Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Phenol-Formaldehyde (PF)
2.3. Lignin Phenolation
2.4. Synthesis of the Lignin-Phenol-Formaldehyde (LPF)
2.5. Resin Properties
2.5.1. Viscosity
2.5.2. pH
2.5.3. Solids Content
2.5.4. Gel Time
2.6. Mechanical Test
2.7. Thermal Tests
2.7.1. Differential Scanning Calorimetry (DSC)
2.7.2. Thermal Gravimetric Analysis (TGA)
2.8. Fourier Transform Infrared Spectrometry (FTIR)
2.9. Statistical Analysis
3. Results
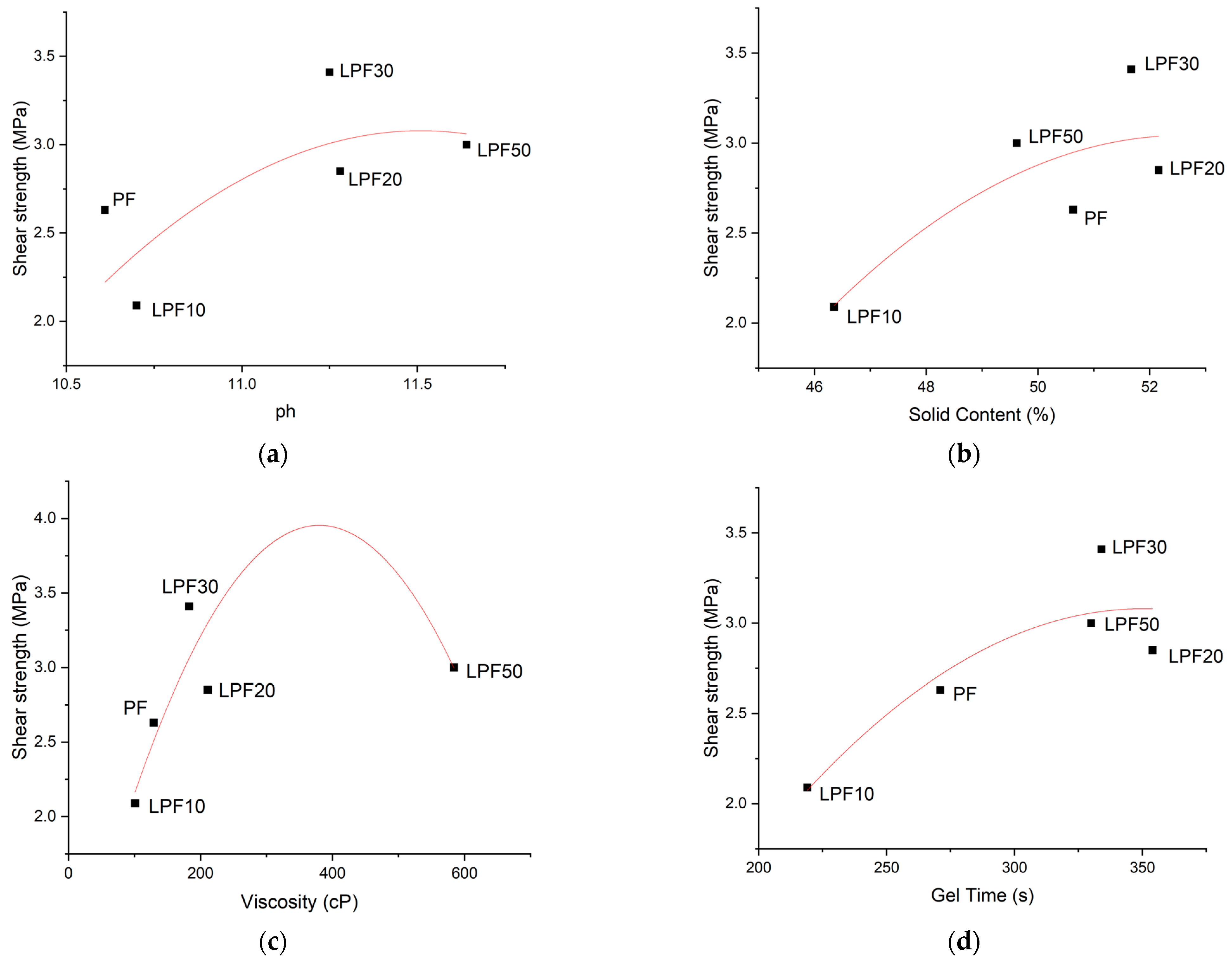
3.1. Physicochemical Properties
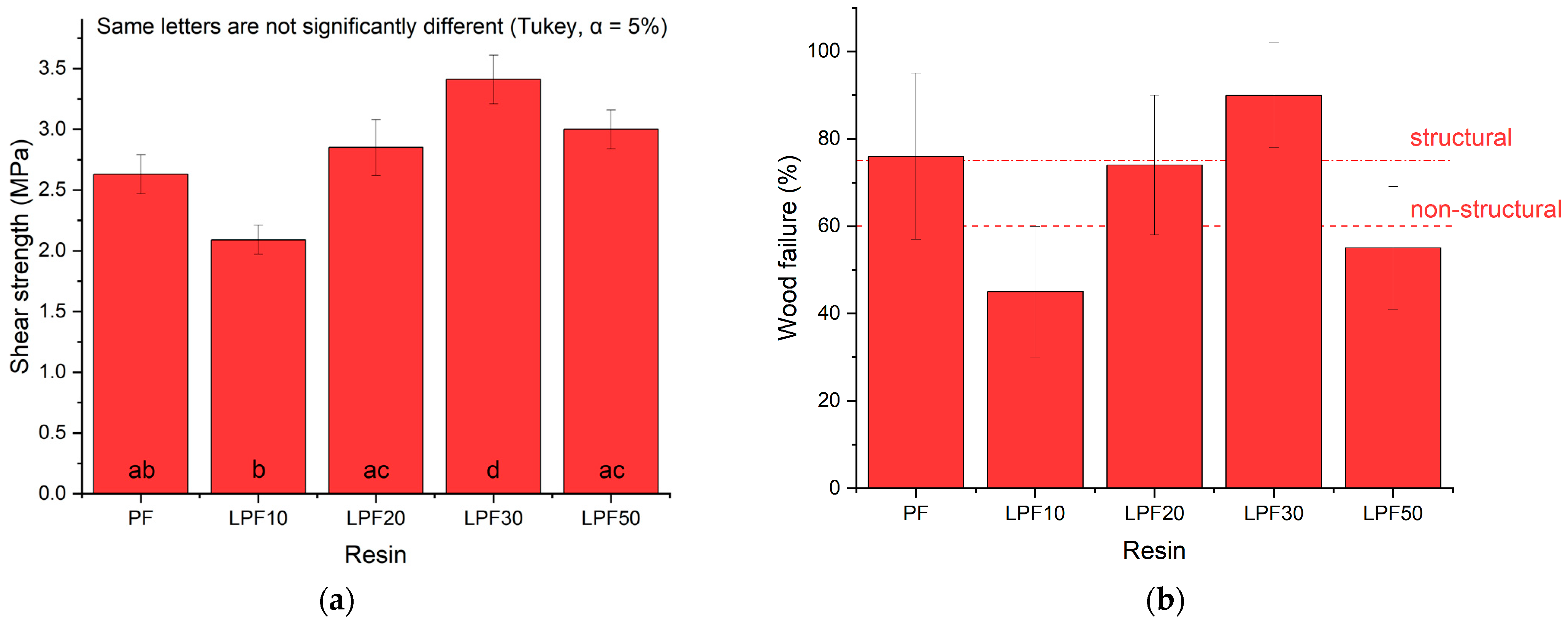
3.2. Mechanical Properties
3.3. Thermal Analyses
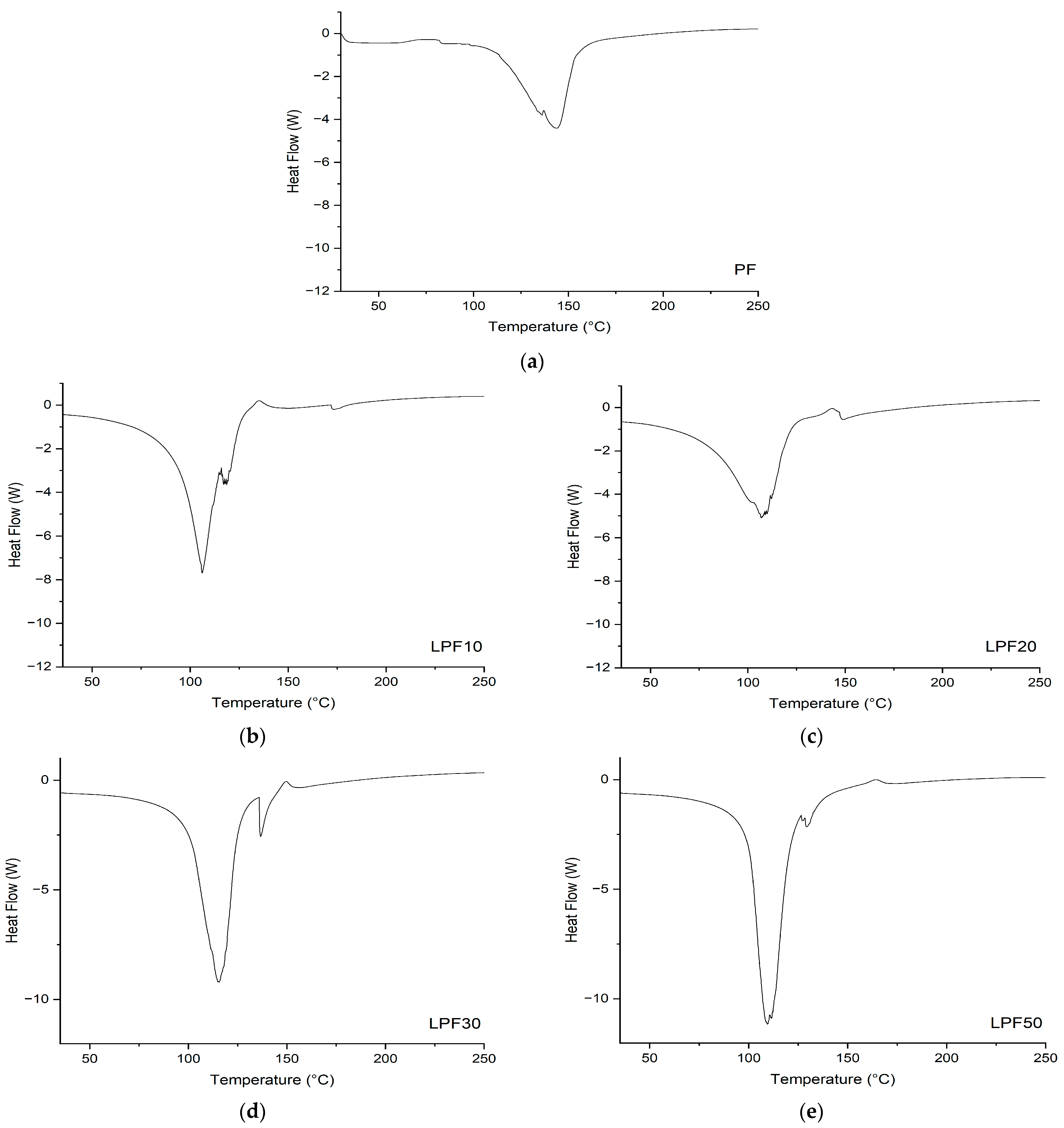
3.3.1. Differential Scanning Calorimetry (DSC)
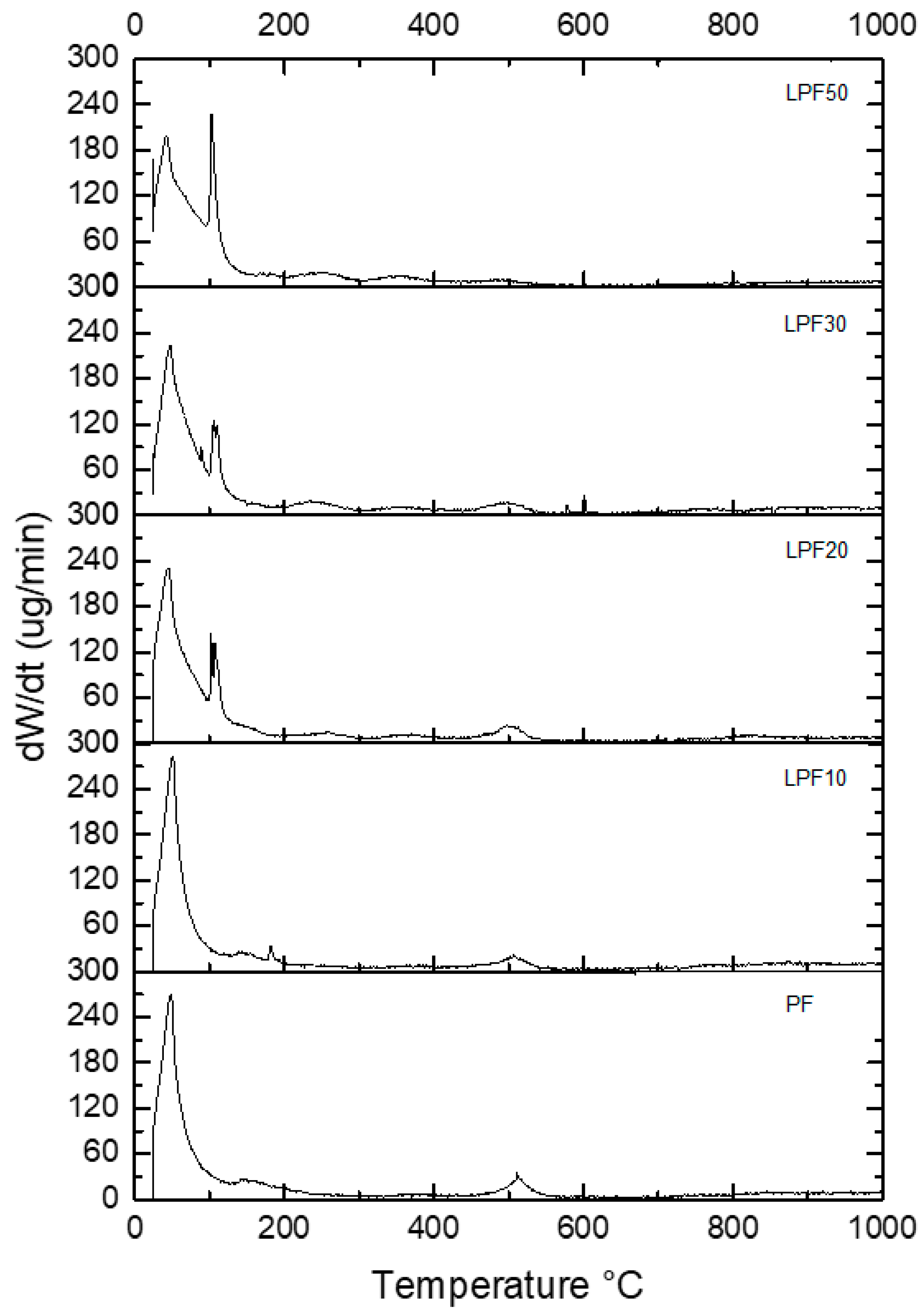
3.3.2. Thermal Gravimetric Analysis (TGA)
3.4. FTIR Analysis
4. Discussion
5. Conclusions
- Lignin-based resins can be synthesized in shorter periods such as 120 min.
- The physical tests of the four resin formulations indicated that the addition of phenol-lignin tends to influence in a more pronounced way the characteristics of the resins from the substitution percentage of 20%.
- Differential Scanning Calorimetry analysis showed that the curing of lignin-based resins occurs at lower temperatures with greater enthalpies compared to PF resin.
- Shear strength in the glue line of LPF resins is compatible with the PF resin in a replacement percentage of 30%, a condition considered ideal as it meets the specifications for structural use.
- Considering the overall performance in thermal tests, the replacement of phenol by lignin results in a slight decrease in resistance to high temperatures, being compensated by the reduction in ignition of LPF resins in the initial stages.
- For future works, we suggest the performance of studies towards the increase of substitution levels and the degree of reticulation of resins. In addition, there is the possibility to investigate the behavior of structural panels glued with LPF resins under the effective simulation of fire tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilato, L. Phenolic Resins: 100 Years and Still Going Strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Ren, H. Prospects of Phenolic Resin. RSC Adv. Open 2019, 50, 28924–28935. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and Properties of Lignin-Phenol-Formaldehyde Resins Based on Different Biorefinery Residues of Agricultural Biomass. Ind. Crops Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Zheng, Z. Bioresource Technology Preparation and Characterization of Phenol—Formaldehyde Adhesives Modified with Enzymatic Hydrolysis Lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Washburn, N.R. Chemistry of Lignin-Based. Green Mater. 2013, 1, 137–160. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Hussin, M.H.; Aziz, A.A.; Iqbal, A.; Nasir, M.; Ibrahim, M.; Hanis, N.; Latif, A. International Journal of Biological Macromolecules Development and Characterization Novel Bio-Adhesive for Wood Using Kenaf Core (Hibiscus Cannabinus) Lignin and Glyoxal. Int. J. Biol. Macromol. 2019, 122, 713–722. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-Based Fl Ame Retardants: When Nature Meets Fi Re Protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current Advancement on the Isolation, Characterization and Application of Lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.; Laoutid, F.; Brohez, S.; Delvosalle, C.; Dubois, P. Phytic Acid—Lignin Combination: A Simple and efficient Route for Enhancing Thermal and Flame Retardant Properties of Polylactide. Eur. Polym. J. 2017, 94, 270–285. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Labib, A.; Sudarmanto; Akbar, F.; Nuryawan, A.; Antov, P.; Kristak, L.; Papadopoulos, A.N. Influence of Lignin Content and Pressing Time on Plywood Properties Bonded with Cold-Setting Adhesive Based on Poly (Vinyl Alcohol), Lignin, and Hexamine. Polymers 2022, 14, 2111. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A.; Niyatzade, G. Reduction of Formaldehyde Emission from Particleboard by Phenolated Kraft Lignin. J. Adhes. 2016, 92, 485–497. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. (Charles) Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Younesi-kordkheili, H.; Pizzi, A. A Comparison among Lignin Modification Methods on the Properties of Lignin-Phenol-Formaldehyde Resin as Wood Adhesive. Polymers 2021, 13, 3502. [Google Scholar] [CrossRef]
- Kamarudin, N.; Biak, D.R.A.; Abidin, Z.Z.; Cardona, F.; Sapuan, S.M. Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application. Materials 2020, 13, 2578. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Eucalyptus Bark Lignin Substituted Phenol Formaldehyde Adhesives: A Study on Optimization of Reaction Parameters and Characterization. J. Appl. Polym. Sci. 2004, 92, 3514–3523. [Google Scholar] [CrossRef]
- Park, B.D.; Riedl, B.; Kim, Y.S.; So, W.T. Effect of Synthesis Parameters on Thermal Behavior of Phenol-Formaldehyde Resol Resin. J. Appl. Polym. Sci. 2002, 83, 1415–1424. [Google Scholar] [CrossRef]
- Ghorbani, M.; Liebner, F.; Van Herwijnen, H.W.G.; Pfungen, L.; Krahofer, M.; Budjav, E.; Konnerth, J. Lignin Phenol Formaldehyde Resoles: The Impact of Lignin Type on Adhesive Properties. BioResources 2012, 11, 6727–6741. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast Curing Bio-Based Phenolic Resins via Lignin Demethylated under Mild Reaction Condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Solt, P.; Rößiger, B.; Konnerth, J.; van Herwijnen, H.W.G. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Development and Characterization of a Wood Adhesive Using Bagasse Lignin. Int. J. Adhes. Adhes. 2004, 24, 485–493. [Google Scholar] [CrossRef]
- Abdelwahab, N.A. Preparation, Optimisation and Characterisation of Lignin Phenol Formaldehyde Resin as Wood Adhesive. Pigment. Resin Technol. 2008, 40, 169–174. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Chrissafis, K. Thermochimica Acta Thermal Study of Phenol—Formaldehyde Resin Modified with Cashew Nut Shell Liquid. Thermochim. Acta 2011, 512, 105–109. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Charles, C. Synthesis of Phenol—Formaldehyde Resol Resins Using Organosolv Pine Lignins. Eur. Polym. J. 2009, 45, 3380–3388. [Google Scholar] [CrossRef]
- Solt, P.; Jääskeläinen, A.S.; Lingenfelter, P.; Konnerth, J.; Herwijnen, H.W.G. Van Impact of Molecular Weight of Kraft Lignin on Adhesive Performance of Lignin-Based Phenol-Formaldehyde Resins. For. Prod. J. 2019, 68, 365–371. [Google Scholar]
- Gu, Y.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Preparation, Characterization and Properties of Starch-Based Adhesive for Wood-Based Panels. Int. J. Biol. Macromol. 2019, 134, 247–254. [Google Scholar] [CrossRef]
- Koley, R.; Kasilingam, R.; Sahoo, S.; Chattopadhyay, S.; Bhowmick, A.K. Synthesis and Characterization of Phenol Furfural Resin from Moringa Oleifera Gum and Biophenol and Its Application in Styrene Butadiene Rubber. Ind. Eng. Chem. Res. 2019, 58, 18519–18532. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers 2020, 12, 2805. [Google Scholar] [CrossRef]
- Dongre, P.; Driscoll, M.; Amidon, T.; Bujanovic, B. Lignin-Furfural Based Adhesives. Energies 2015, 8, 7897. [Google Scholar] [CrossRef]
- De Araujo, V.; Vasconcelos, J.; Lahr, F.; Christoforo, A. Timber forest products: A way to intensify global bioeconomy from bio-materials. Acta Fac. Xylologiae Zvolen 2022, 64, 99–111. [Google Scholar] [CrossRef]



| Resin | Lignin (g) | Phenol (g) | Formaldehyde (g) |
|---|---|---|---|
| PF | 0 | 50 | 90 |
| LPF10 | 5 | 45 | 90 |
| LPF20 | 10 | 40 | 90 |
| LPF30 | 15 | 35 | 90 |
| LPF50 | 25 | 25 | 90 |
| Resin | pH | Solids Content (%) | Viscosity (cP) | Gel Time (s) |
|---|---|---|---|---|
| PF | 10.61 | 50.63 (0.68 1) ab | 129 (3.05) a | 271 (10.8) ab 2 |
| LPF10 | 10.70 | 46.35 (0.09) c | 101 (1.47) a | 219 (8.82) a |
| LPF20 | 11.28 | 52.16 (0.13) d | 211 (1.49) b | 354 (17.7) c |
| LPF30 | 11.25 | 51.67 (0.13) ad | 183 (3.92) b | 334 (21.8) bc |
| LPF50 | 11.64 | 49.62 (0.11) b | 584 (23.56) c | 330 (22.2) bc |
| Resin | Tonset 1 (°C) | TPeak 2 (°C) | ΔT 3 (°C) | ΔH 4 (J.g−1) |
|---|---|---|---|---|
| PF | 122.2 | 144.1 | 21.9 | 623 |
| LPF10 | 92.2 | 106.1 | 13.9 | 984 |
| LPF20 | 84.5 | 107.0 | 22.5 | 873 |
| LPF30 | 98.1 | 115.6 | 17.5 | 1126 |
| LPF50 | 99.2 | 109.5 | 10.3 | 1211 |
| Resin | T5% (°C) | T10% (°C) | T30% (°C) | T60% (°C) | Residue150°C (%) | Residue300°C (%) | Residue600°C (%) | Residue900°C (%) |
|---|---|---|---|---|---|---|---|---|
| PF | 34.6 | 38.9 | 59.8 | 519.9 | 53.8 | 47.1 | 37.7 | 31.2 |
| LPF10 | 35.2 | 38.0 | 56.6 | 487.4 | 52.0 | 45.4 | 35.9 | 27.2 |
| LPF20 | 35.0 | 40.7 | 68.1 | 414.5 | 50.2 | 43.6 | 33.4 | 26.8 |
| LPF30 | 34.2 | 40.7 | 68.4 | 366.3 | 49.8 | 42.2 | 32.4 | 25.0 |
| LPF50 | 34.6 | 40.5 | 71.8 | 260.1 | 46.7 | 38.0 | 29.0 | 24.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdino, D.S.; Kondo, M.Y.; De Araujo, V.A.; Ferrufino, G.L.A.A.; Faustino, E.; Santos, H.F.d.; Christoforo, A.L.; Luna, C.M.R.; Campos, C.I.d. Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites. Polymers 2023, 15, 357. https://doi.org/10.3390/polym15020357
Galdino DS, Kondo MY, De Araujo VA, Ferrufino GLAA, Faustino E, Santos HFd, Christoforo AL, Luna CMR, Campos CId. Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites. Polymers. 2023; 15(2):357. https://doi.org/10.3390/polym15020357
Chicago/Turabian StyleGaldino, Danilo Soares, Marcel Yuzo Kondo, Victor Almeida De Araujo, Gretta Larisa Aurora Arce Ferrufino, Emerson Faustino, Herisson Ferreira dos Santos, André Luis Christoforo, Carlos Manuel Romero Luna, and Cristiane Inácio de Campos. 2023. "Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites" Polymers 15, no. 2: 357. https://doi.org/10.3390/polym15020357
APA StyleGaldino, D. S., Kondo, M. Y., De Araujo, V. A., Ferrufino, G. L. A. A., Faustino, E., Santos, H. F. d., Christoforo, A. L., Luna, C. M. R., & Campos, C. I. d. (2023). Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites. Polymers, 15(2), 357. https://doi.org/10.3390/polym15020357











