Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Microorganisms, Growth Assay, Cell Line and Storage
2.2. Isolation and Identification of the Microorganism
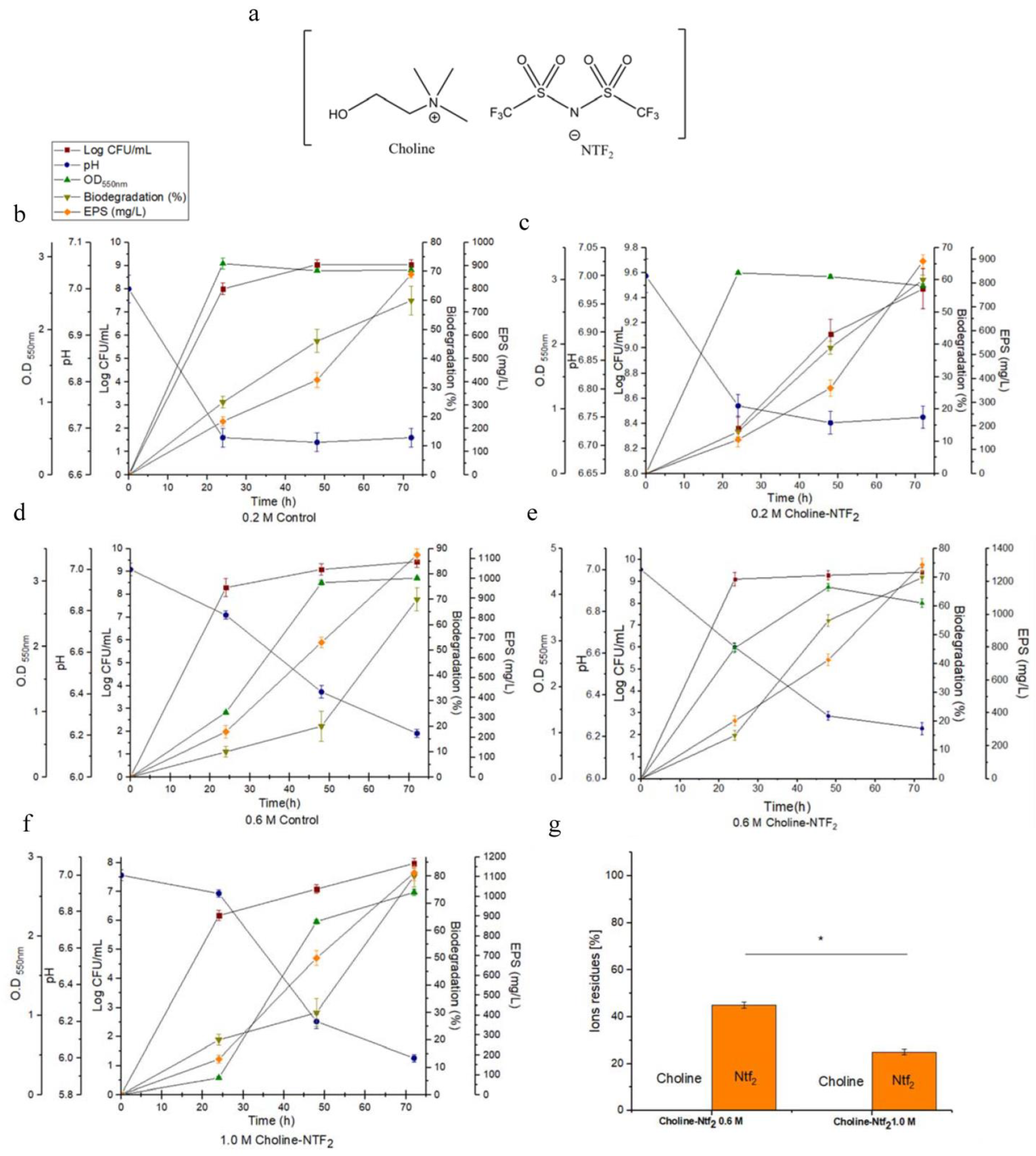
2.3. Aerobic Biodegradation, Bacterial Growth, Microbial Cells, and pH. Analysis of Ion Residues Choline NTF2
2.4. Production, Extraction and Purification of Exopolysaccharides
2.5. Transmission Electron Microscopy (TEM) Analysis and Scanning Electronic Microscopy (SEM)
2.6. Exopolysaccharide Molecular Weight and Monosaccharides Identification
2.7. Attenuated Total Reflectance/FT-Infrared Spectroscopy (ATR FTIR). Thermogravimetric (TGA) and Differential Scanning Calorimetric (DSC) Analysis
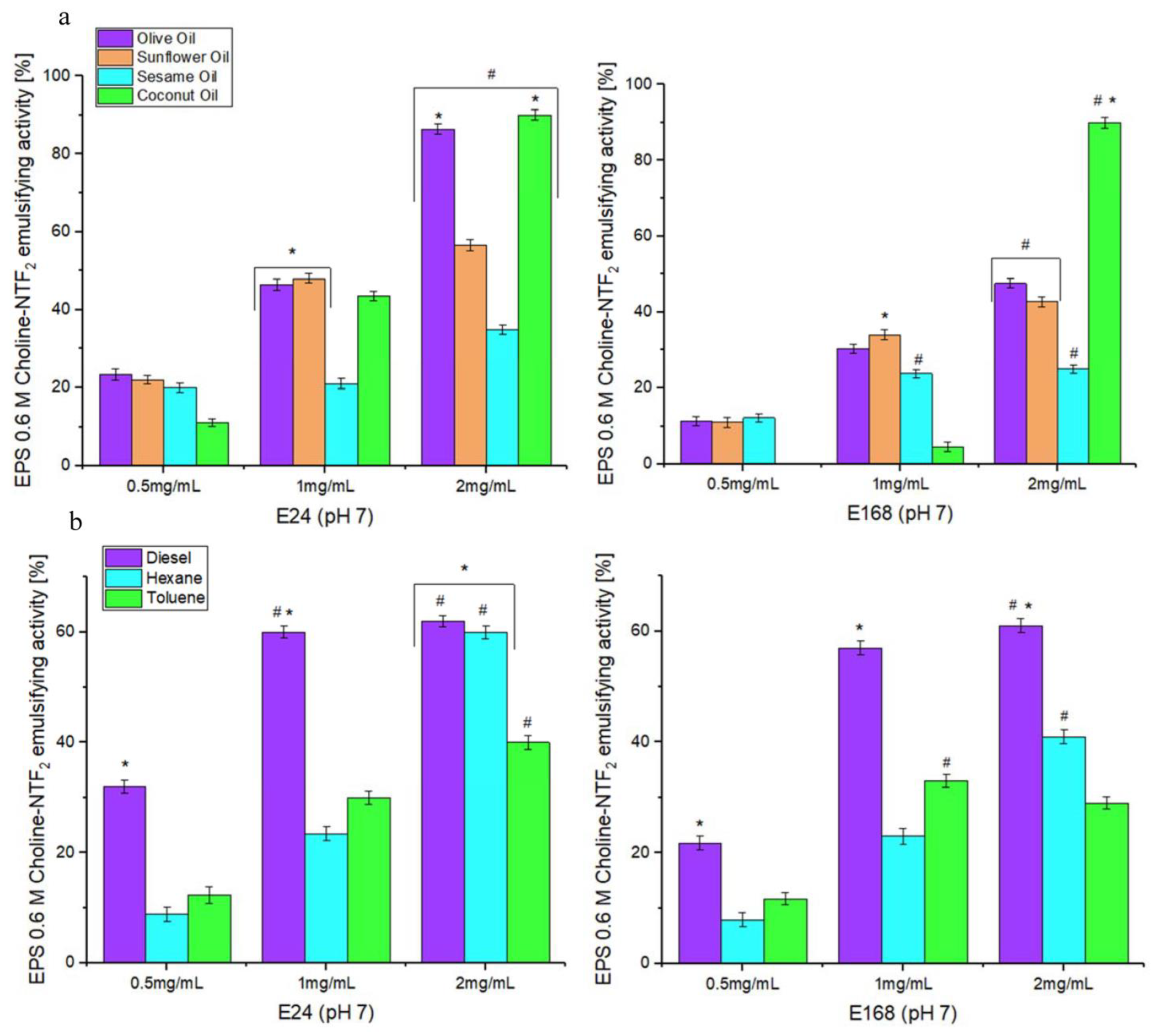
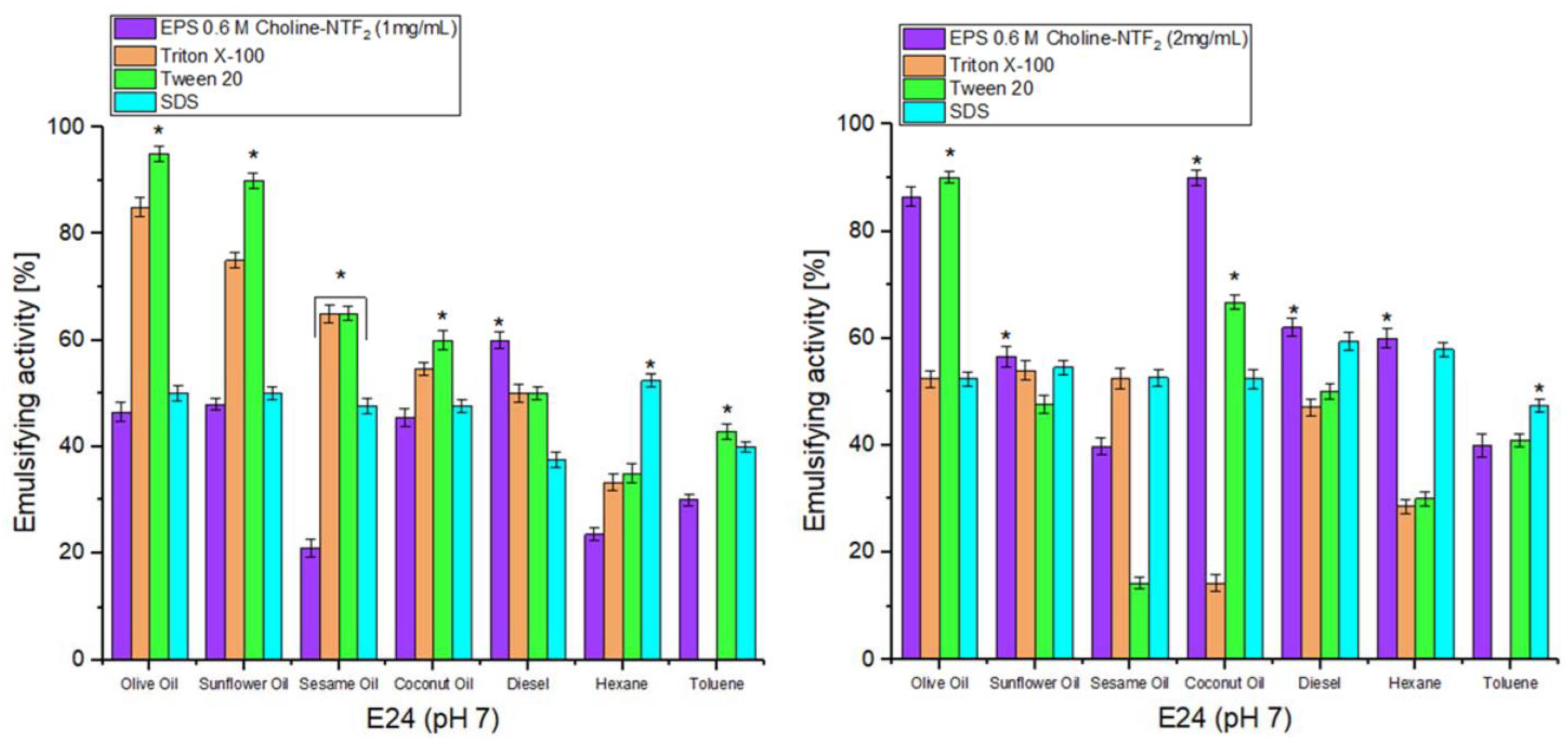
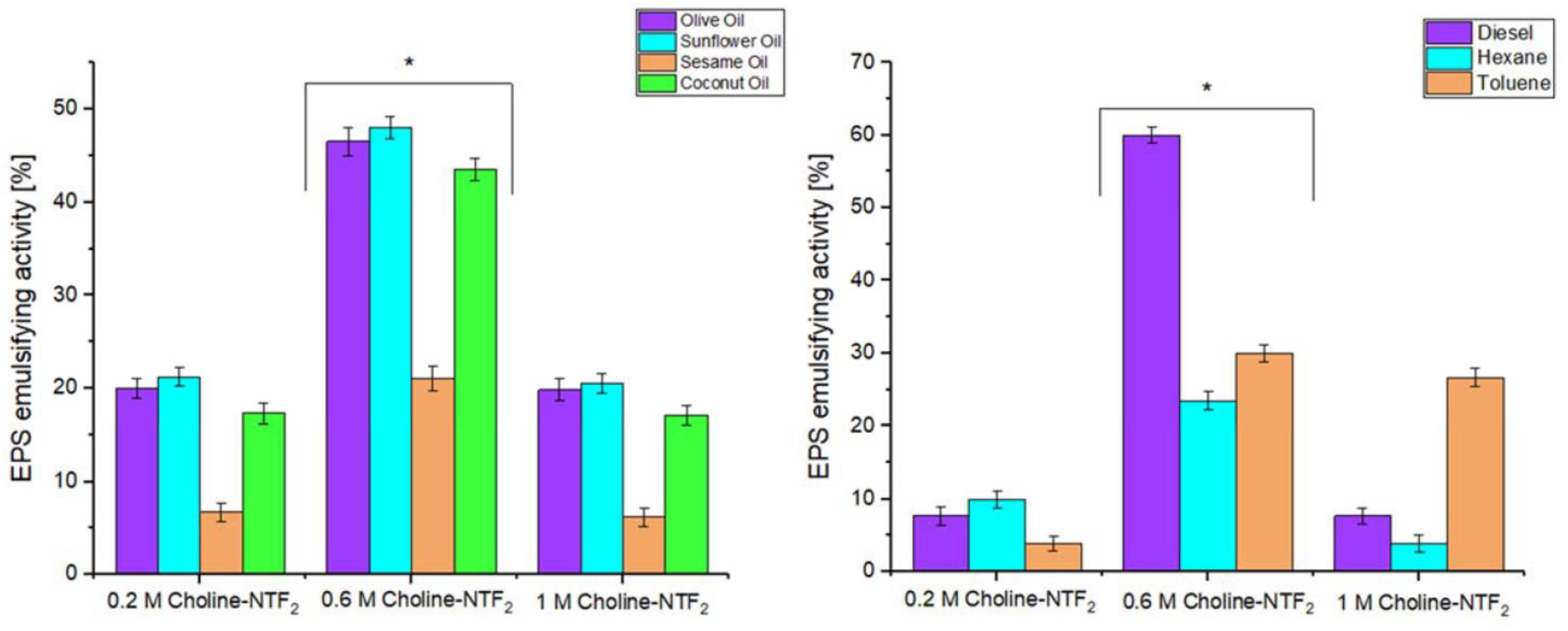
2.8. Emulsifying Activity
2.9. Nonenzymatic Antioxidants Assays
2.10. Toxicity Assay
2.11. Measurement of the Non-Radical H2O2, Morphological Cell and Catalase (CAT) Assay
2.11.1. Measurement of the Non-Radical H2O2
Damage Inducement
Protective Effect of EPS against Non-Radical H2O2
2.11.2. Morphological Cell
2.11.3. Catalase (CAT) Assay
2.12. Chelating Activity
- A0 = OD562nm of the deionized water.
- A1 = OD562nm of the reaction mixture.
- A2 = OD562nm of the reaction mixture but without FeCl2.
2.13. Antibacterial Activity by Agar Well Diffusion Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Bacterial Strain, Morphological and Biochemical Characterization
3.2. Monitoring of Growth and Biodegradation Parameters. Production of Exopolysaccharides
3.3. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
3.4. Characterization of the Exopolysaccharides
3.5. Environmental Implications of the Produced Exopolysaccharides
3.5.1. Emulsifying Study
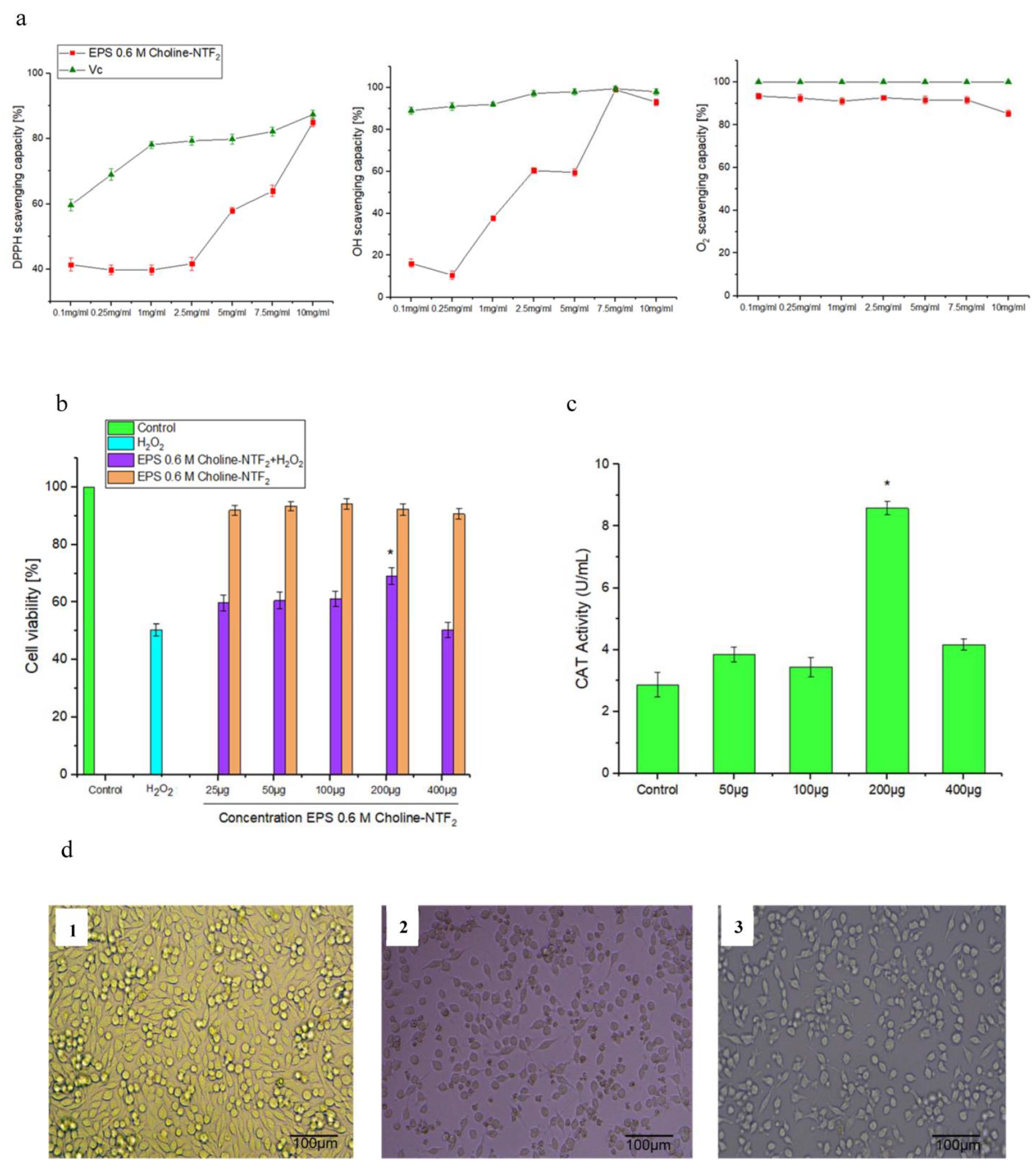
3.5.2. Nonenzymatic Antioxidant, Toxicity, Non-Radical H2O2, Morphological Cell and Catalase (CAT) Assay
3.5.3. Chelating Activity and Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akoh, C.C.; Chang, S.W.; Lee, G.C.; Shaw, J.F. Biocatalysis for the Production of Industrial Products and Functional Foods from Rice and Other Agricultural Produce. J. Agric. Food Chem. 2008, 56, 10445–10451. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ruhoy, I.S. Green Pharmacy and PharmEcovigilance: Prescribing and the Planet. Expert Rev. Clin. Pharmacol. 2011, 4, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, N.S.; Asimakopoulos, A.G.; Bletsou, A.A. Emerging Contaminants: A Tutorial Mini-Review. Glob. NEST J. 2012, 14, 72–79. [Google Scholar] [CrossRef]
- Rudnicka-Kępa, P.; Bełdowska, M.; Zaborska, A. Enhanced Heavy Metal Discharges to Marine Deposits in Glacial Bays of Two Arctic Fjords (Hornsund and Kongsfjorden). J. Mar. Syst. 2024, 241, 103915. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; He, Y.; Liang, D.; Shen, Y.; Gu, Q.; Zeng, Y. How Microplastic Loads Relate to Natural Conditions and Anthropogenic Activities in the Yangtze River Basin. Chemosphere 2023, 342, 140146. [Google Scholar] [CrossRef]
- Marimuthu, T.; Sidat, Z.; Kumar, P.; Choonara, Y.E. An Imidazolium-Based Ionic Liquid as a Model to Study Plasticization Effects on Cationic Polymethacrylate Films. Polymers 2023, 15, 1239. [Google Scholar] [CrossRef]
- Cho, C.W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.S. Review of the Toxic Effects of Ionic Liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Leitch, A.C.; Nevjestić, I.; Ibrahim, I.; Miwa, S.; Wilson, C.; Heutz, S.; Wright, M.C. Emerging Risk from “Environmentally-Friendly” Solvents: Interaction of Methylimidazolium Ionic Liquids with the Mitochondrial Electron Transport Chain Is a Key Initiation Event in Their Mammalian Toxicity. Food Chem. Toxicol. 2020, 145, 111593. [Google Scholar] [CrossRef]
- Tan, Z.Q.; Liu, J.F.; Pang, L. Advances in Analytical Chemistry Using the Unique Properties of Ionic Liquids. TrAC—Trends Anal. Chem. 2012, 39, 218–227. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, S.; Lai, Y.; Wang, Z.; Ni, N.; Dai, F.; Xu, Y.; Yang, X. Ionic Liquids Facilitate the Dispersion of Branched Polyethylenimine Grafted ZIF-8 for Reinforced Epoxy Composites. Polymers 2023, 15, 1837. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Radošević, K.; Radojčić Redovniković, I.; Halambek, J.; Gaurina Srček, V. A Brief Overview of the Potential Environmental Hazards of Ionic Liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef]
- Matzke, M.; Thiele, K.; Müller, A.; Filser, J. Sorption and Desorption of Imidazolium Based Ionic Liquids in Different Soil Types. Chemosphere 2009, 74, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Miquel, M.; Bedia, J.; Abrusci, C.; Palomar, J.; Rodriguez, F. Anion Effects on Kinetics and Thermodynamics of CO2 Absorption in Ionic Liquids. J. Phys. Chem. B 2013, 117, 3398–3406. [Google Scholar] [CrossRef]
- Palomar, J.; Gonzalez-Miquel, M.; Polo, A.; Rodriguez, F. Understanding the Physical Absorption of CO2 in Ionic Liquids Using the COSMO-RS Method. Ind. Eng. Chem. Res. 2011, 50, 3452–3463. [Google Scholar] [CrossRef]
- An, Y.X.; Zong, M.H.; Wu, H.; Li, N. Pretreatment of Lignocellulosic Biomass with Renewable Cholinium Ionic Liquids: Biomass Fractionation, Enzymatic Digestion and Ionic Liquid Reuse. Bioresour. Technol. 2015, 192, 165–171. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced Applications of Ionic Liquids in Polymer Science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Environmental Impact of Ionic Liquids: Recent Advances in (Eco)Toxicology and (Bio)Degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, X.; Lu, X. Choline-Based Deep Eutectic Solvents for Mitigating Carbon Dioxide Emissions; Elsevier B.V.: Amsterdam, The Netherlands, 2015; ISBN 9780444632593. [Google Scholar]
- Abrusci, C.; Palomar, J.; Pablos, J.L.; Rodriguez, F.; Catalina, F. Efficient Biodegradation of Common Ionic Liquids by Sphingomonas paucimobilis Bacterium. Green. Chem. 2011, 13, 709–717. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of Ionic Liquids-a Critical Review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Liu, Q.P.; Smith, T.J.; Li, N.; Zong, M.H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J. Role of Pseudomonas aeruginosa Biofilm in the Initial Adhesion, Growth and Detachment of Escherichia coli in Porous Media. Environ. Sci. Technol. 2008, 42, 443–449. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, N.; Marathe, S.J.; Singhal, R.S. Food Polysaccharides: A Review on Emerging Microbial Sources, Bioactivities, Nanoformulations and Safety Considerations. Carbohydr. Polym. 2022, 287, 119355. [Google Scholar] [CrossRef]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of Photo-Degraded Mulching Films Based on Polyethylenes and Stearates of Calcium and Iron as pro-Oxidant Additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Abrusci, C.; Martín-González, A.; Del Amo, A.; Corrales, T.; Catalina, F. Biodegradation of Type-B Gelatine by Bacteria Isolated from Cinematographic Films. A Viscometric Study. Polym. Degrad. Stab. 2004, 86, 283–291. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Corrales, T.; Pablos, J.L.; Marin, I.; Abrusci, C. New Blends of Ethylene-Butyl Acrylate Copolymers with Thermoplastic Starch. Characterization and Bacterial Biodegradation. Carbohydr. Polym. 2016, 149, 68–76. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Abrusci, C.; Marquina, D.; Del Amo, A.; Catalina, F. Biodegradation of Cinematographic Gelatin Emulsion by Bacteria and Filamentous Fungi Using Indirect Impedance Technique. Int. Biodeterior. Biodegrad. 2007, 60, 137–143. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and Characterization of an Exopolymer Produced by Bacillus licheniformis: In Vitro Antiviral Activity against Enveloped Viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, P.; Slaninova, E.; Koller, M.; Nebesarova, J.; Marova, I.; Krzyzanek, V.; Obruca, S. PHA Granules Help Bacterial Cells to Preserve Cell Integrity When Exposed to Sudden Osmotic Imbalances. N. Biotechnol. 2019, 49, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Morro, A.; Catalina, F.; Pablos, J.L.; Corrales, T.; Marin, I.; Abrusci, C. Surface Modification of Poly(ε-Caprolactone) by Oxygen Plasma for Antibacterial Applications. Biocompatibility and Monitoring of Live Cells. Eur. Polym. J. 2017, 94, 405–416. [Google Scholar] [CrossRef]
- Sánchez-león, E.; Huang-lin, E.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers 2023, 15, 1550. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Sanchez-León, E.; Abrusci, C. Photodegradation and Biodegradation Under Thermophile Conditions of Mulching Films Based on Poly(Butylene Adipate-Co-Terephthalate) and Its Blend with Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 352–363. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Pérez-Blanco, C.; Huang-Lin, E.; Abrusci, C. Characterization, Biodegradation and Cytotoxicity of Thermoplastic Starch and Ethylene-Vinyl Alcohol Copolymer Blends. Carbohydr. Polym. 2022, 298, 120085. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, L.; Jia, K.; Zhan, H.; Zhang, Z.; Shah, N.P.; Tao, X.; Wei, H. Sulfonation of Lactobacillus plantarum WLPL04 Exopolysaccharide Amplifies Its Antioxidant Activities in Vitro and in a Caco-2 Cell Model. J. Dairy Sci. 2019, 102, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Parveen, Z. Pantoea. Encycl. Food Microbiol. 1999, 3, 1623–1630. [Google Scholar]
- Delétoile, A.; Decré, D.; Courant, S.; Passet, V.; Audo, J.; Grimont, P.; Arlet, G.; Brisse, S. Phylogeny and Identification of Pantoea Species and Typing of Pantoea agglomerans Strains by Multilocus Gene Sequencing. J. Clin. Microbiol. 2009, 47, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Loch, T.P.; Faisal, M. Isolation of Pantoea agglomerans from Brown Trout (Salmo Trutta) from Gilchrist Creek, Michigan, USA. Bull. Eur. Assoc. Fish Pathol. 2007, 27, 200–204. [Google Scholar]
- Raad, Z.; Hassan, S.; Mohammed, A. Temperature Effects on Growth of the Biocontrol Agent Pantoea agglomerans (An Oval Isolate from Iraqi Soils). J. Adv. Lab. Res. Biol. 2017, 8, 85–88. [Google Scholar]
- Brady, C.L.; Venter, S.N.; Cleenwerck, I.; Engelbeen, K.; Vancanneyt, M.; Swings, J.; Coutinho, T.A. Pantoea vagans sp. Nov., Pantoea eucalypti sp. Nov., Pantoea deleyi sp. Nov. and Pantoea anthophila sp. Nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2339–2345. [Google Scholar] [CrossRef]
- Popp, A.; Cleenwerck, I.; Iversen, C.; De Vos, P.; Stephan, R. Pantoea gaviniae sp. Nov. and Pantoea calida sp. Nov., Isolated from Infant Formula and an Infant Formula Production Environment. Int. J. Syst. Evol. Microbiol. 2010, 60, 2786–2792. [Google Scholar] [CrossRef]
- Suleimanova, A.D.; Itkina, D.L.; Pudova, D.S.; Sharipova, M.R. Identification of Pantoea Phytate-Hydrolyzing Rhizobacteria Based on Their Phenotypic Features and Multilocus Sequence Analysis (MLSA). Microbiology 2021, 90, 87–95. [Google Scholar] [CrossRef]
- Silvi, S.; Barghini, P.; Aquilanti, A.; Juarez-Jimenez, B.; Fenice, M. Physiologic and Metabolic Characterization of a New Marine Isolate (BM39) of Pantoea sp. Producing High Levels of Exopolysaccharide. Microb. Cell Fact. 2013, 12, 10. [Google Scholar] [CrossRef]
- Quesada, E.; Bejar, V.; Calvo, C. Exopolysaccharide Production by Volcaniella eurihalina. Experientia 1993, 49, 1037–1041. [Google Scholar] [CrossRef]
- Gu, D.; Jiao, Y.; Wu, J.; Liu, Z.; Chen, Q. Optimization of EPS Production and Characterization by a Halophilic Bacterium, Kocuria rosea ZJUQH from Chaka Salt Lake with Response Surface Methodology. Molecules 2017, 22, 814. [Google Scholar] [CrossRef] [PubMed]
- Seesuriyachan, P.; Kuntiya, A.; Hanmoungjai, P.; Techapun, C.; Chaiyaso, T.; Leksawasdi, N. Optimization of Exopolysaccharide Overproduction by Lactobacillus confusus in Solid State Fermentation under High Salinity Stress. Biosci. Biotechnol. Biochem. 2012, 76, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Firth, E.; Carpenter, S.D.; Sørensen, H.L.; Collins, R.E.; Deming, J.W. Bacterial Use of Choline to Tolerate Salinity Shifts in Sea-Ice Brines. Elementa 2016, 2016, 000120. [Google Scholar] [CrossRef]
- Kaplan, C.P.; Porter, R.K.; Brand, M.D. The Choline Transporter Is the Major Site of Control of Choline Oxidation in Isolated Rat Liver Mitochondria. FEBS Lett. 1993, 321, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Landfald, B.; Strom, A.R. Choline-Glycine Betaine Pathway Confers a High Level of Osmotic Tolerance in Escherichia coli. J. Bacteriol. 1986, 165, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Stahl, J.; Waclawska, I.; Ziegler, C.; Averhoff, B. Identification of an Osmo-Dependent and an Osmo-Independent Choline Transporter in Acinetobacter baylyi: Implications in Osmostress Protection and Metabolic Adaptation. Environ. Microbiol. 2014, 16, 1490–1502. [Google Scholar] [CrossRef]
- Sydow, M.; Owsianiak, M.; Framski, G.; Woźniak-Karczewska, M.; Piotrowska-Cyplik, A.; Ławniczak, Ł.; Szulc, A.; Zgoła-Grześkowiak, A.; Heipieper, H.J.; Chrzanowski, Ł. Biodiversity of Soil Bacteria Exposed to Sub-Lethal Concentrations of Phosphonium-Based Ionic Liquids: Effects of Toxicity and Biodegradation. Ecotoxicol. Environ. Saf. 2018, 147, 157–164. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.K.; Golec, M.; Milanowski, J. Pantoea agglomerans: A Mysterious Bacterium of Evil and Good. Part IV. Beneficial Effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef]
- El-Bestawy, E.; Mansy, A.H.; Attia, A.M.; Zahran, H. Biodegradation of Persistent Chlorinated Hydrocarbons Using Selected Freshwater Bacteria. J. Bioremediation Biodegrad. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Boch, J.; Kempf, B.; Bremer, E. Osmoregulation in Bacillus subtilis: Synthesis of the Osmoprotectant Glycine Betaine from Exogenously Provided Choline. J. Bacteriol. 1994, 176, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Rath, H.; Reder, A.; Hoffmann, T.; Hammer, E.; Seubert, A.; Bremer, E.; Völker, U.; Mäder, U. Management of Osmoprotectant Uptake Hierarchy in Bacillus subtilis via a SigB-Dependent Antisense RNA. Front. Microbiol. 2020, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Le Marrec, C.; Blanco, C.; Jebbar, M. Glycine Betaine, Carnitine, and Choline Enhance Salinity Tolerance and Prevent the Accumulation of Sodium to a Level Inhibiting Growth of Tetragenococcus halophila. Appl. Environ. Microbiol. 2000, 66, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.L.; Dohnalkova, A.C.; Nachimuthu, P.; Kennedy, D.W.; Saffarini, D.A.; Arey, B.W.; Shi, L.; Wang, Z.; Moore, D.; McLean, J.S.; et al. Role of Outer-Membrane Cytochromes MtrC and OmcA in the Biomineralization of Ferrihydrite by Shewanella oneidensis MR-1. Geobiology 2010, 8, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, K.B.; Ozcan, Y.; Dogan, N.M.; Bozkaya, O.; Ide, S. Characterization and Production of Extracellular Polysaccharides (EPS) by Bacillus pseudomycoides U10. Environments 2018, 5, 63. [Google Scholar] [CrossRef]
- de Brito, M.M.; Bundeleva, I.; Marin, F.; Vennin, E.; Wilmotte, A.; Plasseraud, L.; Visscher, P.T. Effect of Culture PH on Properties of Exopolymeric Substances from Synechococcus PCC7942: Implications for Carbonate Precipitation. Geosciences 2022, 12, 210. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Liu, H.; Zang, J.; Wang, P.; Jiang, X. Purification, Structural Characterization and Anti-UVB Irradiation Activity of an Extracellular Polysaccharide from Pantoea agglomerans. Int. J. Biol. Macromol. 2019, 137, 1002–1012. [Google Scholar] [CrossRef]
- Eudes, A.; Juminaga, D.; Baidoo, E.E.K.; Collins, F.W.; Keasling, J.D.; Loqué, D. Production of Hydroxycinnamoyl Anthranilates from Glucose in Escherichia coli. Microb. Cell Fact. 2013, 12, 62. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. A Novel Exopolysaccharide from Marine Bacterium Pantoea sp. YU16-S3 Accelerates Cutaneous Wound Healing through Wnt/β-Catenin Pathway. Carbohydr. Polym. 2020, 238, 116191. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Mao, W.J.; Chen, Y.; Guo, S.D.; Li, H.Y.; Qi, X.H.; Chen, Y.L.; Xu, J. Isolation, Chemical Characteristics and Antioxidant Properties of the Polysaccharides from Marine Fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Xiu, W.; Wang, X.; Yu, S.; Na, Z.; Li, C.; Yang, M.; Ma, Y. Structural Characterization, In Vitro Digestion Property, and Biological Activity of Sweet Corn Cob Polysaccharide Iron (III) Complexes. Molecules 2023, 28, 2961. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, H.; Park, B.D.; Khanjanzadeh, H.; Park, H.J.; Cho, Y.J. Nanocellulose-Based Ammonia Sensitive Smart Colorimetric Hydrogels Integrated with Anthocyanins to Monitor Pork Freshness. Food Control 2023, 147, 109595. [Google Scholar] [CrossRef]
- Shang, M.; Zhang, X.; Dong, Q.; Yao, J.; Liu, Q.; Ding, K. Isolation and Structural Characterization of the Water-Extractable Polysaccharides from Cassia Obtusifolia Seeds. Carbohydr. Polym. 2012, 90, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, Y.; Lei, P.; Li, S.; Xu, H.; Wang, R.; Qiu, Y.; Zhang, W. Structure Characterization, Antioxidant and Emulsifying Capacities of Exopolysaccharide Derived from Pantoea alhagi NX-11. Carbohydr. Polym. 2021, 261, 117872. [Google Scholar] [CrossRef] [PubMed]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide Production of Pantoea sp. BCCS 001 GH: Physical Characterizations, Emulsification, and Antioxidant Activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Kianpour, S.; Jafarzadeh, S.; Alishahi, M.; Najafpour Darzi, G.; Morowvat, M.H.; Ghasemi, Y.; Shavandi, A. Biosynthesis of Exopolysaccharide from Waste Molasses Using Pantoea sp. BCCS 001 GH: A Kinetic and Optimization Study. Sci. Rep. 2022, 12, 10128. [Google Scholar] [CrossRef]
- Maalej, H.; Hmidet, N.; Boisset, C.; Bayma, E.; Heyraud, A.; Nasri, M. Rheological and Emulsifying Properties of a Gel-like Exopolysaccharide Produced by Pseudomonas stutzeri AS22. Food Hydrocoll. 2016, 52, 634–647. [Google Scholar] [CrossRef]
- Furuhashi, H.; Higashiyama, M.; Okada, Y.; Kurihara, C.; Wada, A.; Horiuchi, K.; Hanawa, Y.; Mizoguchi, A.; Nishii, S.; Inaba, K.; et al. Dietary Emulsifier Polysorbate-80-Induced Small-Intestinal Vulnerability to Indomethacin-Induced Lesions via Dysbiosis. J. Gastroenterol. Hepatol. 2020, 35, 110–117. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential Commercial Applications of Microbial Surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Chen, B.Y.; Shen, F.T.; Young, C.C. Optimization of Exopolysaccharide Production and Diesel Oil Emulsifying Properties in Root Nodulating Bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and Antibacterial Activities of Exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, J.R.; An, F.; Xu, J.; Munchdrigel; Wei, L.; Li, M.; Bilige, M.; Wu, R. Structure Characterization, Antioxidant and Emulsifying Capacities of Exopolysaccharide Derived from Tetragenococcus halophilus SNTH-8. Int. J. Biol. Macromol. 2022, 208, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hu, X.; Li, J.; Sun, X.; Luo, C.; Zhang, X.; Li, H.; Lu, J.; Li, Y.; Bao, M. Purification, Structural Characterization, Antioxidant and Emulsifying Capabilities of Exopolysaccharide Produced by Rhodococcus qingshengii QDR4-2. J. Polym. Environ. 2023, 31, 64–80. [Google Scholar] [CrossRef]
- Govindan, S.; Johnson, E.E.R.; Christopher, J.; Shanmugam, J.; Thirumalairaj, V.; Gopalan, J. Antioxidant and Anti-Aging Activities of Polysaccharides from Calocybe indica Var. APK2. Exp. Toxicol. Pathol. 2016, 68, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Hu, P.; Liao, Q.; Luo, Y.; Li, J.; Feng, D.; Zhang, J.; Wu, Z.; Xu, H. Extraction, Purification, and Antioxidant Activity of Exopolysaccharides Produced by Lactobacillus kimchi SR8 from Sour Meat in Vitro and in Vivo. CYTA—J. Food 2021, 19, 228–237. [Google Scholar] [CrossRef]
- Pan, D.; Mei, X. Antioxidant Activity of an Exopolysaccharide Purified from Lactococcus lactis Subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Jiang, Y.Y.; Zhao, X.; Hao, X.N.; Li, L.; Yang, Z.N. Characterization and Antioxidant Activity of the Exopolysaccharide Produced by Bacillus amyloliquefaciens GSBa-1. J. Microbiol. Biotechnol. 2018, 28, 1282–1292. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay As. Processes 2022, 10, 312. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on Antibacterial Activity and Antibacterial Mechanism of a Novel Polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concepción, A.; Ricardo, A.; Enrique, S.-L. Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide. Polymers 2023, 15, 3974. https://doi.org/10.3390/polym15193974
Concepción A, Ricardo A, Enrique S-L. Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide. Polymers. 2023; 15(19):3974. https://doi.org/10.3390/polym15193974
Chicago/Turabian StyleConcepción, Abrusci, Amils Ricardo, and Sánchez-León Enrique. 2023. "Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide" Polymers 15, no. 19: 3974. https://doi.org/10.3390/polym15193974
APA StyleConcepción, A., Ricardo, A., & Enrique, S.-L. (2023). Biodegradation of Choline NTF2 by Pantoea agglomerans in Different Osmolarity. Characterization and Environmental Implications of the Produced Exopolysaccharide. Polymers, 15(19), 3974. https://doi.org/10.3390/polym15193974






