Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems
Abstract
1. Introduction
2. Materials and Methods
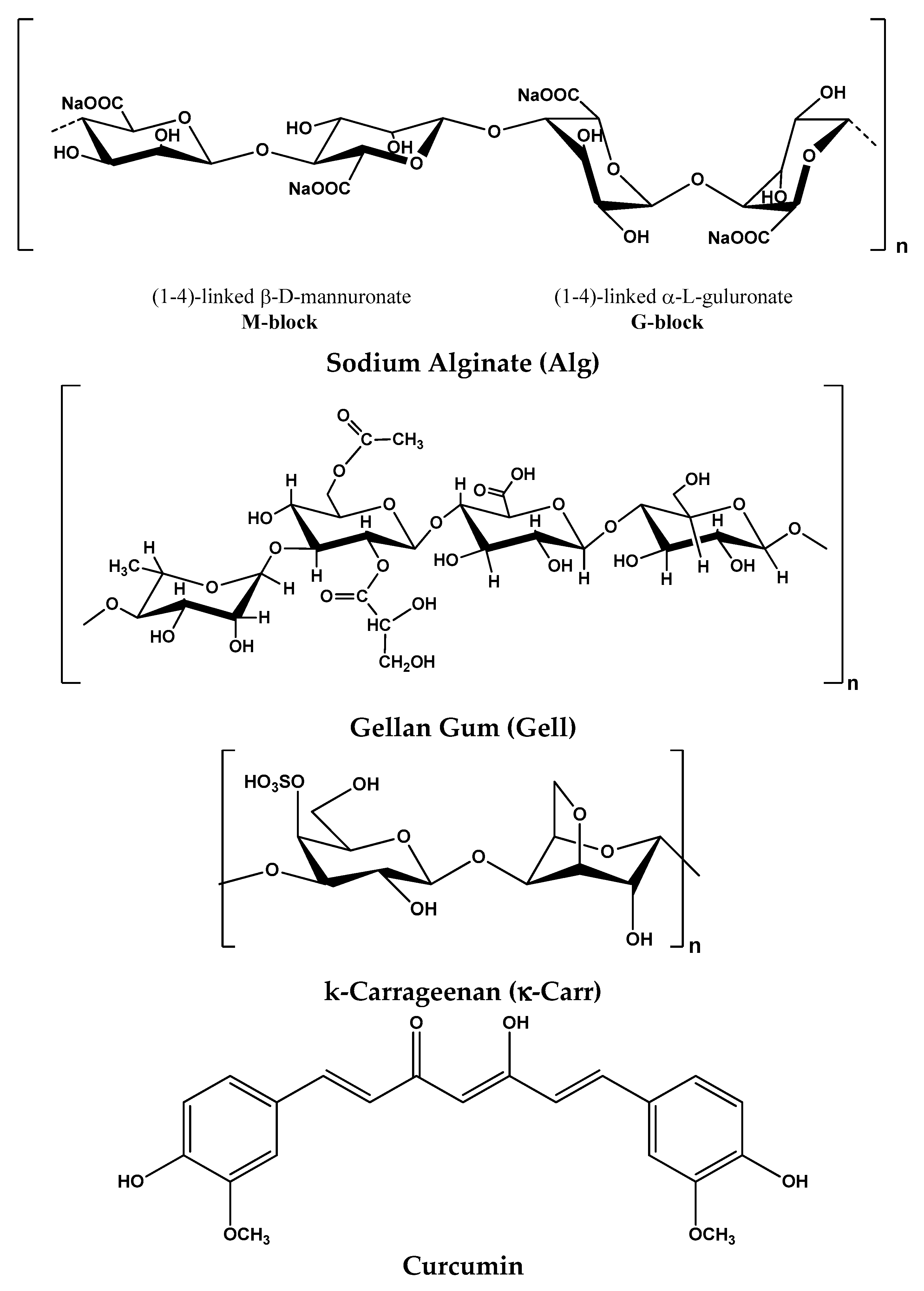
2.1. Materials
2.2. Methods
2.2.1. Rheological Measurements
2.2.2. Antimicrobial Activity
3. Results
3.1. Rheological Behavior
3.1.1. Rheological Behavior of Polysaccharides in Solution
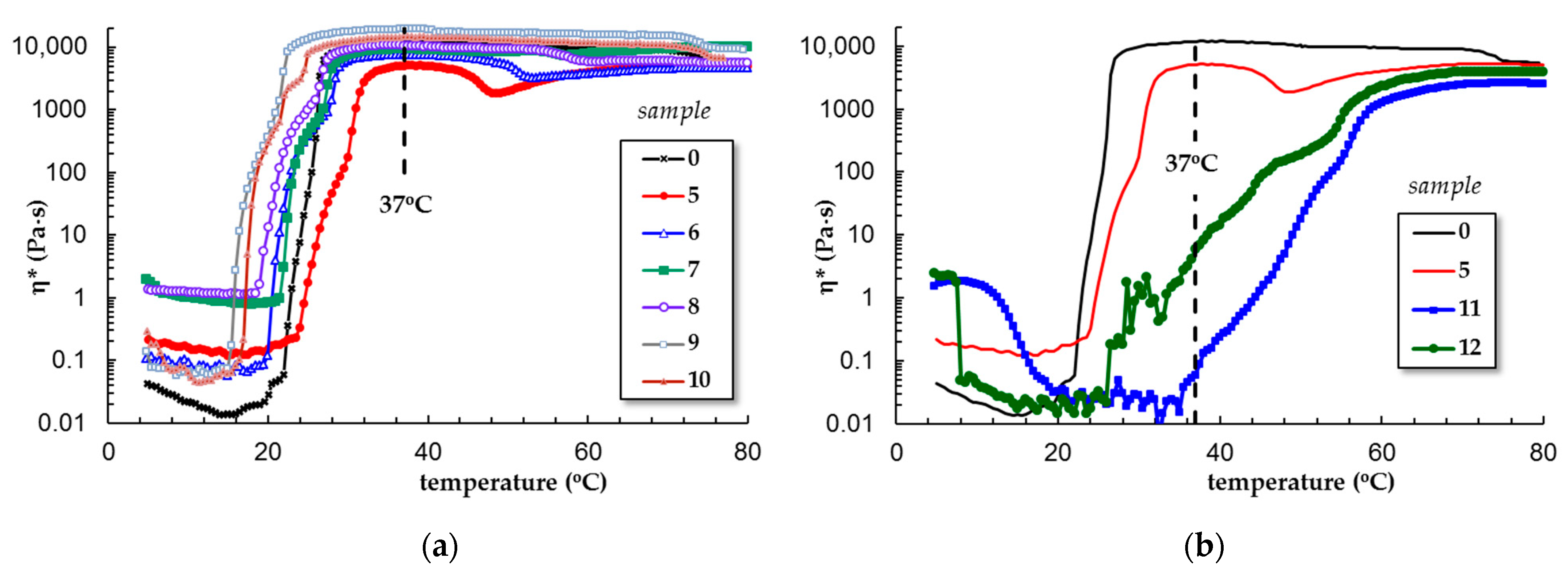
3.1.2. Temperature-Induced Gelation for Various PL-Based Gels
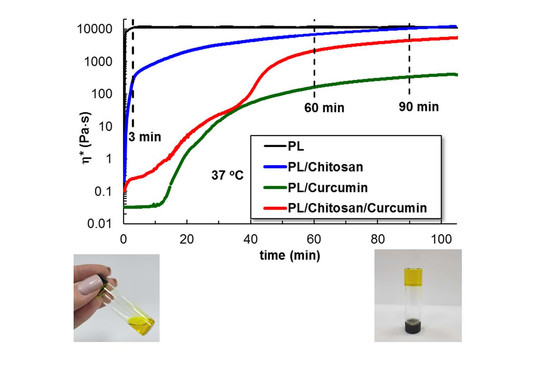
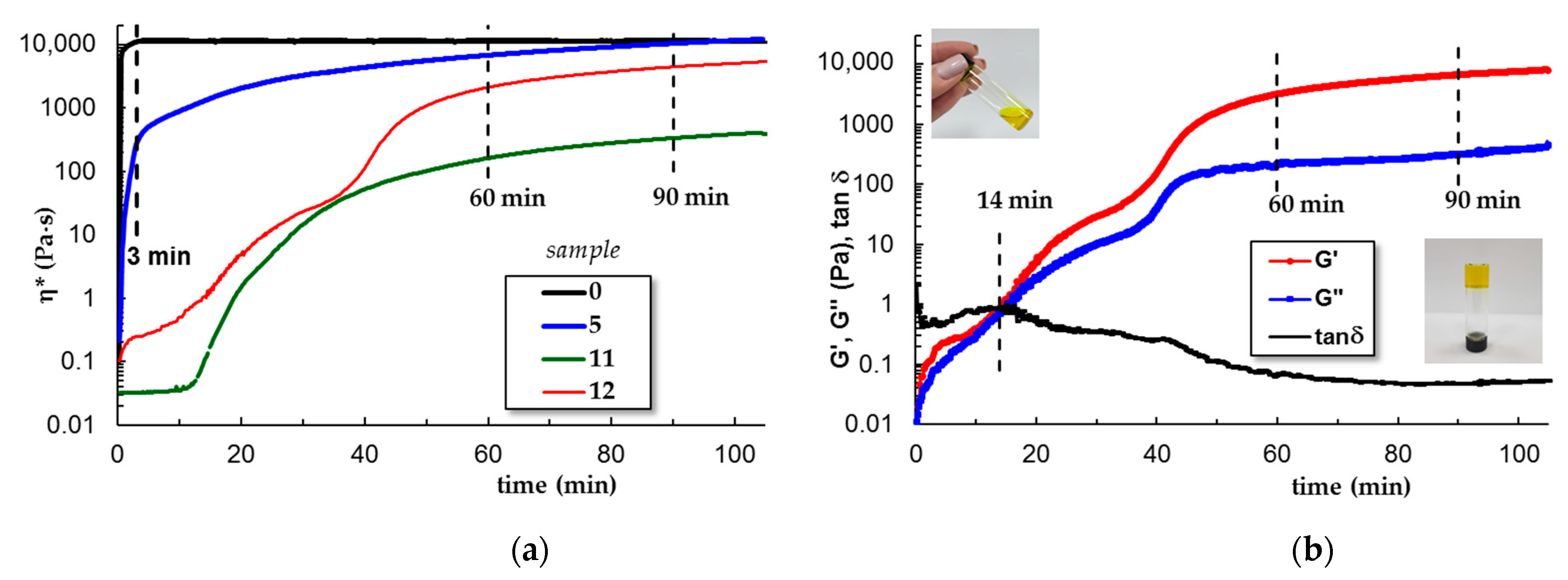
3.1.3. Gelation Kinetics at 37 °C
3.1.4. Viscoelastic Behavior of Gels at 37 °C
3.1.5. Flow Behavior of Gels at 37 °C
3.2. The Antimicrobial Behavior of the Pristine Polysaccharides and Pluronic F127-Based Gels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Contr. Rel. 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug delivery systems based on pluronic micelles with antimicrobial activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a cell encapsulation material: Utilization of membrane-stabilizing agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Cidade, M.T.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable hydrogels based on Pluronic/water systems filled with alginate microparticles for biomedical applications. Materials 2019, 12, 1083. [Google Scholar] [CrossRef]
- Muller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. J. Visualized Exp. 2013, 77, 50632. [Google Scholar] [CrossRef]
- Mosallanezhad, P.; Nazockdast, H.; Ahmadi, Z.; Rostami, A. Fabrication and characterization of polycaprolactone/chitosan nanofibers containing antibacterial agents of curcumin and ZnO nanoparticles for use as wound dressing. Front. Bioeng. Biotechnol. 2022, 10, 1027351. [Google Scholar] [CrossRef]
- Suman, K.; Sourav, S.; Joshi, Y.M. Rheological signatures of gel–glass transition and a revised phase diagram of an aqueous triblock copolymer solution of Pluronic F127. Phys. Fluids 2021, 33, 073610. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Park, E.-K.; Hyun, K. Rheological analysis of core-stabilized Pluronic F127 by semi-interpenetrating network (sIPN) in aqueous solution. J. Rheol. 2018, 62, 107. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Said dos Santos, R.; Bassi da Silva, M.; Braga, G.; Cook, M.T.; Bruschi, M.L. Interaction between mucoadhesive cellulose derivatives and Pluronic F127: Investigation on the micelle structure and mucoadhesive performance. Mater. Sci. Eng. C 2021, 119, 111643. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Locarno, S.; Gallo, S.; Orsini, F.; Pini, C.; Farronato, M.; Thomaz, D.V.; Lenardi, C.; Piazzoni, M.; Tartaglia, G. Poloxamer-based hydrogel as drug delivery system: How polymeric excipients influence the chemical-physical properties. Polymers 2022, 14, 3624. [Google Scholar] [CrossRef]
- da Silva, L.C.E.; Borges, A.C.; de Oliveira, M.G.; de Farias, M.A. Visualization of supramolecular structure of Pluronic F127 micellar hydrogels using cryo-TEM. MethodsX 2020, 7, 101084. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.-L.; Calejo, A.M.; Zhu, K.; Nyström, B.; Sande, S.A. Stabilization of pluronic gels in the presence of different polysaccharides. J. Appl. Polym. Sci. 2014, 131, 40465. [Google Scholar] [CrossRef]
- Bercea, M.; Darie, R.N.; Nita, L.E.; Morariu, S. Temperature responsive gels based on Pluronic F127 and poly(vinyl alcohol). Ind. Eng. Chem. Res. 2011, 50, 4199–4206. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Bercea, M.; Wolf, B.A. Synergistic behavior of poly(aspartic acid) and Pluronic F127 in aqueous solution as studied by viscometry and dynamic light scattering. Colloids Surf. B 2013, 103, 544–549. [Google Scholar] [CrossRef]
- Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive acrylated block copolymers micelles for the delivery of hydrophobic drugs. Colloids Surf. B 2016, 139, 42–51. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-leung, P.; Wat, E.; Kan, C.; Leung, P.C. Drug delivery system of dual-responsive PF127 hydrogel with polysaccharide-based nano-conjugate for textile-based transdermal therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A guide to polysaccharide-based hydrogel bioinks for 3D bioprinting applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef] [PubMed]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 thermosensitive hydrogel as an injectable dexamethasone delivery carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Joung, Y.K.; Choi, J.H.; Moon, H.T.; Park, K.D. Targeting ligand-functionalized and redox-sensitive heparin-Pluronic nanogels forintracellular protein delivery. Biomed. Mat. 2011, 6, 055004. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Yu, Y.; Zheng, Y. Insights into the role of natural polysaccharide-based hydrogel wound dressings in biomedical applications. Gels 2022, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Bertilla, X.J.; Rupachandra, S. Insights into current directions of protein and peptide-based hydrogel drug delivery systems for inflammation. Polym. Bull. 2022; in press. [Google Scholar] [CrossRef]
- Bercea, M. Bioinspired hydrogels as platforms for life-science applications: Challenges and opportunities. Polymers 2022, 14, 2365. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Karthikeyan, C.; Yallapu, M.M.; Sadiku, R. The significance of biomacromolecule alginate for the 3D printing of hydrogels for biomedical applications. Int. J. Biol. Macromol. 2022, 212, 561–578. [Google Scholar] [CrossRef]
- Salay, L.C.; Prazeres, E.A.; Marín Huachaca, N.S.; Lemos, M.; Piccoli, J.P.; Sanches, P.R.S.; Feitosa, E. Molecular interactions between Pluronic F127 and the peptide tritrpticin in aqueous solution. Colloid. Polym. Sci. 2018, 296, 809–817. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, G.H.; Park, H.J. Solid lipid nanoparticles loaded thermoresponsive Pluronic–xanthan gum hydrogel as a transdermal delivery system. J. Appl. Polym. Sci. 2018, 135, 46004. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G. Aggregation behavior of Pluronic F127 solutions in presence of chitosan/clay nanocomposites examined by dynamic light scattering. J. Colloid Interface Sci. 2019, 542, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.; Kan, C.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Zheng, L.; Wu, J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Res. 2022; in press. [Google Scholar] [CrossRef]
- Lin, H.R.; Sung, K.C.; Vong, W.J. In situ gelling of alginate/Pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Choi, J.H.; Lee, S.; Lee, S.W.; Moon, B.K.; Song, J.E.; Khang, G. Pluronic F-127/silk fibroin for enhanced mechanical property and sustained release drug for tissue engineering biomaterial. Materials 2021, 14, 1287. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-W.; Park, M.J.; Hwang, J.; Char, K. Effects of alcohol addition on gelation in aqueous solution of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer. Polym. J. 2001, 33, 404–410. [Google Scholar] [CrossRef]
- Bercea, M.; Constantin, M.; Plugariu, I.A.; Daraba, M.O.; Ichim, D.L. Thermosensitive gels of pullulan and poloxamer 407 as potential injectable biomaterials. J. Mol. Liq. 2022, 362, 119717. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Masson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Singh, R.; Tønnesen, H.H.; Kristensen, S.; Berg, K. The influence of Pluronics® on dark cytotoxicity, photocytotoxicity, localization and uptake of curcumin in cancer cells: Studies of curcumin and curcuminoids XLIX. Photochem. Photobiol. Sci. 2013, 12, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M. Single disc antibiotic sensitivity testing of Staphylococci. AMA Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100 (ISBN 978-1-68440-134-5 [Print]; ISBN 978-1-68440-135-2 [Electronic]); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 1 December 2022).
- XLSTAT|Statistical Software for Excel n.d. Available online: https://www.xlstat.com/en (accessed on 1 December 2022).
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.-S.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer properties of curcumin against colorectal cancer: A review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Thapa, R.K.; Cazzador, F.; Grønlien, K.G.; Tønnesen, H.H. Effect of curcumin and cosolvents on the micellization of Pluronic F127 in aqueous solution. Colloids Surf. B 2020, 195, 111250. [Google Scholar] [CrossRef]
- Ganguly, R.; Kumar, S.; Kunwar, A.; Nath, S.; Sarma, H.D.; Tripathi, A.; Verma, G.; Chaudhari, D.P.; Aswal, V.K.; Melo, J.S. Structural and therapeutic properties of curcumin solubilized Pluronic F127 micellar solutions and hydrogels. J. Mol. Liq. 2020, 314, 113591. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sharma, S.; Shaikh, S.; Ray, D.; Aswal, V.K.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro--inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2019, 2, e1133. [Google Scholar] [CrossRef]
- Dung, T.H.; Huong, L.H.; Yoo, H. Morphological feature of Pluronic F127 and its application in burn treatment. J. Nanosci. Nanotechnol. 2018, 18, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Rył, A.; Owczarz, P. Injectability of thermosensitive, low-concentrated chitosan colloids as flow phenomenon through the capillary under high shear rate conditions. Polymers 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Muszanska, A.K.; Busscher, H.J.; Herrmann, A.; van der Mei, H.C.; Norde, W. Pluronic-lysozyme conjugates as anti-adhesive and antibacterial bifunctional polymers for surface coating. Biomaterials 2011, 32, 6333–6341. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An overview of antimicrobial activity of lysozyme and its functionality in cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Deka, C.; Deka, D.; Bora, M.M.; Jha, D.K.; Kakati, D.K. Synthesis of peppermint oil-loaded chitosan/alginate polyelectrolyte complexes and study of their antibacterial activity. J. Drug Deliv. Sci. Technol. 2016, 35, 314–322. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocoll. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Razali, M.H. Antibacterial study of gellan gum (GG) film incorporated norfloxacin. J. Pure Appl. Microbiol. 2019, 13, 1095–1102. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Wloch, M.; Pamula, E. Injectable gellan gum-based nanoparticles-loaded system for the local delivery of vancomycin in osteomyelitis treatment. J. Mater. Sci. Mater. Med. 2016, 27, 9. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Fang, Y.; Holley, R.A. Inhibition of Campylobacter jejuni on fresh chicken breasts by κ-carrageenan/chitosan-based coatings containing allyl isothiocyanate or deodorized oriental mustard extract. Int. J. Food Microbiol. 2014, 187, 77–82. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.; Assis, O. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based antimicrobial films for food packaging applications. e-Polymers 2008, 93, 1–7. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Ailincai, D.; Rosca, I.; Morariu, S.; Mititelu-Tartau, L.; Marin, L. Iminoboronate—Chitooligosaccharides hydrogels with strong antimicrobial activity for biomedical applications. Carbohydr. Polym. 2022, 276, 118727. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert. Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- David, G.; Bargan, A.I.; Drobota, M.; Bele, A.; Rosca, I. Comparative investigation of collagen-based hybrid 3D structures for potential biomedical applications. Materials 2021, 14, 3313. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Jalaal, M.; Cottrell, G.; Balmforth, N.; Stoeber, B. On the rheology of Pluronic F127 aqueous solutions. J. Rheol. 2017, 61, 139–146. [Google Scholar] [CrossRef]
- Ramy, K.A.; Kodavaty, J.; Dorishetty, P.; Setti, M.; Deshpande, A.P. Characterizing the yielding processes in pluronic-hyaluronic acid thermoreversible gelling systems using oscillatory rheology. J. Rheol. 2019, 63, 215–228. [Google Scholar] [CrossRef]
- Weinand, C.; Pomerantseva, I.; Neville, C.M.; Gupta, R.; Weinberg, E.; Madisch, I.; Shapiro, F.; Abukawa, H.; Troulis, M.J.; Vacanti, J.P. Hydrogel-beta-TCP scaffolds and stem cells for tissue engineering bone. Bone 2006, 38, 555–563. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of Poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Swennen, I.; Girones, J.; Moroni, L.; van Blitterswijk, C.A.; Schacht, E.; Alblas, J.; Dhert, W.J.A. Evaluation of photocrosslinked Lutrol hydrogel for tissue printing applications. Biomacromolecules 2009, 10, 1689–1696. [Google Scholar] [CrossRef]
- Wang, P.-L.; Johnston, T.P. Sustained-release interleukin-2 following intramuscular injection in rats. Int. J. Pharm. 1995, 113, 73–81. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Blanchard, J. Controlled-release delivery system for the a-MSH analog melanotan-I using poloxamer 407. J. Pharm. Sci. 1996, 85, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, W.-L.; Wang, J.-C.; Zhang, X.; Zhang, H.; Wang, X.-Q.; Zhou, T.-Y.; Zhang, Q. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic® F127 hydrogel for subcutaneous administration: In vitro and in vivo characterization. J. Control. Release 2007, 117, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.; Lando, G.; Sosnik, A.; Garty, S.; Levi, A. PEO–PPO–PEO-based poly(ether ester urethane)s as degradable reverse thermo-responsive multiblock copolymers. Biomaterials 2006, 27, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Batrakova, E.V.; Kabanov, A.V. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug. Chem. 2008, 19, 2023–2029. [Google Scholar] [CrossRef]
- Arranja, A.; Schroder, A.P.; Schmutz, M.; Watona, G.; Schosseler, F.; Mendes, E. Cytotoxicity and internalization of Pluronic micelles stabilized by core cross-linking. J. Control. Release 2014, 196, 87–95. [Google Scholar] [CrossRef]
- Sungkhaphan, P.; Risangud, N.; Hankamolsiri, W.; Sonthithai, P.; Janvikul, W. Pluronic-F127 and Click chemistry-based injectable biodegradable hydrogels with controlled mechanical properties for cell encapsulation. React. Funct. Polym. 2022, 181, 105439. [Google Scholar] [CrossRef]
- Dang, L.H.; Huynh, N.T.; Pham, N.O.; Nguyen, C.T.; Vu, M.T.; Dinh, V.T.; Le, V.T.; Tran, N.Q. Injectable nanocurcumin-dispersed gelatin–pluronic nanocomposite hydrogel platform for burn wound treatment. Bull. Mater. Sci. 2019, 42, 1–10. [Google Scholar] [CrossRef]
- Boonlai, W.; Hirun, N.; Suknuntha, K.; Tantishaiyakul, V. Development and characterization of pluronic F127 and methylcellulose based hydrogels for 3D bioprinting. Polym. Bull. 2022; in press. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, S.; Lin, L.; Cao, Y.; Xie, X.; Yu, H.; Chen, M.; Li, H. Redox-sensitive Pluronic F127-tocopherol micelles: Synthesis, characterization, and cytotoxicity evaluation. Int. J. Nanomedicine 2017, 12, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Lu, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Feng, C.; Liu, M. Injectable shell-crosslinked F127 micelle/hydrogel composites with pH and redox sensitivity for combined release of anticancer drugs. Chem. Eng. J. 2016, 287, 20–29. [Google Scholar] [CrossRef]







| Sample Code | Sample Composition | G′ (Pa) (10 rad/s) | G″ (Pa) (10 rad/s) | ηo (Shear) (Pa·s) |
|---|---|---|---|---|
| 0 * | Pluronic® F127 (PL) | 13,200 | 2600 | 74,000 |
| 1 ** | Chitosan (CS) | 0.0254 | 0.0622 | 0.0488 |
| 2 ** | Sodium Alginate (Alg) | 0.3067 | 1.5612 | 0.0976 |
| 3 ** | Gellan Gum (GG) | 0.1718 | 0.2914 | 0.0318 |
| 4 ** | k-Carrageenan (κ-Carr) | 0.1554 | 0.5511 | 0.0446 |
| 5 * | PL/CS | 8800 | 3300 | 23,300 |
| 6 * | PL/Alg | 8990 | 2450 | 47,400 |
| 7 * | PL/Gell | 8680 | 2310 | 28,600 |
| 8 * | PL/k-Carr | 10,300 | 2990 | 27,900 |
| 9 * | PL/ZnO | 19,500 | 3670 | 54,600 |
| 10 * | PL/Lysozyme | 42,500 | 5850 | 35,200 |
| 11 * | PL/Curcumin | 21,100 | 1320 | 61,500 |
| 12 * | PL/CS/Curcumin | 57,583 | 6733 | 45,500 |
| Sample Code | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | E. faecalis | K. pneumoniae | P. aeruginosa | C. albicans | C. glabrata | |
| 0 | -* | -* | -* | -* | -* | -* | -* |
| 1 | -* | 8.45 ± 0.35 | 8.40 ± 0.14 | 8.30 ± 0.14 | 8.80 ± 0.14 | 7.90 ± 0.14 | 7.50 ± 0.42 |
| 2 to 10 | -* | -* | -* | -* | -* | -* | -* |
| 11 | 21.35 ± 0.63 | 25.00 ± 0.14 | 21.30 ± 0.14 | 21.00 ± 0.14 | 33.30 ± 0.14 | 14.80 ± 0.57 | 11.60 ± 0.99 |
| 12 | 22.60 ± 0.28 | 26.95 ± 0.21 | 25.40 ± 2.68 | 22.25 ± 0.07 | 34.15 ± 0.91 | 15.30 ± 0.56 | 12.60 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, A.; Rosca, I.; Gradinaru, V.R.; Bercea, M. Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems. Polymers 2023, 15, 355. https://doi.org/10.3390/polym15020355
Lupu A, Rosca I, Gradinaru VR, Bercea M. Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems. Polymers. 2023; 15(2):355. https://doi.org/10.3390/polym15020355
Chicago/Turabian StyleLupu, Alexandra, Irina Rosca, Vasile Robert Gradinaru, and Maria Bercea. 2023. "Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems" Polymers 15, no. 2: 355. https://doi.org/10.3390/polym15020355
APA StyleLupu, A., Rosca, I., Gradinaru, V. R., & Bercea, M. (2023). Temperature Induced Gelation and Antimicrobial Properties of Pluronic F127 Based Systems. Polymers, 15(2), 355. https://doi.org/10.3390/polym15020355