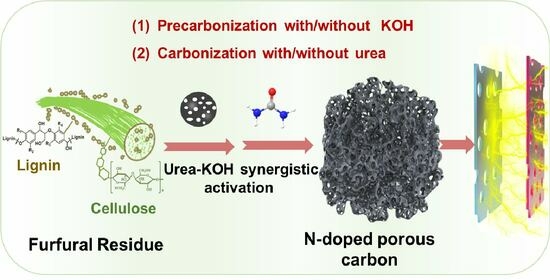
Fabrication of N-Doped Porous Carbon with Micro/Mesoporous Structure from Furfural Residue for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PCs
2.2.2. Characterization of PCs
2.2.3. Electrochemical Analysis
3. Results and Discussion
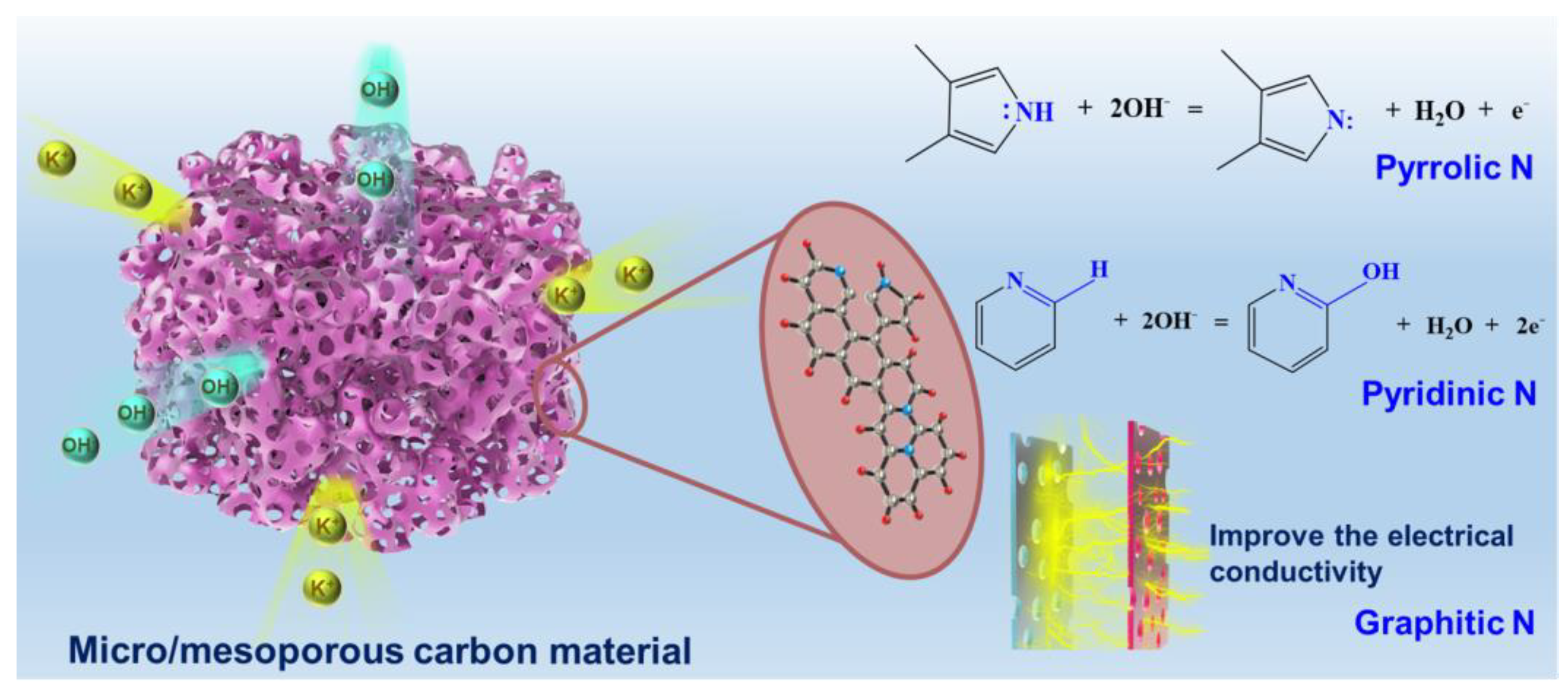
3.1. Characterization of PCs
3.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Wang, H.; Wang, X.; Liu, Y.; Liu, Z.; Wang, S.; Lin, Y. Synthesis of mesopore-dominated porous carbon with ultra-high surface area via urea-assisted KOH activation. J. Wood Chem. Technol. 2023, 43, 78–91. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Qi, F.; Shi, H.; Zhang, Y.; Ma, P. Efficient preparation of P-doped carbon with ultra-high mesoporous ratio from furfural residue for dye removal. Sep. Purif. Technol. 2022, 292, 120954. [Google Scholar] [CrossRef]
- Ren, S.; Qu, X.; Zhang, X.; Dong, L.; Yang, Y.; Lee, D.; Cao, Q.V.; Wu, Q.; Lei, T. Valorization of biomass furfural residue: Nitrogen-doped porous carbon towards electrocatalytic reaction. Ind. Crop. Prod. 2023, 193, 116251. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Wang, Y.; Tian, X.; Qiao, Y. Converting furfural residue wastes to carbon materials for high performance supercapacitor. Green Energy Environ. 2022, 7, 1270–1280. [Google Scholar] [CrossRef]
- Wang, G.; Bi, J.; Lei, M.; Liu, J.; Zhang, W. Hierarchical porous carbon obtained from directly carbonizing carex meyeriana for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 21278–21287. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Wu, S.; Li, Z.; Fan, B.; Li, Y.; Zhou, Y. Facile synthesis of N/B co-doped hierarchically porous carbon materials based on threonine protic ionic liquids for supercapacitor. Electrochim. Acta 2021, 380, 138230. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Hu, C.-C.; Wang, C.-C. Effects of pore structure and electrolyte on the capacitive characteristics of steam-and KOH-activated carbons for supercapacitors. J. Power Sources 2005, 144, 302–309. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H.; Yang, Z.; Zhang, Y.; Yu, B.; Liu, Z. Highly mesoporous carbons derived from biomass feedstocks templated with eutectic salt ZnCl2/KCl. J. Mater. Chem. A 2014, 2, 19324–19329. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Hui, L.; Ma, L. Biomass-derived porous carbon materials for supercapacitor electrodes: A review. Pap. Biomater. 2020, 5, 60–75. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Requejo, A.; Peleteiro, S.; Rodríguez, A.; Garrote, G.; Parajó, J.C. Second-generation bioethanol from residual woody biomass. Energ. Fuel 2011, 25, 4803–4810. [Google Scholar] [CrossRef]
- Correa, C.R.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2017, 97, 53–64. [Google Scholar] [CrossRef]
- Jin, J.; Geng, X.; Chen, Q.; Ren, T.-L. A better Zn-ion storage device: Recent progress for Zn-ion hybrid supercapacitors. Nano-Micro Lett. 2022, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.; Sun, L.; Wan, P.; Zhang, X.; Qiu, J. Sustainable synthesis of phosphorus-and nitrogen-co-doped porous carbons with tunable surface properties for supercapacitors. J. Power Sources 2013, 239, 81–88. [Google Scholar] [CrossRef]
- Zou, K.; Deng, Y.; Chen, J.; Qian, Y.; Yang, Y.; Li, Y.; Chen, G. Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors. J. Power Sources 2018, 378, 579–588. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Ouyang, T.; Cheng, K.; Gao, Y.; Kong, S.; Ye, K.; Wang, G.; Cao, D. Molten salt synthesis of nitrogen doped porous carbon: A new preparation methodology for high-volumetric capacitance electrode materials. J. Mater. Chem. A 2016, 4, 9832–9843. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, K.-H.; Hu, C.-C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Zhang, L.; Jiao, S.; Zhang, H.; Zhang, J.; Li, P.; Tao, Y.; Zhao, X. Rice hull-derived carbon for supercapacitors: Towards sustainable silicon-carbon supercapacitors. Polymers 2021, 13, 4463. [Google Scholar] [CrossRef]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X.; Wang, J. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2018, 261, 49–57. [Google Scholar] [CrossRef]
- Zhang, D.; He, C.; Zhao, J.; Wang, J.; Li, K. Facile synthesis of hierarchical mesopore-rich activated carbon with excellent capacitive performance. J. Colloid Interface Sci. 2019, 546, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yu, C.; Yang, J.; Ling, Z.; Hu, C.; Zhang, M.; Qiu, J. A layered-nanospace-confinement strategy for the synthesis of two-dimensional porous carbon nanosheets for high-rate performance supercapacitors. Adv. Energy Mater. 2015, 5, 1401761. [Google Scholar] [CrossRef]
- Tian, H.; Lin, Z.; Xu, F.; Zheng, J.; Zhuang, X.; Mai, Y.; Feng, X. Quantitative control of pore size of mesoporous carbon nanospheres through the self-assembly of diblock copolymer micelles in solution. Small 2016, 12, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, Y.; Su, J.; Wang, M.; Wei, X.; Jiang, T.; Wang, Z. Triboelectric nanogenerator powered electrochemical degradation of organic pollutant using Pt-free carbon materials. ACS Nano 2017, 11, 3965–3972. [Google Scholar] [CrossRef]
- Tian, X.; Li, X.; Yang, T.; Wang, K.; Wang, H.; Song, Y.; Liu, Z.; Guo, Q.; Chen, C. Flexible carbon nanofiber mats with improved graphitic structure as scaffolds for efficient all-solid-state supercapacitor. Electrochim. Acta 2017, 247, 1060–1071. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Hu, K.; Wang, S.; Liu, Q.; Kong, X. A facile synthesis of bare biomass derived holey carbon absorbent for microwave absorption. Appl. Surf. Sci. 2021, 544, 148891. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, Z.; Li, X.; Wang, F.; Yan, Y.; Feng, L. Nitrogen-doped carbon derived from reindeer manure for tetracycline hydrochloride removal: Synergetic effects of adsorption and catalysis. J. Environ. Chem. Eng. 2022, 10, 108286. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Rehman, A.; Munir, M.A.M.; Irshad, S.; Rashid, M.S.; Cheema, A.I. Understanding the synergy between N-doped ultra-microporous carbonaceous adsorbent and nitrogen functionalities for high performance of CO2 sorption. J. Environ. Chem. Eng. 2021, 9, 104646. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Gao, S. Biomass-derived interconnected carbon nanoring electrochemical capacitors with high performance in both strongly acidic and alkaline electrolytes. J. Mater. Chem. A 2017, 5, 181–188. [Google Scholar] [CrossRef]
- Li, X.; Xin, E.; Zhang, J. Effects of Hf incorporation on indium zinc oxide thin-film transistors using solution process. Electron. Mater. Lett. 2015, 11, 143–148. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shimanovska, V.; Chashechnikova, I.; Khalyavka, T.; Baran, J. Pyridine-TiO2 surface interaction as a probe for surface active centers analysis. Appl. Surf. Sci. 2003, 214, 222–231. [Google Scholar] [CrossRef]
- Kim, C.; Zhu, C.; Aoki, Y.; Habazaki, H. Heteroatom-doped porous carbon with tunable pore structure and high specific surface area for high performance supercapacitors. Electrochim. Acta 2019, 314, 173–187. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yan, Y.; Li, A.; Ren, L. Fabrication of heteroatom-self-doped hierarchical porous carbon from soy protein isolate hydrogel for high-performance supercapacitors via a double-effect strategy of template-activation. Microporous Mesoporous Mater. 2022, 338, 111912. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Ban, C.-L.; Xu, Z.; Wang, D.; Liu, Z.; Zhang, H. Porous layered carbon with interconnected pore structure derived from reed membranes for supercapacitors. ACS Sustain. Chen. Eng. 2019, 7, 10742–10750. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Jia, X.; Gao, S. Why and how to tailor the vertical coordinate of pore size distribution to construct ORR-active carbon materials? Nano Energy 2019, 58, 384–391. [Google Scholar] [CrossRef]
- Guan, L.; Pan, L.; Peng, T.; Gao, C.; Zhao, W.; Yang, Z.; Hu, H.; Wu, M. Synthesis of Biomass-Derived Nitrogen-Doped Porous Carbon Nanosheests for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 8405–8412. [Google Scholar] [CrossRef]
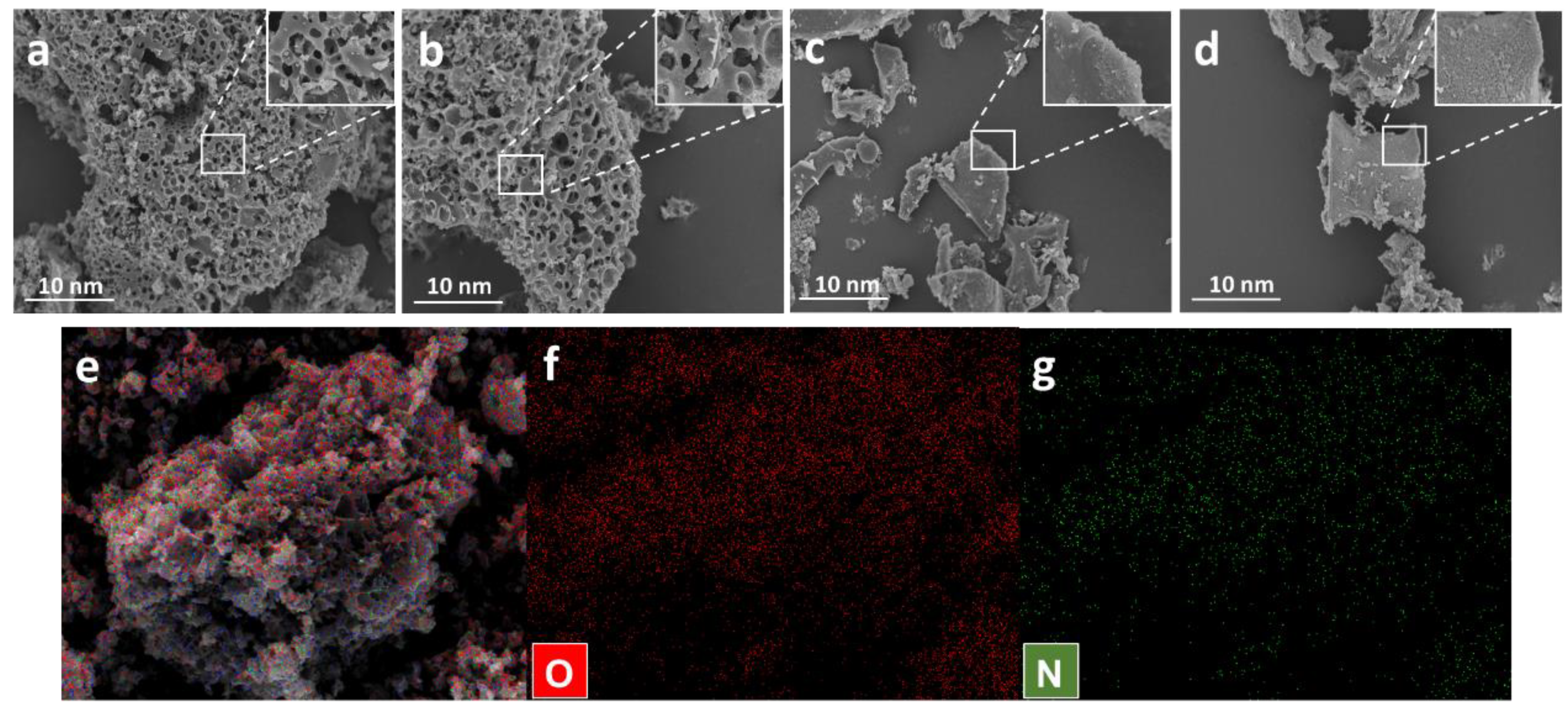
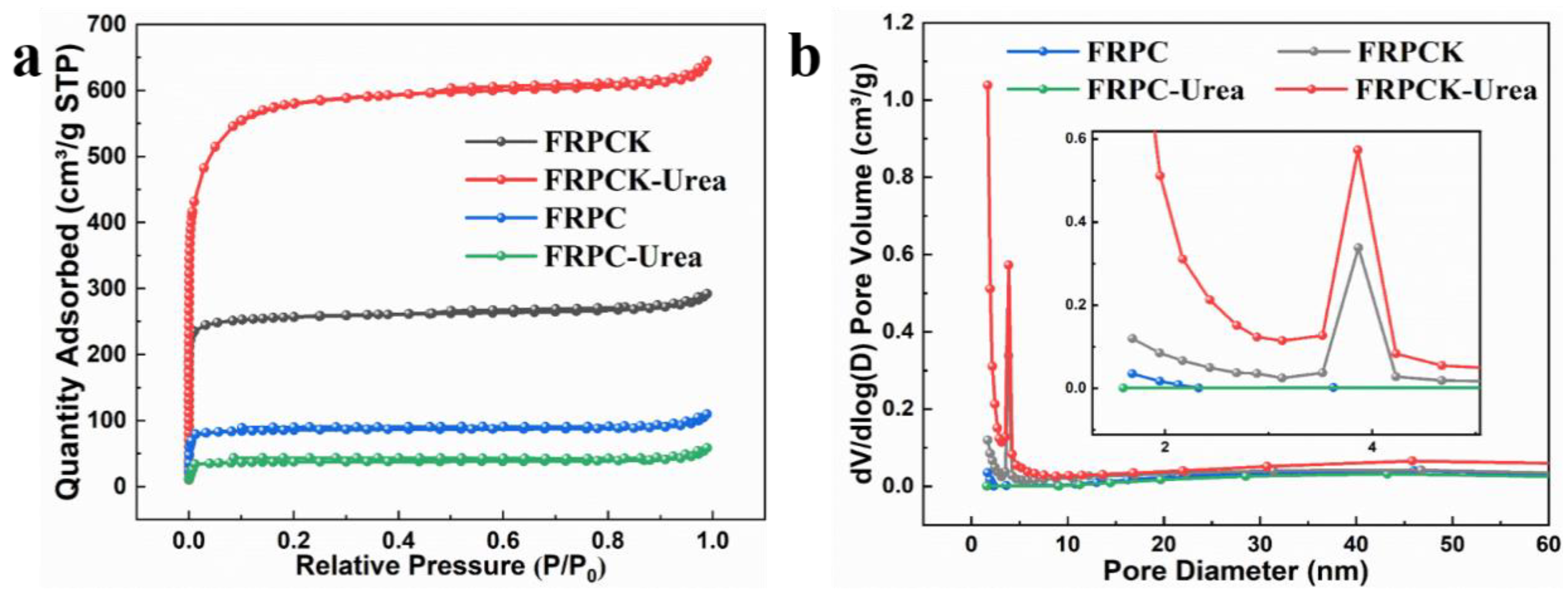


| Samples | SSA (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | VT (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vmeso/VT |
|---|---|---|---|---|---|---|---|
| FRPC | 274.9 | 246.6 | 28.34 | 0.1695 | 0.1203 | 0.0492 | 29.03 |
| FRPC-Urea | 120.7 | 104.6 | 16.03 | 0.0908 | 0.0511 | 0.0397 | 43.87 |
| FRPCK | 824.2 | 743.8 | 80.39 | 0.4520 | 0.3621 | 0.0899 | 19.89 |
| FRPCK-Urea | 1850 | 1496 | 354.1 | 0.9973 | 0.7432 | 0.2541 | 25.48 |
| Samples | C | N | O | S | The Content of Carbon Species, % | The Content of Nitrogen Species, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Graphitic C | C–N/C–O | C=O | Pyridinic N | Pyrrolic N | Graphitic N | Oxidic N | |||||
| FRPC | 76.55 | 1.091 | 19.01 | 1.770 | 49.46 | 44.11 | 6.429 | − | − | − | − |
| FRPC-Urea | 77.72 | 1.813 | 19.28 | − | 64.96 | 27.53 | 7.516 | − | − | − | − |
| FRPCK | 81.72 | 0.7706 | 14.46 | 0.014 | 70.55 | 24.39 | 5.061 | − | − | − | − |
| FRPCK-Urea | 84.43 | 5.313 | 8.445 | 0.6053 | 67.58 | 26.58 | 5.847 | 35.99 | 15.55 | 44.89 | 3.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, X.; Li, W.; Kong, F.; Zhang, F. Fabrication of N-Doped Porous Carbon with Micro/Mesoporous Structure from Furfural Residue for Supercapacitors. Polymers 2023, 15, 3976. https://doi.org/10.3390/polym15193976
Meng X, Wang X, Li W, Kong F, Zhang F. Fabrication of N-Doped Porous Carbon with Micro/Mesoporous Structure from Furfural Residue for Supercapacitors. Polymers. 2023; 15(19):3976. https://doi.org/10.3390/polym15193976
Chicago/Turabian StyleMeng, Xia, Xiaohui Wang, Wei Li, Fangong Kong, and Fengshan Zhang. 2023. "Fabrication of N-Doped Porous Carbon with Micro/Mesoporous Structure from Furfural Residue for Supercapacitors" Polymers 15, no. 19: 3976. https://doi.org/10.3390/polym15193976
APA StyleMeng, X., Wang, X., Li, W., Kong, F., & Zhang, F. (2023). Fabrication of N-Doped Porous Carbon with Micro/Mesoporous Structure from Furfural Residue for Supercapacitors. Polymers, 15(19), 3976. https://doi.org/10.3390/polym15193976