Dendrimer-Based Coatings on a Photonic Crystal Surface for Ultra-Sensitive Small Molecule Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photonic Crystal Surface Mode Detection System
2.3. Fabrication of the PC Biochip
2.3.1. PC Activation and Functionalization
2.3.2. Functionalization of the PC chip
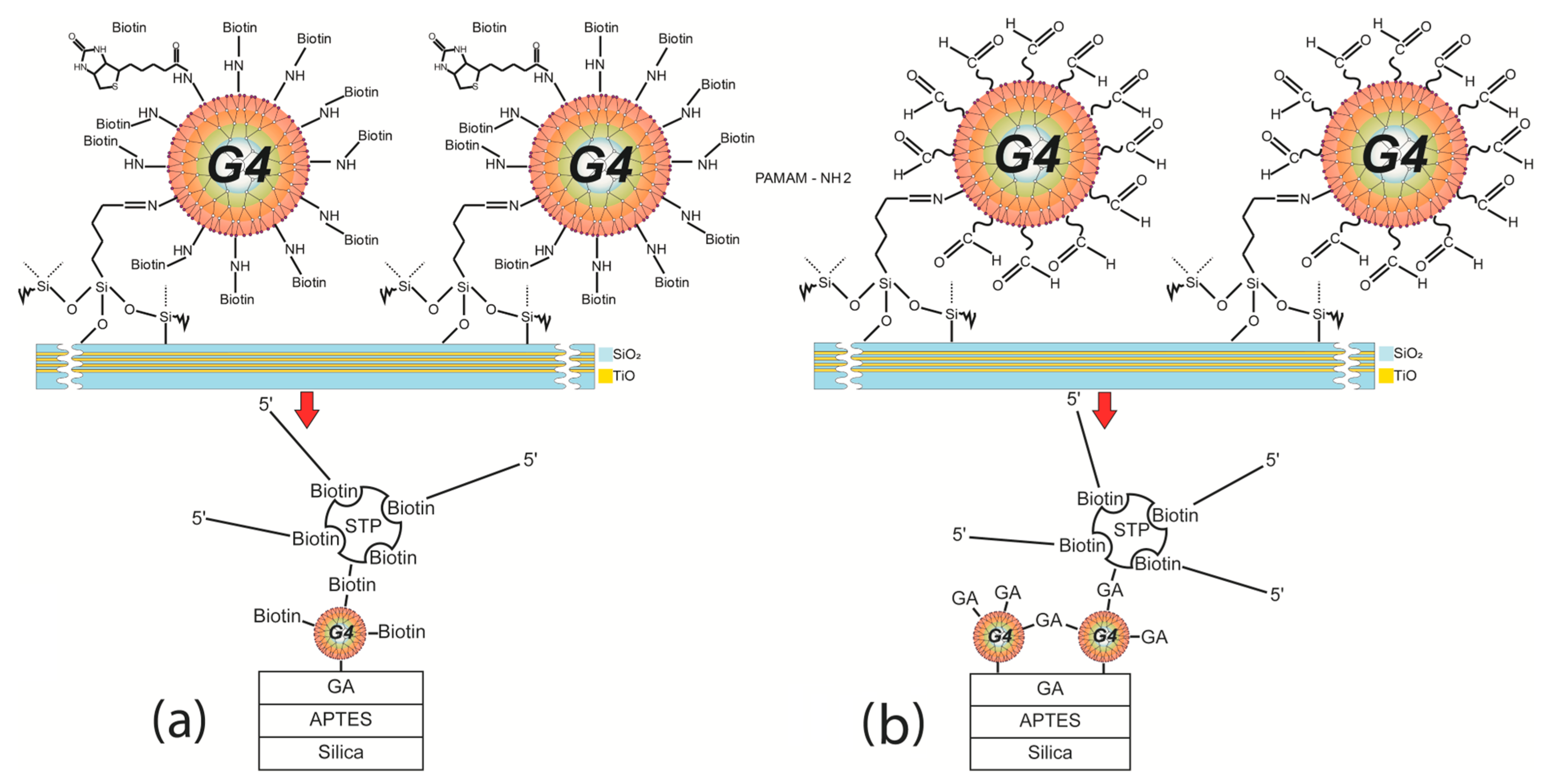
Functionalization with PAMAM G4-GA (First Method)
Functionalization with Biotinylated PAMAM G4 (Second Method)
2.4. Protein and Low-Molecular-Weight Biomolecule Binding Detection
2.4.1. Protein Detection
2.4.2. Low-Molecular-Weight Biomolecule Detection
2.5. Atomic Force Microscopy
3. Results
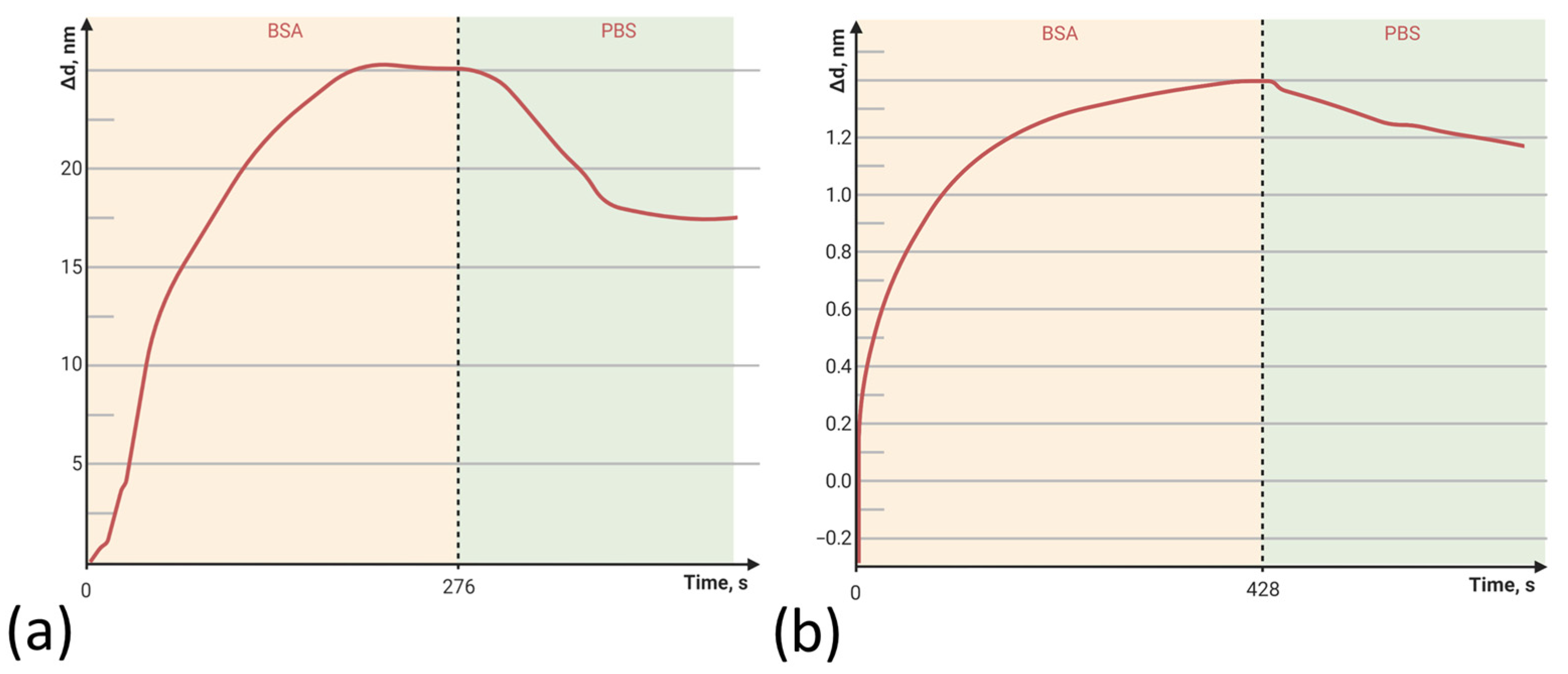
3.1. Model Protein-Binding Capacity on the PAMAM G4-GA PC Chip
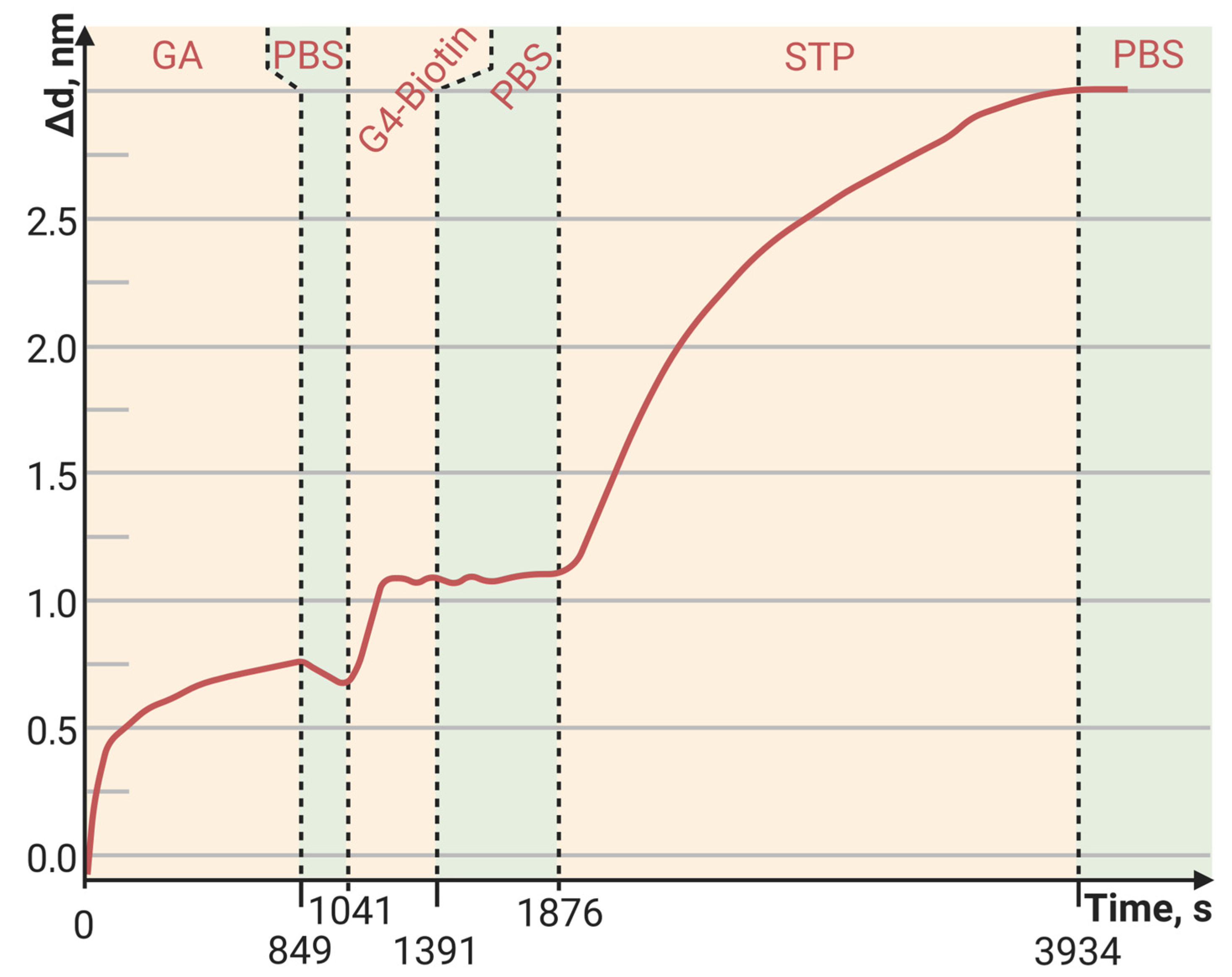
3.2. Streptavidin Binding Capacity on the PAMAM G4-GA PC Chip
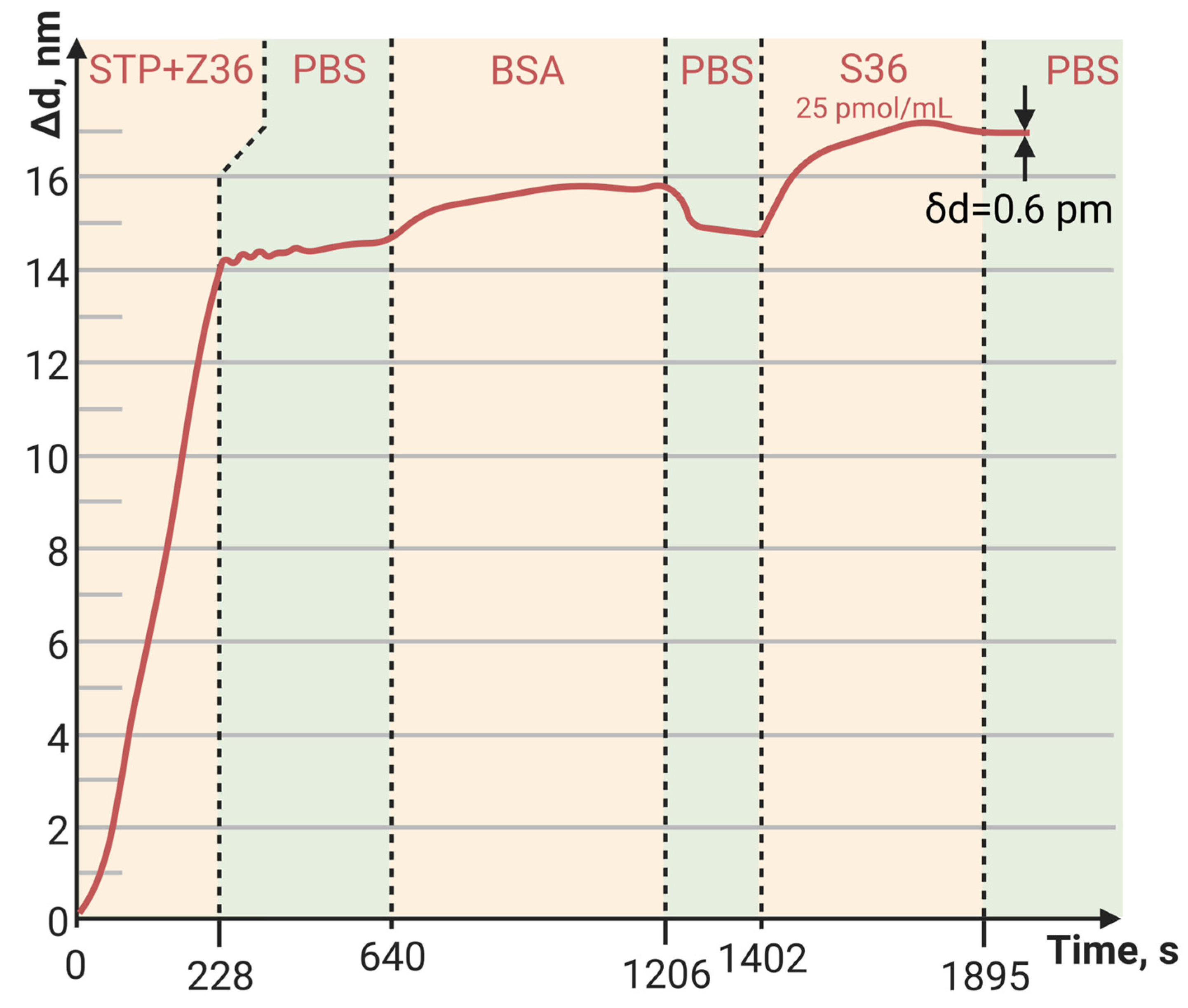
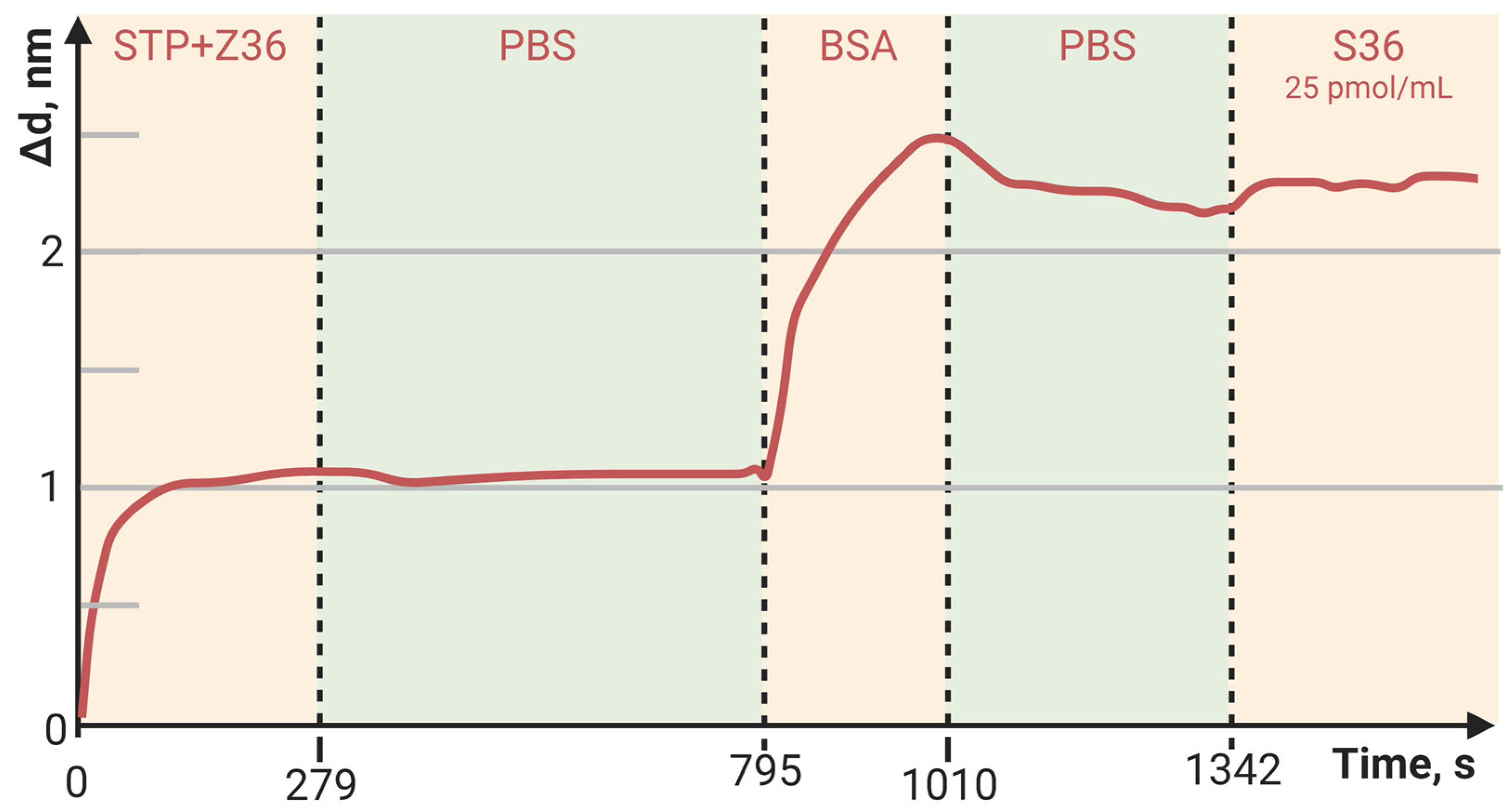
3.3. Detection of Low-Molecular-Weight Biomolecule Interaction with PC SM Biosensor
3.4. Sensor Baseline Noise, LoD, LoQ, and Dynamic Range
3.5. Atomic Force Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, S.; Lu, Y.; Li, X.; Cao, S.; Pei, Y.; Aastrup, T.; Pei, Z. Optimization of 3D surfaces of dextran with different moleculeweights for real-time detection of biomolecular interactions by a QCM biosensor. Polymers 2017, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Montagut, Y.; García, J.V.; Jiménez, Y.; March, C.; Montoya, Á.; Arnau, A. 9 QCM Technology in Biosensors. Biosens.-Emerg. Mater. Appl. 2011, 153–178. Available online: www.intechopen.com (accessed on 22 April 2023).
- Löfås, S.; Johnsson, B. A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc.Chem. Commun. 1990, 21, 1526–1528. [Google Scholar] [CrossRef]
- Kanoh, N.; Kyo, M.; Inamori, K.; Ando, A.; Asami, A.; Nakao, A.; Osada, H. SPR imaging of photo-cross-linked small-molecule arrays on gold. Anal. Chem. 2006, 78, 2226–2230. [Google Scholar] [CrossRef]
- Christau, S.; Thurandt, S.; Yenice, Z.; von Klitzing, R. Stimuli-responsive polyelectrolyte brushes as a matrix for the attachment of gold nanoparticles: The effect of brush thickness on particle distribution. Polymers 2014, 6, 1877–1896. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Fang, Y.; Ferrie, A.M.; Fontaine, N.H.; Mauro, J.; Balakrishnan, J. Resonant waveguide grating biosensor for living cell sensing. Biophys. J. 2006, 91, 1925–1940. [Google Scholar] [CrossRef]
- Quaranta, G.; Basset, G.; Martin, O.J.F.; Gallinet, B. Recent Advances in Resonant Waveguide Gratings. Laser Photonics Rev. 2018, 12, 1800017. [Google Scholar] [CrossRef]
- Zourob, M.; Elwary, S.; Fan, X.; Mohr, S.; Goddard, N.J. Label-free detection with the resonant mirror biosensor. Methods Mol. Biol. 2009, 503, 89–138. [Google Scholar] [CrossRef]
- De Tommasi, E.; De Stefano, L.; Rea, I.; Di Sarno, V.; Rotiroti, L.; Arcari, P.; Lamberti, A.; Sanges, C.; Rendina, I. Porous silicon based resonant mirrors for biochemical sensing. Sensors 2008, 8, 6549–6556. [Google Scholar] [CrossRef]
- Vollmer, F.; Yang, L.; Fainman, S. Label-free detection with high-Q microcavities: A review of biosensing mechanisms for integrated devices. Nanophotonics 2012, 1, 267–291. [Google Scholar] [CrossRef]
- Subramanian, S.; Wu, H.Y.; Constant, T.; Xavier, J.; Vollmer, F. Label-Free Optical Single-Molecule Micro- and Nanosensors. Adv. Mater. 2018, 30, e1801246. [Google Scholar] [CrossRef]
- Guo, H.; Qi, W. New materials and designs for 2D-based infrared photodetectors. Nano Res. 2023, 16, 3074–3103. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Lin, L.; Cai, J.; Chen, J.; Wang, S.; Gou, X.; Ye, J.; Luo, Z.; Huang, W. Enhanced-performance self-powered photodetector based on multi-layer MoS2 sandwiched between two asymmetric graphene contacts. Sci. China Technol. Sci. 2022, 65, 2658–2666. [Google Scholar] [CrossRef]
- Guo, J.; Lin, L.; Li, S.; Chen, J.; Wang, S.; Wu, W.; Cai, J.; Zhou, T.; Liu, Y.; Huang, W. Ferroelectric superdomain controlled graphene plasmon for tunable mid-infrared photodetector with dual-band spectral selectivity. Carbon N. Y. 2022, 189, 596–603. [Google Scholar] [CrossRef]
- Guo, J.; Lin, L.; Li, S.; Chen, J.; Wang, S.; Wu, W.; Cai, J.; Liu, Y.; Ye, J.; Huang, W. WSe2/MoS2 van der Waals Heterostructures Decorated with Au Nanoparticles for Broadband Plasmonic Photodetectors. ACS Appl. Nano Mater. 2022, 5, 587–596. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, J.; Liang, L. Two dimensional photonic crystal slab biosensors using label free refractometric sensing schemes: A review. Prog. Quantum. Electron. 2021, 77, 100298. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, C.; Guo, Z.; Wang, B.; Wu, X.; Fei, Y. Highly sensitive label-free detection of small molecules with an optofluidic microbubble resonator. Micromachines 2018, 9, 274. [Google Scholar] [CrossRef]
- Park, J.-H.; Cho, Y.-W.; Kim, T.-H. Recent Advances in Surface Plasmon Resonance Sensors for Sensitive Optical Detection of Pathogens. Biosensors 2022, 12, 180. [Google Scholar] [CrossRef]
- Konopsky, V.N.; Karakouz, T.; Alieva, E.V.; Vicario, C.; Sekatskii, S.K.; Dietler, G. Photonic crystal biosensor based on optical surfacewaves. Sensors 2013, 13, 2566–2578. [Google Scholar] [CrossRef]
- Konopsky, V.; Mitko, T.; Aldarov, K.; Alieva, E.; Basmanov, D.; Moskalets, A.; Matveeva, A.; Morozova, O.; Klinov, D. Photonic crystal surface mode imaging for multiplexed and high-throughput label-free biosensing. Biosens. Bioelectron. 2020, 168, 112575. [Google Scholar] [CrossRef]
- Konopsky, V.N.; Alieva, E.V. Photonic crystal surface waves for optical biosensors. Anal. Chem. 2007, 79, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Konopsky, V.; Nabiev, I.; Sukhanova, A. Label-Free Flow Multiplex Biosensing via Photonic Crystal Surface Mode Detection. Sci. Rep. 2019, 9, 8745. [Google Scholar] [CrossRef] [PubMed]
- Olyaee, S.; Seifouri, M.; Mohsenirad, H. Label-free detection of glycated haemoglobin in human blood using silicon-based photonic crystal nanocavity biosensor. J. Mod. Opt. 2016, 63, 1274–1279. [Google Scholar]
- Kawasaki, D.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Imprinted Photonic Crystal-Film-Based Smartphone-Compatible Label-Free Optical Sensor for SARS-CoV-2 Testing. Biosensors 2022, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Sizova, S.; Shakurov, R.; Mitko, T.; Shirshikov, F.; Solovyeva, D.; Konopsky, V.; Alieva, E.; Klinov, D.; Bespyatykh, J.; Basmanov, D. The Elaboration of Effective Coatings for Photonic Crystal Chips in Optical Biosensors. Polymers 2022, 14, 152. [Google Scholar] [CrossRef]
- Mori, T.; Yamanouchi, G.; Han, X.; Inoue, Y.; Shigaki, S.; Yamaji, T.; Sonoda, T.; Yasui, K.; Hayashi, H.; Niidome, T.; et al. Signal-to-noise ratio improvement of peptide microarrays by using hyperbranched-polymer materials. J. Appl. Phys. 2009, 105, 102020. [Google Scholar] [CrossRef]
- Trummer, N.; Adányi, N.; Váradi, M.; Szendrö, I. Modification of the surface of integrated optical wave-guide sensors for immunosensor applications. Anal. Bioanal. Chem. 2001, 371, 21–24. [Google Scholar] [CrossRef]
- Wong, A.K.Y.; Krull, U.J. Surface characterization of 3-glycidoxypropyltrimethoxysilane films on silicon-based substrates. Anal. Bioanal. Chem. 2005, 383, 187–200. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Falamaki, C. A novel method for the fabrication of ATPES silanized SPR sensor chips: Exclusion of Cr or Ti intermediate layers and optimization of optical/adherence properties. Appl. Surf. Sci. 2014, 301, 544–550. [Google Scholar] [CrossRef]
- Kurunczi, S.; Hainard, A.; Juhasz, K.; Patko, D.; Orgovan, N.; Turck, N.; Sanchez, J.C.; Horvath, R. Polyethylene imine-based receptor immobilization for label free bioassays. Sens. Actuators B Chem. 2013, 181, 71–76. [Google Scholar] [CrossRef]
- Morozova, O.V.; Levchenko, O.A.; Cherpakova, Z.A.; Prokhorov, V.V.; Barinov, N.A.; Obraztsova, E.A.; Belova, A.M.; Prusakov, K.A.; Aldarov, K.G.; Basmanov, D.V.; et al. Surface modification with polyallylamines for adhesion of biopolymers and cells. Int. J. Adhes. Adhes. 2019, 92, 125–132. [Google Scholar] [CrossRef]
- Sonawane, M.D.; Nimse, S.B. Surface Modification Chemistries of Materials Used in Diagnostic Platforms with Biomolecules. J. Chem. 2016, 2016, 9241378. [Google Scholar] [CrossRef]
- Kuhlmeier, D.; Rodda, E.; Kolarik, L.O.; Furlong, D.N.; Bilitewski, U. Application of Atomic Force Microscopy and Grating Coupler for the Characterization of Biosensor Surfaces. Biosens. Bioelectron. 2002, 18, 925–936. [Google Scholar] [CrossRef]
- Kurunczi, S.; Horvath, R.; Yeh, Y.P.; Muskotál, A.; Sebestýn, A.; Vonderviszt, F.; Ramsden, J.J. Self-assembly of rodlike receptors from bulk solution. J. Chem. Phys. 2009, 130, 11101. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chauhan, S.; Thakur, A. Chitosan-Based Biosensors—A Comprehensive Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Singh, S.; Sanwaria, S.; Gurave, P.M. Design and development of advanced glucose biosensors via tuned interactions between marine polysaccharides and diagnostic elements—A survey. Sens. Int. 2022, 3, 100170. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity 497 Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Rosenfeldt, S.; Karpuk, E.; Lehmann, M.; Meier, H.; Lindner, P.; Harnau, L.; Ballauff, M. The Solution Structure of Stilbenoid Dendrimers: A Small-Angle Scattering Study. ChemPhysChem 2006, 7, 2097–2104. [Google Scholar] [CrossRef]
- Kretzmann, J.A.; Ho, D.; Evans, C.W.; Plani-Lam, J.H.C.; Garcia-Bloj, B.; Mohamed, A.E.; O’Mara, M.L.; Ford, E.; Tan, D.E.K.; Lister, R.; et al. Synthetically controlling dendrimer flexibility improves delivery of large plasmid DNA. Chem. Sci. 2017, 8, 2923–2930. [Google Scholar] [CrossRef]
- An, M.; Parkin, S.R.; DeRouchey, J.E. Intermolecular forces between low generation PAMAM dendrimer condensed DNA helices: Role of cation architecture. Soft Matter. 2014, 10, 590–599. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. The dendritic effect. In Dendrimers, Dendrons, and Dendritic Polymers; Cambridge University Press: Cambridge, UK, 2012; pp. 276–292. [Google Scholar] [CrossRef]
- Tomalia, D.A. Dendritic effects: Dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J. Chem. 2012, 36, 264–281. [Google Scholar] [CrossRef]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. Pamam dendrimer nanomolecules utilized as drug delivery systems for potential treatment of glioblastoma: A systematic review. Int. J. Nanomed. 2020, 15, 2789–2808. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, A.A.A.; Abraham, G.A.; Aparecida, M.; Camillo, P.; Higa, O.Z.; Silva, G.S.; Fernández, D.M.; Román, J.S. Physicochemical and antimicrobial properties of boron-complexed polyglycerol-chitosan dendrimers. J. Biomater. Sci. Polym. Ed. 2006, 17, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, S.A.; Shifrina, Z.B. Dendrimers as Antiamyloid Agents. Pharmaceutics 2022, 14, 760. [Google Scholar] [CrossRef] [PubMed]
- Soršak, E.; Valh, J.V.; Urek, S.K.; Lobnik, A. Application of PAMAM dendrimers in optical sensing. Analyst 2015, 140, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive Detection of Dengue Virus Type 2 E-Proteins Signals Using Self-Assembled Monolayers/Reduced Graphene Oxide-PAMAM Dendrimer Thin Film-SPR Optical Sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Konopsky, V.N. Plasmon-polariton waves in nanofilms on one-dimensional photonic crystal surfaces. New J. Phys. 2010, 12, 093006. [Google Scholar] [CrossRef]
- Konopsky, V. Design of 1D Photonic Crystals Sustaining Optical Surface Modes. Coatings 2022, 12, 1489. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Kumar, A.; Maher, D.M.; Chauhan, S.C.; Vangara, K.K.; Palakurthi, S. Biotinylated PAMAM Dendrimers for Intracellular Delivery of Cisplatin to Ovarian Cancer: Role of SMVT. Anticancer Res. 2011, 31, 897. Available online: http://ar.iiarjournals.org/content/31/3/897.abstract (accessed on 22 April 2023).
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Yadav, A.R.; Sriram, R.; Carter, J.A.; Miller, B.L. Comparative study of solution-phase and vapor-phase deposition of aminosilanes on silicon dioxide surfaces. Mater. Sci. Eng. C 2014, 35, 283–290. [Google Scholar] [CrossRef]
- Sareło, Z.; Białońska, K.; Walczak, U.; Wołowiec, A. Mixed-Generation PAMAM G3-G0 Megamer as a Drug Delivery System for Nimesulide: Antitumor Activity of the Conjugate Against Human Squamous Carcinoma and Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 4998. [Google Scholar] [CrossRef]
- Li, J.; Swanson, D.R.; Qin, D.; Brothers, H.M.; Piehler, L.T.; Tomalia, D.; Meier, D.J. Characterizations of Core−Shell Tecto-(Dendrimer) Molecules by Tapping Mode Atomic Force Microscopy. Langmuir 1999, 15, 7347–7350. [Google Scholar] [CrossRef]




| Name | Sequence 5′→3′ | Length, Nt | Mw, Da |
|---|---|---|---|
| S36, oligotarget | TTCGGGAGCATGCCGCAGCTGCGGATGTGGTGCTGGATTTCGA | 43 | 13,321 |
| Z36, oligosensor | CGAAATCCAGCACCACATCCGCAGCTG-(Biotin) | 27 | 8157 |
| S37, oligotarget | CTGAAAGGGGGACTGTGGACGAGTTCGCGCTCAAAAT | 37 | 11,467 |
| Z37, oligosensor | GAGCGCGAACTCGTCCACAGTCCC-(Biotin) | 24 | 7261 |
| Outer PC Surface | APTES | PAMAM G4-GA |
|---|---|---|
| Δd, nm | 1.2 | 16.5 |
| Outer PC Surface | Biotin | PAMAM G4-Biotin (1:8) | PAMAM G4-Biotin (1:64) |
|---|---|---|---|
| Δd, nm | 0.8 | 1.9 | 15 |
| Outer PC Surface | APTES | PAMAM G4 GA | PAMAM G4-Biotin |
|---|---|---|---|
| Δd, nm | 0.11 | 2.1 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakurov, R.; Sizova, S.; Dudik, S.; Serkina, A.; Bazhutov, M.; Stanaityte, V.; Tulyagin, P.; Konopsky, V.; Alieva, E.; Sekatskii, S.; et al. Dendrimer-Based Coatings on a Photonic Crystal Surface for Ultra-Sensitive Small Molecule Detection. Polymers 2023, 15, 2607. https://doi.org/10.3390/polym15122607
Shakurov R, Sizova S, Dudik S, Serkina A, Bazhutov M, Stanaityte V, Tulyagin P, Konopsky V, Alieva E, Sekatskii S, et al. Dendrimer-Based Coatings on a Photonic Crystal Surface for Ultra-Sensitive Small Molecule Detection. Polymers. 2023; 15(12):2607. https://doi.org/10.3390/polym15122607
Chicago/Turabian StyleShakurov, Ruslan, Svetlana Sizova, Stepan Dudik, Anna Serkina, Mark Bazhutov, Viktorija Stanaityte, Petr Tulyagin, Valery Konopsky, Elena Alieva, Sergey Sekatskii, and et al. 2023. "Dendrimer-Based Coatings on a Photonic Crystal Surface for Ultra-Sensitive Small Molecule Detection" Polymers 15, no. 12: 2607. https://doi.org/10.3390/polym15122607
APA StyleShakurov, R., Sizova, S., Dudik, S., Serkina, A., Bazhutov, M., Stanaityte, V., Tulyagin, P., Konopsky, V., Alieva, E., Sekatskii, S., Bespyatykh, J., & Basmanov, D. (2023). Dendrimer-Based Coatings on a Photonic Crystal Surface for Ultra-Sensitive Small Molecule Detection. Polymers, 15(12), 2607. https://doi.org/10.3390/polym15122607










