1. Introduction
In contemporary times, the utilization of chemical dyes has witnessed a notable surge across a spectrum of industries including textiles, paper, plastics, printing, tanning, pigments, food, and pharmaceuticals. This proliferation, while catering to diverse industrial needs, has concurrently raised substantial concerns regarding its ecological ramifications presenting serious risks to the environment due to the inevitable leakage of effluents containing dangerous substances for soil and natural waters [
1,
2,
3,
4].
In addition to their well-documented harmful effects on health, the presence of dyes in aquatic environments leads to water discoloration and reduces oxygen levels, causing significant harm to aquatic life. This is especially concerning as most dyes used in industries are synthetic and contain aromatic rings, making their degradation in the environment a slow process. Moreover, the degradation of these synthetic dyes can produce secondary toxic substances that further damage the environment. Consequently, the effective removal of these hazardous dyes from industrial wastewater is an urgent issue, leading to the protection of aquatic ecosystems and also carries economic benefits [
1,
2,
3,
4,
5,
6,
7].
Crystal violet, a versatile dye used in a wide range of industries, including textiles, printing, and pharmaceuticals, is also used in human and veterinary medicine [
8,
9]. However, crystal violet is a toxic and non-biodegradable dye, and its presence in residual effluents can have a detrimental impact on the health of aquatic ecosystems and the photosynthesis of aquatic plants. Even though it poses little threat to human health, it can still have negative consequences such as increased heart rate, eye irritation, respiratory problems, cyanosis, kidney failure, and cancer. Therefore, treatment of wastewater before discharge to remove the dye is imperative [
4,
7,
8].
Numerous physical, chemical, electrochemical, and biological techniques are commonly used to remove dyes from waters: chemical precipitation, microbial degradation, coagulation/flocculation, advanced oxidation processes, membrane techniques, electrochemical treatments, and adsorption. However, the industrial application of many of these methods are often limited due to their high cost, energy consumption, and operation time. Additionally, some methods can generate large amounts of secondary sludge that require supplementary treatment. To select the best removal method, it is important to consider not only the process efficiency but also the raw materials and associated costs in order to achieve a balance between environmental and economic considerations. Among these, adsorption has been identified as one of the most effective, being primarily preferred due to its low cost, ease of use, simple design, adaptability, and high separation efficiency even at low dye concentrations [
1,
2,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19].
Many operating variables influence adsorption efficiency, including contaminant concentration, adsorbent dosage, pH, contact time, adsorbate–adsorbent interaction, and adsorbent type. As a result, there is a need for new adsorbents that are widely available, reusable, and cost-effective. Due to their clean, biodegradable, and rotatable qualities, lignocellulosic biomasses have attracted special attention in obtaining adsorbent materials. These materials are mainly composed of macromolecules, especially lignocellulose, which includes cellulose, lignin, and hemicellulose. These materials possess a number of interesting properties such as: abundance, renewability, high porosity, and very low costs. In addition, they have favorable mechanical properties and are easily modifiable. As a result, these materials represent a significant resource for the production of eco-friendly bio-polymeric adsorbents with a high adsorption capacity [
20,
21,
22,
23,
24].
Hart’s-tongue fern (
Asplenium scolopendrium) is a fern species that grows in Europe, North America, North Africa, and East Asia, either as a terrestrial plant or as an epiphyte on rotting and moist bark. It is a perennial, evergreen plant that prefers shady and humid areas, with leaves having a length of 40–70 cm and a width of 8–12 cm. In addition to its exceptional beauty, it also has numerous pharmaceutical attributes, being used in traditional medicine to treat lung and biliary problems. It has diuretic, astringent, and anticancer effects, being used to treat wounds, seborrhea, and acne [
25,
26].
The objective of this paper was to propose the hart’s-tongue fern (Asplenium scolopendrium) leaves as a new adsorbent for crystal violet dye from aqueous solutions. The surface characteristics of the adsorbent were investigated using scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and color analysis. Equilibrium, kinetics, and thermodynamic parameters were calculated and analyzed to elucidate the adsorption process mechanism. The Taguchi method was used to optimize the parameters that influenced the adsorption process to obtain maximum dye removal efficiency.
2. Materials and Methods
The mature leaves of hart’s-tongue fern, dried and shredded, were purchased from a company whose activity is the processing and packaging of medicinal plants, StefMar (Ramnicu Valcea, Romania). These were grounded using an electric mill; the resulting powder was then washed with distilled water to remove color and turbidity. The wet material was dried in a laboratory oven at 105 °C for 24 h.
In order to determine the characteristics of the adsorbent surface, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and color analysis (in
CIEL*a*b* system) were used and the point of zero charge (pH
PZC) was determined. FTIR analysis was performed with a Shimadzu Prestige-21 FTIR spectrophotometer (Shimadzu, Kyoto, Japan) using a pellet obtained by pressing a mixture of KBr with the adsorbent powder. SEM analysis was carried out with a Quanta FEG 250 microscope (FEI, Eindhoven, The Netherlands) at 800 × magnification and the color analysis with a Cary-Varian 300 Bio UV-VIS colorimeter (Varian Inc., Mulgrave, Australia) under D65 (natural light) illumination and with 10 observer angles. The point of zero charge (pH
PZC) was calculated according to the solid addition method [
27] using a WTW Ino-lab pH-meter (model 7310, Xylem Analytics Germany, Weilheim, Germany). The granulometric analysis of the powder adsorbent was carried out according to standard ASTM D6913-04(2009)e1 [
28].
All adsorption experiments were carried out in batch mode using three independent replicates, using a volume of dye solution of 50 mL. The values of the main parameters that affect the process varied as follows: pH = 2–12, stirring time = 1–60 min, initial dye concentration = 25–700 (mg L−1), adsorbent dose = 1–6 (g L−1), temperature = 285–319 K, and ionic strength = 0–0.25 (mol L−1). Dilute solutions of HCl 0.1 (mol dm−3) and NaOH 0.1 (mol dm−3) were used for pH adjustment and the ionic strength was modified by adding solid NaCl. The crystal violet concentration was measured with a Specord 200 PLUS UV-VIS spectrophotometer (Analytik Jena, Jena, Germany) at a wavelength of 590 nm.
In all the experimental determinations carried out, a control sample was also used in which the adsorbent material was not introduced. The absorbance and concentration of the control sample remained practically constant throughout the determinations, which proves that there are no degradation processes of the dye in the solution.
Equilibrium and kinetics of adsorption were analyzed by modeling the experimental data using several adsorption isotherms and kinetic models. In the
Supplementary Materials, Table S1, these isotherms and models along with their corresponding non-linear equations are presented [
29,
30]. In order to establish which isotherm and kinetic model is the most appropriate to characterize the dye adsorption process on adsorbent material obtained from hart’s-tongue fern, the values for determination coefficient (R
2), sum of square error (SSE), chi-square (χ
2), and average relative error (ARE) were calculated. The calculation equations of these parameters are detailed in the
Supplementary Materials, Table S2 [
30].
The Taguchi method, a powerful experimental design technique, was used to optimize the critical factors influencing the adsorption process, maximizing the removal efficiency of the dye. For this purpose, the L27 orthogonal array with six factors at three levels was used and the signal-to-noise ratio (S/N) was analyzed to assess the experimental results.
ANOVA (general linear model) analysis was used to evaluate the Taguchi method results and to calculate the percentage contribution of each controllable factor on the crystal violet removal efficiency. The required mathematical calculations were conducted with the Minitab 19 Software (version 19.1.1, Minitab LLC, State College, PA, USA).
In the desorption study, the dye-loaded adsorbent was stirred continuously for two hours with three different desorbing agents: distilled water, HCl (0.1 M), and NaOH (0.1 M) and then the amount of the dye released into the solution was measured. The equation used to calculate the desorption efficiency is shown in the
Supplementary Materials, Table S4.
3. Results and Discussion
3.1. Adsorbent Surface Characterisation
Figure 1 shows the FTIR spectrum for adsorbent material obtained from hart’s-tongue fern. The analysis of the peaks highlighted in this spectrum (
Table 1) indicates that they belong to functional groups that can interact with crystal violet dye; these groups are specific to the main constituents of the adsorbent, namely, cellulose, hemicellulose, and lignin. The correspondence of the peaks identified with the specific functional groups is as follows: 3715 cm
−1—OH-stretching mode of the free OH of water [
31], 3568 cm
−1, 3482 cm
−1, and 3400 cm
−1—OH- stretching vibration related to cellulose and hemicellulose [
32,
33,
34]; 3224 cm
−1—N−H stretching [
35]; 2918 cm
−1 –CH stretching vibration in cellulose and hemicellulose [
36]; 2862 cm
−1—CH
2 asymmetric stretching [
37]; 2350 cm
−1 and 2024 cm
−1—stretching of aromatic ring C=C [
38,
39]; 1728 cm
−1—C=O stretching vibration of the carboxylic group from lignin and hemicellulose [
40]; 1615 cm
−1—stretching vibration of carboxyl group [
41]; 1522 cm
−1—aromatic C=C/C=N stretching [
42]; 1435 cm
−1—CH bending in lignin [
43]; 1247 cm
−1—C–O stretching vibration in lignin [
44]; 1035 cm
−1—C-O, C=C, and C-C-O stretching in cellulose, hemicellulose, and lignin [
45], and 615 cm
−1—the vibration elongation of C-H bonds in aromatics [
46].
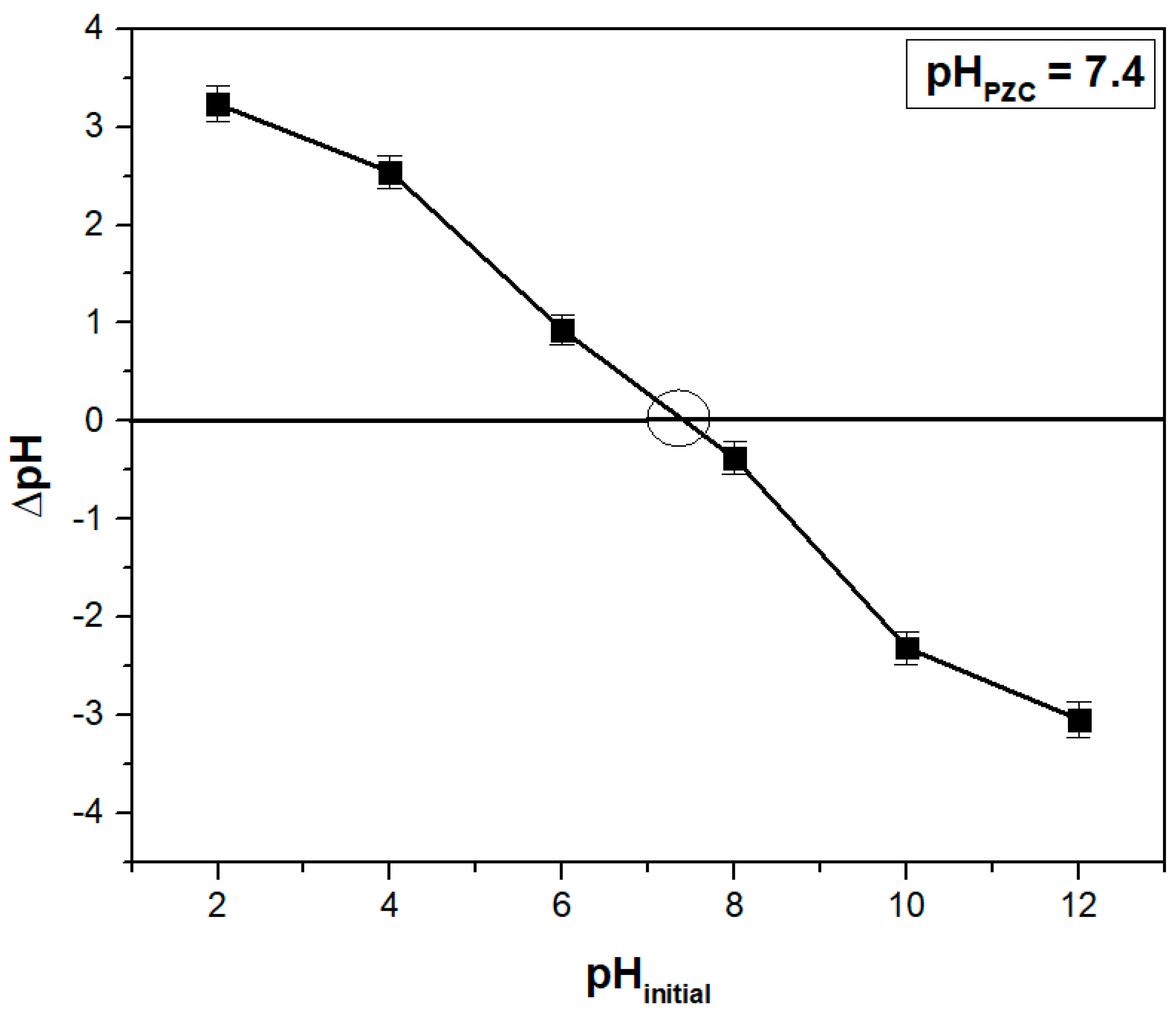
Another critical parameter in adsorption studies is the point of zero charge (pH
PZC), which is the pH at which the net surface charge of an adsorbent material is zero. At pH values higher than pH
PZC, the surface is negatively charged, favoring the adsorption of cationic dyes, while at lower values, the effect is the opposite [
19,
47]. The value determined for the adsorbent obtained from hart’s-tongue fern leaves was 7.4 (
Figure 2), being comparable to the values determined in other studies for similar adsorbents such as 6.98 for weeping willow (
Salix babylonica) leaves [
47], 7.1 for chicory (
Cichorium intybus) leaves [
48], 7.5 for gulmohar (
Delonix regia) leaves [
49], and 7.7 boxwood (
Buxus sempervirens) leaves [
50].
Figure S1, in the Supplementary Materials, shows the granulometric distribution of the hart’s-tongue fern leaves powder. As the dry leaves are brittle and easy to grind, the granulometric distribution of the powder is very narrow after grinding with an average particle size of 0.056 mm.
Scanning electron microscopy (SEM) images of the adsorbent surface before and after dye adsorption are shown in
Figure 3. Initially, the surface is heterogeneous, with many irregularities and different pores, which may provide a large number of adsorption sites (
Figure 3A). After adsorption, the surface is more compact and uniform because the adsorbed dye molecules fill and cover the pores and irregularities (
Figure 3B).
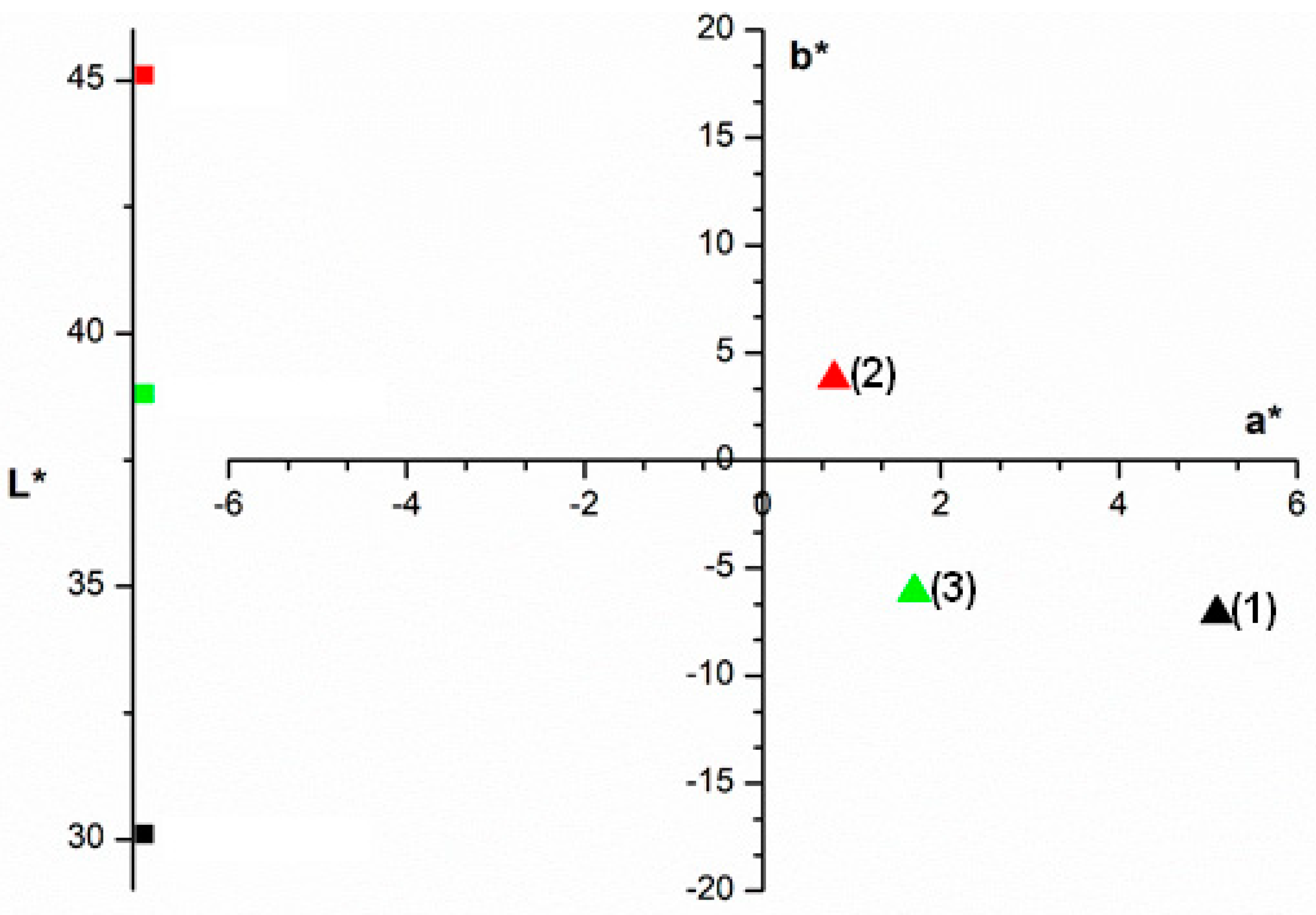
Figure 4 shows the CIELab* color analysis results for the studied adsorbent before and after dye adsorption. The analysis reveals changes in the L*, a*, and b* parameters after the adsorption of crystal violet dye. Point (1) represents the color of the cationic dye, and points (2) and (3) represent the color of the adsorbent before and after adsorption, respectively. The point representing the adsorbent color moves towards the color quadrant of the crystal violet dye after adsorption, indicating that the adsorbent color is modified by the dye adsorption.
3.2. The Influence of pH, Ionic Strength, and Adsorbent Dose on Adsorption Capacity
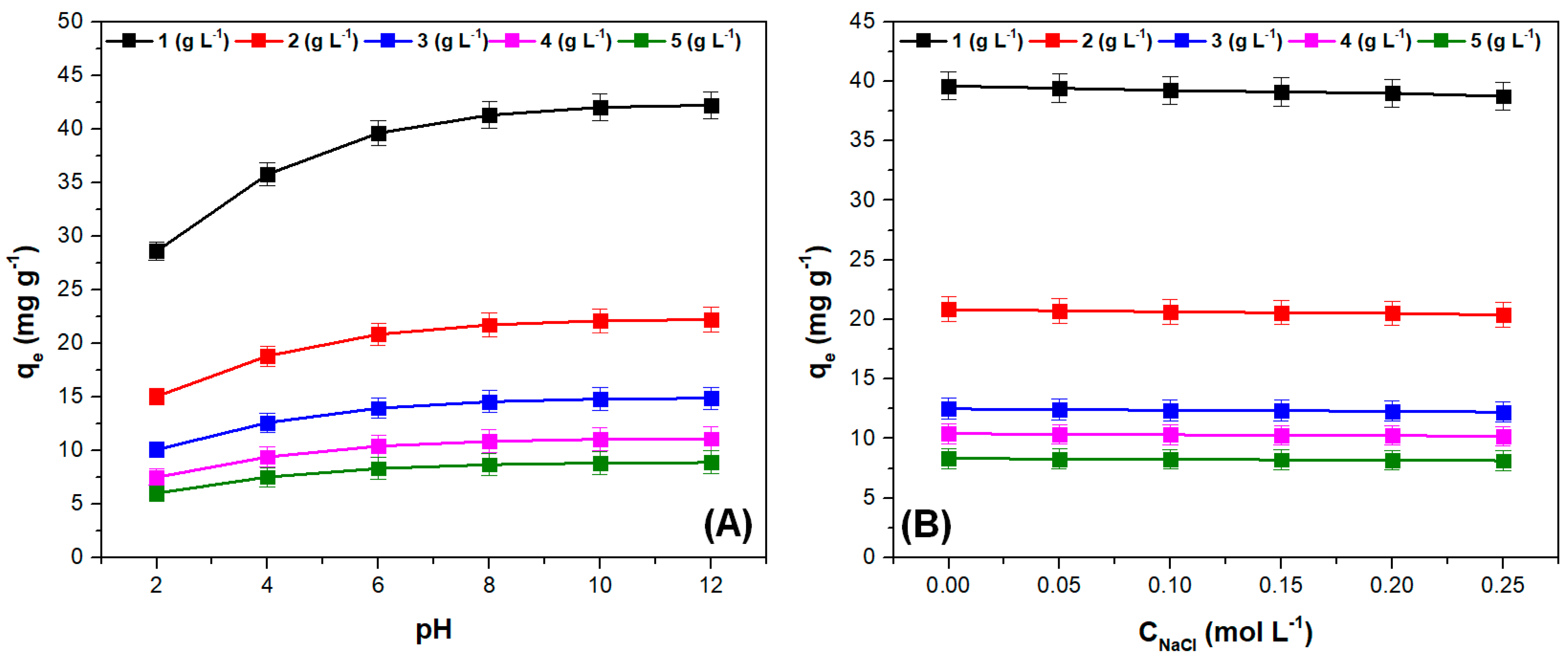
The influence of pH and ionic strength on adsorption capacity at different adsorbent doses is illustrated in
Figure 5. As expected, at pH values higher than pH
PZC, the adsorption capacity had the highest values, highlighting the positive effect of the electrostatic attraction between the adsorbate surface and the cationic dye [
51,
52]. Increasing the adsorbent dose increases the number of available adsorption sites, but a significant portion of these sites remained unsaturated. This fact, along with the agglomeration of the adsorbent particles that can appear when the dose increases, lead to a decrease of the adsorption capacity [
46,
53,
54]. Similar effects of pH and adsorbent dose were reported in other studies where the same type of adsorbent materials were used to retain crystal violet dye [
51,
55,
56,
57]. The increase in ionic strength results in a negligible decrease in adsorption capacity, indicating the adsorbent material’s affinity for the cationic dye.
While increasing the ionic strength of the solution may introduce competition between crystal violet cations and sodium ions for the occupation of adsorption sites on the adsorbate surface, this does not significantly affect the retention of the dye. This suggests that the adsorption process is not solely driven by electrostatic attraction. This is further supported by the observation that the adsorption capacity does not increase significantly at pH values above the point of zero charge (pHPZC).
These results indicate that the proposed adsorbent material is suitable for practical applications, such as the removal of cationic dyes from wastewaters containing other salts together with the considered dye.
3.3. Equilibrum Isotherms
The adsorption equilibrium was examined by modeling the experimental data using the isotherm models described in
Table 1, which lists the isotherms’ constants and the corresponding error parameter. The information in the table indicates that the Sips isotherm best describes the process, this isotherm having the highest value for R
2 and the lowest values for SSE, χ
2 and ARE.
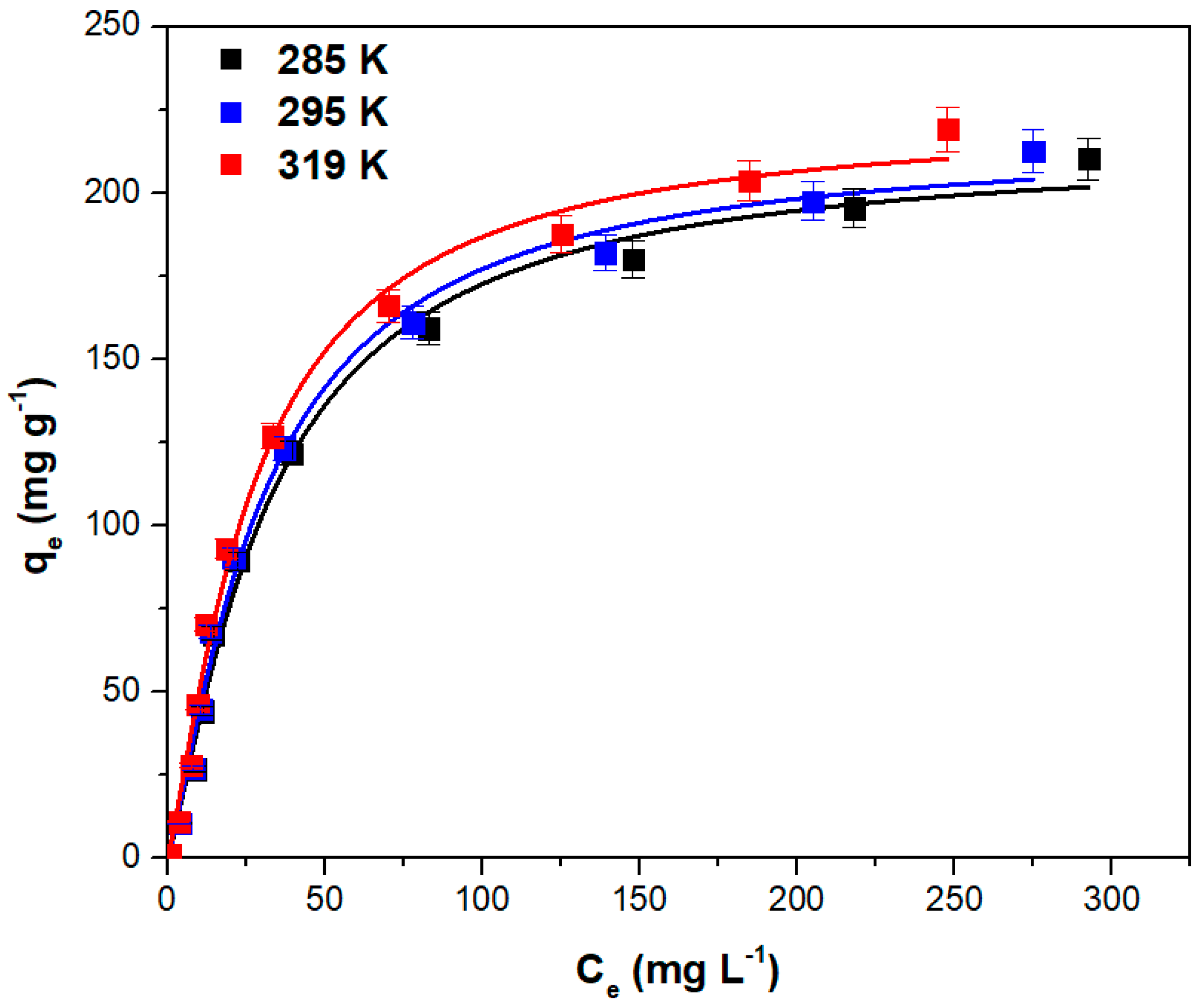
Figure 6 depicts the fitted Sips isotherm curves at various temperatures. According to the figure, the adsorption capacity increases with temperature. The solutions’ viscosity decreases as the temperature rises, therefore the mobility of the dye molecules increases. This has a beneficial impact on the adsorption capacity with the process being endothermic [
58,
59,
60].
The maximum absorption capacities of various similar adsorbents obtained from plant leaves and used for crystal violet adsorption are compared in
Table 2. The data indicate that the adsorbent obtained from hart’s-tongue fern (
Asplenium scolopendrium) leaves has an adsorption capacity higher than other adsorbents, demonstrating the usefulness of the new adsorbent proposed in this work.
3.4. Kinetic Study
The effect of contact time on the ability of the adsorbent material to retain the dye at different initial dye concentrations is illustrated in
Figure 7. The adsorption capacity increases quickly in the first minutes, due to a large number of adsorption sites accessible for the dye retention, and then more slowly as it approaches equilibrium. This is reached after 20 min when it is assumed that the adsorbent surface is almost completely coated by dye molecules [
55,
60]. The obtained equilibrium time is lower than those reported in the scientific literature for other adsorbents based on plant leaves (
Table 3).
Increasing the initial concentration of the dye has a positive effect on the adsorption capacity due to the increase in the concentration gradient and the number of collisions between the dye molecules and the adsorbent material particles [
8,
46,
51,
53,
55].
Five kinetic models were tested to model the experimental results.
Table 4 presents these models together with their constants and the corresponding error parameter. The highest value for R
2 and the lowest values for SSE, χ
2, and ARE indicate that the general- order model is the proper model to describe the crystal violet adsorption.
Figure 7 shows the fitted curves of this model at various initial dye concentrations. In the scientific literature, it is mentioned that the general-order kinetic model characterizes the adsorption of the crystal violet dye on similar adsorbent materials such as motherwort biomass [
67] and sour cherry leaf [
68].
3.5. Thermodynamic Study
The thermodynamic parameters listed in
Table 5 were calculated from the slope and intercept of the plot of ln K
L versus 1/T, which is shown in
Figure S2 of the Supplementary Materials, based on experimental data collected at three different temperatures: 282, 293, and 307 K. The standard Gibbs free energy change (ΔG
0) is negative and varies with increasing temperature, indicating that the adsorption process is spontaneous and favorable. The standard enthalpy change (ΔH
0) and standard entropy change (ΔS
0) are both positive, indicating that the adsorption process is endothermic and increases randomness at the solid–liquid interface [
55,
58].
The value of ∆H
0 lower than 20 (kJ mol
−1) indicates that the main mechanism is physical adsorption, with van der Waals interaction implied in the process [
69,
70]. The standard Gibbs free energy change (ΔG
0) of the adsorption process is between −80 and −20 (kJ mol
−1), but closer to −20 (kJ mol
−1). This suggests that there is a small chemical effect that may enhance the adsorption [
43,
71].
3.6. Optimization Using the Taguchi Method
The Taguchi method was used to determine the optimal adsorption conditions, which were based on an L27 orthogonal array experimental design. The six controllable factors that formed the basis of this array, together with their levels, are shown in
Table 6.
The Taguchi method is a powerful way to design experiments. It focuses on finding the signal-to-noise ratio (S/N), which is a measure of how accurate and reliable the results are. The S/N ratio is calculated by comparing the response to the noise, which is any factor that can affect the accuracy of the results.
The Taguchi method has two main advantages: it minimizes the number of experiments needed and it provides a visual representation of the best conditions.
In this study, the Taguchi method was used to improve the efficiency of dye removal. The “larger is the better” option for the S/N ratio was used [
72,
73,
74].
Table 7 details the L27 orthogonal array experimental design, the results of the experiments, and the S/N ratio for each experiment.
Table 8 shows the signal-to-noise (S/N) ratios for each factor at each level and their significant ranks. These ratios indicate how much each factor affects the effectiveness of dye removal. The higher the S/N ratio, the greater the impact of the factor. Based on the S/N ratios and significant ranks, pH has the most impact on dye removal efficiency, while temperature has the least. The Taguchi approach leads to the following optimal adsorption conditions: pH of 12, contact time of 60 min, adsorbent dose of 6 (g L
−1), initial dye concentration of 200 (mg L
−1), temperature of 319 K, and ionic strength of 0.0 (mol L
−1).
Table 8 also shows the ANOVA analysis results and the contribution percent of each controllable factor on crystal violet removal efficiency. Their value indicates the same hierarchy of influence of the controllable factors as the Taguchi technique.
By correlating the experimental dye removal efficiency values to those predicted by optimization, the validity of the Taguchi experimental design was confirmed (
Figure 8). The value of determination coefficient R
2 obtained for linear regression demonstrates a high degree of accuracy of the Taguchi approach.
3.7. Desorption Study
The desorption of crystal violet dye from the absorbent material was inefficient, regardless of the desorbing agent used, suggesting that it is not practical to reuse it. The desorption efficiencies were 7.83%, 29.38%, and 17.35% for distilled water, HCl, and NaOH, respectively. However, the low cost and abundance of hart’s-tongue fern leaves offset this drawback. Additionally, the absorbent material can be incinerated to generate energy, which is a simple and efficient way to reuse it.
4. Conclusions
Within this study, a novel lignocellulosic adsorbent was proposed to remove crystal violet dye from water. The source material for this adsorbent was derived from the leaves of the hart’s-tongue fern (Asplenium scolopendrium), having undergone a procedure of minimal processing that deliberately avoided both chemical and thermal treatments.
FTIR analysis identified different functional groups specific to the main constituents of the adsorbent (cellulose, hemicellulose, and lignin) that can interact with the crystal violet dye. SEM and color analysis, before and after adsorption, revealed changes in the morphology and color of the adsorbent, confirming the retention of the dye on its surface. The augmentation of specific parameters, such as pH, contact time, initial dye concentration, and temperature, exerts a favorable impact on the enhancement of the adsorption capacity value. The increase of the ionic strength resulted in a nearly negligible reduction in the adsorption capacity, underscoring the inherent affinity of the adsorbent towards crystal violet dye. Sips isotherm was the most suitable to characterize the process compared to other isotherms tested: Langmuir, Freundlich, Temkin, Sips, and Redlich–Peterson. The adsorbent exhibits an adsorption capacity (224.2 mg g−1) surpassing that of comparable adsorbents, suggesting the efficacy and practical value of the new proposed adsorbent. Equilibrium is reached after 20 min and the general-order model is the most proper model to describe the crystal violet adsorption. The thermodynamic parameters indicate a spontaneous and favorable process, the main mechanism being physical adsorption, with van der Waals interaction involved, along with a small chemical effect that may enhance adsorption. The Taguchi method and ANOVA analysis were used to determine the best conditions for adsorption (using an L27 orthogonal array experimental design) and the relative importance of each controllable factor on the removal efficiency of crystal violet, respectively. pH had the greatest impact on dye removal efficiency (75.84%), while temperature had the least (0.22%). The Taguchi method had good accuracy, with a good match between the experimental dye removal efficiency values and those predicted by the optimization.
The comprehensive assessment of the acquired data suggests that the hart’s-tongue fern (Asplenium scolopendrium) leaves serve as a cost-effective, readily accessible, and efficient adsorbent for eliminating crystal violet dye from aqueous solutions.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/polym15193923/s1, Table S1: The non-linear equations of the adsorption isotherms and kinetic models used to assess the adsorption process; Table S2: The calculation equations for error parameters R
2, χ
2, SSE, and ARE; Table S3: The equations of specific thermodynamic parameters; Table S4: The equation used to calculate the desorption efficiency; Figure S1: The particle size distribution of hart’s-tongue fern (
Asplenium scolopendrium) leaves powder; Figure S2: Plot of ln K
L vs. 1/T for the dye adsorption onto hart’s-tongue fern (
Asplenium scolopendrium) leaves powder.
Author Contributions
Conceptualization, G.M., M.D. and S.B.; methodology, G.M. and M.D.; software, G.M. and C.V.; validation, G.M. and M.D.; formal analysis, G.M., C.V. and S.B.; investigation, G.M., S.P., M.D. and S.B.; resources, G.M. and M.D.; data curation, G.M. and S.P.; writing—original draft preparation, G.M., C.V. and S.B.; writing—review and editing, G.M., M.D. and S.B.; visualization, G.M.; supervision, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Research, Innovation and Digitalization, project number PFE 26/30.12.2021, PERFORM-CDI@UPT100—The increasing of the performance of the Polytechnic University of Timisoara by strengthening the research, development and technological transfer capacity in the field of “Energy, Environment and Climate Change” at the beginning of the second century of its existence, within Program 1—Development of the national system of Research and Development, Subprogram 1.2—Institutional PerformanceInstitutional Development Projects—Excellence Funding Projects in RDI, PNCDI III.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All the experimental data obtained are presented in the form of tables and/or figures, in the article, and in the
Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mennas, N.; Lahreche, S.; Chouli, F.; Sabantina, L.; Benyoucef, A. Adsorption of Methylene Blue Dye by Cetyltrimethylammonium Bromide Intercalated Polyaniline-Functionalized Montmorillonite Clay Nanocomposite: Kinetics, Isotherms, and Mechanism Study. Polymers 2023, 15, 3518. [Google Scholar] [CrossRef] [PubMed]
- Shkliarenko, Y.; Halysh, V.; Nesterenko, A. Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal. Water 2023, 15, 1536. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Gul, S.; Gul, H.; Khitab, F.; Khattak, R.; Khan, M.S.; Ullah, R.; Ullah, R.; Wasil, Z.; Krauklis, A.E.; et al. Dried Leaves Powder of Adiantum capillus-veneris as an Efficient Biosorbent for Hazardous Crystal Violet Dye from Water Resources. Separations 2023, 10, 165. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar]
- Thamer, B.M.; Al-Enizi, A.; Altaleb, H.A.; AlAnazi, N.B.; Ubaidullah, M.; El-Newehy, M.H. Hollow carbon fibers and flakes derived from Calotropis procera as adsorbents for dye removal from aqueous solutions. Mater. Chem. Phys. 2022, 279, 125752. [Google Scholar] [CrossRef]
- Abril, D.; Ferrer, V.; Mirabal-Gallardo, Y.; Cabrera-Barjas, G.; Segura, C.; Marican, A.; Pereira, A.; Durán-Lara, E.F.; Valdés, O. Comparative Study of Three Dyes’ Adsorption onto Activated Carbon from Chenopodium quinoa Willd and Quillaja saponaria. Materials 2022, 15, 4898. [Google Scholar] [CrossRef]
- Zamouche, M.; Habib, A.; Saaidia, K.; Lehocine, M.B. Batch mode for adsorption of crystal violet by cedar cone forest waste. SN Appl. Sci. 2020, 2, 198. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Sie Yon, J. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agriculturals solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Enache, A.-C.; Samoila, P.; Cojocaru, C.; Apolzan, R.; Predeanu, G.; Harabagiu, V. An Eco-Friendly Modification of a Walnut Shell Biosorbent for Increased Efficiency in Wastewater Treatment. Sustainability 2023, 15, 2704. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Technol. 2021, 6, 2000955. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent advances in graphene based TiO2 nanocomposites (GTiO2Ns) for photocatalytic degradation of synthetic dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and removal of crystal violet dye from aqueous solution by modified rice husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef]
- Kosar Hashemi, Y.; Tavakkoli Yaraki, M.; Ghanbari, S.; Heidarpoor Saremi, L.; Givianrad, M.H. Photodegradation of organic water pollutants under visible light using anatase F, N co-doped TiO2/SiO2 nanocomposite: Semi-pilot plant experiment and density functional theory calculations. Chemosphere 2021, 275, 129903. [Google Scholar] [CrossRef]
- Maleš, L.; Fakin, D.; Bračič, M.; Gorgieva, S. Efficiency of Differently Processed Membranes Based on Cellulose as Cationic Dye Adsorbents. Nanomaterials 2020, 10, 642. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- González-Delgado, A.D.; Villabona-Ortíz, A.; Tejada-Tovar, C. Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water 2022, 14, 408. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Nindjio, G.F.K.; Tagne, R.F.T.; Jiokeng, S.L.Z.; Fotsop, C.G.; Bopda, A.; Doungmo, G.; Temgoua, R.C.T.; Doench, I.; Njoyim, E.T.; Tamo, A.K.; et al. Lignocellulosic-Based Materials from Bean and Pistachio Pod Wastes for Dye-Contaminated Water Treatment: Optimization and Modeling of Indigo Carmine Sorption. Polymers 2022, 14, 3776. [Google Scholar] [CrossRef]
- Rosales, E.; Escudero, S.; Pazos, M.; Sanromán, M.A. Sustainable Removal of Cr(VI) by Lime Peel and Pineapple Core Wastes. Appl. Sci. 2019, 9, 1967. [Google Scholar] [CrossRef]
- Robledo-Peralta, A.; Torres-Castañón, L.A.; Rodríguez-Beltrán, R.I.; Reynoso-Cuevas, L. Lignocellulosic Biomass as Sorbent for Fluoride Removal in Drinking Water. Polymers 2022, 14, 5219. [Google Scholar] [CrossRef] [PubMed]
- Yekefallah, M.; Raofie, F. Preparation of stable nanosuspensions from Asplenium scolopendrium leaves via rapid expansion of supercritical solution into aqueous solutions (RESSAS). J. Drug Deliv. Sci. Technol. 2021, 64, 102566. [Google Scholar] [CrossRef]
- Bremer, P.; Eelke Jongejans, E. Frost and Forest Stand Effects on the Population Dynamics of Asplenium scolopendrium. Popul. Ecol. 2009, 52, 211–222. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Gupta, N.; Chattopadhyaya, M.C. Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J. Saudi Chem. Soc. 2014, 18, 200–207. [Google Scholar] [CrossRef]
- ASTM D6913-04(2009)e1; Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM International: West Conshohocken, PA, USA, 2017.
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–76. [Google Scholar]
- Lee, Y.F.; Kelterer, A.M.; Matisz, G.; Kunsági-Máté, S.; Chung, C.Y.; Lee, Y.P. Infrared absorption of methanol-water clusters (CH3OH)n(H2O), n = 1–4, recorded with the VUV-ionization/IR-depletion technique. J. Chem. Phys. 2017, 146, 144308. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Chen, X.; Yan, J.K.; Wu, J.Y. Characterization and antibacterial activity of silver nanoparticles prepared with a fungal exopolysaccharide in water. Food Hydrocoll. 2016, 53, 69–74. [Google Scholar] [CrossRef]
- Shi, J.; Xing, D.; Li, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Zhou, T.; Liu, X.; Yuan, Q.; Zhang, A. New Understanding on the Reaction Pathways of the Polyacrylonitrile Copolymer Fiber Pre-Oxidation: Online Tracking by Two-Dimensional Correlation FTIR Spectroscopy. RSC Adv. 2016, 6, 4397–4409. [Google Scholar] [CrossRef]
- Liu, W.; Mohanty, A.K.; Drzal, L.T.; Askel, P.; Misra, M. Effects of alkali treatment on the structure, morphology and thermal properties of native grass fibers as reinforcements for polymer matrix composites. J. Mater. Sci. 2004, 39, 1051–1054. [Google Scholar] [CrossRef]
- Wang, Y. Application of Fourier Transform Infrared Microspectroscopy (FTIR) and Thermogravimetric Analysis (TGA) for quick identification of Chinese herb Solanum lyratum. Plant Omics 2012, 5, 508–513. [Google Scholar]
- Ruiz, H.A.; Ruzene, D.S.; Silva, D.P.; Macieira da Silva, F.F.; Vicente, A.A.; Teixeira, J.A. Development and Characterization of an Environmentally Friendly Process Sequence (Autohydrolysis and Organosolv) for Wheat Straw Delignification. Appl. Biochem. Biotechnol. 2011, 164, 629–641. [Google Scholar] [CrossRef]
- Salleh, M.N.; Aziz, R.A.; Razak, M.F.S.A.; Musa, L.; Ying, H.L. Effect of Surface Treatment on Mechanical Properties of Rice Husk Reinforced Recycled High Density Polyethylene (rHDPE) Composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 957, 012011. [Google Scholar] [CrossRef]
- Plermjai, K.; Phoohinkong, W.; Pavasupree, S.; Mekprasart, W.; Pecharapa, W.; Boonyarattanakalin, K. UV shielding properties of cellulose/TiO2 composite film. Curr. J. Appl. Sci. Technol. 2018, 18, 111–118. [Google Scholar]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anita, B. Vibrational Spectroscopic Investigation of Ornidazole—An Antiprotozoan Agent. Asian J. Chem. 2009, 21, 7241–7248. [Google Scholar]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers 2022, 14, 1966. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Grassi, P.; Reis, C.; Drumm, F.C.; Georgin, J.; Tonato, D.; Escudero, L.B.; Kuhn, R.; Jahn, S.L.; Dotto, G.L. Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci Technol. 2019, 79, 709–717. [Google Scholar] [CrossRef]
- Khodabandehloo, A.; Rahbar-Kelishami, A.; Shayesteh, H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Haghighizadeh, M.; Zare, K.; Aghaie, H.; Monajjemi, M. Preparation and characterization of Chicory leaf powder and its application as a nano-native plant sorbent for removal of Acid Blue 25 from aqueous media: Isotherm, kinetic and thermodynamic study of the adsorption phenomenon. J. Nanostruct. Chem. 2020, 10, 75–86. [Google Scholar] [CrossRef]
- Ponnusami, V.; Gunasekar, V.; Srivastava, S.N. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: Multivariate regression analysis. J. Hazard. Mater. 2009, 169, 119–127. [Google Scholar] [CrossRef]
- Rahman-Setayesh, M.R.; Kelishami, A.R.; Shayesteh, H. Equilibrium, kinetic, and thermodynamic applications for methylene blue removal using Buxus sempervirens leaf powder as a powerful low-cost adsorbent. J. Part. Sci. Technol. 2019, 5, 161–170. [Google Scholar]
- Chakraborty, S.; Chowdhury, S.; Das, P. Insight into biosorption equilibrium, kinetics and thermodynamics of crystal violet onto Ananas comosus (pineapple) leaf powder. Appl. Water Sci. 2012, 2, 135–141. [Google Scholar] [CrossRef][Green Version]
- Ghazali, A.; Shirani, M.; Semnania, A.; Zare-Shahabadic, V.; Nekoeiniad, M. Optimization of crystal violet adsorption onto Date palm leaves as a potent biosorbent from aqueous solutions using response surface methodology and ant colony. J. Environ. Chem. Eng. 2018, 6, 3942–3950. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Das, P. Removal of Crystal Violet from Aqueous Solution by Adsorption onto Eggshells: Equilibrium, Kinetics, Thermodynamics and Artificial Neural Network Modeling. Waste Biomass Valorization 2012, 4, 655–664. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of crystal violet onto functionalized multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Nasar, A. Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Groundw. Sustain. Dev. 2018, 7, 30–38. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Dotto, G.L. Evaluation of Ocotea puberula bark powder (OPBP) as an effective adsorbent to uptake crystal violet from colored effluents: Alternative kinetic approaches. Environ. Sci. Pollut. Res. 2020, 27, 25727–25739. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef]
- Zehra, T.; Priyantha, N.; Lim, L.B.L. Removal of crystal violet dye from aqueous solution using yeast-treated peat as adsorbent: Thermodynamics, kinetics, and equilibrium studies. Environ. Earth Sci. 2016, 75, 357. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Bathurst Burr (Xanthium spinosum) Powder—A New Natural Effective Adsorbent for Crystal Violet Dye Removal from Synthetic Wastewaters. Materials 2021, 14, 5861. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Optimization of crystal violet adsorption on common lilac tree leaf powder as natural adsorbent material. Glob. Nest J. 2022, 24, 87–96. [Google Scholar]
- Das, P.; Chakraborty, S.; Chowdhury, S. Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloids Surf. B Colloid Surf. B 2012, 92, 262–270. [Google Scholar]
- Mehmood, A.; Bano, S.; Fahim, A.; Parveen, R.; Khurshid, S. Efficient removal of crystal violet and eosin B from aqueous solution using Syzygium cumini leaves: A comparative study of acidic and basic dyes on a single adsorbent. Korean J. Chem. Eng. 2015, 32, 882–895. [Google Scholar] [CrossRef]
- Khan, F.A.; Ahad, A.; Shah, S.S.; Farooqui, M. Adsorption of crystal violet dye using Platanus orientalis (Chinar tree) leaf powder and its biochar: Equilibrium, kinetics and thermodynamics study. Int. J. Environ. Anal. Chem. 2021, 103, 4820–4840. [Google Scholar] [CrossRef]
- Alsenani, G. Studies on adsorption of crystal violet dye from aqueous solution onto Calligonum comosum leaf powder (CCLP). J. Am. Sci. 2013, 9, 30–35. [Google Scholar]
- Ali, H.; Muhammad, S.K. Biosorption of Crystal Violet from Water on Leaf Biomass of Calotropis procera. J. Environ. Sci. Technol. 2008, 1, 143–150. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. A Novel High-Efficiency Natural Biosorbent Material Obtained from Sour Cherry (Prunus cerasus) Leaf Biomass for Cationic Dyes Adsorption. Materials 2023, 16, 4252. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. Crystal Violet Adsorption on Eco-Friendly Lignocellulosic Material Obtained from Motherwort (Leonurus cardiaca L.) Biomass. Polymers 2022, 14, 3825. [Google Scholar] [CrossRef]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A.; Jabri, M.; Kali, A.; Chraibi, S.; Hadey, C.; Aziz, F. Adsorption of Crystal Violet onto an Agricultural Waste Residue: Kinetics, Isotherm, Thermodynamics, and Mechanism of Adsorption. Sci. World. J. 2020, 2020, 5873521. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2018, 276, 105–114. [Google Scholar] [CrossRef]
- Zhai, Q.-Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81, 2092–2108. [Google Scholar] [CrossRef]
- Abbasi, F.; Tavakkoli Yaraki, M.; Farrokhnia, A.; Bamdad, M. Keratin nanoparticles obtained from human hair for removal of crystal violet from aqueous solution: Optimized by Taguchi method. Int. J. Biol. Macromol. 2020, 143, 492–500. [Google Scholar] [CrossRef]
- Rahmani, M.; Kaykhaii, M.; Sasani, M. Application of Taguchi L16 design method for comparative study of ability of 3A zeolite in removal of Rhodamine B and Malachite green from environmental water samples. Spectrochim. Acta. Part A 2018, 188, 164–169. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]
Figure 1.
The FTIR spectra of adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves.
Figure 1.
The FTIR spectra of adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves.
Figure 2.
Determination of point of zero charge (pHPZC) for the studied adsorbent using the solid addition method.
Figure 2.
Determination of point of zero charge (pHPZC) for the studied adsorbent using the solid addition method.
Figure 3.
SEM images of adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves: (A) Before adsorption and (B) After adsorption.
Figure 3.
SEM images of adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves: (A) Before adsorption and (B) After adsorption.
Figure 4.
Color analysis for the adsorbent material, before and after adsorption, using CIEL*a*b* color parameters of: (1) Crystal violet dye, (2) Adsorbent before adsorption, and (3) Adsorbent after adsorption.
Figure 4.
Color analysis for the adsorbent material, before and after adsorption, using CIEL*a*b* color parameters of: (1) Crystal violet dye, (2) Adsorbent before adsorption, and (3) Adsorbent after adsorption.
Figure 5.
The influence of pH (A) and ionic strength (B), on adsorption capacity, at different adsorbent doses (Adsorption conditions: (A) contact time = 20 min, adsorbent dose = 2 (g L−1), crystal violet concentration = 50 (mg L−1), temperature = 295 K, ionic strength = 0 (mol L−1); (B) pH = 6, others identical to (A)).
Figure 5.
The influence of pH (A) and ionic strength (B), on adsorption capacity, at different adsorbent doses (Adsorption conditions: (A) contact time = 20 min, adsorbent dose = 2 (g L−1), crystal violet concentration = 50 (mg L−1), temperature = 295 K, ionic strength = 0 (mol L−1); (B) pH = 6, others identical to (A)).
Figure 6.
The experimental data points and the fitted Sips isotherm curves at various temperatures (Adsorption conditions: pH = 6, contact time = 20 min, adsorbent dose = 2 (g L−1), ionic strength = 0 (mol L−1)).
Figure 6.
The experimental data points and the fitted Sips isotherm curves at various temperatures (Adsorption conditions: pH = 6, contact time = 20 min, adsorbent dose = 2 (g L−1), ionic strength = 0 (mol L−1)).
Figure 7.
The experimental data points and the fitted general-order kinetic model at various initial dye concentration (Adsorption conditions: pH = 6, adsorbent dose = 2 (g L−1), temperature = 295 K, ionic strength = 0 (mol L−1)).
Figure 7.
The experimental data points and the fitted general-order kinetic model at various initial dye concentration (Adsorption conditions: pH = 6, adsorbent dose = 2 (g L−1), temperature = 295 K, ionic strength = 0 (mol L−1)).
Figure 8.
The relationship between the dye removal efficiency values measured in the experiment and those predicted by the Taguchi method.
Figure 8.
The relationship between the dye removal efficiency values measured in the experiment and those predicted by the Taguchi method.
Table 1.
The constants and the corresponding error parameters for the tested adsorption isotherms.
Table 1.
The constants and the corresponding error parameters for the tested adsorption isotherms.
| Isotherm Model | Parameters | Value |
|---|
| 285 K | 295 K | 319 K |
|---|
| Langmuir | KL (L mg−1) | 0.023 ± 0.004 | 0.025 ± 0.003 | 0.028 ± 0.003 |
| qmax (mg g−1) | 237.2 ± 9.1 | 239.8 ± 8.4 | 247.3 ± 9.2 |
| R2 | 0.9916 | 0.9916 | 0.9915 |
| χ2 | 13.64 | 13.78 | 14.21 |
| SSE | 557.2 | 569.5 | 605.4 |
| ARE (%) | 13.15 | 13.14 | 13.16 |
| Freundlich | Kf (mg g−1) | 21.82 ± 3.21 | 22.06 ± 2.74 | 22.74 ± 0.92 |
| 1/n | 0.41 ± 0.07 | 0.41 ± 0.05 | 0.42 ± 0.04 |
| R2 | 0.9446 | 0.9446 | 0.9944 |
| χ2 | 51.18 | 51.73 | 53.33 |
| SSE | 3401 | 3476 | 3694 |
| ARE (%) | 22.32 | 22.34 | 22.35 |
| Temkin | KT (L mg−1) | 0.265 ± 0.043 | 0.265 ± 0.048 | 0.266 ± 0.057 |
| b (kJ g−1) | 48.85 ± 5.43 | 43.32 ± 2.41 | 46.87 ± 4.81 |
| R2 | 0.9923 | 0.9923 | 0.9923 |
| χ2 | 24.15 | 24.38 | 25.16 |
| SSE | 451.5 | 464.2 | 493.4 |
| ARE (%) | 30.99 | 30.94 | 30.97 |
| Sips | Qsat (mg g−1) | 215.1 ± 8.4 | 217.5 ± 5.3 | 224.2 ± 7.3 |
| KS (L mg−1) | 0.014 ± 0.003 | 0.014 ± 0.002 | 0.016 ± 0.002 |
| n | 1.22 ± 0.15 | 1.23 ± 0.14 | 1.24 ± 0.16 |
| R2 | 0.9939 | 0.9939 | 0.9939 |
| χ2 | 6.99 | 7.06 | 7.29 |
| SSE | 371.7 | 379.7 | 403.5 |
| ARE (%) | 10.02 | 10.02 | 10.03 |
| Redlich–Peterson | KRP (L g−1) | 5.54 | 5.60 ± 0.84 | 5.78 |
| aRP (L mg−1) | 0.016 | 0.016 ± 0.003 | 0.017 |
| ΒRP | 1.05 | 1.05 ± 0.09 | 1.06 |
| R2 | 0.9918 | 0.9918 | 0.9918 |
| χ2 | 12.19 | 12.32 | 12.70 |
| SSE | 524.7 | 536.3 | 569.4 |
| ARE (%) | 12.72 | 12.71 | 12.72 |
Table 2.
The maximum absorption capacities of various similar adsorbents obtained from plant leaves and used for crystal violet adsorption.
Table 2.
The maximum absorption capacities of various similar adsorbents obtained from plant leaves and used for crystal violet adsorption.
| Adsorbent | Maximum Adsorption
Capacity (mg g−1) | Reference |
|---|
| hart’s-tongue fern (Asplenium scolopendrium) leaves | 224.2 | This study |
| lilac tree leaf | 196.7 | [61] |
| pineapple leaf | 78.22 | [51] |
| jackfruit leaf | 43.39 | [62] |
| Syzygium cumini leaves | 38.75 | [63] |
| date palm leaves | 37.73 | [52] |
| Platanus orientalis leaf | 25.88 | [64] |
| Adiantum capillus-veneris leaves | 18.51 | [4] |
| Calligonum comosum leaf | 5.00 | [65] |
| Calotropis procera leaf | 4.14 | [66] |
Table 3.
The equilibrium times reported in the scientific literature for various adsorbents based on plant leaves and used for crystal violet adsorption.
Table 3.
The equilibrium times reported in the scientific literature for various adsorbents based on plant leaves and used for crystal violet adsorption.
| Adsorbent | Equilibrium Time (min) | Reference |
|---|
| hart’s-tongue fern (Asplenium scolopendrium) leaves | 20 | This study |
| lilac tree leaf | 40 | [61] |
| date palm leaves | 21 | [52] |
| Platanus orientalis leaf | 30 | [64] |
| Syzygium cumini leaves | 60 | [63] |
| Calotropis procera leaf | 60 | [66] |
| Adiantum capillus-veneris leaves | 90 | [4] |
| jackfruit leaf | 120 | [62] |
Table 4.
The constants and the corresponding error parameters for the tested kinetic models.
Table 4.
The constants and the corresponding error parameters for the tested kinetic models.
| Kinetic Model | Parameters | Initial Dye Concentration (mg L−1) |
|---|
| 25 | 50 | 100 | 200 |
|---|
| Pseudo-first order | k1 (min−1) | 1.82 ± 0.21 | 1.83 ± 0.24 | 1.81 ± 0.31 | 1.82 ± 0.34 |
| qe,calc (mg g−1) | 10.33 ± 0.27 | 20.45 ± 0.62 | 44.33 ± 1.78 | 89.04 ± 3.75 |
| R2 | 0.9916 | 0.9912 | 0.9914 | 0.9915 |
| χ2 | 0.07 | 0.16 | 0.34 | 0.68 |
| SSE | 0.78 | 3.21 | 14.88 | 58.93 |
| ARE (%) | 13.38 | 13.51 | 13.41 | 13.39 |
| Pseudo-second order | k2 (min−1) | 0.42 ± 0.08 | 0.21 ± 0.03 | 0.09 ± 0.02 | 0.04 ± 0.01 |
| qe,calc (g mg−1 min−1) | 10.58 ± 0.34 | 20.93 ± 0.82 | 45.60 ± 1.34 | 91.16 ± 4.62 |
| R2 | 0.9991 | 0.9988 | 0.9991 | 0.9991 |
| χ2 | 0.007 | 0.02 | 0.03 | 0.07 |
| SSE | 0.07 | 0.42 | 1.48 | 5.96 |
| ARE (%) | 0.89 | 10.5 | 0.90 | 0.90 |
| Elovich | a (g mg−1) | 1.21 ± 0.08 | 0.61 ± 0.07 | 0.34 ± 0.08 | 0.14 ± 0.03 |
| b (mg g−1 min−1) | 1483 ± 214 | 7926 ± 347 | 14,523 ± 1347 | 25,523 ± 2436 |
| R2 | 0.9653 | 0.9657 | 0.9684 | 0.9653 |
| χ2 | 1.11 | 2.20 | 7.87 | 9.63 |
| SSE | 3.35 | 12.99 | 38.56 | 248.2 |
| ARE (%) | 15.77 | 15.66 | 21.32 | 15.75 |
| Avrami | kAV (min−1) | 1.63 ± 0.11 | 1.63 ± 0.09 | 1.62 ± 0.08 | 1.63 ± 0.07 |
| qAV (mg g−1) | 10.33 ± 0.73 | 20.45 ± 0.87 | 44.53 ± 1.73 | 89.04 ± 3.56 |
| nAV | 1.12 | 1.12 | 1.11 | 1.12 |
| R2 | 0.9916 | 0.9912 | 0.9914 | 0.9915 |
| χ2 | 0.07 | 0.17 | 0.34 | 0.68 |
| SSE | 0.78 | 3.24 | 14.88 | 58.93 |
| ARE (%) | 13.38 | 14.58 | 13.41 | 13.39 |
| General-order | kn (min−1 (g mg−1) n−1) | 0.0005 ± 0.0001 | 0.0005 ± 0.0001 | 0.0006 ± 0.0001 | 0.0004 ± 0.0001 |
| qn (mg g−1) | 10.70 ± 0.81 | 21.11 ± 0.78 | 44.21 ± 1.64 | 91.65 ± 4.15 |
| n | 4.42 | 3.85 | 3.51 | 3.17 |
| R2 | 0.9998 | 0.9996 | 0.9996 | 0.9997 |
| χ2 | 0.001 | 0.006 | 0.008 | 0.02 |
| SSE | 0.01 | 0.13 | 0.15 | 2.06 |
| ARE (%) | 0.30 | 0.42 | 0.63 | 0.43 |
Table 5.
The thermodynamic parameters for the dye adsorption on the adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves.
Table 5.
The thermodynamic parameters for the dye adsorption on the adsorbent obtained from hart’s-tongue fern (Asplenium scolopendrium) leaves.
| ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) |
|---|
| 285 K | 295 K | 319 K |
|---|
| −21.75 | −22.65 | −24.78 | 0.44 | 10.74 |
Table 6.
Controllable factors and their levels used to realize the L27 orthogonal array experimental design.
Table 6.
Controllable factors and their levels used to realize the L27 orthogonal array experimental design.
| Factor | Level 1 | Level 2 | Level 3 |
|---|
| pH | 2 | 6 | 12 |
| Time (min) | 1 | 20 | 60 |
| Adsorbent dose (mg L−1) | 1 | 3 | 6 |
| Initial dye concentration (mg L−1) | 25 | 100 | 200 |
| Temperature (K) | 285 | 295 | 319 |
| Ionic strength (mol L−1) | 0 | 0.10 | 0.25 |
Table 7.
The L27 orthogonal array experimental design, the experimental value obtained for dye removal efficiency, and corresponding S/N ratios after each run.
Table 7.
The L27 orthogonal array experimental design, the experimental value obtained for dye removal efficiency, and corresponding S/N ratios after each run.
| pH | Time | Adsorbent
Dose | Initial Dye
Concentration | Temperature | Ionic
Strength | Removal Efficiency | S/N
Ratio |
|---|
| 2 | 1 | 1 | 25 | 285 | 0.00 | 47.63 | 33.55 |
| 2 | 1 | 1 | 25 | 295 | 0.10 | 47.74 | 33.57 |
| 2 | 1 | 1 | 25 | 319 | 0.25 | 48.12 | 33.64 |
| 2 | 20 | 3 | 100 | 285 | 0.00 | 63.48 | 36.05 |
| 2 | 20 | 3 | 100 | 295 | 0.10 | 63.62 | 36.07 |
| 2 | 20 | 3 | 100 | 319 | 0.25 | 64.12 | 36.13 |
| 2 | 60 | 6 | 200 | 285 | 0.00 | 65.71 | 36.35 |
| 2 | 60 | 6 | 200 | 295 | 0.10 | 65.85 | 36.37 |
| 2 | 60 | 6 | 200 | 319 | 0.25 | 66.38 | 36.44 |
| 6 | 1 | 3 | 200 | 285 | 0.10 | 74.12 | 37.39 |
| 6 | 1 | 3 | 200 | 295 | 0.25 | 74.06 | 37.39 |
| 6 | 1 | 3 | 200 | 319 | 0.00 | 77.31 | 37.76 |
| 6 | 20 | 6 | 25 | 285 | 0.10 | 83.22 | 38.40 |
| 6 | 20 | 6 | 25 | 295 | 0.25 | 83.15 | 38.39 |
| 6 | 20 | 6 | 25 | 319 | 0.00 | 86.80 | 38.77 |
| 6 | 60 | 1 | 100 | 285 | 0.10 | 83.68 | 38.45 |
| 6 | 60 | 1 | 100 | 295 | 0.25 | 83.61 | 38.44 |
| 6 | 60 | 1 | 100 | 319 | 0.00 | 87.28 | 38.81 |
| 12 | 1 | 6 | 100 | 285 | 0.25 | 77.74 | 37.81 |
| 12 | 1 | 6 | 100 | 295 | 0.00 | 80.44 | 38.10 |
| 12 | 1 | 6 | 100 | 319 | 0.10 | 81.33 | 38.20 |
| 12 | 20 | 1 | 200 | 285 | 0.25 | 88.45 | 38.93 |
| 12 | 20 | 1 | 200 | 295 | 0.00 | 91.53 | 39.23 |
| 12 | 20 | 1 | 200 | 319 | 0.10 | 92.54 | 39.32 |
| 12 | 60 | 3 | 25 | 285 | 0.25 | 87.47 | 38.83 |
| 12 | 60 | 3 | 25 | 295 | 0.00 | 90.51 | 39.13 |
| 12 | 60 | 3 | 25 | 319 | 0.10 | 91.51 | 39.22 |
Table 8.
Response table for signal-to-noise S/N ratios (larger is better) and the ANOVA results.
Table 8.
Response table for signal-to-noise S/N ratios (larger is better) and the ANOVA results.
| Level | pH | Time | Adsorbent
Dose | Initial Dye Concentration | Temperature | Ionic
Strength |
|---|
| 1 | 35.36 | 36.38 | 37.11 | 37.06 | 37.31 | 37.53 |
| 2 | 38.20 | 37.93 | 37.56 | 37.57 | 37.41 | 37.45 |
| 3 | 38.76 | 38.01 | 37.65 | 37.69 | 37.59 | 37.34 |
| Delta | 3.40 | 1.62 | 0.54 | 0.63 | 0.28 | 0.19 |
| Rank | 1 | 2 | 4 | 3 | 5 | 6 |
| Contribution (%) | 75.84 | 19.04 | 1.91 | 2.53 | 0.46 | 0.22 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).