2019, 20, 1631–1640. [Google Scholar] [CrossRef]
- Chen, Y.; Zhan, J.; Zhang, P.; Nie, S.; Lu, H.; Song, L.; Hu, Y. Preparation of intumescent flame retardant poly(butylene succinate) using fumed silica as synergistic agent. Ind. Eng. Chem. Res. 2010, 49, 8200–8208. [Google Scholar] [CrossRef]
- Wang, J.; Xue, L.; Zhao, B.; Lin, G.; Jin, X.; Liu, D.; Zhu, H.; Yang, J.; Shang, K. Flame retardancy, fire behavior, and flame retardant mechanism of intumescent flame retardant EPDM containing ammonium polyphosphate/pentaerythritol and expandable graphite. Materials 2019, 12, 4035. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jin, F.; Mao, Z.; Guan, Y.; Zheng, A. Effect of ammonium polyphosphate to pentaerythritol ratio on composition and properties of carbonaceous foam deriving from intumescent flame-retardant polypropylene. Polym. Degrad. Stab. 2014, 107, 64–73. [Google Scholar] [CrossRef]
- Ding, S.; Liu, P.; Zhang, S.; Ding, Y.; Wang, F.; Gao, C.; Yang, M. A green intumescent flame retardant system using an inositol-based carbon source: Preparation and characteristics in polypropylene. Polym. Int. 2021, 70, 1559–1569. [Google Scholar] [CrossRef]
- Chen, C.; Gu, X.; Jin, X.; Sun, J.; Zhang, S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of green lignin-based flame retardants for enhancing the thermal and fire retardancy properties of polypropylene/wood composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Yue, X.; Li, J.; Liu, P.; Lin, Y.; Du, X. Study on the performance of flame retardant esterified starch-modified cassava dregs-PBS composites. J. Appl. Polym. Sci. 2018, 135, 46210. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Wang, B.; Wen, P.; Song, L.; Hu, Y.; Zhang, P. Comparative study on the effect of beta-cyclodextrin and polypseudorotaxane as carbon sources on the thermal stability and flame retardance of polylactic acid. Ind. Eng. Chem. Res. 2013, 52, 3287–3294. [Google Scholar] [CrossRef]
- Jin, X.; Cui, S.; Sun, S.; Sun, J.; Zhang, S. The preparation and characterization of polylactic acid composites with chitin-based intumescent flame retardants. Polymer 2021, 13, 3513. [Google Scholar] [CrossRef]
- Shukor, F.; Hassan, A.; Saiful Islam, M.; Mokhtar, M.; Hasan, M. Effect of ammonium polyphosphate on flame retardancy, thermal stability and mechanical properties of alkali treated kenaf fiber filled PLA biocomposites. Mater. Des. 2014, 54, 425–429. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Wu, K.; Dai, G.; Hu, Y. Intumescent flame retardation of polypropylene/bamboo fiber semi-biocomposites. J. Therm. Anal. Calorim. 2013, 111, 425–430. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Lai, J.; Lu, C.; Wu, X.; Cai, Y.; Gu, L.; Yang, L.; Zhang, G.; Shi, G. Soy proteins and halloysite nanotubes-assisted preparation of environmentally friendly intumescent flame retardant for poly(butylene succinate). Polym. Test. 2020, 81, 106174. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Gabr, A.A. Evaluation of water hyacinth as a feed for ruminants. Arch. Tierernaehr. 1991, 41, 745–756. [Google Scholar] [CrossRef]
- Flores Ramirez, N.; Sanchez Hernandez, Y.; Cruz de Leon, J.; Vasquez Garcia, S.R.; Domratcheva Lvova, L.; Garcia Gonzalez, L. Composites from water hyacinth (Eichhornea crassipe) and polyester resin. Fibers Polym. 2015, 16, 196–200. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcille, S.; Fontaine, G. Poly(isosorbide carbonate): A ‘green’ char forming agent in polybutylene succinate intumescent formulation. Compos. Part B Eng. 2020, 184, 107675. [Google Scholar] [CrossRef]
- ASTM D3801-20a; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D635-22; Standard Test Method for Rate of Burning and/or Extent and Time of Burning of Plastics in a Horizontal Position. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM: West Conshohocken, PA, USA, 2019.
- ASTM D128-98(2019); Standard Test Methods for Analysis of Lubricating Grease. ASTM: West Conshohocken, PA, USA, 2019.
- ISO 5660-1:2015; Reaction-to-Fire Tests Heat Release, Smoke Production and Mass Loss Rate Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Chen, Z.; Yu, Y.; Zhang, Q.; Chen, Z.; Chen, T.; Li, C.; Jiang, J. Surface-modified ammonium polyphosphate with (3-aminopropyl) triethoxysilane, pentaerythritol and melamine dramatically improve flame retardancy and thermal stability of unsaturated polyester resin. J. Therm. Anal. Calorim. 2021, 143, 3479–3488. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Chen, Q.; Luo, F.; Cao, C. Effect of bamboo flour on flame retardancy and smoke suppression of polypropylene/ammonium polyphosphate composites. Front. Mater. 2020, 7, 574924. [Google Scholar] [CrossRef]
- Yu, B.; Yuen, A.C.Y.; Xu, X.; Zhang, Z.-C.; Yang, W.; Lu, H.; Fei, B.; Yeoh, G.; Song, P.; Wang, H. Engineering MXene surface with POSS for reducing fire hazards of polystyrene with enhanced thermal stability. J. Hazard. Mater. 2021, 401, 123342. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame retardancy index for thermoplastic composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef]
- Vahabi, H.; Movahedifar, E.; Kandola, B.K.; Saeb, M.R. Flame retardancy index (FRI) for polymer materials ranking. Polymers 2023, 15, 2422. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Li, X.; Zhang, T.; Xu, H. An intumescent flame-retardant layer with cyclodextrin as charring agent and its flame retardancy in jute/polypropylene composites. Polym. Bull. 2021, 78, 4281–4296. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.Y.; Feng, X.M.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Li, J. Effects of maleic anhydride grafted polypropylene on the physical, mechanical and flammability properties of wood-flour/polypropylene/ammonium polyphosphate composites. Fibers Polym. 2021, 22, 1137–1144. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Yu, B.; Kandola, B.; Deli, D. Comparative study on the synergistic effect of POSS and graphene with melamine phosphate on the flame retardance of poly(butylene succinate). Thermochim. Acta 2012, 543, 156–164. [Google Scholar] [CrossRef]
- Monika Mulchandani, N.; Katiyar, V. Generalized kinetics for thermal degradation and melt rheology for poly(lactic acid)/poly(butylene succinate)/functionalized chitosan based reactive nanobiocomposite. Int. J. Biol. Macromol. 2019, 141, 831–842. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Liu, H.; Evrendilek, F.; Buyukada, M. Pyrolysis of water hyacinth biomass parts: Bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers. Manag. 2020, 207, 112552. [Google Scholar] [CrossRef]




| Sample | WHF:APP Ratio | Total IFR (wt%) | PBS (wt%) | PBS-g-GMA (wt%) | WHF (wt%) | APP (wt%) |
|---|---|---|---|---|---|---|
| PBS | - | - | 100 | - | - | - |
| 30APP/PBS | - | 30 | 65 | 5 | - | 30 |
| 30WHF/PBS | - | 30 | 65 | 5 | 30 | - |
| 9WHF/36APP/PBS | 1:4 | 45 | 50 | 5 | 9 | 36 |
| 11.3WHF/33.7APP/PBS | 1:3 | 45 | 50 | 5 | 11.3 | 33.7 |
| 15WHF/30APP/PBS | 1:2 | 45 | 50 | 5 | 15 | 30 |
| 13WHF/27APP/PBS | 1:2 | 40 | 55 | 5 | 13.3 | 26.7 |
| 12WHF/23APP/PBS | 1:2 | 35 | 60 | 5 | 11.7 | 23.3 |
| 10WHF/20APP/PBS | 1:2 | 30 | 65 | 5 | 10 | 20 |
| Sample | LOI (%) | UL-94 | Horizontal Burning Rate (mm/min) | |||||
|---|---|---|---|---|---|---|---|---|
| WHF: APP Ratio | Total IFR Content (wt%) | t1 (s) | Dripping | Ignite the Absorbent Cotton | Rating | |||
| PBS | - | - | 23.3 | >30 | Yes | Yes | NC | 16.39 |
| 30APP/PBS | - | 30 | 32.0 | - | Yes | No | V-0 | - |
| 30WHF/PBS | - | 30 | 20.6 | >30 | Yes | Yes | NC | 14.45 |
| 9WHF/36APP/PBS | 1:4 | 45 | 25.5 | 4 | Yes | No | V-0 | - |
| 11.3WHF/33.7APP/PBS | 1:3 | 45 | 27.4 | 2 | No | No | V-0 | - |
| 15WHF/30APP/PBS | 1:2 | 45 | 28.8 | - | No | No | V-0 | - |
| 13WHF/27APP/PBS | 1:2 | 40 | 24.5 | 28 | Yes | Yes | V-2 | 9.75 |
| 12WHF/23APP/PBS | 1:2 | 35 | 24.4 | 20 | Yes | Yes | V-2 | 8.25 |
| 10WHF/20APP/PBS | 1:2 | 30 | 23.7 | 22 | Yes | Yes | V-2 | 3.75 |
| Sample | WHF:APP Ratio | Total IFR Content (wt%) | T5% (°C) | T50% (°C) | Tmax1 (°C) | Rate at Tmax (%/min) | Tmax2 (°C) | W800 (wt%) |
|---|---|---|---|---|---|---|---|---|
| PBS | - | - | 341.8 | 395.8 | 401.8 | 0.40 | - | - |
| 30APP/PBS | - | 30 | 330.2 | 352.5 | 352.3 | 1.77 | 613.3 | 4.04 |
| 30WHF/PBS | - | 30 | 271.2 | 391.2 | 396.3 | 0.28 | - | 10.93 |
| 9WHF/36APP/PBS | 1:4 | 45 | 298.8 | 347.5 | 347.2 | 1.53 | 632.5 | 7.54 |
| 11.3WHF/33.7APP/PBS | 1:3 | 45 | 280.7 | 346.8 | 345.8 | 1.39 | - | 13.79 |
| 15WHF/30APP/PBS | 1:2 | 45 | 220.2 | 351.3 | 349.7 | 0.62 | - | 20.93 |
| 13WHF/27APP/PBS | 1:2 | 40 | 245.7 | 346.2 | 345.2 | 1.34 | - | 10.98 |
| 12WHF/23APP/PBS | 1:2 | 35 | 249.2 | 346.0 | 345.5 | 1.68 | - | 9.24 |
| 10WHF/20APP/PBS | 1:2 | 30 | 253.3 | 344.7 | 344.8 | 1.75 | - | 7.53 |
| Sample | Time to | pHRR (kW/m2) | FPI (m2s/kW) | FIG (kW/m2s) | FRI | THR (MJ/m2) | TSP (m2) | Residue (wt%) | |
|---|---|---|---|---|---|---|---|---|---|
| Ignition (s) | pHRR (s) | ||||||||
| PBS | 75 | 120 | 528.59 | 0.14 | 4.40 | - | 71.7 | 2.9 | 0.73 |
| 15WHF/30APP/PBS | 65 | 75 | 250.10 | 0.26 | 3.33 | 3.14 | 41.8 | 3.0 | 37.42 |
| 10WHF/20APP/PBS | 45 | 60 | 380.93 | 0.13 | 6.35 | 1.06 | 56.0 | 5.3 | 23.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwanniroj, A.; Suppakarn, N. Water Hyacinth Fiber as a Bio-Based Carbon Source for Intumescent Flame-Retardant Poly (Butylene Succinate) Composites. Polymers 2023, 15, 4211. https://doi.org/10.3390/polym15214211
Suwanniroj A, Suppakarn N. Water Hyacinth Fiber as a Bio-Based Carbon Source for Intumescent Flame-Retardant Poly (Butylene Succinate) Composites. Polymers. 2023; 15(21):4211. https://doi.org/10.3390/polym15214211
Chicago/Turabian StyleSuwanniroj, Anothai, and Nitinat Suppakarn. 2023. "Water Hyacinth Fiber as a Bio-Based Carbon Source for Intumescent Flame-Retardant Poly (Butylene Succinate) Composites" Polymers 15, no. 21: 4211. https://doi.org/10.3390/polym15214211
APA StyleSuwanniroj, A., & Suppakarn, N. (2023). Water Hyacinth Fiber as a Bio-Based Carbon Source for Intumescent Flame-Retardant Poly (Butylene Succinate) Composites. Polymers, 15(21), 4211. https://doi.org/10.3390/polym15214211







