A Hyperbranched Polyol Process for Designing and Manufacturing Nontoxic Cobalt Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Synthesis of Colloidal-Cobalt-Containing Nanocomposite CoNPs Using the Hyperbranched Polyol Process
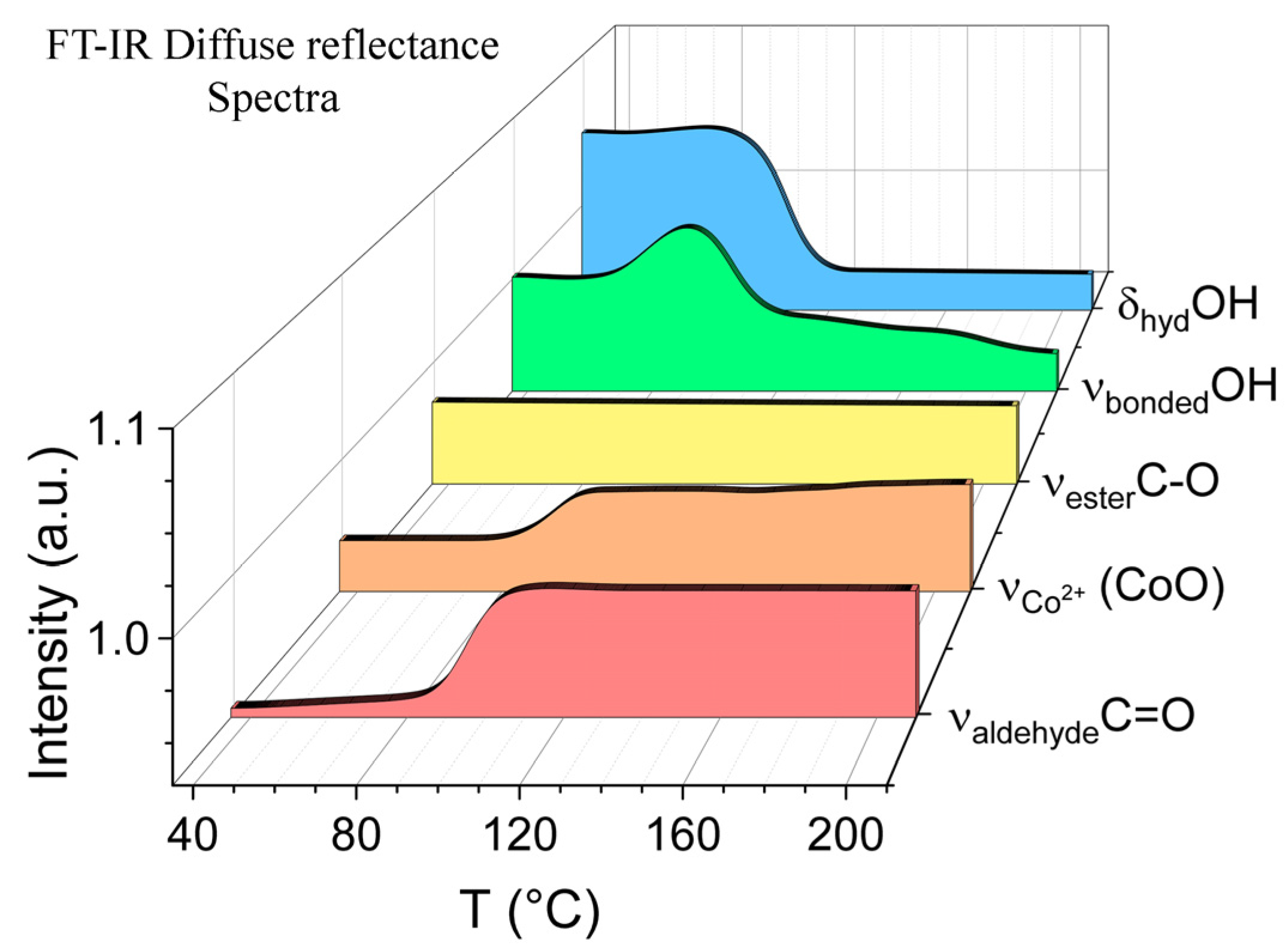
- CoNPs-1. IR spectrum, ν, cm−1: 3447 (OHbonded), 2978 (CH3), 2943 (CH2), 2885 (CH2), 1733 (C=Oester.), 1713 (C=Oaldehyde), 1470 [δ (CH3)], 1397 [δ (CH2)], 1373 [δ (CH2)], 1298 [δ (OH)], 1239 (C-Oester.), 1125 (O-Cester), 1048 (O–Chyd), 1090 (Co-O-H), 1008 (C–O), 579 (Co3+Co3O4), 668 (Co2+CoO).
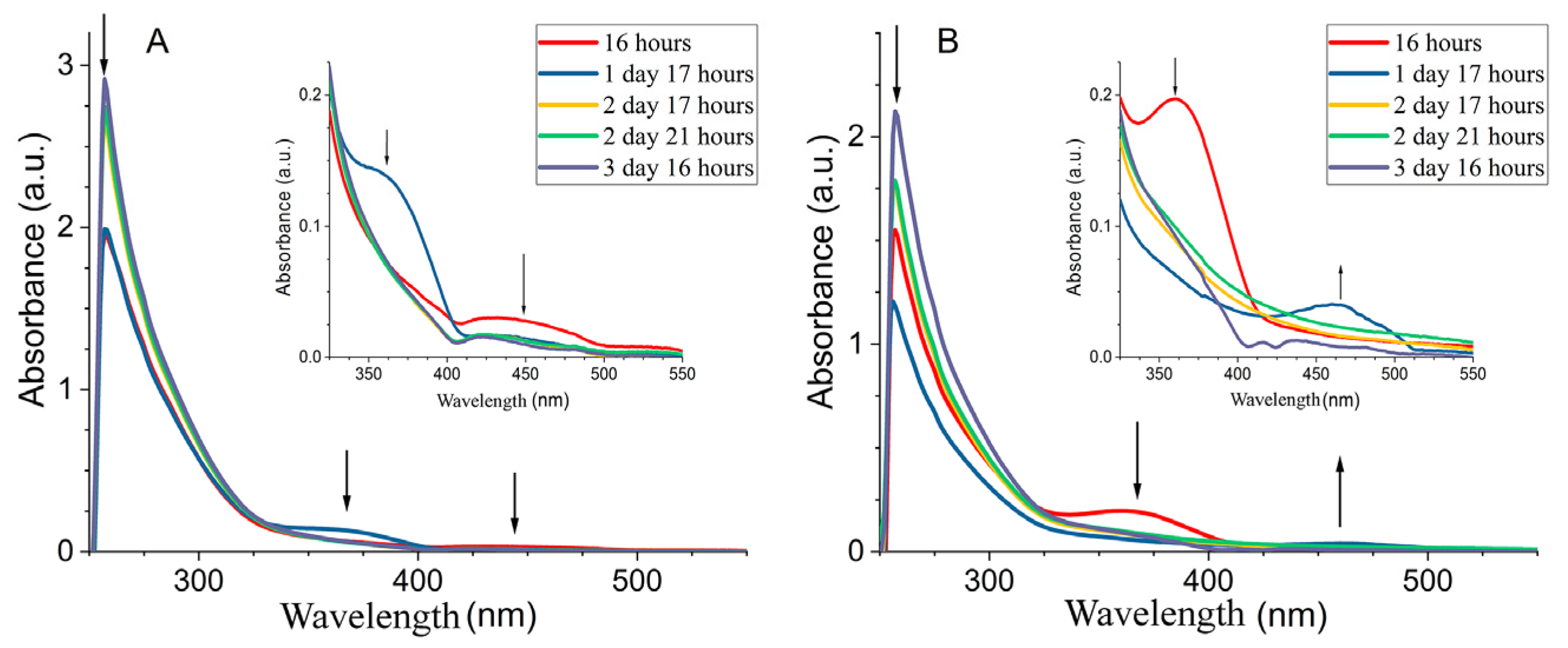
- UV-Vis spectrum, λmax, nm: 250 (Co0 cluster), 273 (Co0), 396 (Co3O4).
- CoNPs-2. IR spectrum, ν, cm−1: 3394 (OHbonded), 2986 (CH3), 2943 (CH2), 2838 (CH2), 1727 (C=Oester.), 1707 (C=Oaldehyde), 1470 [δ (CH3)], 1447 [δ (CH2)], 1369 [δ (CH2)], 1307 [δ (OH)], 1232 (C-Oester.), 1127 (O-Cester), 1048 (O–Chyd), 1028 (Co-O-H), 1013 (C–O), 570 (Co3+ Co3O4), 656 (Co2+CoO).
- UV-Vis spectrum, λmax, nm: 276 (Co0), 390 (Co3O4).
2.4. Sorption Studies
2.5. Estimation of Cobalt Concentration in Nanocomposite CoNPs
2.6. Hemolysis Assay
2.7. Enzymatic Activity Studies
2.8. Antimycotic Study
3. Results
3.1. Synthesis of Cobalt-Containing Nanocomposite CoNPs
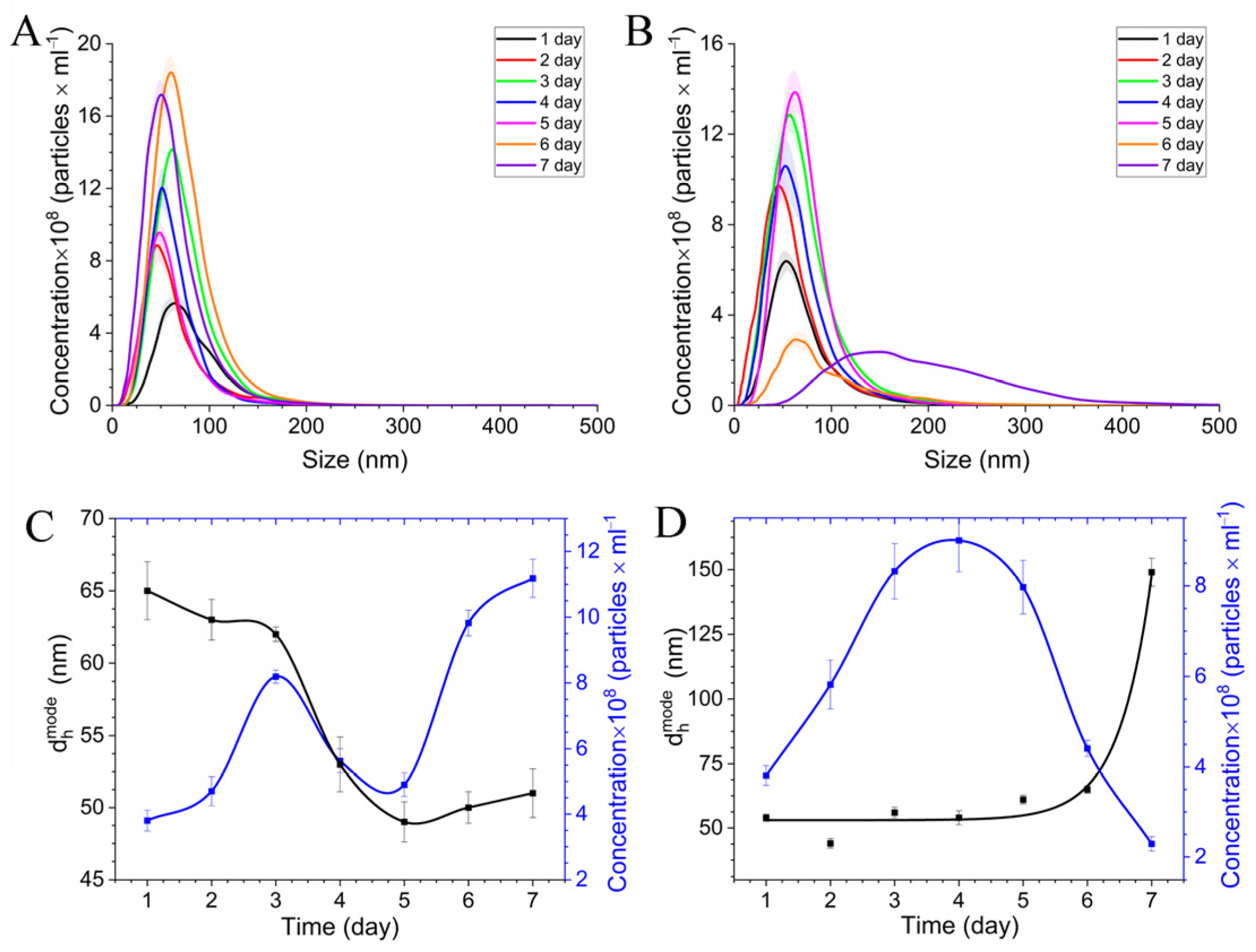
Studying the Stabilization of Cobalt Nanoparticles
3.2. Spectral Properties of Nanocomposite CoNPs
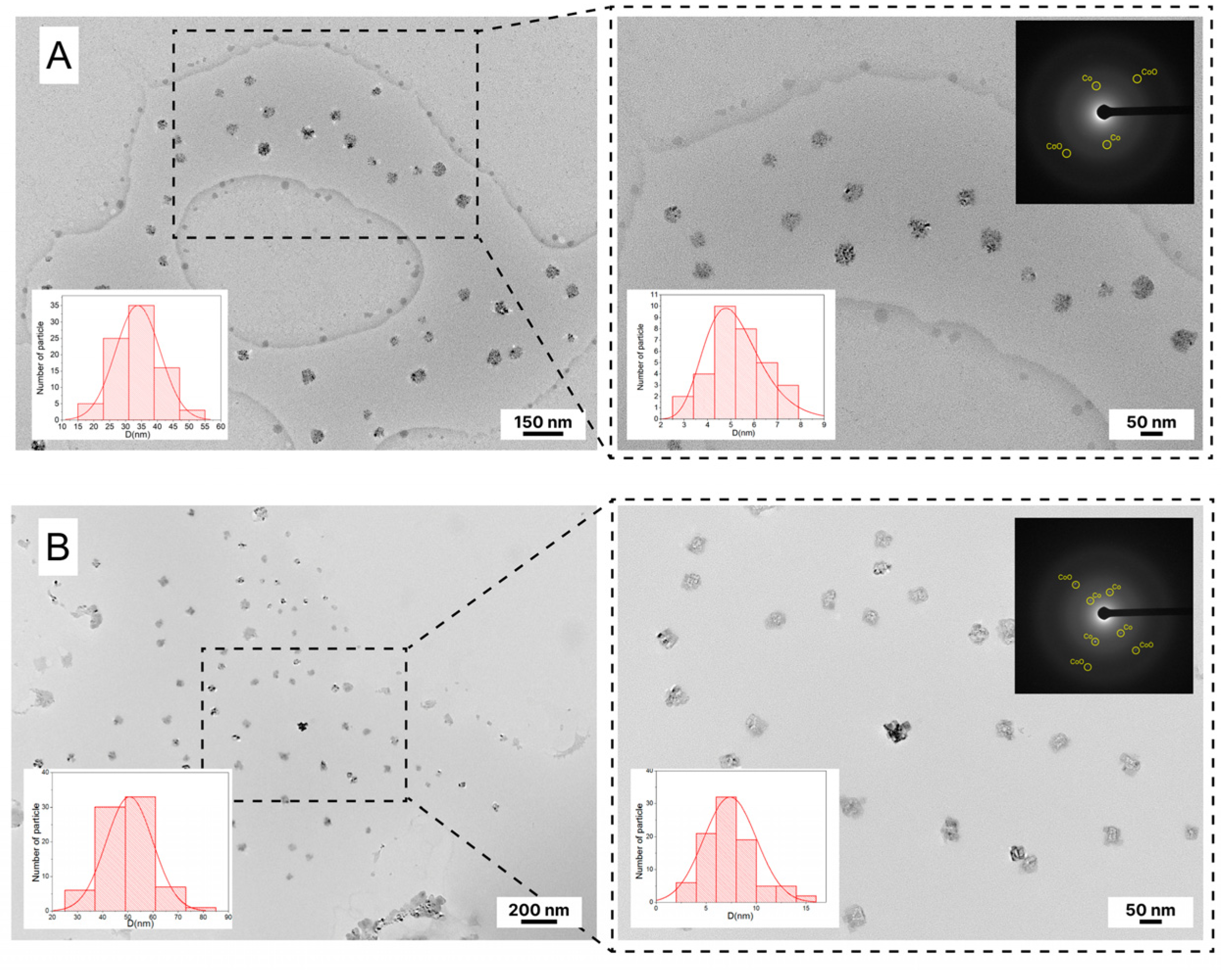
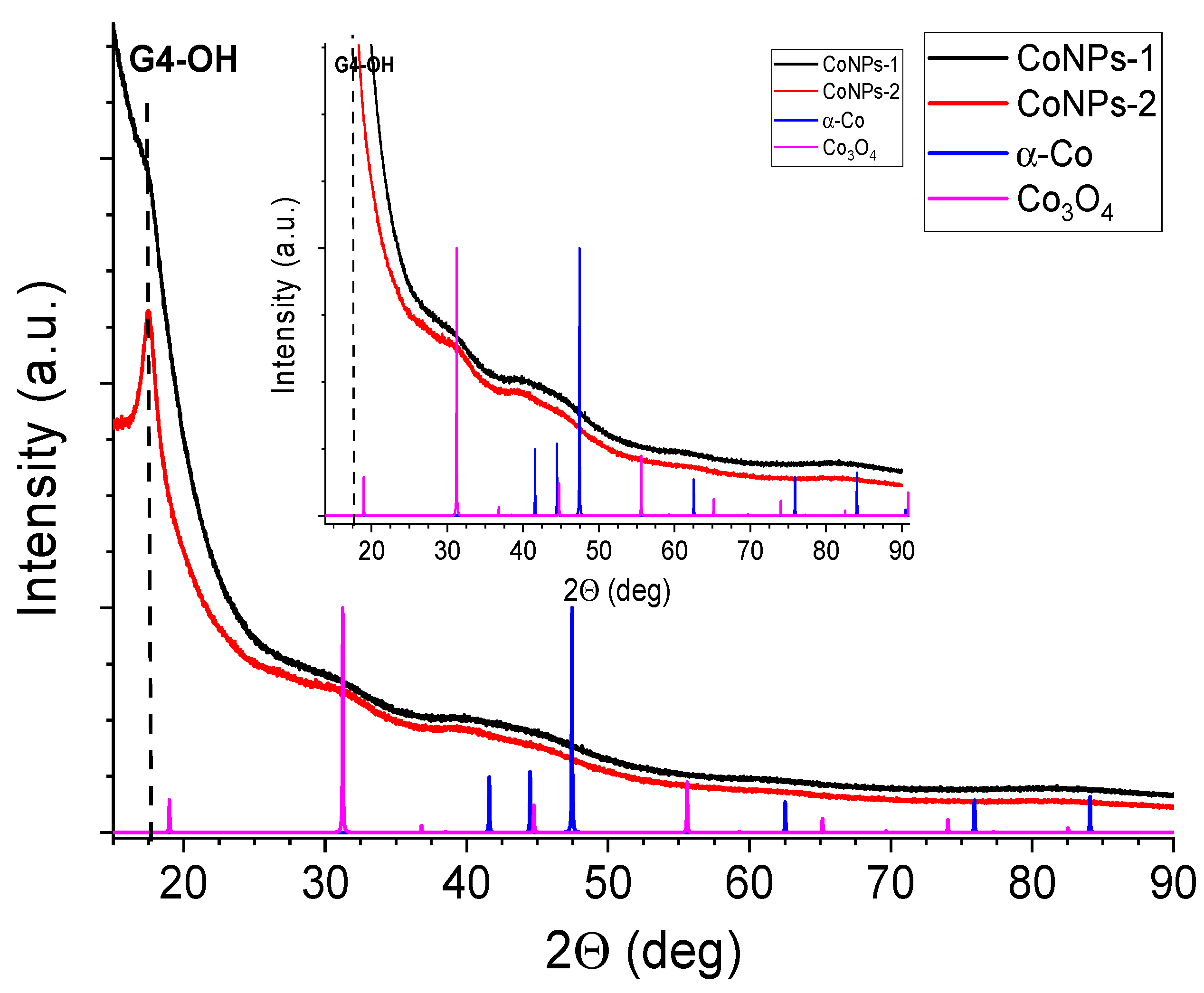
3.3. Morphology of Nanocomposite CoNPs
3.4. Thermal Stability of Nanocomposite CoNPs
3.5. Magnetic Properties of Nanocomposite CoNPs
3.6. Biological Properties
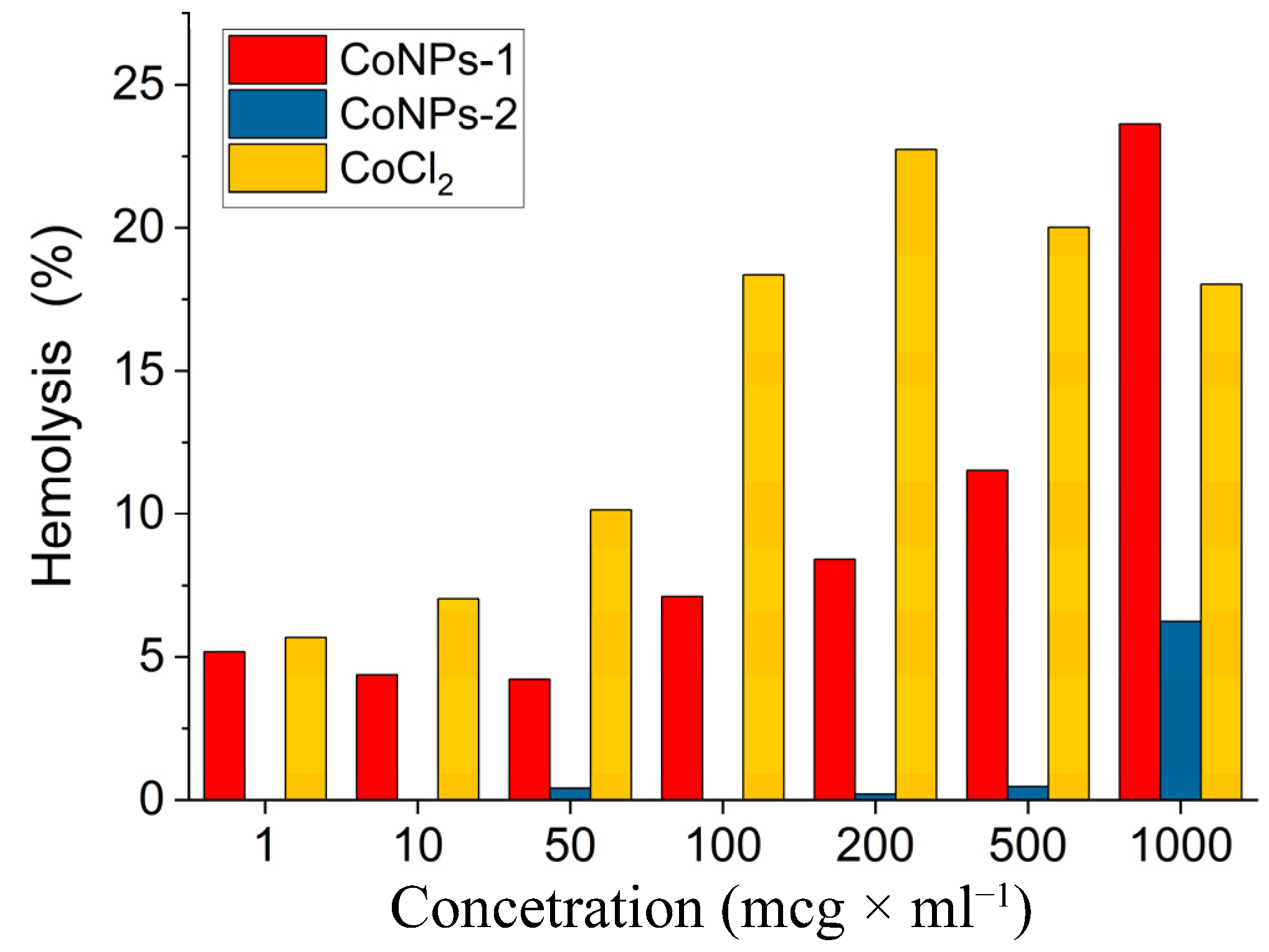
3.6.1. Hemolytic Activity of Nanocomposite CoNPs
3.6.2. Antiproteinase Activity of Nanocomposite CoNPs
3.6.3. Antimycotic Activity of Nanocomposite CoNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Wei, J.; Ge, Q.; Xing, D.; Zhou, X.; Qian, Y.; Jiang, G. The optimized drug delivery systems of treating cancer bone metastatic osteolysis with nanomaterials. Drug Deliv. 2021, 28, 37–53. [Google Scholar] [CrossRef]
- Petrarca, C.; Poma, A.M.; Vecchiotti, G.; Bernardini, G.; Niu, Q.; Cattaneo, A.G.; Di Gioacchino, M.; Sabbioni, E. Cobalt magnetic nanoparticles as theranostics: Conceivable or forgettable? Nanotechnol. Rev. 2020, 9, 1522–1538. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Ulrih, N.P. Multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Lett. 2013, 336, 8–17. [Google Scholar] [CrossRef]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef]
- Jaison, D.; Mothilal, M. Iron Oxide Nanoparticles and Nano-Composites: An Efficient Tool for Cancer Theranostics. In Iron Oxide Nanoparticles; Huang, X.L., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 4. [Google Scholar]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Indian Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Chauhan, P.; Kushwaha, P. Applications of Iron Oxide Nanoparticles in Magnetic Resonance Imaging (MRI). Nanosci. Nanotechnol.-Asia 2021, 11, 290–299. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Attaran, N.; Sazgarnia, A.; Shaegh, S.A.M.; Montazerabadi, A. Superparamagnetic cobalt ferrite nanoparticles as T 2 contrast agent in MRI: In vitro study. IET Nanobiotechnol. 2020, 14, 396–404. [Google Scholar] [CrossRef]
- Tufani, A.; Qureshi, A.; Niazi, J.H. Iron oxide nanoparticles based magnetic luminescent quantum dots (MQDs) synthesis and biomedical/biological applications: A review. Mater. Sci. Eng. C 2021, 118, 111545. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic Iron Oxide Nanoparticle Probes for Molecular Imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Garanina, A.S.; Nikitin, A.A.; Abakumova, T.O.; Semkina, A.S.; Prelovskaya, A.O.; Naumenko, V.A.; Erofeev, A.S.; Gorelkin, P.V.; Majouga, A.G.; Abakumov, M.A.; et al. Cobalt Ferrite Nanoparticles for Tumor Therapy: Effective Heating versus Possible Toxicity. Nanomaterials 2022, 12, 38. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ion. 1989, 32–33, 198–205. [Google Scholar] [CrossRef]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T.K. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef] [Green Version]
- Cheah, P.; Qu, J.; Li, Y.; Cao, D.; Zhu, X.; Zhao, Y. The key role of reaction temperature on a polyol synthesis of water-dispersible iron oxide nanoparticles. J. Magn. Magn. Mater. 2021, 540, 168481. [Google Scholar] [CrossRef]
- Kim, C.W.; Cha, H.G.; Kim, Y.H.; Jadhav, A.P.; Ji, E.S.; Kang, D.I.; Kang, Y.S. Surface Investigation and Magnetic Behavior of Co Nanoparticles Prepared via a Surfactant-Mediated Polyol Process. J. Phys. Chem. C 2009, 113, 5081–5086. [Google Scholar] [CrossRef]
- Takahashi, K.; Yokoyama, S.; Matsumoto, T.; Cuya Huaman, J.L.; Kaneko, H.; Piquemal, J.-Y.; Miyamura, H.; Balachandran, J. Towards a designed synthesis of metallic nanoparticles in polyols—Elucidation of the redox scheme in a cobalt–ethylene glycol system. New J. Chem. 2016, 40, 8632–8642. [Google Scholar] [CrossRef]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- Viau, G.; Fiévet-Vincent, F.; Fiévet, F. Monodisperse iron-based particles: Precipitation in liquid polyols. J. Mater. Chem. 1996, 6, 1047–1053. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Wang, T.; Wang, C.; Zhang, Q.; Ma, L. Synthesis of shape-controllable cobalt nanoparticles and their shape-dependent performance in glycerol hydrogenolysis. RSC Adv. 2015, 5, 4861–4871. [Google Scholar] [CrossRef]
- Viau, G.; Piquemal, J.-Y.; Esparrica, M.; Ung, D.; Chakroune, N.; Warmont, F.; Fiévet, F. Formation of assembled silver nanowires by reduction of silver thiolate in polyol/toluene medium. Chem. Commun. 2003, 17, 2216–2217. [Google Scholar] [CrossRef]
- Bae, S.; Han, H.; Bae, J.G.; Lee, E.Y.; Im, S.H.; Kim, D.H.; Seo, T.S. Growth of Silver Nanowires from Controlled Silver Chloride Seeds and Their Application for Fluorescence Enhancement Based on Localized Surface Plasmon Resonance. Small 2017, 13, 1603392. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; DuanMu, Y.; Xu, L.; Xie, S.; Gu, N. Facile synthesis of micrometer-sized gold nanoplates through an aniline-assisted route in ethylene glycol solution. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 33–38. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kamada, K.; Enomoto, N.; Hojo, J. Polyhedral Gold Nanoplate: High Fraction Synthesis of Two-Dimensional Nanoparticles through Rapid Heating Process. Cryst. Growth Des. 2008, 8, 2638–2645. [Google Scholar] [CrossRef]
- Soumare, Y.; Garcia, C.; Maurer, T.; Chaboussant, G.; Ott, F.; Fiévet, F.; Piquemal, J.-Y.; Viau, G. Kinetically Controlled Synthesis of Hexagonally Close-Packed Cobalt Nanorods with High Magnetic Coercivity. Adv. Funct. Mater. 2009, 19, 1971–1977. [Google Scholar] [CrossRef]
- Couto, G.G.; Klein, J.J.; Schreiner, W.H.; Mosca, D.H.; de Oliveira, A.J.A.; Zarbin, A.J.G. Nickel nanoparticles obtained by a modified polyol process: Synthesis, characterization, and magnetic properties. J. Colloid Interface Sci. 2007, 311, 461–468. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Shinoda, K.; Kodama, D.; Jeyadevan, B. Size controlled Fe nanoparticles through polyol process and their magnetic properties. Mater. Chem. Phys. 2010, 123, 487–493. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis and characterization of nickel nanoparticles by hydrazine reduction in ethylene glycol. J. Colloid Interface Sci. 2003, 259, 282–286. [Google Scholar] [CrossRef]
- Xiong, Y.; Siekkinen, A.R.; Wang, J.; Yin, Y.; Kim, M.J.; Xia, Y. Synthesis of silver nanoplates at high yields by slowing down the polyol reduction of silver nitrate with polyacrylamide. J. Mater. Chem. 2007, 17, 2600–2602. [Google Scholar] [CrossRef]
- Izu, N.; Matsubara, I.; Uchida, T.; Itoh, T.; Shin, W. Synthesis of spherical cobalt oxide nanoparticles by a polyol method. J. Ceram. Soc. Jpn. 2017, 125, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Shen, C.; Song, N.; Wang, Y.; Yang, T.; Gao, H.; Cheng, Z. Facile synthesis of hollow nano-spheres and hemispheres of cobalt by polyol reduction. Nanotechnology 2010, 21, 375602. [Google Scholar] [CrossRef]
- Chakroune, N.; Viau, G.; Ricolleau, C.; Fiévet-Vincent, F.; Fiévet, F. Cobalt-based anisotropic particles prepared by the polyol process. J. Mater. Chem. 2003, 13, 312–318. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Polyol Synthesis of Silver Nanostructures: Control of Product Morphology with Fe(II) or Fe(III) Species. Langmuir 2005, 21, 8077–8080. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, M. Aliphatic hyperbranched polyesters based on 2,2-bis(methylol)propionic acid—Determination of structure, solution and bulk properties. Prog. Polym. Sci. 2011, 36, 53–88. [Google Scholar] [CrossRef]
- Carlmark, A.; Hawker, C.; Hult, A.; Malkoch, M. New methodologies in the construction of dendritic materials. Chem. Soc. Rev. 2009, 38, 352–362. [Google Scholar] [CrossRef] [Green Version]
- Balogh, L.; Valluzzi, R.; Laverdure, K.S.; Gido, S.P.; Hagnauer, G.L.; Tomalia, D.A. Formation of Silver and Gold Dendrimer Nanocomposites. J. Nanopart. Res. 1999, 1, 353–368. [Google Scholar] [CrossRef]
- Medvedeva, O.I.; Kambulova, S.S.; Ulakhovich, N.A.; Vorobev, V.V.; Evtyugin, V.G.; Khaldeeva, E.V.; Kutyreva, M.P. Formation of copper nanoparticles with hyperbranched polyesterpolyols as template. Russ. J. Gen. Chem. 2017, 87, 1985–1994. [Google Scholar] [CrossRef]
- Khannanov, A.A.; Rossova, A.A.; Ignatyeva, K.A.; Ulakhovich, N.A.; Gerasimov, A.V.; Boldyrev, A.E.; Evtugyn, V.G.; Rogov, A.M.; Cherosov, M.A.; Gilmutdinov, I.F.; et al. Superparamagnetic cobalt nanoparticles in hyperbranched polyester polyol matrix with anti-protease activity. J. Magn. Magn. Mater. 2021, 547, 168808. [Google Scholar] [CrossRef]
- Medvedeva, O.I.; Kambulova, S.S.; Bondar, O.V.; Gataulina, A.R.; Ulakhovich, N.A.; Gerasimov, A.V.; Evtugyn, V.G.; Gilmutdinov, I.F.; Kutyreva, M.P. Magnetic Cobalt and Cobalt Oxide Nanoparticles in Hyperbranched Polyester Polyol Matrix. J. Nanotechnol. 2017, 2017, 7607658. [Google Scholar] [CrossRef] [Green Version]
- Khannanov, A.; Burmatova, A.; Ignatyeva, K.; Vagizov, F.; Kiiamov, A.; Tayurskii, D.; Cherosov, M.; Gerasimov, A.; Vladimir, E.; Kutyreva, M. Effect of the Synthetic Approach on the Formation and Magnetic Properties of Iron-Based Nanophase in Branched Polyester Polyol Matrix. Int. J. Mol. Sci. 2022, 23, 14764. [Google Scholar] [CrossRef] [PubMed]
- Khannanov, A.; Rossova, A.; Ulakhovich, N.; Evtugyn, V.; Valiullin, L.; Nabatov, A.; Kutyrev, G.; Kutyreva, M. Doxorubicin-Loaded Hybrid Micelles Based on Carboxyl-Terminated Hyperbranched Polyester Polyol. ACS Appl. Polym. Mater. 2022, 4, 2553–2561. [Google Scholar] [CrossRef]
- Khannanov, A.A.; Kutyreva, M.P.; Ulakhovich, N.A.; Gataulina, A.R.; Bondar, O.V.; Zakharova, L.Y.; Kutyrev, G.A. Hyperbranched polyester polyacids and their binary systems with surfactants for doxorubicin encapsulation. Fluid Phase Equilibria 2016, 411, 93–100. [Google Scholar] [CrossRef]
- Solodov, A.N.; Shayimova, J.R.; Burilova, E.A.; Shurtakova, D.V.; Zhuravleva, Y.I.; Cherosov, M.A.; Tian, Y.; Kiiamov, A.G.; Amirov, R.R. Understanding the Nucleation and Growth of Iron Oxide Nanoparticle Formation by a “Heating-Up” Process: An NMR Relaxation Study. J. Phys. Chem. C 2021, 125, 20980–20992. [Google Scholar] [CrossRef]
- Klajnert, B.; Walach, W.; Bryszewska, M.; Dworak, A.; Shcharbin, D. Cytotoxicity, haematotoxicity and genotoxicity of high molecular mass arborescent polyoxyethylene polymers with polyglycidol-block-containing shells. Cell Biol. Int. 2006, 30, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M. Hyperbranched polymers: Phase behavior and new applications in the field of chemical engineering. Fluid Phase Equilibria 2006, 241, 155–174. [Google Scholar] [CrossRef]
- Duggan, J.N.; Bozack, M.J.; Roberts, C.B. The synthesis and arrested oxidation of amorphous cobalt nanoparticles using DMSO as a functional solvent. J. Nanopart. Res. 2013, 15, 2089. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Matsumoto, T.; Takahashi, H.; Kodama, D.; Tohji, K.; Jeyadevan, B. Designed synthesis of cobalt and its alloys by polyol process. J. Solid State Chem. 2007, 180, 3008–3018. [Google Scholar] [CrossRef]
- Alrehaily, L.M.; Joseph, J.M.; Biesinger, M.C.; Guzonas, D.A.; Wren, J.C. Gamma-radiolysis-assisted cobalt oxide nanoparticle formation. Phys. Chem. Chem. Phys. 2013, 15, 1014–1024. [Google Scholar] [CrossRef]
- Aranishi, K.; Zhu, Q.-L.; Xu, Q. Dendrimer-Encapsulated Cobalt Nanoparticles as High-Performance Catalysts for the Hydrolysis of Ammonia Borane. ChemCatChem 2014, 6, 1375–1379. [Google Scholar] [CrossRef]
- Karahan, S.; Özkar, S. Poly(4-styrenesulfonic acid-co-maleic acid) stabilized cobalt(0) nanoparticles: A cost-effective and magnetically recoverable catalyst in hydrogen generation from the hydrolysis of hydrazine borane. Int. J. Hydrogen Energy 2015, 40, 2255–2265. [Google Scholar] [CrossRef]
- Stepanov, A.; Fedorenko, S.; Mendes, R.; RÜMmeli, M.; Giebeler, L.; Weise, B.; Gemming, T.; Dutz, S.; Zahn, D.; Ismaev, I.; et al. T2- and T1 relaxivities and magnetic hyperthermia of iron-oxide nanoparticles combined with paramagnetic Gd complexes. J. Chem. Sci. 2021, 133, 43. [Google Scholar] [CrossRef]






| Sample | AE, % | Effect | CCoNPs, μg/mL | AC50, mg/mL | IC50, mg/mL |
|---|---|---|---|---|---|
| CoNPs-1 | 210 ± 5 | Activation | 0.1–2 | 0.0047 | - |
| 54 ± 3 | Inhibition | 5000–10,000 | - | 10 | |
| CoNPs-2 | 134 ± 5 | Activation | 500–10,000 | 12.8 | - |
| CoCl2 | 53 ± 8 | Inhibition | 400–10,000 | - | 0.6 |
| Sample | Zone of Inhibition (mm) (Figure S10) | |
|---|---|---|
| Candida albicans ATCC 10201 | Aspergillus fumigatus F-753 | |
| G4-OH | 0 | 0 |
| CoNPs-2 | 13.6 | 25.2 |
| Nystatin | 15.8 | 13.5 |
| Ketoconazole | 34.7 | 11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmatova, A.; Khannanov, A.; Gerasimov, A.; Ignateva, K.; Khaldeeva, E.; Gorovaia, A.; Kiiamov, A.; Evtugyn, V.; Kutyreva, M. A Hyperbranched Polyol Process for Designing and Manufacturing Nontoxic Cobalt Nanocomposite. Polymers 2023, 15, 3248. https://doi.org/10.3390/polym15153248
Burmatova A, Khannanov A, Gerasimov A, Ignateva K, Khaldeeva E, Gorovaia A, Kiiamov A, Evtugyn V, Kutyreva M. A Hyperbranched Polyol Process for Designing and Manufacturing Nontoxic Cobalt Nanocomposite. Polymers. 2023; 15(15):3248. https://doi.org/10.3390/polym15153248
Chicago/Turabian StyleBurmatova, Anastasia, Artur Khannanov, Alexander Gerasimov, Klara Ignateva, Elena Khaldeeva, Arina Gorovaia, Airat Kiiamov, Vladimir Evtugyn, and Marianna Kutyreva. 2023. "A Hyperbranched Polyol Process for Designing and Manufacturing Nontoxic Cobalt Nanocomposite" Polymers 15, no. 15: 3248. https://doi.org/10.3390/polym15153248
APA StyleBurmatova, A., Khannanov, A., Gerasimov, A., Ignateva, K., Khaldeeva, E., Gorovaia, A., Kiiamov, A., Evtugyn, V., & Kutyreva, M. (2023). A Hyperbranched Polyol Process for Designing and Manufacturing Nontoxic Cobalt Nanocomposite. Polymers, 15(15), 3248. https://doi.org/10.3390/polym15153248








