Anticancer Drug-Loaded Chitosan Nanoparticles for In Vitro Release, Promoting Antibacterial and Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Drug-Loaded Chitosan Nanoparticles
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. X-ray Powder Diffraction (XRD)
2.5. Morphology
2.6. Size Measurements
2.7. Drug Entrapment
2.8. In Vitro Kinetic Release
2.9. Antimicrobial Activity
2.10. Cell Cytotoxicity
2.11. Live/Dead Cell Assay
2.12. Statistical Analysis
3. Results
3.1. Surface Analysis of Drug-Loaded CSNPs
3.2. Morphology of Drug-Loaded CSNPs
3.3. Size Measurements of Drug-Loaded CSNPs
3.4. Encapsulation Efficiency
3.5. In Vitro Drug Release from CSNPs
3.6. Antimicrobial Activity of Drug-Loaded CSNPs
3.7. Anticancer Properties
3.7.1. Cell Cytotoxicity of Drug-Loaded CSNPs
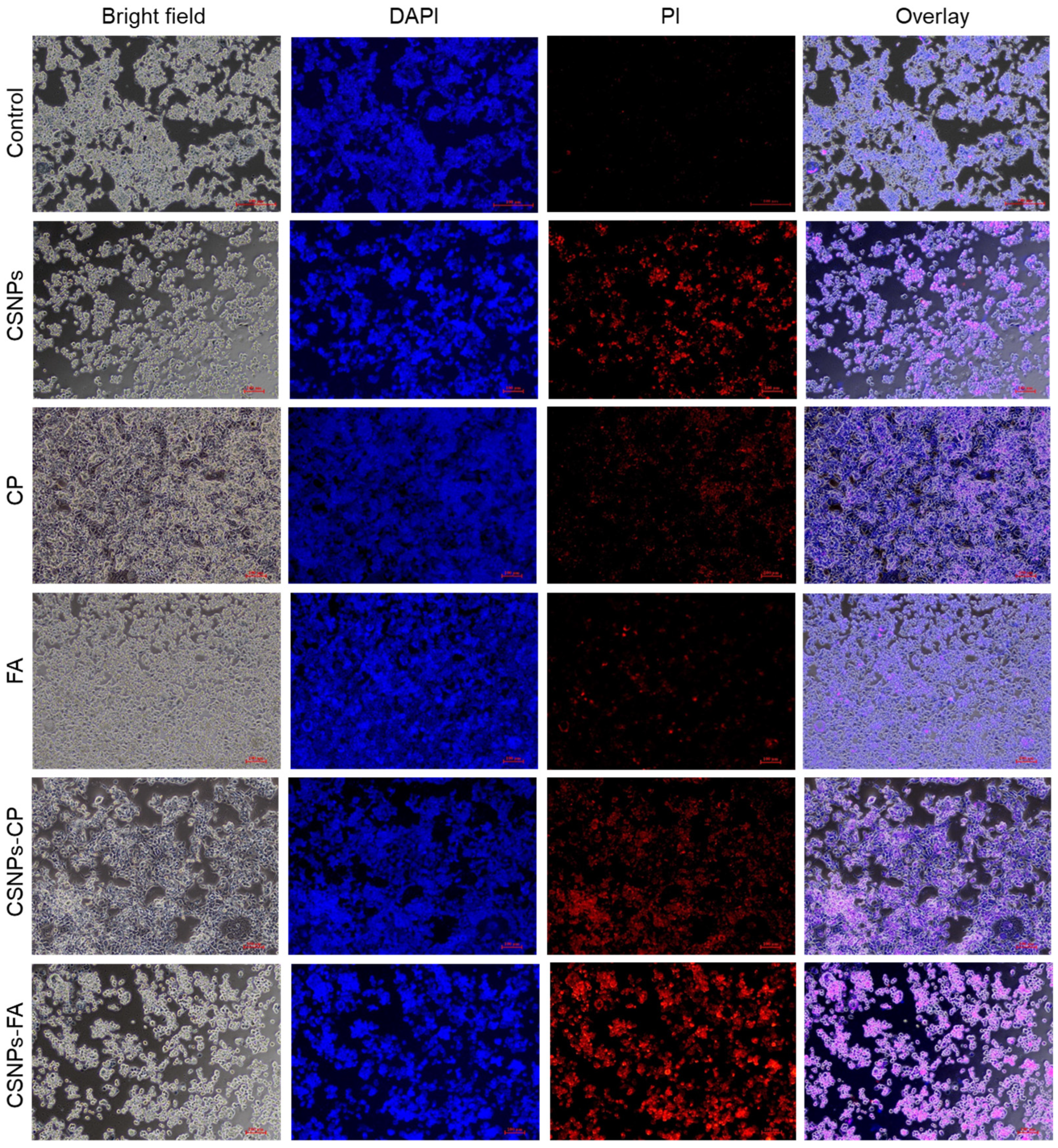
3.7.2. Live/Dead Cell Assay of Drug-Loaded CSNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, C.; Neid, M. Cancer Invasion and Metastasis. Oncology 2005, 69, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 205031212110343. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Shepelytskyi, Y.; Fox, M.S.; Davenport, K.; Li, T.; Albert, M.S.; Davenport, E. In-Vivo Retention of 5-Fluorouracil Using 19F Magnetic Resonance Chemical Shift Imaging in Colorectal Cancer in a Murine Model. Sci. Rep. 2019, 9, 13244. [Google Scholar] [CrossRef]
- Kemp, J.A.; Kwon, Y.J. Cancer Nanotechnology: Current Status and Perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, A.W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Boros, B.V.; Dascalu, D.; Ostafe, V.; Isvoran, A. Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna Minor. Molecules 2022, 27, 6123. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.U.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Stability of Chitosan Nanoparticles Cross-Linked with Tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B.; Mohamed, H.I.; Al-Subaie, A.M.; Al-Ohali, A.I.; Mahmoud, N.M.R. Investigation of the Antimicrobial Activity and Hematological Pattern of Nano-Chitosan and Its Nano-Copper Composite. Sci. Rep. 2021, 11, 9450. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A.; et al. Characterization of Cisplatin-Loaded Chitosan Nanoparticles and Rituximab-Linked Surfaces as Target-Specific Injectable Nano-Formulations for Combating Cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef]
- Dongsar, T.T.; Dongsar, T.S.; Gupta, N.; Almalki, W.H.; Sahebkar, A.; Kesharwani, P. Emerging Potential of 5-Fluorouracil-Loaded Chitosan Nanoparticles in Cancer Therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104371. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-Fluorouracil-Loaded Chitosan Nanoparticles and Study of the Sustained Release in Vitro and in Vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816. [Google Scholar] [CrossRef]
- Maliyakkal, N.; Appadath Beeran, A.; Udupa, N. Nanoparticles of Cisplatin Augment Drug Accumulations and Inhibit Multidrug Resistance Transporters in Human Glioblastoma Cells. Saudi Pharm. J. 2021, 29, 857–873. [Google Scholar] [CrossRef]
- Christensen, S.; Van der Roest, B.; Besselink, N.; Janssen, R.; Boymans, S.; Martens, J.W.M.; Yaspo, M.L.; Priestley, P.; Kuijk, E.; Cuppen, E.; et al. 5-Fluorouracil Treatment Induces Characteristic T>G Mutations in Human Cancer. Nat. Commun. 2019, 10, 4571. [Google Scholar] [CrossRef]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef] [PubMed]
- Bangun, H.; Tandiono, S.; Arianto, A. Preparation and Evaluation of Chitosan-Tripolyphosphate Nanoparticles Suspension as an Antibacterial Agent. J. Appl. Pharm. Sci. 2018, 8, 147–156. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Fan, J.; Guo, H.; Gou, L.; Yang, H.; Liang, C. Preparation Nanoparticle by Ionic Cross-Linked Emulsified Chitosan and Its Antibacterial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 362–370. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Y.; Meng, X. Chitosan Nanolayered Cisplatin-Loaded Lipid Nanoparticles for Enhanced Anticancer Efficacy in Cervical Cancer. Nanoscale Res. Lett. 2016, 11, 524. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor Efficacy of Cisplatin-Loaded Glycol Chitosan Nanoparticles in Tumor-Bearing Mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Othayoth, R.; Mathi, P.; Bheemanapally, K.; Kakarla, L.; Botlagunta, M. Characterization of Vitamin-Cisplatin-Loaded Chitosan Nano-Particles for Chemoprevention and Cancer Fatigue. J. Microencapsul. 2015, 32, 578–588. [Google Scholar] [CrossRef]
- Reddy, A.B.; Manjula, B.; Jayaramudu, T.; Sadiku, E.R.; Anand Babu, P.; Periyar Selvam, S. 5-Fluorouracil Loaded Chitosan–PVA/Na+MMT Nanocomposite Films for Drug Release and Antimicrobial Activity. Nano-Micro Lett. 2016, 8, 260–269. [Google Scholar] [CrossRef]
- Tiǧli Aydin, R.S.; Pulat, M. 5-Fluorouracil Encapsulated Chitosan Nanoparticles for PH-Stimulated Drug Delivery: Evaluation of Controlled Release Kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, Characterization, and Antibacterial Activity of Diclofenac-Loaded Chitosan Nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Tomaz, A.F.; de Carvalho, S.M.S.; Barbosa, R.C.; Silva, S.M.L.; Gutierrez, M.A.S.; de Lima, A.G.B.; Fook, M.V.L. Ionically Crosslinked Chitosan Membranes Used as Drug Carriers for Cancer Therapy Application. Materials 2018, 11, 2051. [Google Scholar] [CrossRef] [PubMed]
- Vhora, I.; Khatri, N.; Desai, J.; Thakkar, H.P. Caprylate-Conjugated Cisplatin for the Development of Novel Liposomal Formulation. AAPS PharmSciTech 2014, 15, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Olukman, M.; Şanlı, O.; Solak, E.K. Release of Anticancer Drug 5-Fluorouracil from Different Ionically Crosslinked Alginate Beads. J. Biomater. Nanobiotechnol. 2012, 3, 469–479. [Google Scholar] [CrossRef]
- Tsong, Y.; Hammerstrom, T.; Chen, J.J. Multipoint Dissolution Specification and Acceptance Sampling Rule Based on Profile Modeling and Principal Component Analysis. J. Biopharm. Stat. 1997, 7, 423–439. [Google Scholar] [CrossRef]
- Beňová, E.; Hornebecq, V.; Zeleňák, V.; Huntošová, V.; Almáši, M.; Máčajová, M.; Bergé-Lefranc, D. PH-Responsive Mesoporous Silica Drug Delivery System, Its Biocompatibility and Co-Adsorption/Co-Release of 5-Fluorouracil and Naproxen. Appl. Surf. Sci. 2021, 561, 150011. [Google Scholar] [CrossRef]
- Sethi, A.; Ahmad, M.; Huma, T.; Khalid, I.; Ahmad, I. Evaluation of Low Molecular Weight Cross Linked Chitosan Nanoparticles, to Enhance the Bioavailability of 5-Flourouracil. Dose-Response 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of Hybrid Copper-Chitosan Nanoparticles with Antibacterial Activity against Cariogenic Streptococcus Mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Z.; Mo, Z.; Zou, B.; Yang, Y.; Sun, R.; Ma, W.; Yu, M.; Zhang, S.; Yu, Z. Synergetic Delivery of Triptolide and Ce6 with Light-Activatable Liposomes for Efficient Hepatocellular Carcinoma Therapy. Acta Pharm. Sin. B 2021, 11, 2004–2015. [Google Scholar] [CrossRef]
- Ogutüzün, S.; Tandogan, N.; Turk, M.; Karahan, S.; Suludere, Z. The Apoptotic and Necrotic Effects of Cisplatin Loaded Chitosan Nanoparticles on Hela Cell Lines. Acad. J. Cancer Res. 2015, 8, 58–68. [Google Scholar] [CrossRef]
- Anand Raj, L.F.A.; Jonisha, R.; Revathi, B.; Jayalakshmy, E. Preparation and Characterization of BSA and Chitosan Nanopartices for Sustainable Delivery System for Quercetin. J. Appl. Pharm. Sci. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Alishahi, A. Antibacterial Effect of Chitosan Nanoparticle Loaded with Nisin for the Prolonged Effect. J. Food Saf. 2014, 34, 111–118. [Google Scholar] [CrossRef]
- Porras-Gómez, M.; Vega-Baudrit, J.; Núñez-Corrales, S. Ampicillin-Loaded Chitosan Nanoparticles for In Vitro Antimicrobial Screening on Escherichia Coli. In Chitin-Chitosan—Myriad Functionalities in Science and Technology; Intechopen: London, UK, 2018. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric Nanoparticles Containing Diazepam: Preparation, Optimization, Characterization, in-Vitro Drug Release and Release Kinetic Study. Nano Converg. 2016, 3, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Arias-Alpizar, G.; Kong, L.; Vlieg, R.C.; Rabe, A.; Papadopoulou, P.; Meijer, M.S.; Bonnet, S.; Vogel, S.; van Noort, J.; Kros, A.; et al. Light-Triggered Switching of Liposome Surface Charge Directs Delivery of Membrane Impermeable Payloads in Vivo. Nat. Commun. 2020, 11, 3638. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin Loaded Chitosan Nanoparticles Augments the PH-Dependent Mitochondrial-Mediated Intrinsic Apoptosis in Human Oral Cancer Cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, A.; Majumder, S.; Lariya, N.; Khaya, A.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Mannosylated Solid Lipid Nanoparticles as Vectors for Site-Specific Delivery of an Anti-Cancer Drug. J. Control. Release 2010, 148, 359–367. [Google Scholar] [CrossRef]
- Zaman, M.; Butt, M.H.; Siddique, W.; Iqbal, M.O.; Nisar, N.; Mumtaz, A.; Nazeer, H.Y.; Alshammari, A.; Riaz, M.S. Fabrication of PEGylated Chitosan Nanoparticles Containing Tenofovir Alafenamide: Synthesis and Characterization. Molecules 2022, 27, 8401. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic Acid Loaded Chitosan Nanoparticles with Sustained Release Property, Retained Antioxidant Activity and Enhanced Bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil Loaded Chitosan/Polyacrylic Acid/Fe3O4 Magnetic Nanocomposite Hydrogel as a Potential Anticancer Drug Delivery System. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Singh, V.; Brecik, M.; Mukherjee, R.; Evans, J.C.; Svetlíková, Z.; Blaško, J.; Surade, S.; Blackburn, J.; Warner, D.F.; Mikušová, K.; et al. The Complex Mechanism of Antimycobacterial Action of 5-Fluorouracil. Chem. Biol. 2015, 22, 63–75. [Google Scholar] [CrossRef]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous Core Epigallocatechin Gallate PLGA Nanocapsules: Characterization, Antibacterial Activity against Uropathogens, and in Vivo Reno-Protective Effect in Cisplatin Induced Nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Manikandakrishnan, M.; Anjali, R.; Rajasekar, P.; Marudhupandi, T.; Manikandan, R.; Vaseeharan, B.; Prabhu, N.M. Investigation of Antioxidant and Anticancer Potential of Fucoidan from Sargassum Polycystum. Int. J. Biol. Macromol. 2018, 116, 151–161. [Google Scholar] [CrossRef] [PubMed]
- England, K.; O’Driscoll, C.; Cotter, T.G. Carbonylation of Glycolytic Proteins Is a Key Response to Drug-Induced Oxidative Stress and Apoptosis. Cell Death Differ. 2004, 11, 252–260. [Google Scholar] [CrossRef] [PubMed]

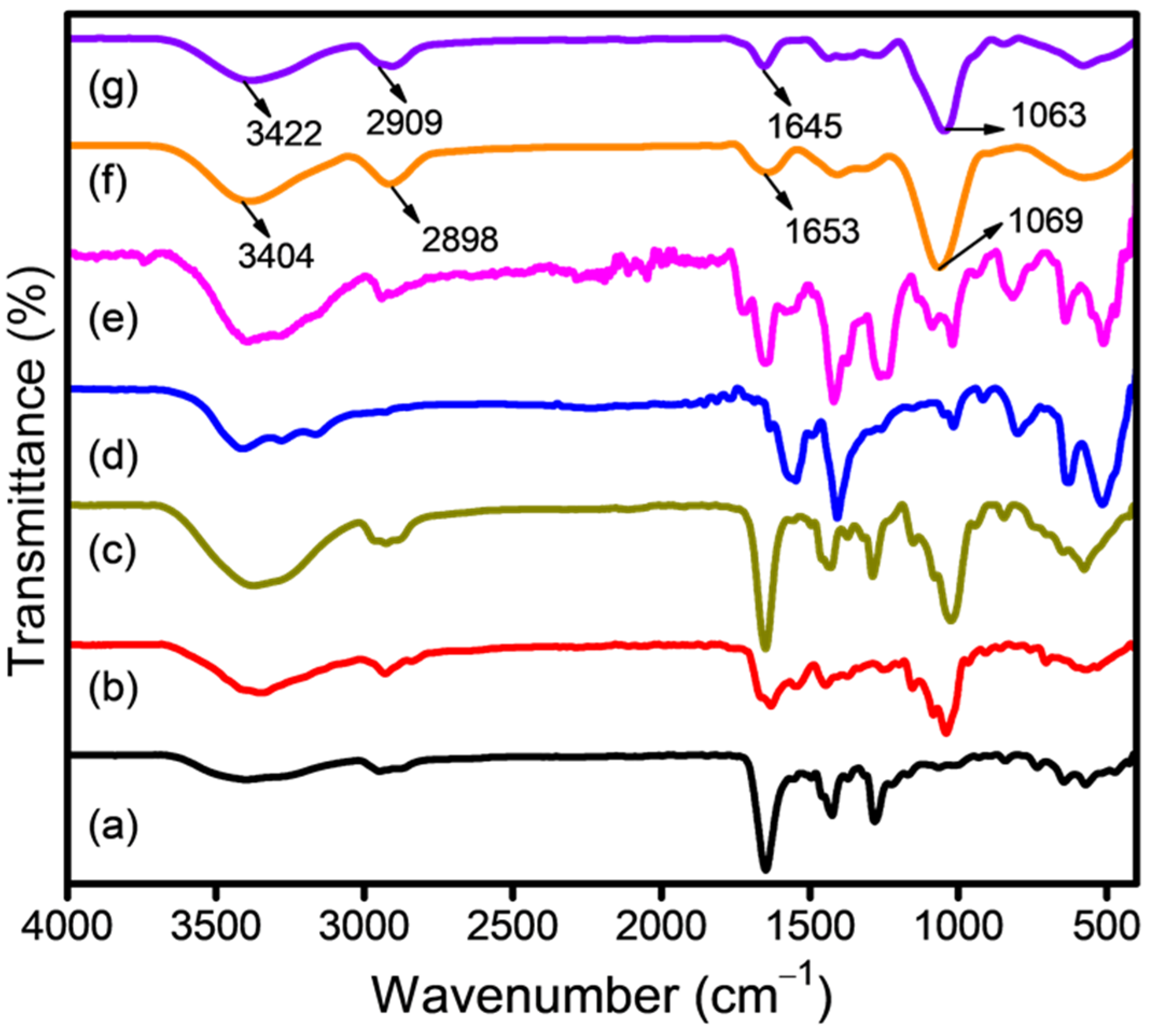



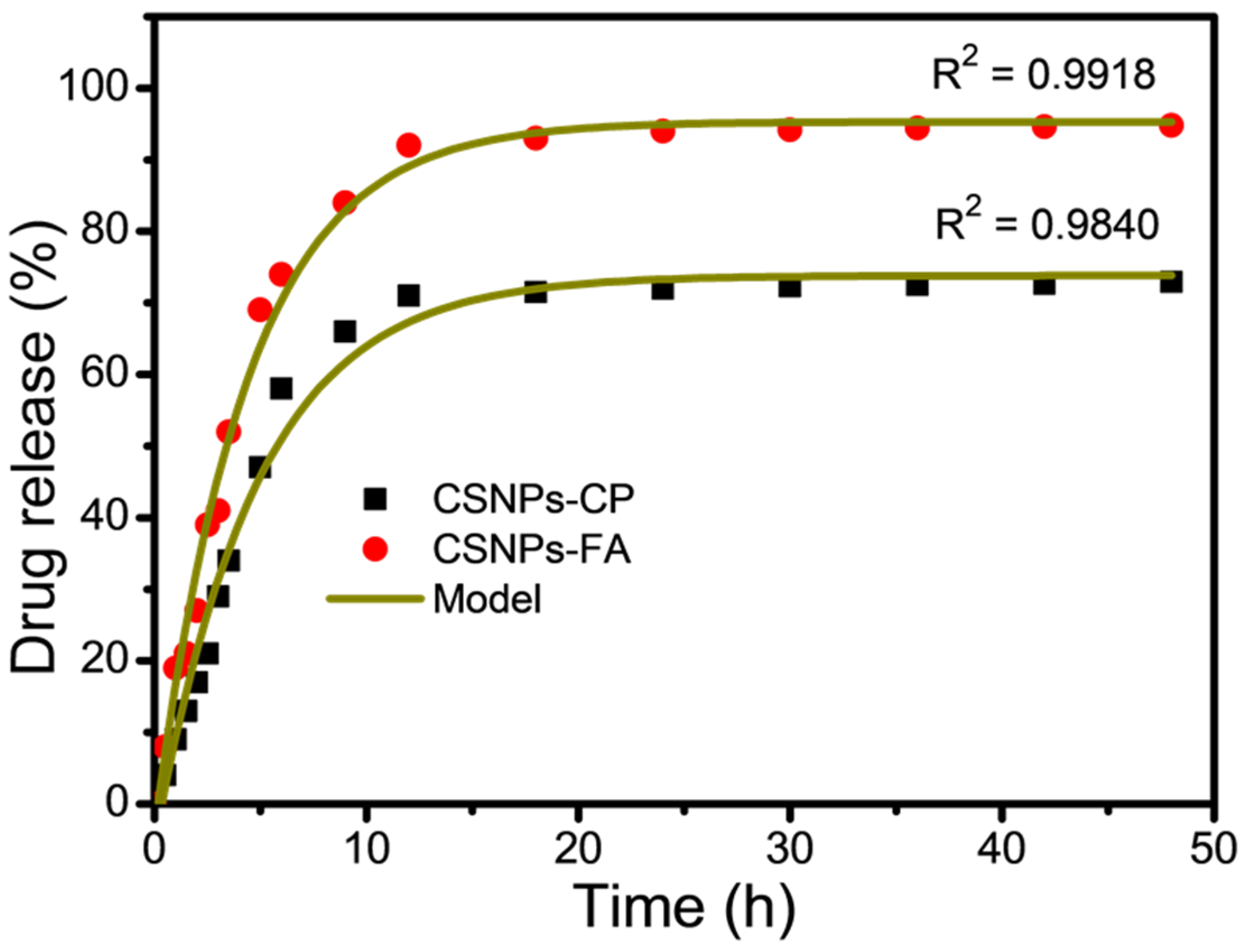
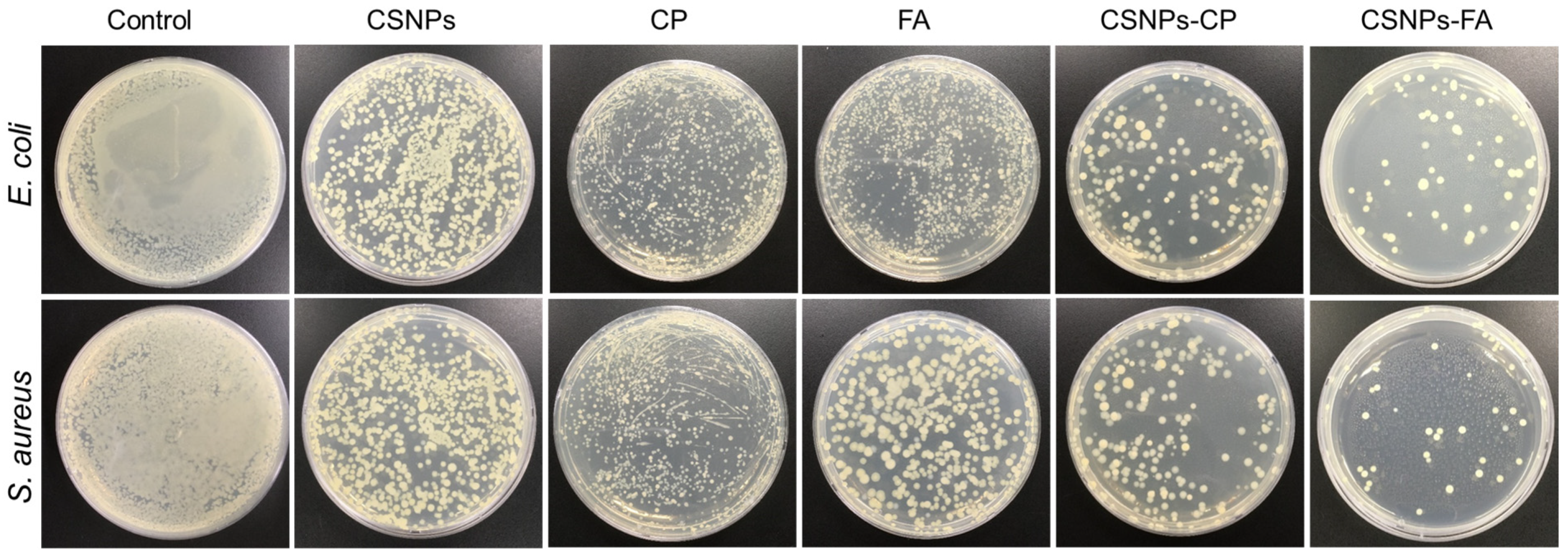
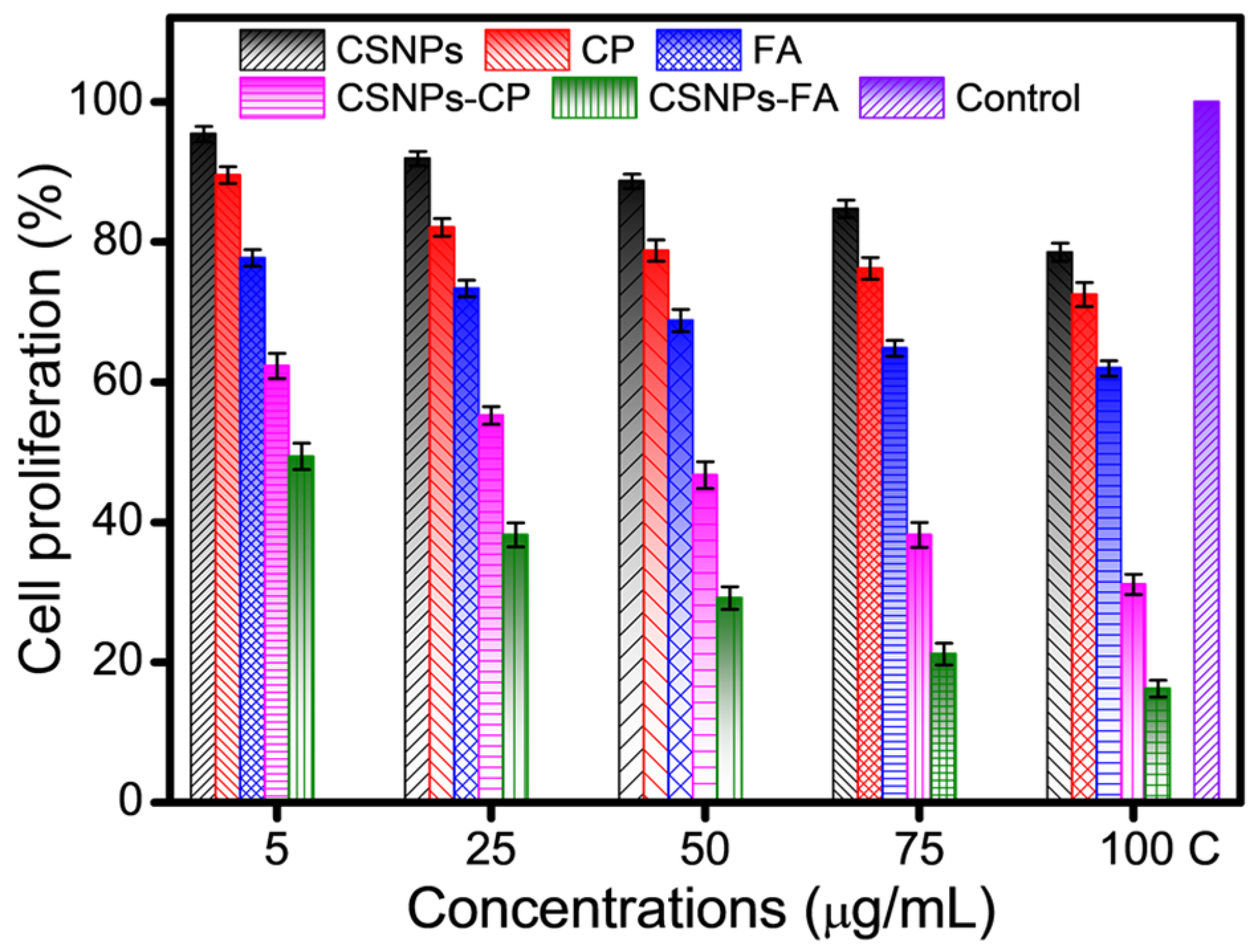
| Parameters | CP | FA |
|---|---|---|
| Fmax | 72.9 | 94.8 |
| k | 0.0798 | 0.1038 |
| R2 | 0.9840 | 0.9918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Khan, M.R.; Palanisamy, S.; Mohandoss, S. Anticancer Drug-Loaded Chitosan Nanoparticles for In Vitro Release, Promoting Antibacterial and Anticancer Activities. Polymers 2023, 15, 3925. https://doi.org/10.3390/polym15193925
Ahmad N, Khan MR, Palanisamy S, Mohandoss S. Anticancer Drug-Loaded Chitosan Nanoparticles for In Vitro Release, Promoting Antibacterial and Anticancer Activities. Polymers. 2023; 15(19):3925. https://doi.org/10.3390/polym15193925
Chicago/Turabian StyleAhmad, Naushad, Mohammad Rizwan Khan, Subramanian Palanisamy, and Sonaimuthu Mohandoss. 2023. "Anticancer Drug-Loaded Chitosan Nanoparticles for In Vitro Release, Promoting Antibacterial and Anticancer Activities" Polymers 15, no. 19: 3925. https://doi.org/10.3390/polym15193925
APA StyleAhmad, N., Khan, M. R., Palanisamy, S., & Mohandoss, S. (2023). Anticancer Drug-Loaded Chitosan Nanoparticles for In Vitro Release, Promoting Antibacterial and Anticancer Activities. Polymers, 15(19), 3925. https://doi.org/10.3390/polym15193925








