Chemorheology of a Si/Al > 3 Alkali Activated Metakaolin Paste through Parallel Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Analysis (DMA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation Procedures
2.2. Thermoanalysis Methods and Testing Procedures
2.2.1. Differential Scanning Calorimetry
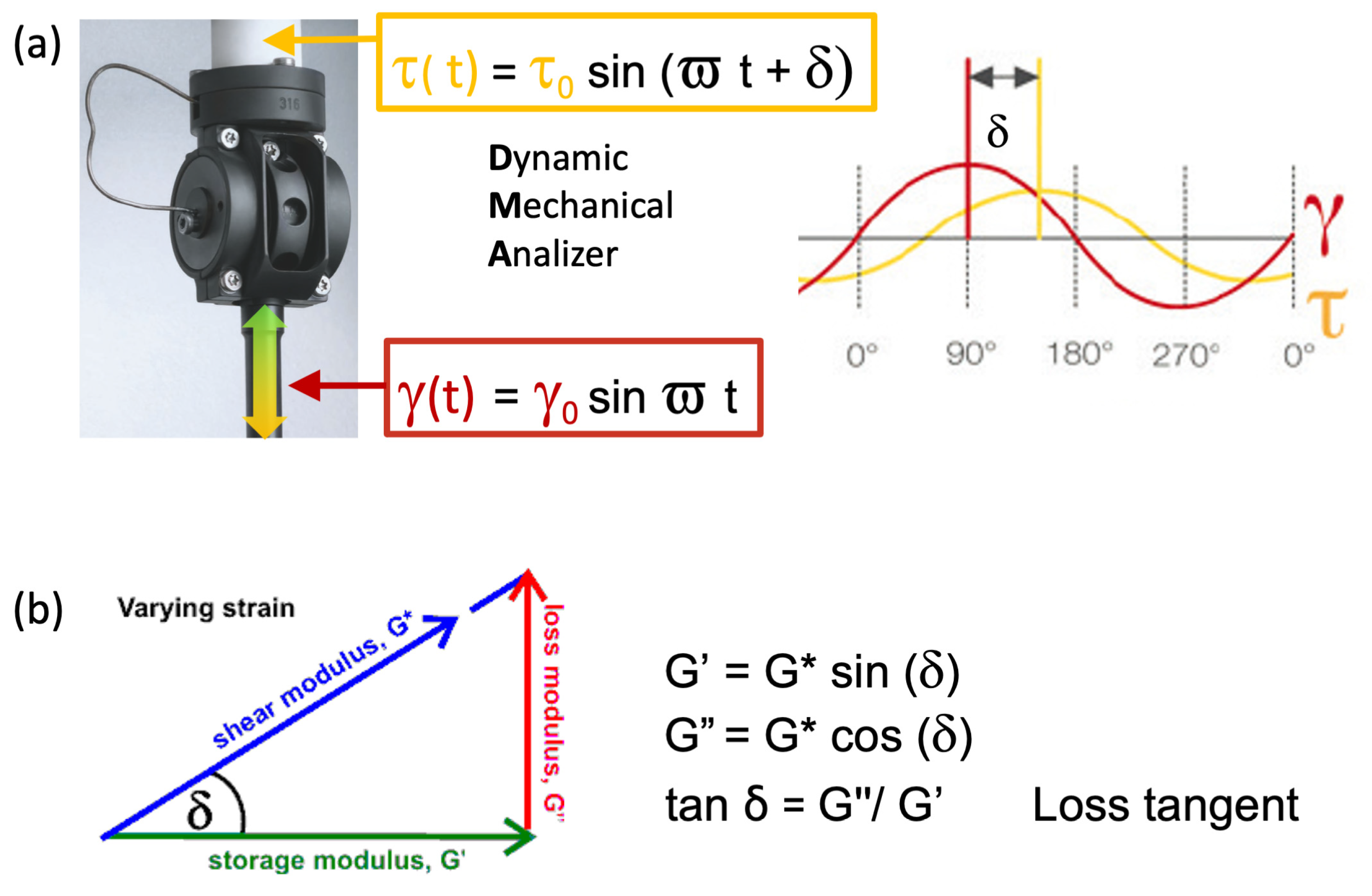
2.2.2. Dynamic Mechanical Analysis Test Procedures and Theoretical Bases
G″= G* cos(δ) Loss modulus
Eta″ = G′/ϖ
3. Results
3.1. Parallel Differential Scanning Calorimetry and Dynamic Mechanical Analysis
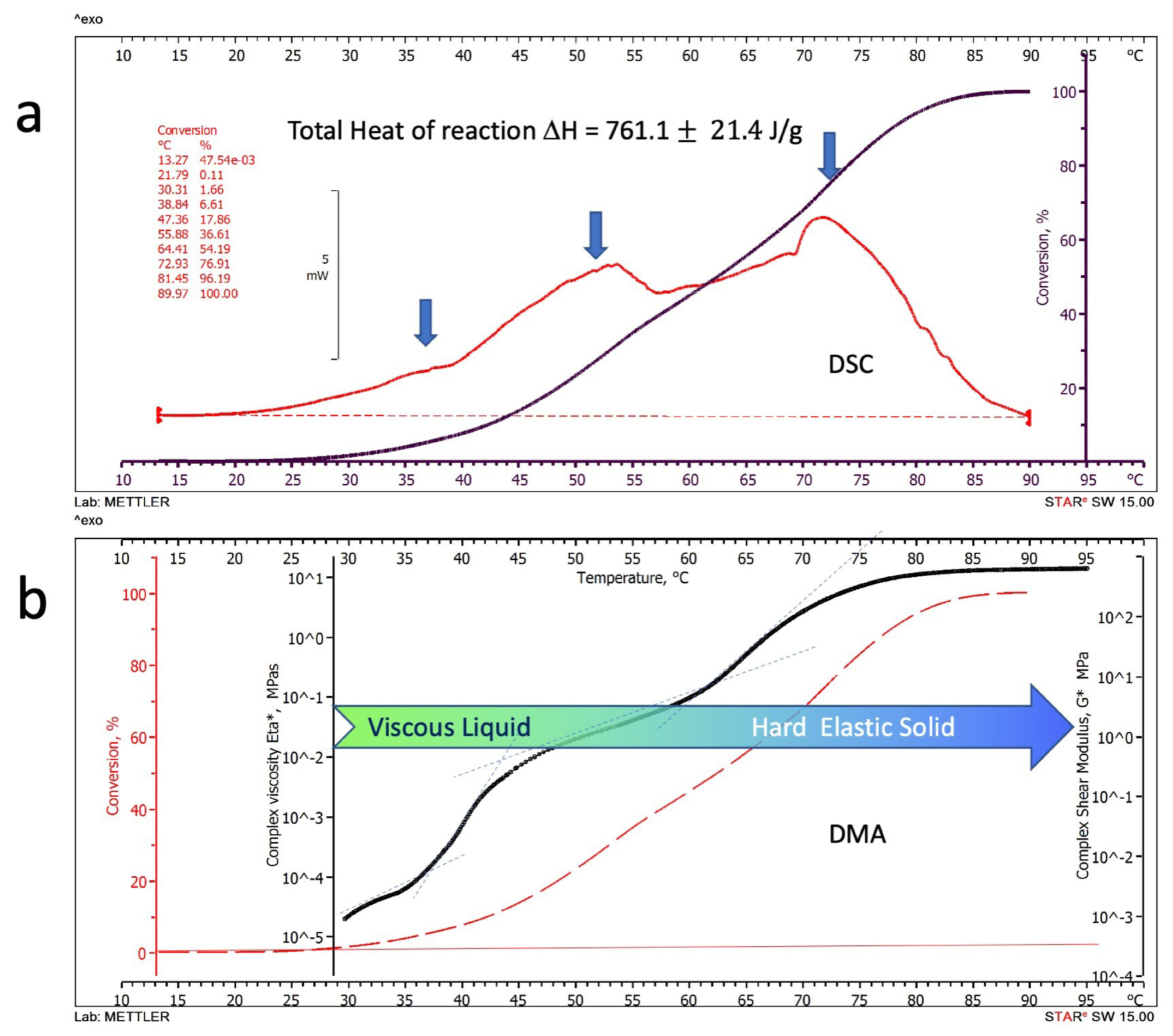
3.1.1. Differential Scanning Calorimetry
3.1.2. Dynamic Mechanical Analysis
3.2. Viscoelasticity Investigations
3.2.1. Elastic (Eta″, G′) and Viscous (Eta′, G″) Shear Behaviour during Geo-Polymerisation
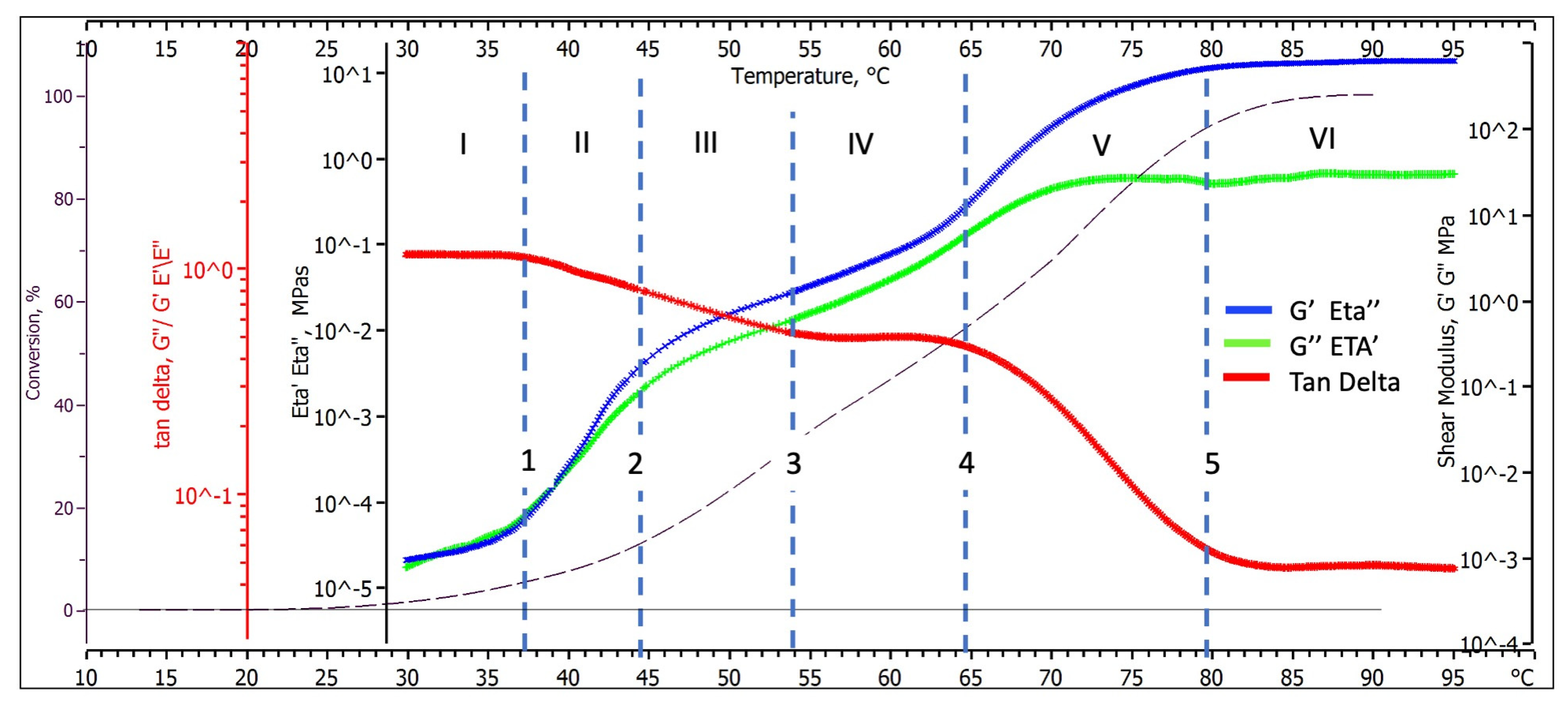
- A first thermogram inflexion occurs around 37 °C and has been represented by the dotted line 1;
- A second thermogram inflection follows around 44 °C (dotted line 2).
- A third inflection is observed around 65 °C (dotted line 4);
- Both loss and storage shear moduli finally stabilised their values at temperatures higher than 80 °C (line 5 in Figure 3).
3.2.2. Loss Tangent (Tan Delta) Behaviour during Geo-Polymerisation
- A first inflexion occurs around 37 °C in correspondence with the change noted for the shear moduli (dotted line 1), where the loss tangent starts to decrease from unity;
- A second inflexion follows around 54 °C (dotted line 3), where the thermogram flattens at a value of about 0.5.
- A third inflexion, in correspondence to that of the shear moduli thermogram, occurs around 65 °C (dotted line 4), where the loss tangent starts again to decrease from its steady value of 0.5.
- The last transition occurs at 80 °C (dotted line 5), where the loss tangent thermogram stabilises to its final value of 0.04.
3.2.3. Chemorheology and Viscoelastic Behavioural Zones
- Zone I—The paste behaves as a viscoelastic liquid (the loss tangent is near unity) that smoothly increases its viscosity as the polycondensation reactions proceed up to a conversion of about 3.9%.
- Zone II—The paste still behaves as a viscoelastic liquid with an increased elastic character (the loss tangent is 0.75). The mechanical properties rapidly increase as chemical conversion rises from 3.9 to 12.8.
- Zone III—The paste behaves as a highly elastic fluid (loss tangent decreases from 0.75 to 0.51). The rate at which the mechanical properties increase as chemical conversion proceeds from 12.4 to 32.5% is slower than for zone II;
- Zone IV—The geopolymer reaches a constant loss tangent of 0.51 while smoothly increasing loss and storage mechanical properties. According to these values, the paste behaves as a solid viscoelastic rubber;
- Zone V—The mechanical property storage component increases at a significantly higher rate than the loss one. Accordingly, the loss tangent rapidly decreases from 0.5 to 0.04, assuming the values characteristic of a rigid glassy polymer when conversions rise from 54.7 to 91.3%;
- Zone VI—The loss tangent (0.04) and modulus components (Table 2) in this zone are characteristic of an essentially elastic solid and remain almost constant up to the test end.
4. Discussion
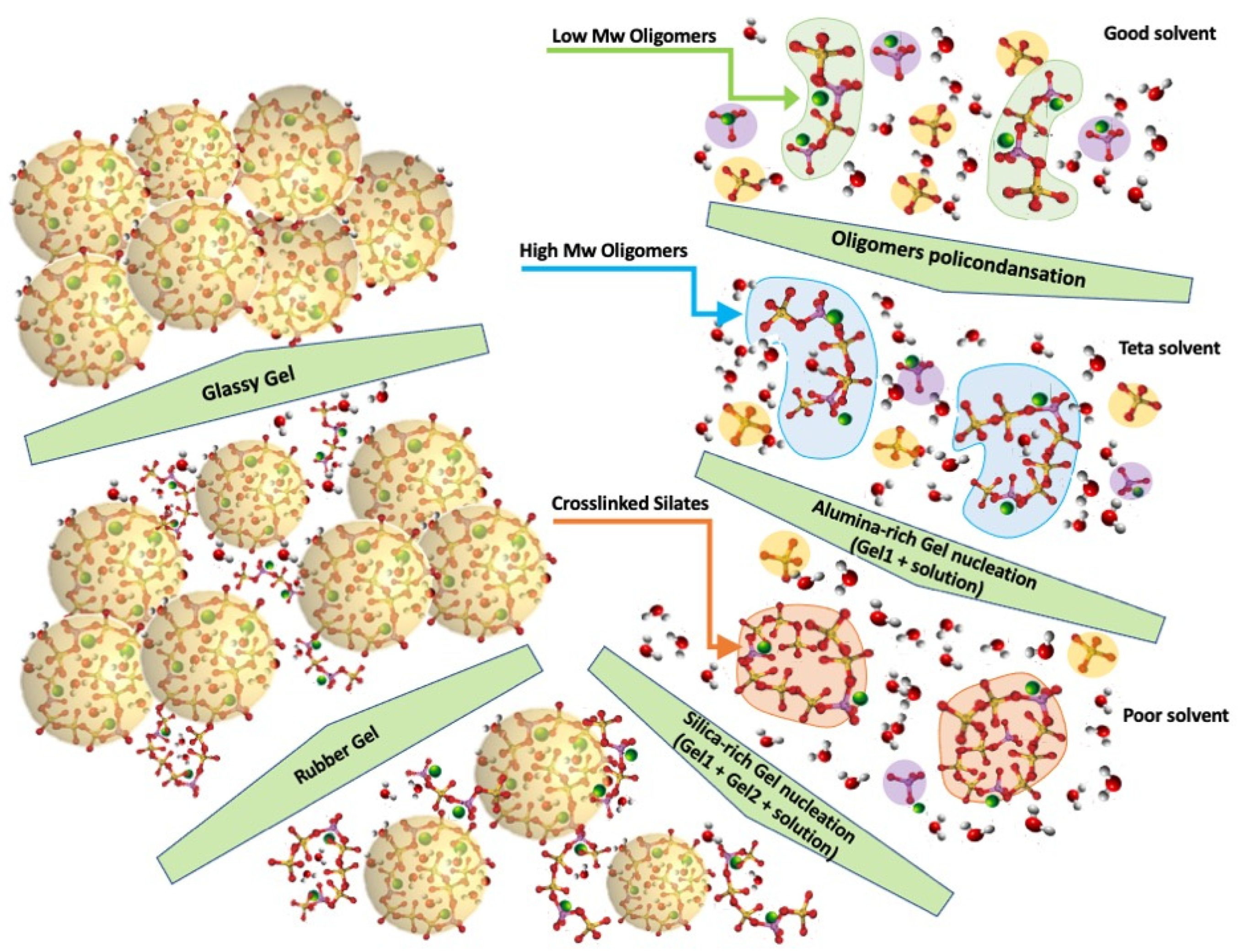
- Silate dimers with a Si/Al = 1
- Silate siloxo trimers with Si/Al = 2
- Silate di-siloxo tetramers with Si/Al = 3
- Branched higher oligomers with Si/Al > 3
- Zone I: Kaolin deconstruction and silico-aluminate oligomers formation—viscoelastic liquid;
- Zone II: Nucleation of Alumina-rich gel particles—a viscoelastic liquid solution containing Alumina-rich gel particles (Gel1);
- Zone III: Nucleation of Silica-rich gel particles—a viscoelastic liquid solution containing Alumina-rich (Gel1) and silica-rich (Gel2) gel particles;
- Zone IV: Silico-aluminate rubber gel—amorphous viscoelastic rubber;
- Zone V: Silico-aluminate glassy gel (still reactive);
- Zone VI: Silico-aluminate glass geopolymer (fully polymerised)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Mikulčić, H.; Klemeš, J.J.; Vujanović, M.; Urbaniec, K.; Duić, N. Reducing greenhouse gasses emissions by fostering the deployment of alternative raw materials and energy sources in the cleaner cement manufacturing process. J. Clean. Prod. 2016, 136, 119–132. [Google Scholar] [CrossRef]
- The European Cement Industry Association (CEMBUERAU). The Role of Cement in the 2050 Low Carbon Economy—Full Report; CEMBUERAU: Brussels, Belgium, 2014. [Google Scholar]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Al-Qutaifi, S.; Nazari, A.; Bagheri, A. Mechanical properties of layered geopolymer structures applicable in concrete 3D-printing. Constr. Build. Mater. 2018, 176, 690–699. [Google Scholar] [CrossRef]
- Singh, N.B. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, M. 3D printing geopolymers: A review. Cem. Concr. Compos. 2022, 128, 104455. [Google Scholar] [CrossRef]
- Provis, J.L.; Duxson, P.; Van Deventer, J.S.J.; Lukey, G.C. The Role of Mathematical Modelling and Gel Chemistry in Advancing Geopolymer Technology. Chem. Eng. Res. Des. 2005, 83, 853–860. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Łach, M.; Gądek, S.; Lin, W.T.; Tran, D.H.; Korniejenko, K. A brief overview of the use of additive manufacturing of con-create materials in construction. Acta Innov. 2023, 48, 22–37. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, L.; Liu, C. Mechanical anisotropy evolution of 3D-printed alkali-activated materials with different GGBFS/FA combinations. J. Build. Eng. 2022, 501, 104126. [Google Scholar] [CrossRef]
- Khan, S.A.; İlcan, H.; Aminipour, E. Buildability analysis on effect of structural design in 3D concrete printing (3DCP): An experimental and numerical study. Case Stud. Constr. Mater. 2023, 19, e02295. [Google Scholar] [CrossRef]
- Marczyk, J.; Ziejewska, C.; Łach, M. Possibilities of using the 3D printing process in the concrete and geopolymers application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012019. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; Fernández-González, A.; Fernández-García, D.A.; Mejía de Gutiérrez, R. Valorization of a low-quality coal ash, in the preparation of alkali activated inks for applications in 3D additive manufacturing. Constr. Build. Mater. 2023, 399, 132598. [Google Scholar] [CrossRef]
- Duan, Z.; Deng, Q.; Liang, C.; Ma, Z.; Wu, H. Upcycling of recycled plastic fiber for sustainable cementitious composites: A critical review and new perspective. Cem. Concr. Compos. 2023, 142, 105192. [Google Scholar] [CrossRef]
- Dilawar Riaz, R.; Usman, M.; Ali, A.; Majid, U.; Faizan, M.; Jalil Malik, U. Inclusive characterization of 3D printed concrete (3DPC) in additive manufacturing: A detailed review. Constr. Build. Mater. 2023, 394, 132229. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.V.; Petrescu, F.I.T.; Apicella, A. Biomimetic and evolutionary design driven innovation in sustainable products development. Am. J. Eng. Appl. Sci. 2016, 9, 1027–1036. [Google Scholar] [CrossRef]
- Buchwald, A.; Zellmann, H.; Kaps, C. Condensation of aluminosilicate gels—Model system for geopolymer binders. J. Non-Cryst. Solids 2011, 357, 1376–1382. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.; Lukey, G.; Mallicoat, S.; Kriven, W.; van Deventera, J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- de Jong, B.H.W.S.; Brown, G.E. Polymerization of silicate and aluminate tetrahedra in glasses, melts, and aqueous solutions: Electronic structure of H6Si2O7, H6AlSiO7, and H6Al2O7. Geochim. Cosmochim. Acta 1980, 44, 491–511. [Google Scholar] [CrossRef]
- Koleżyński, A.; Król, M.; Żychowicz, M. The structure of geopolymers. Theoretical studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Walkley, B.; Rees, G.J.; San Nicolas, R.; van Deventer, J.S.; Hanna, J.V.; Provis, J.L. New structural model of sodium aluminosilicate gels and the role of charge balancing extra-framework. Al. J. Phys. Chem. C 2018, 122, 5673–5685. [Google Scholar] [CrossRef]
- Apicella, A.; Nicolais, L.; Halpin, J.C. Role of the processing chemo-rheology on the ageing behaviour of high performance epoxy matrices. In Proceedings of the 28th National SAMPE Symposium and Exhibition, Anheim, CA, USA, 12–14 April 1983; pp. 518–527. [Google Scholar]
- Halpin, J.C.; Apicella, A.; Nicolais, L. Processing of Thermosets. In Polymer Processing and Properties; Astarita, G., Nicolais, L., Eds.; Springer: Boston, MA, USA, 1984. [Google Scholar] [CrossRef]
- Apicella, A.; Nicolais, L.; Iannone, M.; Passerini, P. Thermokinetics and chemorheology of the cure reactions of the tetraglycidyl diamino diphenyl methane–diamino diphenyl sulfone epoxy systems. J. Appl. Polym. Sci. 1984, 29, 2083–2096. [Google Scholar] [CrossRef]
- Apicella, A. Effect of Chemorheology on Epoxy Resin Properties. In Developments in Reinforced Plastics—5; Pritchard, G., Ed.; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar] [CrossRef]
- Nicolais, L.; Apicella, A. Processing of composite structures. Pure Appl. Chem. 1985, 57, 1701–1706. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.V.; Apicella, A.; Petrescu, F.I.T. One can slow down the aging through antioxidants. Am. J. Eng. Appl. Sci. 2016, 9, 1112–1126. [Google Scholar] [CrossRef]
- ASTM C-618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and Characterization of Novel Epoxy Geopolymer Hybrid Composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef] [PubMed]
- Aversa, R.; Petrescu, R.V.V.; Petrescu, F.I.T.; Apicella, A. Smart-factory: Optimization and process control of composite centrifuged pipes. Am. J. Appl. Sci. 2016, 13, 1330–1341. [Google Scholar] [CrossRef]
- Annunziata, M.; Aversa, R.; Apicella, A.; Annunziata, A.; Apicella, D.; Buonaiuto, C.; Guida, L. In vitro biological response to a light-cured composite when used for cementation of composite inlays. Dent. Mater. 2006, 22, 1081–1085. [Google Scholar] [CrossRef]
- Mark, J.E. (Ed.) Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31235-4. [Google Scholar] [CrossRef]
- Rifaai, Y.; Yahia, A.; Mostafa, A.; Aggoun, S.; Kadri, E.H. Rheology of fly ash-based geopolymer: Effect of NaOH concentration. Constr. Build. Mater. 2019, 223, 583–594. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Chen, X.; Kim, E.; Suraneni, P.; Struble, L. Quantitative Correlation between the Degree of Reaction and Compressive Strength of Metakaolin-Based Geopolymers. Materials 2020, 13, 5784. [Google Scholar] [CrossRef] [PubMed]
- Kryven, I.; Duivenvoorden, J.; Hermans, J.; Iedema, P.D. Random Graph Approach to Multifunctional Molecular Networks. Macromol. Theory Simul. 2016, 25, 449–465. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Görhan, G.; Aslaner, R.; Şinik, O. The effect of curing on the properties of metakaolin and fly ash-based geopolymer paste. Compos. Part B Eng. 2016, 97, 329–335. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterization of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers I. Gelation. J. Am. Chem. Soc. 1941, 63, 3083. [Google Scholar] [CrossRef]
- Stockmayer, W.H. Theory of Molecular Size Distribution and Gel Formation in Branched Polymers II. General Cross Linking. J. Chem. Phys. 1944, 12, 125. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers II. Trifunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3091. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers III. Tetrafunctional Branching Units. J. Am. Chem. Soc. 1941, 63, 3096. [Google Scholar] [CrossRef]
- Sahini, M.; Sahimi, M. Applications of Percolation Theory; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-22153-2. [Google Scholar]
- Kryven, I. Emergence of the giant weak component in directed random graphs with arbitrary degree distributions. Phys. Rev. E 2016, 94, 012315. [Google Scholar] [CrossRef] [PubMed]
- Mierzwiński, D.; Walter, J.; Wanat, D. Possibilities of Checking Water Content in Porous Geopolymer Materials Using Impedance Spectroscopy Methods. Materials 2023, 16, 5190. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Uthayakumar, M.; Balamurugan, P.; Mierzwiński, D. The Variable Frequency Conductivity of Geopolymers during the Long Agieng Period. Materials 2021, 14, 5648. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Manzano, H.; Provis, J.L.; Bignozzi, M.C.; Masoero, E. Atomistic Simulations of Geopolymer Models: The Impact of Disorder on Structure and Mechanics. ACS Appl. Mater. Interfaces 2018, 10, 22809–22820. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M. Polymer Physics; Colby, R.H., Ed.; Oxford University Press: Oxford, UK, 2003; p. 284. ISBN 019852059X. [Google Scholar]


| Oxide | Metakaolin | Sodium Silicate |
|---|---|---|
| Al2O3 | 45.20 | - |
| SiO2 | 52.30 | 28.36 |
| K2O | 0.15 | - |
| Fe2O3 | 0.42 | - |
| Na2O | - | 8.60 |
| MgO | 0.04 | |
| H2O | - | 63.04 |
| ZONE | Temperature, °C | Loss Viscosity, MPa·s | Storage Viscosity, MPa·s | Loss Modulus, MPa | Storage Modulus MPa | Loss Tangent | % of Conversion |
|---|---|---|---|---|---|---|---|
| I | 30.0 | × 10−5 | × 10−5 | × 10−3 | × 10−3 | 0 | |
| 37.4 | × 10−5 | ×10−5 | × 10−3 | × 10−3 | |||
| II | 37.4 | - | - | - | - | - | - |
| 4 4.6 | × 10−3 | × 10−3 | × 10−1 | × 10−1 | |||
| III | 44.6 | - | - | - | - | - | - |
| 53.9 | × 10−2 | × 10−2 | × 10−1 | × 10 | |||
| IV | 53.9 | - | - | - | - | - | - |
| 64.8 | × 10−2 | × 10−1 | × 10 | ||||
| V | 64.8 | - | - | - | - | - | - |
| 79.9 | × 10−1 | × 10 | × 10 | × 102 | |||
| VI | 79.9 | - | - | - | - | - | - |
| 95.0 | × 10−1 | × 10 | × 10 | × 102 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aversa, R.; Ricciotti, L.; Perrotta, V.; Apicella, A. Chemorheology of a Si/Al > 3 Alkali Activated Metakaolin Paste through Parallel Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Analysis (DMA). Polymers 2023, 15, 3922. https://doi.org/10.3390/polym15193922
Aversa R, Ricciotti L, Perrotta V, Apicella A. Chemorheology of a Si/Al > 3 Alkali Activated Metakaolin Paste through Parallel Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Analysis (DMA). Polymers. 2023; 15(19):3922. https://doi.org/10.3390/polym15193922
Chicago/Turabian StyleAversa, Raffaella, Laura Ricciotti, Valeria Perrotta, and Antonio Apicella. 2023. "Chemorheology of a Si/Al > 3 Alkali Activated Metakaolin Paste through Parallel Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Analysis (DMA)" Polymers 15, no. 19: 3922. https://doi.org/10.3390/polym15193922
APA StyleAversa, R., Ricciotti, L., Perrotta, V., & Apicella, A. (2023). Chemorheology of a Si/Al > 3 Alkali Activated Metakaolin Paste through Parallel Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Analysis (DMA). Polymers, 15(19), 3922. https://doi.org/10.3390/polym15193922









