Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Cassava Starch SBPE Films
2.3. Characterization of Cassava Starch SBPE Films
2.3.1. Characterization via Fourier Transform Infrared Spectrometry
2.3.2. Characterization via Thermal Analysis
2.3.3. Characterization by Cyclic Voltammetry
2.3.4. Characterization via Electrochemical Impedance Spectroscopy
3. Results and Discussion
3.1. FTIR Characterization of Cassava Starch SBPE Films
3.2. Thermal Characterization of Cassava Starch SBPE Films
3.3. Electrochemistry Characterization of Cassava Starch SBPE Films via Cyclic Voltammetry
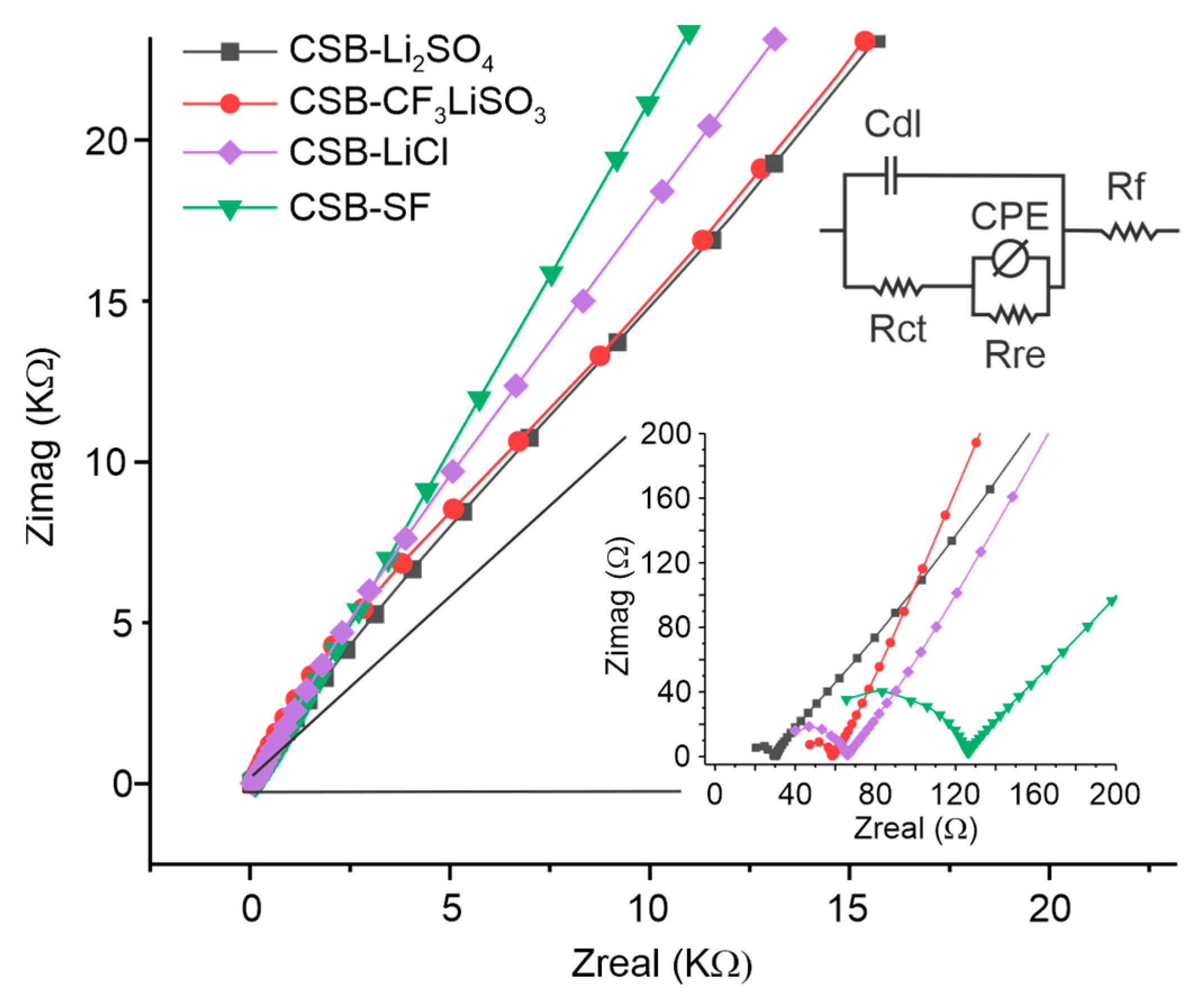
3.4. Electrochemistry Characterization of Cassava Starch SBPE Films by Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Koga, M.; Hashimoto, K.; Tominaga, Y. Ion-Conductive Properties of Propylene Carbonate/Propylene Oxide Copolymers. Kobunshi Ronbunshu 2017, 74, 577–583. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, Y.; Guo, W.; Zhu, S.; Fan, J.; Min, Y. Reactive Aramid Nanofiber-Reinforced Polyvinyl-Alcohol-Based Solid Polymer Electrolyte for High-Performance Li Metal Batteries. ACS Appl. Energy Mater. 2023, 6, 4922–4933. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Sameela, T.P.; Meera, K.; Bahuleyan, B.K.; Verma, M. In situ emulsion polymerization of poly (vinyl acetate) and asparagus racemosus biopolymer composites for flexible energy storage applications. Polym. Compos. 2023, 1, 4168–4177. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Ding, J.; Liu, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Poly(acrylic acid)-Based Composite Gel Polymer Electrolytes with High Mechanical Strength and Ionic Conductivity toward Flexible Zinc–Air Batteries with Long Cycling Lifetime. ACS Appl. Mater. Interfaces 2022, 14, 49801–49810. [Google Scholar] [CrossRef]
- Larrabide, A.; Rey, I.; Lizundia, E. Environmental Impact Assessment of Solid Polymer Electrolytes for Solid-State Lithium Batteries. Adv. Energy Sustain. Res. 2022, 3, 2200079. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Gañán, P.F.; Márquez, S.E.; Zuluaga, R. Electrically Conductive Bioplastics from Cassava Starch. J. Braz. Chem. Soc. 2011, 22, 1170–1176. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brevik, I.; Hamsan, M.H.; Brza, M.A.; Nofal, M.M.; Abdullah, A.M.; Rostam, S.; Al-Zangana, S.; Muzakir, S.K.; Kadir, M.F.Z. Compatible Solid Polymer Electrolyte Based on Methyl Cellulose for Energy Storage Application: Structural, Electrical, and Electrochemical Properties. Polymers 2020, 12, 2257. [Google Scholar] [CrossRef]
- Xue, X.; Wan, L.; Li, W.; Tan, X.; Du, X.; Tong, Y. A Self-Healing Gel Polymer Electrolyte, Based on a Macromolecule Cross-Linked Chitosan for Flexible Supercapacitors. Gels 2023, 9, 8. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E.; Durairaj, R. Utilisation of corn starch in production of ‘green’ polymer electrolytes. Mater. Res. Innov. 2011, 15, s13-s8. [Google Scholar] [CrossRef]
- Perumal, P.; Christopher Selvin, P. Boosting the performance of electric double layer capacitor via engaging pectin macromolecular electrolyte with elevated ionic conductivity and potential window stability. Chem. Eng. J. Adv. 2021, 8, 100178. [Google Scholar] [CrossRef]
- Chitra, R.; Sathya, P.; Selvasekarapandian, S.; Monisha, S.; Moniha, V.; Meyvel, S. Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 2019, 25, 2147–2157. [Google Scholar] [CrossRef]
- Blesstina, S.R.K.; Mathavan, T.; Buvaneshwari, P.; Joel, T.; Benial, A.M.F. Conducting behaviour of a novel solid biopolymer electrolyte for electrochemical application. Ionics 2023, 29, 3437–3450. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Structure, functionality and applications of debranched starch: A review. Trends Food Sci. Technol. 2017, 63, 70–79. [Google Scholar] [CrossRef]
- Agarwal, S. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Abral, H.; Hagabean, M.; Hartono, J.; Efendi, R.; Asrofi, M.; Sugiarti, E.; Sapuan, S.M.; Park, J.W.; Kim, H.J. Characterization of Tapioca Starch Biopolymer Composites Reinforced with Micro Scale Water Hyacinth Fibers. Starch 2018, 70, 1700287. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Xie, F.; Zan, K.; Wange, S.; Wang, S. Applications of ionic liquids in starch chemistry: A review. Green Chem. 2020, 7, 2162–2183. [Google Scholar] [CrossRef]
- Arrieta, A.; Mendoza, J.; Barrera, I. Thermal and electrochemical properties of solid biopolymer electrolytes from starch of different botanical origin. Periódico Tchê Química 2021, 38, 137–148. [Google Scholar] [CrossRef]
- Tiwari, T.; Srivastava, N.; Srivastava, P. Electrical transport study of potato starch-based electrolyte system. Ionics 2011, 17, 353–360. [Google Scholar] [CrossRef]
- Kumar, L.S.; Selvin, P.C.; Selvasekarapandian, S. Impact of lithium triflate (LiCF3SO3) salt on tamarind seed polysaccharide-based natural solid polymer electrolyte for application in electrochemical device. Polym. Bull. 2021, 78, 1797–1819. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Effect of different iodide salts on ionic conductivity and structural and thermal behavior of rice-starch-based polymer electrolytes for dye-sensitized solar cell application. Ionics 2015, 21, 2383–2391. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 2014, 20, 977–999. [Google Scholar] [CrossRef]
- Selvanathan, V.; Yahya, R.; Ruslan, M.H.; Sopian, K.; Amin, N.; Nour, M.; Sindi, H.; Rawa, M.; Akhtaruzzaman, M. Organosoluble Starch-Cellulose Binary Polymer Blend as a Quasi-Solid Electrolyte in a Dye-Sensitized Solar Cell. Polymers 2020, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Noor Shah, A.; Ahmad Anjum, S.; Alam Cheema, S.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Mendoza, J.M.; Palencia, M. Composite material elaborated from conducting biopolymer cassava starch and polyaniline. Mex. J. Chem. Eng. 2019, 19, 707–715. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Iqbal, M.; Hussain, F.; Sarim, F.M. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef]
- Chandrasekhar, T.; Sasidhar, N.; Devidas, G.B.; Ravikiran, Y.T.; Raghavendra, S.C. AC conduction studies on biodegradable Poly vinyl alcohol-starch/NaI blend at room temperature. In Proceedings of the 2020 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 4 February–9 April 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N,N-Dimethylacetamide/Lithium Chloride: Revisiting through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef]
- Krishna Koduru, H.; Georgiev Marinov, Y.; Scaramuzza, N. Review on Microstructural and Ion-conductivity Properties of Biodegradable Starch-Based Solid Polymer Electrolyte Membranes. Starch 2022, 74, 2100172. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.-S.; Ramesh, S. Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement 2014, 48, 87–95. [Google Scholar] [CrossRef]
- Chia, M.-R.; Ahmad, I.; Phang, S.-W. Starch/Polyaniline Biopolymer Film as Potential Intelligent Food Packaging with Colourimetric Ammonia Sensor. Polymers 2022, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Yuan, C.; Dong, D.; Zou, F.; Tao, H.; Guo, L.; Lu, L.; Zhao, M.; Yang, N.; Liu, P.; et al. Effects of primary, secondary and tertiary structures on functional properties of thermoplastic starch biopolymer blend films. J. Int. J. Biol. Macromol. 2023, 236, 124006. [Google Scholar] [CrossRef]
- Subban, R.H.Y.; Arof, A.K. Plasticiser interactions with polymer and salt in PVC–LiCF3SO3–DMF electrolytes. Eur. Polym. J. 2004, 40, 1841–1847. [Google Scholar] [CrossRef]
- Ramesh, S.; Chiam Wen, L. Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionic 2010, 16, 255–262. [Google Scholar] [CrossRef]
- Vernon-Carter, E.; Alvarez-Ramirez, J.; Bello-Perez, L.; Roldan-Cruz, C.; Garcia-Hernandez, A.; Huerta, L. The order of addition of corn starch/lithium perchlorate/glycerol affects the optical, mechanical, and electrical properties of a solid polymer electrolyte. Ionics 2017, 23, 3111–3123. [Google Scholar] [CrossRef]
- Beck, M.; Jekle, M.; Becker, T. Starch re-crystallization kinetics as a function of various cations. Starch 2011, 63, 792–800. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. Thermal Decomposition of Corn Starch with Different Amylose/Amylopectin Ratios in Open and Sealed Systems. Cereal Chem. 2009, 86, 383–385. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch Stärke 2013, 65, 48–60. [Google Scholar] [CrossRef]
- Ayala, G.; Agudelo, A.; Vargas, R. Effect of glycerol on the electrical properties and phase behavior of cassava starch biopolymers. Dyna 2012, 79, 138–147. Available online: http://www.scielo.org.co/pdf/dyna/v79n171/a18v79n171.pdf (accessed on 5 June 2023).
- Park, S.; Boo, H.; Dong Chung, T. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Hernandez-Jaimes, C.; Lobato-Calleros, C.; Sosa, E.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Electrochemical characterization of gelatinized starch dispersions: Voltammetry and electrochemical impedance spectroscopy on platinum surface. Carbohydr. Polym. 2015, 124, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Liew, C.-W.; Arof, A.Ñ. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Non-Cryst. Solids 2011, 357, 3654–3660. [Google Scholar] [CrossRef]
- Bementa, E.; Jothi Rajan, M.A. Polymer Crust Electrolyte Based on Starch-Chitosan Blend Mixed with KI by Gelation Method. J. Appl. Sci. Eng. Methodol. 2016, 2, 336–341. Available online: http://www.jasem.in/2016/23336341.html (accessed on 23 September 2023).
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The Effect of Plasticization on Conductivity and Other Properties of Starch/Chitosan Blend Biopolymer Electrolyte Incorporated with Ammonium Iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
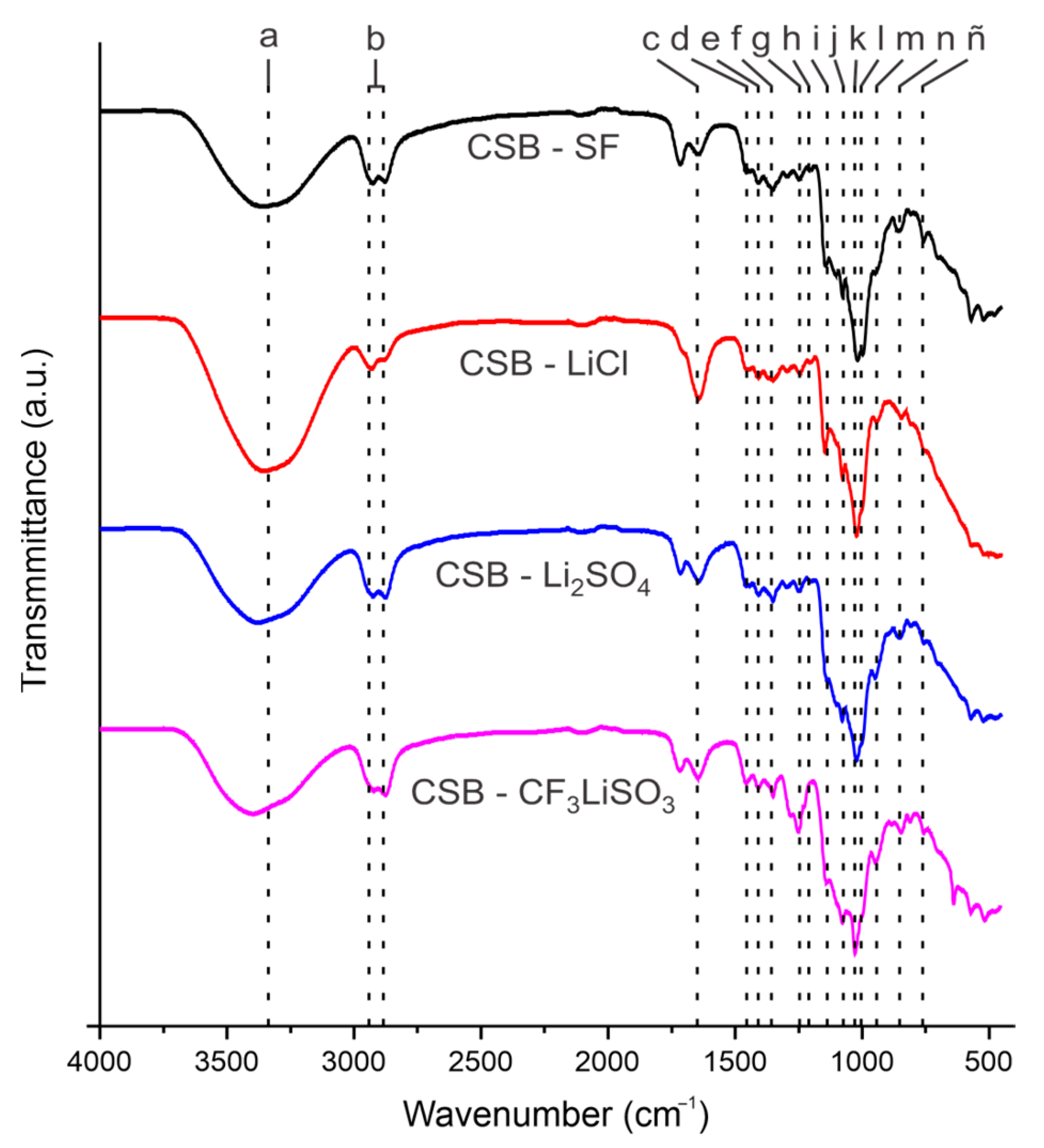
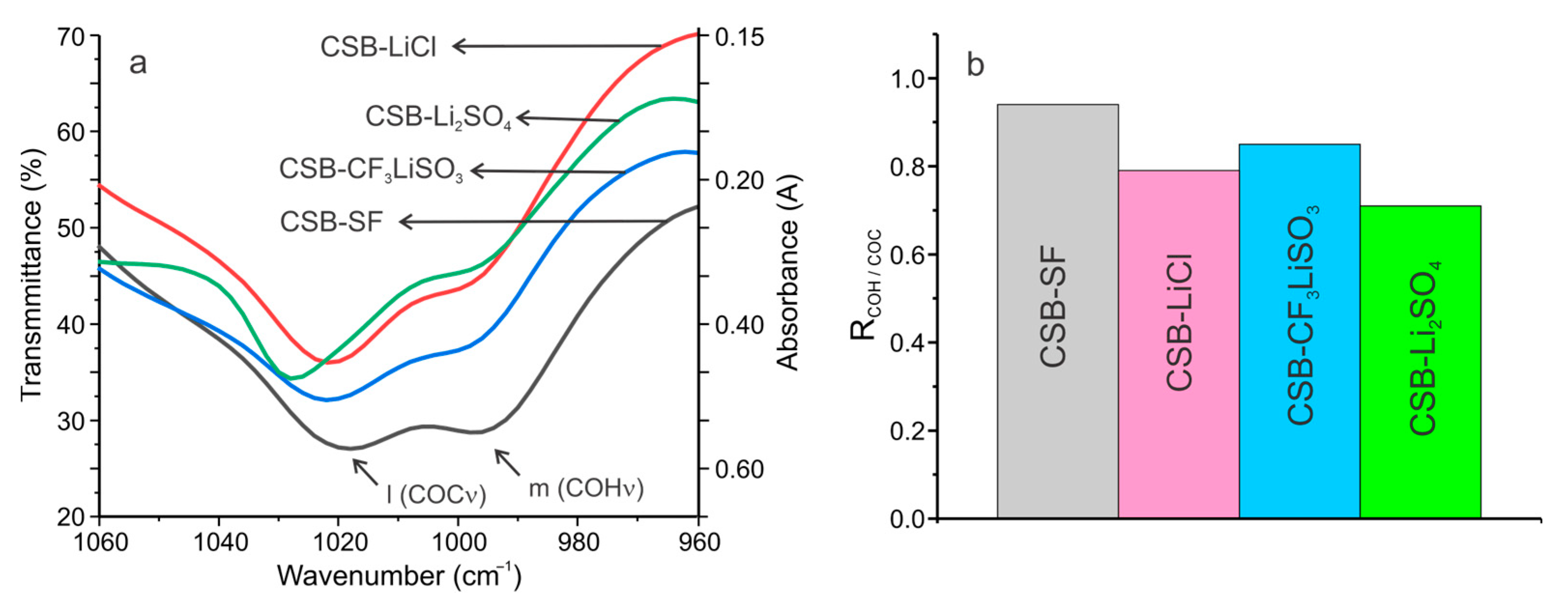


| Band | Assignments | Wavenumber (cm−1) | |||
|---|---|---|---|---|---|
| CSB-SF | CSB-CF3LiSO3 | CSB-Li2SO4 | CSB-LiCl | ||
| a | O-H stretching | 3364 | 3398 | 3373 | 3350 |
| b | C-H stretching | 2926, 2878 | 2922, 2876 | 2925, 2877 | 2931, 2877 |
| c | O-H (water) bending | 1649 | 1647 | 1642 | 1641 |
| d | C-H bending | 1456 | 1456 | 1456 | 1456 |
| e | O-H bending | 1407 | 1407 | 1407 | 1407 |
| f | COH bending/S=O stretching | 1350 | 1350 | 1350 | 1350 |
| g | CH2OH related modes/SO3 stretching | 1247 | 1250 | 1247 | 1245 |
| h | COH deformation | 1201 | 1223 | 1203 | 1204 |
| i | CO antisymmetric bridge stretching | 1146 | 1141 | 1142 | 1145 |
| j | COH antisymmetric stretching in plane ring | 1103 | 1102 | 1103 | 1104 |
| k | C-OH bending | 1077 | 1077 | 1078 | 1077 |
| l | COC ring vibration of carbohydrate/ CF3 stretching | 1018 | 1028 | 1021 | 1021 |
| m | COH solved | 995 | 997 | 998 | 997 |
| n | C-H bending modes | 844 | 844 | 843 | 845 |
| ñ | CH2 rocking | 757 | 757 | 757 | 757 |
| Process | Peak | Solid Biopolymer Electrolytes | ||||
|---|---|---|---|---|---|---|
| CSB | CSB-CF3LiSO3 | CSB-Li2SO4 | CSB-LiCl | |||
| I | Anodic | Ep (V) | 1.52 | 0.86 | 1.16 | 1.05 |
| Ip (µA) | 0.096 | 0.113 | 0.248 | 0.155 | ||
| Cathodic | Ep (V) | - | 0.80 | 1.00 | 0.81 | |
| Ip (µA) | - | −0.010 | −0.188 | −0.013 | ||
| II | Anodic | Ep (V) | 0.57 | −0.22 | 0.19 | - |
| Ip (µA) | 0.056 | 0.016 | 0.117 | - | ||
| Cathodic | Ep (V) | 0.31 | 0.13 | −0.04 | - | |
| Ip (µA) | −0.028 | −0.038 | 0.050 | - | ||
| III | Anodic | Ep (V) | −0.19 | −0.87 | −0.22 | −0.66 |
| Ip (µA) | 0.032 | 0.006 | 0.074 | 0.004 | ||
| Cathodic | Ep (V) | −0.66 | −0.91 | −1.88 | −0.45 | |
| Ip (µA) | −0.070 | −0.148 | −0.848 | −0.128 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta, A.A.; Calabokis, O.P.; Mendoza, J.M. Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes. Polymers 2023, 15, 4150. https://doi.org/10.3390/polym15204150
Arrieta AA, Calabokis OP, Mendoza JM. Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes. Polymers. 2023; 15(20):4150. https://doi.org/10.3390/polym15204150
Chicago/Turabian StyleArrieta, Alvaro A., Oriana Palma Calabokis, and Jorge Mario Mendoza. 2023. "Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes" Polymers 15, no. 20: 4150. https://doi.org/10.3390/polym15204150
APA StyleArrieta, A. A., Calabokis, O. P., & Mendoza, J. M. (2023). Effect of Lithium Salts on the Properties of Cassava Starch Solid Biopolymer Electrolytes. Polymers, 15(20), 4150. https://doi.org/10.3390/polym15204150








