Abstract
Microbial polysaccharides are natural carbohydrates that can confer adhesion capacity to cells and protect them from harsh environments. Due to their various physiological activities, these macromolecules are widely used in food, medicine, environmental, cosmetic, and textile applications. Microbial co-culture is an important strategy that is used to increase the production of microbial polysaccharides or produce new polysaccharides (structural alterations). This is achieved by exploiting the symbiotic/antagonistic/chemo-sensitive interactions between microbes and stimulating the expression of relevant silent genes. In this article, we review the performance of polysaccharides produced using microbial co-culture in terms of yield, antioxidant activity, and antibacterial, antitumor, and anti-inflammatory properties, in addition to the advantages and application prospects of co-culture. Moreover, the potential for microbial polysaccharides to be used in various applications is discussed.
1. Introduction
Polysaccharides, as natural polymers, are widely found in plants, animals, marine organisms, and microorganisms, and their significant biological activity has attracted a lot of attention []. Many studies have demonstrated that polysaccharides possess a variety of biological activities, such as antitumor, antioxidant, immunomodulatory, antibacterial, hepatoprotective, and anti-inflammatory [], which are in turn linked to their molecular weight, monosaccharide composition, glycosidic bonds, and branching degree. However, the low yields of polysaccharides and difficulties in their production and purification have led to limited research on them; this can be improved through co-culture. For example, the co-culture of Lactobacillus rhamnosus with Saccharomyces cerevisiae increases the yield of polysaccharides by approximately 40%; in addition, co-culture has been shown to increase the levels of metabolites [].
Microbial co-culture is the process of directly or indirectly cultivating different types of microbial cells in a limited environment (anaerobic or aerobic conditions), simulating the interactions between microorganisms in natural environment []. Many biosynthetic genes are usually not expressed or expressed at low levels under laboratory conditions which prevents the realization of the full metabolic potential of these microorganisms []. However, co-culture has been proved to be a practical strategy in inducing the biosynthesis of secondary metabolites by activating the silent genes. (Figure 1) []. To date, 33 novel secondary metabolites have been successfully obtained from actinomycetes through co-culture with mycolic acid-containing bacteria []. Further, the co-culture-induced production of extracellular polymeric substances (EPSs) by cyanobacteria, microalgae, and basidiomycetes was found to be associated with a decrease in the fermentation time and an increase in EPSs production. Co-cultured EPS is a combination of monosaccharides from two purely cultured EPS, resulting in a new structure []. Here, we summarized the classification and applications of polysaccharides and co-cultures, compared the EPSs between microbial co-cultures with pure cultures, in addition to discussing the yield of co-cultured EPSs and their effects in antioxidant, antibacterial, antitumor, and anti-inflammatory properties.
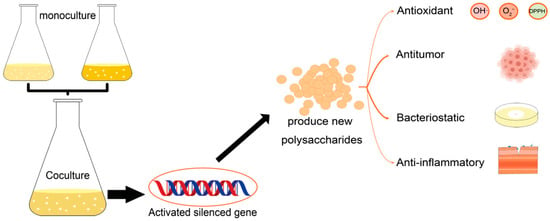
Figure 1.
Polysaccharides production by co-culture and its physiological activities.
2. Classification and Application of Polysaccharides
Polysaccharides are classified according to their biological origin as either plant, animal, or microbial polysaccharides. Plant polysaccharides have the advantages of biodegradability, ease of modification, low immunogenicity, and low toxicity []. Moreover, they can be conjugated, cross-linked, or functionally modified and then used as nanocarrier materials for polysaccharide delivery systems based on the therapeutic effects of traditional Chinese medicine []. Animal polysaccharides can be prepared as nanofibers via electrospinning technology and then applied to tissue engineering, wound healing, and drug delivery []. Microbial polysaccharides are carbohydrate polymers with high molecular weights. They are classified as capsular polysaccharides, lipopolysaccharides (LPSs), and EPSs []. Microbial EPSs, with shorter production times, easy extraction operations, and the ability to be applied to numerous fields, are becoming popular for research, and these include EPSs produced by endophytic bacteria, extremophiles, microalgae, cyanobacteria, and mixed microbial communities. In food, the EPS of Lactobacillus plantarum BR2, with high antioxidant activity and antidiabetic and cholesterol-lowering potential, shows great promise for use in functional foods []. From an environmental perspective, EPS produced by Bacillus sp. MC3B-22 and Microbacterium sp. MC3B-10 (Microbactan), which has biosorption potential when cadmium is involved, offers an alternative approach to heavy metal remediation, especially in industrial waste water []. In health care, EPSs produced by Rhodotorula mucilaginosa UANL−001L co-cultured with Escherichia coli can inhibit biofilm formation and be a promising antimicrobial agent []. Further, edible fungal polysaccharides can reach the distal intestine and be assimilated to remodel the gut microbiome (GM) []. For example, mushroom polysaccharides improve intestinal inflammation and barrier functions by altering the GM composition and increasing the synthesis of short-chain fatty acids []. Moreover, Ganoderma polysaccharides can result in remodeling of the disordered GM in rats with type 2 diabetes mellitus and improve host metabolism to achieve antidiabetic effects []. The market for microbial polysaccharides is expanding, as they can be produced using industrial waste as a substrate and have various desired properties. This can overcome challenges in production and purification and improve product quality, which could help in the path to their commercialization.
3. Microbial Co-Culture
3.1. Advantages and Applications of Co-Culture
Microbial co-culture comprises a group of natural microorganisms that is formed by different species or the same species but different strains, in which members can interact with each other symbiotically/antagonistically/chemo-sensitively. This can induce the synthesis of natural products, which can occur via the activation of silent biosynthetic genes through the modulation of signaling molecules and also by providing precursors to promote the biosynthesis of natural products [,,]. Co-culture methods include growth in liquid media, solid−liquid interface systems, membrane separations, spatial separations, and microfluidic systems different co-culture methods are suitable for different microorganisms and objectives [].
The advantages of co-culture can be maximized by designing co-culture substrates and strains. Co-culture advantages include direct cross-feeding [] and modularity []. The former comprises the conversion of inexpensive, widely available substrate/energy sources into intermediate metabolites, which can subsequently be converted to higher value products through sequential and/or parallel conversion. S. cerevisiae and Scheffersomyces stipitis are useful strains for ethanol production. Here, the former cannot break down xylose, whereas the latter can absorb both sugars and produce bioethanol efficiently in glucose−xylose mixtures []. Modularity reduces the metabolic burdens on each component strain, thus contributing to improved overall bioproduction/biotransformation performance []. Different modules of the rosmarinic acid pathway were previously adapted by developing co-cultures of three metabolically engineered E. coli strains []. The glucose catabolic pathway was disrupted in two strains such that they grew only on xylose, whereas the third strain grew only on glucose. The stability of the co-culture system was improved by reducing competition for carbon sources, and the end product concentration was found to be significantly higher. Co-cultivation also allows for a diverse environment, which might be more suitable for the expression of genes in specific pathways than the homogeneous environment provided by individual strains. This also reduces the adverse effects of byproducts, thus improving biosynthetic performance []. Therefore, microbial co-culture has a wide range of application prospects.
3.2. Metabolites
The co-culture of microorganisms can be achieved in solid or liquid media and is widely used to study natural interactions and discover new active metabolites [,,]. Co-culture not only resulted in the production of new metabolic products, but also increased the yield of the original metabolites. Five metabolites were isolated after the co-culture of Streptomyces rochei MB037 with the fungus Rhinocladiella similis 35. Compounds 1 and 2 (Figure 2) were obtained only from co-culture, and compound 1 showed significant antibacterial activity against methicillin-resistant Staphylococcus aureus. Compound 3 was present in both co-culture and monoculture, but its yield was significantly increased through co-culture []. Six new isoprenylated chromene derivatives and two new isoprenylated phenol glucoside derivatives were produced via the co-culture of Pestalotiopsis sp. and Penicillium bialowiezense, and compounds 1a and 1b (Figure 2) were found to have potent β-glucuronidase-inhibitory effects [].
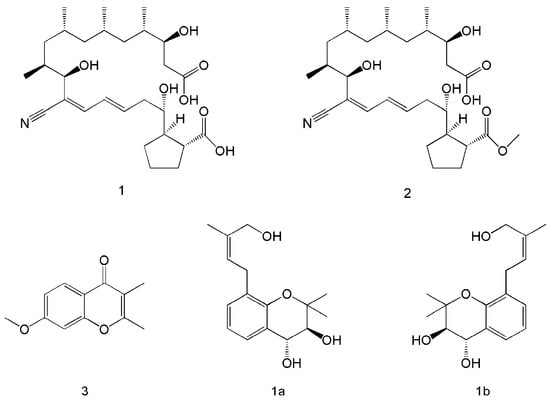
Figure 2.
Co-culture to produce metabolites with new structural formulae. Compounds 1, 2, and 3 were produced through the co-culture of Streptomyces rochei MB037 and Rhinocladiella similis 35 []. Compounds 1a and 1b were produced via the co-culture of Pestalotiopsis sp. and Penicillium bialowiezense [].
In terms of polysaccharide production through co-culture, it is also possible to obtain new EPSs or increase their yield. When Cellulomonas sp. (KYM-7) was co-cultured with Agrobacterium tumefaciens (KYM-8), KYM-7 was able to utilize starch but could not produce polysaccharides on its own. Meanwhile, KYM-8 was not able to degrade starch but could utilize the products of KYM-7 to produce polysaccharides. Both can produce only very small amounts of polysaccharides when cultured alone but can produce large amounts of polysaccharides upon co-culture []. Lactobacillus kefiranofaciens that produces EPSs was cultured in pairs with L. bulgaricus and Streptococcus thermophilus that do not produce EPSs. Through Fourier-transform infrared spectroscopy, scanning electron microscopy, and monosaccharide composition analysis, the EPS produced by co-culture have different monosaccharide compositions and surface morphologies compared with L. kefiranofaciens []. Thus, co-culture is a useful strategy to produce natural products with diverse structures and biologically active [], for which the functions and mechanisms are unknown [].
4. Comparison of Co- and Pure Culture-Produced Polysaccharides
Compared to pure culture, the advantages of microbial polysaccharide production by co-culture outweigh the disadvantages. When co-cultures of Sanghuangporus lonicericola and Cordyceps militaris were performed, the EPSs content were higher than that in the pure cultures of both S. lonicericola and C. militaris []. Notably, in pure culture, co-culture, and triple fermentation of Ganoderma lucidum (A), Tricholoma matsutake (B), and Cordyceps sinensis (C): the pure culture of A had the highest EPSs content, A-B co-culture had slightly lower EPSs content than A, and A-B-C triple fermentation had the lowest EPSs content. This indicates that not every co-culture can increase the content of EPSs, and the interaction between strains and the selection of medium need to be considered []. Co-culture needs to consider the interaction between strains as well as the growth rate, which highlights the advantages of pure culture in comparison. However, this can be refined by pre-experiments and by using pre-and post-inoculation.
Co-culture remained stronger than pure culture in terms of biological activity. The EPSs from the co-culture of S. lonicericola and C. militaris showed greater scavenging effects on 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide anions (O2−), and hydroxyl radicals (OH∙) than their pure culture counterparts []. However, there were some exceptions where co-culture with Saccharomyces boulardii was added during the fermentation of G. lucidum, and the EPSs that were produced were comparable to those produced by G. lucidum monoculture in terms of scavenging 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), DPPH, and OH∙ []. In the study of lipid peroxidation, the co- and pure cultures of Marasmius androsaceus and C. militaris were used to extract their EPSs separately and test them with rat liver homogenate; the inhibition rate decreased in the following order: M. androsaceus > co-culture > C. militaris []. This result may be due to the low content of new EPSs produced by co-culture or the new EPSs are inherently less effective in inhibiting lipid peroxidation than M. androsaceus. In the three-fungal co-fermentation test, erythrocyte membranes were used as the experimental material, and the lowest relative oxidation rate was determined for three-fungal co-fermentation, followed by two-fungal co-fermentation []. Although the experimental materials and evaluation methods were different, the obtained results were similar. Based on suitable strain combination, co-culture polysaccharides had comparable biological activities to those from pure culture, while improving the polysaccharide yield; this could be further improved.
In the isolation, purification, and structural characterization of polysaccharides, at least one fraction can be obtained from pure and co-cultured polysaccharides via ion exchange and gel filtration. One fraction (CP1) was isolated from the co-culture of G. lucidum and Flammulina velutipes, two fractions (GP1 and GP2-1) were isolated from the monoculture of G. lucidum, and one fraction (FP1-1) was isolated from the monoculture of F. velutipes. Subsequent infrared spectroscopy revealed that the chemical structure of CP1 was different to that of GP1, GP2-1, and FP1-1 []; in addition, four fractions were isolated from the co-culture of S. lonicericola and C. militaris, and three fractions were isolated from both S. lonicericola and C. militaris []. In summary, alterations in fractions or chemical structure affected the biological activity of polysaccharides. The co-culture technique can interfere with the expression of the secondary metabolite genome through the exchange of signaling molecules, thereby regulating the transcription, translation, and metabolic levels of secondary metabolites, which results in changes in the chemical structure of the secondary metabolites []. Therefore, the co-culture method is better at exploiting the potential of microorganisms and promoting the production of microbial polysaccharides than the pure culture method.
5. Co-Culture-Derived Microbial Polysaccharides
5.1. Polysaccharide Yields
Polysaccharides are highly active and versatile molecules used in the food, chemical, and pharmaceutical industries []; improving polysaccharide yields has become essential for the improvement of their commercialization and industrialization []. Compared to that with monoculture, co-culture fermentation can markedly improve polysaccharide yields. The production of extracellular proteins and EPSs during co-cultivation of one strain of Nostoc sp. (F280) and two strains of Anabaena cylindrica (B1611 and F243) was investigated. Co-cultures of F280 and B1611 not only yielded the highest dry weights, but also higher yields of extracellular proteins and cell-bound polysaccharides compared to that of F280 monoculture; meanwhile, F280 and F243 co-culture resulted in the highest content of released polysaccharides []. EPS production by Inonotus obliquus can also be increased by adding stimulants (VB6, VB1, birchwood, and birch extract), and the addition of different stimulants changes the monosaccharide composition of the polysaccharide, which will affect its physiological activity []. Further, a co-culture approach improves polysaccharide production and some physiological activities owing to the production of some novel polysaccharides and secondary metabolites during the co-culture process; the co-culture of different microorganisms might also change the resulting polysaccharide species and structure. In addition to using microbial co-culture to increase the production of polysaccharides, there are also other ways to increase their production (Table 1).

Table 1.
Methods for increasing polysaccharide yields.
5.2. Antioxidant Activity
The antioxidant activity of polysaccharides is closely related to their chemical properties, such as their molecular weight, degree of branching, monosaccharide type, monosaccharide ratio, glycosidic bonds, and modifications []. For example, with a decrease in molecular weight, the antioxidant activity of chitosan increases significantly []. The basic principle is that molecules with low molecular weights can be incorporated into cells more effectively than those with high molecular weights and donate protons []. In an evaluation of polysaccharides extracted from mushroom culture filtrate, the antioxidant activity of polysaccharides was found to depend on the proportion of different combinations of monosaccharides. In simple sugars, rhamnose is a determinant of antioxidant activity. For glycosidic bonds, the side chains arabinose 1→4 and mannose 1→2 are significantly correlated with reducing power, whereas glucose 1→6 and arabinose 1→4 are correlated with DPPH radical scavenging []. In terms of polysaccharide modification, hydroxymethylated pumpkin polysaccharide has better O2− and OH∙ radical scavenging ability []. Garlic polysaccharide was co-heated with ferric chloride to synthesize a garlic polysaccharide–Fe (III) complex, and its O2− radical scavenging ability was significantly better than that of garlic polysaccharide, showing a good synergistic effect, but the OH∙ radical scavenging activity was similar []. Moreover, the antioxidant capacity of polysaccharides can be improved by heat stress, ultrasound, enzymatic degradation, and sulfonation (Table 2).

Table 2.
Methods for improving the antioxidant activity of polysaccharides.
5.2.1. DPPH Radical Scavenging Activity
DPPH comprises a stable nitrogen centered on a radical with an unpaired electron, which is responsible for strong absorbance at 517 nm. The amount of this radical is easily reduced upon exposure to proton radical scavengers []. DPPH solutions change from dark purple to yellow when antioxidants are present. The co-culture of two strains, Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12, was performed to detect the DPPH free radical scavenging rate using a polysaccharide concentration range of 0–2 mg/mL. With an increase in the concentration, the scavenging rate also increased. Polysaccharides obtained via co-culture also had better DPPH free radical scavenging ability and different heat resistance compared with that of polysaccharides from the LA5 pure culture []. However, this ability is not generated by co-culture, as purely cultured EPSs also have the ability to scavenge DPPH and are heat resistant. Co-cultures are aimed at producing EPSs with structural alterations that allow them to improve their DPPH scavenging ability on top of the original one, but not all co-cultures can improve either.
5.2.2. OH∙ Radical Scavenging Activity
A radical is defined as a molecule or molecular fragment that contains one or more unpaired electrons in an atomic or molecular orbital []. Free radicals produced through normal biological oxidation are beneficial to the body, as they regulate cell signaling and cytogenesis and inhibit the entry of viruses and bacteria into the body to prevent infection []; however, highly reactive OH∙ radicals can react rapidly with biomolecules and can cause serious damage to adjacent organs and tissues []. Therefore, it is necessary to improve the antioxidant capacity of polysaccharides. Photobacterium sp. LYM-1 and Aureobasidium pullulans 2012 were co-cultured and cultured separately; subsequently, the water-soluble polysaccharides (WSPs) produced in fermentations of each strain and the co-culture were extracted and used for OH∙ scavenging assays, separately. With each of the measured concentrations, the WSPs from co-culture exhibited a stronger ability to scavenge OH∙ than the other two WSPs factions [].
5.2.3. O2− Radical Scavenging Activity
Mitochondrial lipid membranes are vulnerable to reactive oxygen species (ROS) attack because mitochondria are the most important source of ROS. O2− is considered to be the major ROS because it is formed by adding one electron to molecular oxygen []. In a radical scavenging assay, 1,2,3-benzenetriol is rapidly autoxidized in alkaline solutions and produces intermediate products; polysaccharides can interfere with 1,2,3-benzenetriol autoxidation. Therefore, the inhibitory activity of 1,2,3-benzenetriol has been used to assess the ability of polysaccharides to scavenge O2− []. Co-culture-generated polysaccharides from Marasmius androsaceus and Cordyceps militaris did not have a good scavenging effect on O2− radicals and no quantitative-effect relationship []. At the same concentration, the ability of polysaccharides extracted from co-cultures to scavenge O2− was not significantly increased compared to that of polysaccharides from monoculture. Notably, the O2− scavenging ability of the co-culture-generated polysaccharides was enhanced via chelation with Fe (III). Moreover, Lepista sordida and Pholiota nameko were co-cultured to produce two polysaccharides (CP-1 and CP-3) and polysaccharides were chelated with iron (III) via –OH and –COOH groups to form stable β-FeOOH structures, CP-1-Fe and CP-3-Fe, which showed antioxidant activities. Furthermore, CP-1-Fe and CP-3-Fe exhibited higher OH∙ and O2− radical scavenging ability than CP-1 and CP-3. When the concentration reached 2.4 mg/mL, the O2− radical clearance rates of CP-1-Fe and CP-3-Fe could reach 96.41% and 92.83%, which were 108.45% and 159.37% higher than those with the same concentration of CP-1 and CP-3, respectively []. Although the co-culture-generated polysaccharides were not effective in O2− scavenging, their antioxidant capacity could be further enhanced after forming chelates with metal ions.
5.3. Antibacterial Activity
The bacteriostatic mechanism of polysaccharides is mainly through their effect on the cell wall, cell membrane, nucleic acids, proteins, and spores, among other targets, which can result in bactericidal or bacteriostatic effects. The polycationic molecules of chitosan can interact with the anionic groups of the cell wall, thus altering the permeability of the bacterial cell wall []. Chitosan can also alter the permeability of the cell membrane, further preventing the entry and exit of nutrients and eventually leading to cell death []. However, gram-positive bacteria are resistant to this as they have peptidoglycan walls []. Moreover, gram-negative bacteria hydrolyze polysaccharides into monosaccharides that can be utilized as nutrient sources owing to the absence of a peptidoglycan wall []. With respect to nucleic acids, which can block the transcription of DNA and interfere with RNA molecules [], S. giganteus H03 polysaccharide exerts antibacterial activity against S. aureus, partly because of its ability to bind to plasmid DNA []. Proteins are important components of the bacterial structure and are involved in the catalysis of many biochemical reactions and bacterial metabolism []; based on this, antimicrobial activity was previously assessed using Cordyceps polysaccharides []. The decrease in total protein concentrations in E. coli cells and the increase in soluble proteins in the cultures indicated a change in membrane permeability, resulting in the inhibition of E. coli growth. Moreover, WSPs from cabbage were found to have a good inhibitory effect on spore formation by gram-positive Bacillus []. In addition to these aspects, polysaccharides can also exert effects on biofilms and intracellular metabolic pathways []. These are mechanisms that could lead to their use as antimicrobial agents (Figure 3).
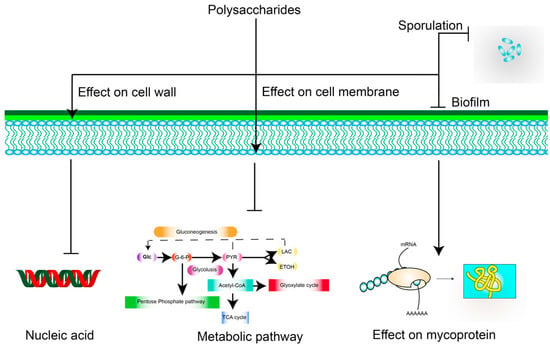
Figure 3.
Possible mechanisms underlying the inhibitory effects of polysaccharides against bacteria. Arrows represent activation; ┴ represent inhibition.
The introduction of quaternary ammonium salts via thiol-Michael addition can impart considerable antibacterial activity to the adducts of polysaccharide for use against S. aureus (gram-positive bacteria) and E. coli (gram-negative bacteria), but the inhibitory effect on gram-positive bacteria is superior to that on gram-negative bacteria []. In addition, the polysaccharides from peony seeds (PSPS) have been modified via sulfation, carboxymethylation, and phosphorylated; the PSPS and their derivatives showed significant antibacterial activity against gram-positive (Bacillus subtilis and S. aureus) and gram-negative (E. coli and S. typhi) bacteria. Further, Enteromorpha prolifera polysaccharide was formed into degraded polysaccharide (LEP), followed by the synthesis of degraded polysaccharide selenide (Se-LEP). Se-LEP showed stronger inhibitory effects on E. coli and weaker inhibitory effects on S. aureus than polysaccharide selenide (Se-EP). Se-LEP also showed better inhibitory effects on plant pathogenic fungi [].
Co- and monocultures were also prepared with Lepista sordida and Pholiota nameko, and L. sordida polysaccharide (LSP), P. nameko polysaccharide (PNP), and co-culture-derived polysaccharide (LPP) were extracted; then, the inhibition effect of the polysaccharides in solid and liquid media was determined via the inhibition circle and 96-well plate method. The three polysaccharides were found to have some inhibitory effects on E. coli, S. aureus, Listeria monocytogenes, Salmonella enteritidis, and Lactobacillus but poorer inhibitory effects on aflatoxin, and the inhibitory effect occurred in the following order: LPP > LSP > PNP. Moreover, co-cultured polysaccharides showed significant inhibitory effects on both gram-positive and gram-negative bacteria. However, the inhibitory effect on mycobacteria was slightly weaker []. In contrast, co-culture can be used to obtain not only new metabolites, but also those with good antibacterial activity, which can be a good way to prepare antibacterial agents.
5.4. Antitumor Activity
Malignant tumors are currently an important disease that endangers human health []; they are mainly treated using surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy. However, these therapies can cause physical and economic burdens to patients, whereas polysaccharides not only have antitumor activity, but also are less expensive and have few toxic side effects []. They are thus gradually becoming a hot spot for antitumor research. Trametes versicolor, fucoidan, and sepia ink polysaccharides can be used as adjuvant therapies for the treatment of tumors [,,]. Polysaccharides mainly exert antitumor effects by blocking the cell cycle or through antitumor angiogenesis, apoptosis-inducing, and immunomodulatory mechanisms (Figure 4) [], and investigating ways to further improve the antitumor activity of polysaccharides is worthwhile.
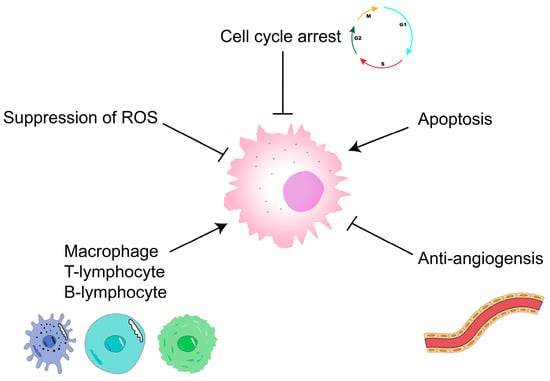
Figure 4.
Antitumor mechanisms of polysaccharides. ┴ represent inhibition.
The antitumor activity of polysaccharides is influenced by various factors, such as the backbone structure, branched chain properties, and molecular advanced structure []. The modification of polysaccharides via sulfation, carboxymethylation, phosphorylation, and acetylation enhances their antitumor activity to some extent []. Combining cisplatin with G. lucidum polysaccharide not only inhibits tumor growth, but also improves spleen and thymus indices and reduces toxic side effects []. Moreover, oridonin in combination with lentinan might enhance the antitumor effect of the latter []. Co-culture fermentation can also improve antitumor activity. Using co-culture fermentation with G. lucidum and T. matsutake, the antitumor activity of co-leavened polysaccharide was found to be superior to that after single bacterial fermentation or with a combination of G. lucidum and T. matsutake polysaccharides []. T. matsutake and C. militaris were also co-cultured and fermented, and the EPSs of single and mixed bacteria were, respectively, extracted and used to treat B16 melanoma for 72 h. The results showed that the EPSs of mixed bacteria had better antitumor activity than those of single bacteria [].
5.5. Anti-Inflammatory Activity
Inflammation is a physiological response of the organism to protect the host from damage triggered by abiotic and biotic factors, such as bacterial infections and harmful stimuli []. Chronic inflammation is closely related to many diseases, such as arthritis, atherosclerosis, and cancer. The treatment of inflammation is focused on steroids and non-steroidal anti-inflammatory drugs []; and the mechanisms through which polysaccharides exert anti-inflammatory effects are mainly via the inhibition of inflammation-related cytokines, inhibition of iNOS and COX-2 expression, modulation of NF-κB-related signaling pathways, effects on the immune system, and other factors [].
A novel polysaccharide (MRP-1) was obtained from Moringa oleifera, and treatment with different concentrations of MRP-1 prevented LPS-induced increases in NO and TNF-α production and caused a significant reduction in LPS-induced iNOS mRNA expression levels, but had no significant effect on COX-2 mRNA expression []. The anti-inflammatory activity of polysaccharides from G. lucidum fermented in co-culture with Flammulina velutipes was also assessed based on the inhibition rate of COX enzymes (both COX-1 and COX-2), which was 74.5% and 75.4%, respectively, for co-cultured EPS at 3 mg/mL, but only 68.7% and 71.6% for G. lucidum polysaccharides and even lower for F. velutipes polysaccharides. In addition, co-cultured EPS also exhibited lower cytotoxicity than G. lucidum polysaccharides [].
6. Summary and Prospect
Polysaccharides, as ubiquitous biomolecules, are used as food supplements and medicines because they are safe and non-toxic and also have physiological activities, such as antitumor, anti-inflammatory, antibacterial, and antioxidant properties. The generation of new metabolites can be achieved via co-culture, which is a feasible strategy to increase polysaccharide production without compromising their biological activity []. However, there are relatively few studies on the use of co-culture to produce polysaccharides, and most of them have only focused on co-culture to improve polysaccharide yields and antioxidant activity, which has limited the development of novel polysaccharides generated through co-culture. Among the aforementioned physiological activities, some mechanisms have been elucidated through in vitro and animal experiments, but more experimental and clinical studies on the practical application of polysaccharides are needed. Together with the continuous optimization of extraction methods and techniques, it is believed that co-culture has obvious significance for promoting the development of polysaccharides.
Author Contributions
Conceptualization, W.P. and X.G.; software and visualization, X.X. and D.Z.; writing—original draft preparation, W.P.; funding acquisition, review and editing, H.Z. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research and Frontier Exploration Foundation of Chongqing (cstc2017jcyjBX00078) and Chengdu University Talent Project (2081922015).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article. No data was used for the research described in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.X.; Yang, J.; Shen, M.Y.; Chen, Y.; Yu, Q.; Xie, J.H. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Shi, M.M.; Yan, Y.Z.; Liu, Y.Q. An insight into the research concerning Panax ginseng C. A. Meyer polysaccharides: A review. Food Rev. Int. 2020, 38, 1149–1165. [Google Scholar] [CrossRef]
- Bertsch, A.; Roy, D.; LaPointe, G. Enhanced exopolysaccharide production by Lactobacillus rhamnosus in co-culture with Saccharomyces cerevisiae. Appl. Sci. 2019, 9, 4026. [Google Scholar] [CrossRef]
- Liu, C.; Kakeya, H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem.-Asian J. 2020, 15, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nicault, M.; Zaiter, A.; Dumarcay, S.; Chaimbault, P.; Gelhaye, E.; Leblond, P.; Bontemps, C. Elicitation of antimicrobial active compounds by Streptomyces-fungus co-cultures. Microorganisms 2021, 9, 178. [Google Scholar] [CrossRef]
- Murakami, S.; Hayashi, N.; Inomata, T.; Kato, H.; Hitora, Y.; Tsukamoto, S. Induction of secondary metabolite production by fungal co-culture of Talaromyces pinophilus and Paraphaeosphaeria sp. J. Nat. Med. 2020, 74, 545–549. [Google Scholar] [CrossRef]
- Hoshino, S.; Onaka, H.; Abe, I. Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria. J. Ind. Microbiol. Biotech. 2019, 46, 363–374. [Google Scholar] [CrossRef]
- Angelis, S.; Novak, A.C.; Sydney, E.B.; Soccol, V.T.; Carvalho, J.C.; Pandey, A.; Noseda, M.D.; Tholozan, J.L.; Lorquin, J.; Soccol, C.R. Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: Process preliminary optimization and partial characterization. Appl. Biochem. Biotech. 2012, 167, 1092–1106. [Google Scholar] [CrossRef]
- Gong, H.X.; Li, W.A.; Sun, J.L.; Jia, L.; Guan, Q.X.; Guo, Y.Y.; Wang, Y.H. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int. J. Biol. Macromol. 2022, 211, 711–728. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, W.; Li, J.; Lin, X.; Wang, Y. Preparation of animal polysaccharides nanofibers by electrospinning and their potential biomedical applications. J. Biomed. Mater. Res. A 2015, 103, 807–818. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Chab, J.C.; Castaneda-Chavez, M.D.; Chan-Bacab, M.J.; Aguila-Ramirez, R.N.; Galaviz-Villa, I.; Bartolo-Perez, P.; Lango-Reynoso, F.; Tabasco-Novelo, C.; Gaylarde, C.; Ortega-Morales, B.O. Biosorption of cadmium by non-toxic extracellular polymeric substances (EPS) synthesized by bacteria from marine intertidal biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Perez, D.B.; Vazquez-Garza, E.; Chapoy-Villanueva, H.; Garcia-Rivas, G.; Garza-Cervantes, J.A.; Gomez-Lugo, J.J.; Gomez-Loredo, A.E.; Gonzalez, M.T.G.; et al. Microbial competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of non-toxic exopolysaccharide with applications as a wide-spectrum antimicrobial. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef]
- Liang, J.J.; Zhang, M.N.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohyd. Polym. 2021, 273, 12. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, W.J.; Wang, L.M.; Yu, C.; Zhang, G.L.; Zhu, H.L.; Wang, C.W.; Zhao, S.J.; Hu, C.A.A.; Liu, Y.L. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef]
- Chen, M.Y.; Xiao, D.; Liu, W.; Song, Y.F.; Zou, B.R.; Li, L.; Li, P.; Cai, Y.; Liu, D.L.; Liao, Q.F.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Knowles, S.L.; Raja, H.A.; Roberts, C.D.; Oberlies, N.H. Fungal-fungal co-culture: A primer for generating chemical diversity. Nat. Prod. Rep. 2022, 39, 1557–1573. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodriguez-Jasso, R.M.; Aguilar, C.N.; Buitron, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 11. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhang, H.R. Utilizing cross-species co-cultures for discovery of novel natural products. Curr. Opin. Biotech. 2021, 69, 252–262. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-culturing microbial consortia: Approaches for applications in biomanufacturing and bioprocessing. Crit. Rev. Biotech. 2022, 42, 46–72. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Olm, I.P.; Sousa, D.Z. Synthetic co-cultures: Novel avenues for bio-based processes. Curr. Opin. Biotech. 2021, 67, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Wang, X.N. Modular co-culture engineering, a new approach for metabolic engineering. Metab. Eng. 2016, 37, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, H.; Liu, Y.X.; Zhuang, L.; Zhang, H.R.; Koffas, M.A.G. Utilization of microbial cocultures for converting mixed substrates to valuable bioproducts. Curr. Opin. Microbiol. 2022, 68, 9. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, X.N.; Zhang, H.R. Balancing the non-linear rosmarinic acid biosynthetic pathway by modular co-culture engineering. Metab. Eng. 2019, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Onaka, H.; Mori, Y.; Igarashi, Y.; Furumai, T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl. Environ. Microbiol. 2011, 77, 400–406. [Google Scholar] [CrossRef]
- Yu, M.L.; Li, Y.X.; Banakar, S.P.; Liu, L.; Shao, C.L.; Li, Z.Y.; Wang, C.Y. New metabolites from the co-culture of marine-derived actinomycete Streptomyces rochei MB037 and fungus Rhinocladiella similis 35. Front. Microbiol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Li, F.L.; Yan, S.; Huang, Z.Y.; Gao, W.X.; Zhang, S.T.; Mo, S.Y.; Lin, S.; Wang, J.P.; Hu, Z.X.; Zhang, Y.H. Inducing new bioactive metabolites production from coculture of Pestalotiopsis sp. and Penicillium bialowiezense. Bioorg. Chem. 2021, 110, 9. [Google Scholar] [CrossRef]
- Kurata, S.; Yamada, K.; Takatsu, K.; Hanada, S.; Koyama, O.; Yokomaku, T.; Kamagata, Y.; Kanagawa, T.; Kurane, R. Extracellular acidic polysaccharide production by a two-membered bacterial coculture. Biosci. Biotech. Biochem. 2003, 67, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Wang, Y.P.; Anjum, N.; Ahmad, H.; Ahmad, A.; Raza, M. Characterization of new exopolysaccharides produced by coculturing of L. kefiranofaciens with yoghurt strains. Int. J. Biol. Macromol. 2013, 59, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhu, L.P.; Xu, X.Y.; Tan, L.L.; Sadilek, M.; Fan, H.; Hu, B.; Shen, X.T.; Yang, J.; Qiao, B.; et al. Discovery of novel xylosides in co-culture of basidiomycetes Trametes versicolor and Ganoderma applanatum by integrated metabolomics and bioinformatics. Sci. Rep. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Research on Co-Fermentation of Fungal Polysaccharides by Sanghuangporus lonicericola and Cordyceps militaris; Central South University of Forestry and Technology: Changsha, China, 2019. [Google Scholar]
- Yin, H. Study on Extraction, Determination of Content and Antioxygenic Activity of Mixed Fermentation Amylase of Ganoderma lucidum; Tricholoma matsutake Fruitbodies and Aweto; Jilin University: Changchun, China, 2008. [Google Scholar]
- Suo, M.Y. Study on the Co-Fermentation of Ganoderma lucidum and Saccharomyces boulardii and Its Effect on Polysaccharides Yield and Activity; Tianjin University of Science and Technology: Tianjin, China, 2020. [Google Scholar]
- Wang, Y.Z. The Comparison of Fermentation, Co-Fermentation and Polysaccharides Activities of Marasmius androsaceus and Cordyceps militaris; Jilin University: Changchun, China, 2007. [Google Scholar]
- Wu, J.Y.; Kaewnarin, K.; Nie, X.M.; Li, Q.B.; He, N.; Huang, J.L.; Geng, A.L. Biological activities of a polysaccharide from the coculture of Ganoderma lucidum and Flammulina velutipes mycelia in submerged fermentation. Process Biochem. 2021, 109, 10–18. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.T.; Li, C.L. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopath. Pharm. 2021, 35, 14. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.B.; Wang, R.; Li, S.; Qiu, Y.B.; Lei, P.; Gao, J.; Xu, H.; Zhang, F.L.; Lv, Y.F. Effects of exopolysaccharide derived from Pantoea alhagi NX-11 on drought resistance of rice and its efficient fermentation preparation. Int. J. Biol. Macromol. 2020, 162, 946–955. [Google Scholar] [CrossRef]
- Xue, C.Z.; Wang, L.B.; Wu, T.; Zhang, S.P.; Tang, T.; Wang, L.; Zhao, Q.Y.; Sun, Y.H. Characterization of co-cultivation of Cyanobacteria on growth, productions of polysaccharides and extracellular proteins, nitrogenase activity, and photosynthetic activity. Appl. Biochem. Biotech. 2017, 181, 340–349. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, Z.Z.; Zhou, X.; Hu, J.R.; Xue, J.; Liu, X.; Zhang, J.S.; Liu, P.; Tong, S.S. Simultaneous use of stimulatory agents to enhance the production and hypoglycaemic activity of polysaccharides from Inonotus obliquus by submerged fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef]
- Wang, X.Y.Z.; Dong, J.J.; Xu, G.C.; Han, R.Z.; Ni, Y. Enhanced curdlan production with nitrogen feeding during polysaccharide synthesis by Rhizobium radiobacter. Carbohydr. Polym. 2016, 150, 385–391. [Google Scholar] [CrossRef]
- Peng, R.; Fu, Y.Z.; Zou, J.; Qiu, H.; Gan, L.T.; Yi, H.L.; Yao, R.Y.; Luo, X.Y. Improvement of polysaccharide and triterpenoid production of Ganoderma lucidum through mutagenesis of protoplasts. Biotechnol. Biotechnol. Equip. 2016, 30, 381–387. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, X.; Chen, P.; Yang, S.L.; Zhang, H. Homologous overexpression of genes in Cordyceps militaris improves the production of polysaccharides. Food Res. Int. 2021, 147, 8. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Shen, S.G.; Wang, H.Y.; Yao, S.Y.; Tan, Z.L.; Zhong, C.; Jia, S.R. Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J. Appl. Phycol. 2017, 29, 55–65. [Google Scholar] [CrossRef]
- Tong, L.L.; Wang, Y.; Yuan, L.; Liu, M.Z.; Du, Y.H.; Mu, X.Y.; Yang, Q.H.; Wei, S.X.; Li, J.Y.; Wang, M.A.; et al. Enhancement of polysaccharides production using microparticle enhanced technology by Paraisaria dubia. Microb. Cell. Fact. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wu, J.Y.; Chang, C.Y.; Yu, S.T.; Liu, Y.C. Enhanced exopolysaccharide production by Cordyceps militaris using repeated batch cultivation. J. Biosci. Bioeng. 2019, 127, 499–505. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.Q.; Baker, P.J.; Yi, M.; Wu, H.; Xu, F.; Teng, Y.; Zheng, Y.G. Enhancement of cordyceps polysaccharide production via biosynthetic pathway analysis in Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 92, 872–880. [Google Scholar] [CrossRef]
- Fan, L.P.; Li, J.W.; Deng, K.Q.; Ai, L.Z. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohydr. Polym. 2012, 87, 1849–1854. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, C.H.; Tsai, G.J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef]
- Redouan, E.; Emmanuel, P.; Michelle, P.; Bernard, C.; Josiane, C.; Cedric, D. Evaluation of antioxidant capacity of ulvan-like polymer obtained by regioselective oxidation of gellan exopolysaccharide. Food Chem. 2011, 127, 976–983. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G.L. The antioxidant activities of carboxymethylated cushaw polysaccharide. Int. J. Biol. Macromol. 2019, 121, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, G.L. Synthesis and antioxidant activities of garlic polysaccharide-Fe(III) complex. Int. J. Biol. Macromol. 2020, 145, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Sun, J.S.; Xu, Z.Y.; Li, H.C.; Hu, J.; Ning, H.J.; Qin, Z.F.; Pei, H.S.; Sun, T.; Zhang, X.Q. Effect of heat stress on production and in-vitro antioxidant activity of polysaccharides in Ganoderma lucidum. Bioprocess. Biosyst. Eng. 2018, 41, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Chen, J.R.; Ren, P.F.; Zhang, Y.W.; Onayango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.L.; Zhou, Q.W.; Hao, S.X.; Zhou, T.; Xie, H.J. Enhanced in vitro antioxidant activity of polysaccharides from Enteromorpha Prolifera by enzymatic degradation. J. Food Biochem. 2016, 40, 275–283. [Google Scholar] [CrossRef]
- Li, S.Q.; Dai, S.H.; Shah, N.P. Sulfonation and antioxidative evaluation of polysaccharides from Pleurotus mushroom and Streptococcus thermophilus bacteria: A review. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 282–294. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 12. [Google Scholar] [CrossRef]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Khaledabad, M.A. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Halliwell, B. Commentary for “oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts”. Arch. Biochem. Biophys. 2022, 718, 2. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.J.; Huang, G.L. Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, M.; Zhang, Y.; Zhang, X.; Liu, X.; Li, F.; Wang, D.; Wei, M.; Yu, X. Antioxidant properties of water-soluble polysaccharides prepared by co-culture fermentation of straw and shrimp shell. Front. Nutr. 2022, 9, 1047932. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Dang, J.; Wang, Q.L.; Yu, M.F.; Jiang, L.; Mei, L.J.; Shao, Y.; Tao, Y.D. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jiang, J.; Li, W. Co-cultured Lepista sordida and Pholiota nameko polysaccharide-iron(iii) chelates exhibit good antioxidant activity. RSC Adv. 2020, 10, 27259–27265. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.T.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118, 881–885. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guo, Y.J.; Guo, L.Y.; Jiang, H.; Ji, Q.H. In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. BioMed Res. Int. 2018, 2018, 8. [Google Scholar] [CrossRef]
- Mazarei, F.; Jooyandeh, H.; Noshad, M.; Hojjati, M. Polysaccharide of caper (Capparis spinosa L.) leaf: Extraction optimization, antioxidant potential and antimicrobial activity. Int. J. Biol. Macromol. 2017, 95, 224–231. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control. 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.X.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Food. 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.Y.; Yuan, W.L.; Jin, S.Y.; Hou, S.T.; Wang, M.; Wang, H.Z. Antifungal activity of nanochitin whisker against crown rot diseases of wheat. J. Agric. Food Chem. 2018, 66, 9907–9913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Sun, Q.; Zhang, H.R.; Wang, J.Q.; Fu, Q.Z.; Qiao, H.Z.; Wang, Q. Insight into antibacterial mechanism of polysaccharides: A review. LWT-Food Sci. Technol. 2021, 150, 6. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ma, X.T.; Edgar, K.J. A versatile method for preparing polysaccharide conjugates via thiol-michael addition. Polymers 2021, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Thakur, K.; Zhang, Y.Y.; Tu, X.F.; Zhang, Y.S.; Zhu, D.Y.; Zhang, J.G.; Wei, Z.J. Effects of different chemical modifications on the antibacterial activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2018, 116, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Biological Activity of Co-Culture Polysaccharides from Lepista sordida and Pholiota nameko and Their Applications; Qingdao Agricultural University: Qingdao, China, 2018. [Google Scholar]
- Cor, D.; Knez, Z.; Hrncic, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.L. Antitumor activity of polysaccharides: An overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes versicolor (synn. Coriolus versicolor) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 18. [Google Scholar] [CrossRef]
- Li, F.P.; Liu, H.Z. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides. Mar. Drugs 2018, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, M.; Ding, Y.Y.; Yang, P.Y.; Wang, M.J.; Zhang, H.H.; He, Y.Q.; Ma, H.L. Polysaccharides as potential anti-tumor biomacromolecules -A review. Front. Nutr. 2022, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Jeya, M.; Moon, H.J.; Lee, K.M.; Kim, I.W.; Kim, J.H.; Lee, J.K. Antitumor activity of methylan polysaccharide derivatives. Biotechnol. Lett. 2010, 32, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Huang, H.L. The derivatization and antitumor mechanisms of polysaccharides. Future Med. Chem. 2017, 9, 1931–1938. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, J.; Jiang, L.L.; Huang, J.Q.; Yan, J.Y.; Chen, Y.W.; Yang, Q. Improved antitumor activity of cisplatin combined with Ganoderma lucidum polysaccharides in U14 cervical carcinoma-bearing mice. Kaohsiung J. Med. Sci. 2019, 35, 222–229. [Google Scholar] [CrossRef]
- Gui, Y.; Cheng, J.; Chen, Z.G. Oridonin improves the therapeutic effect of lentinan on lung cancer. Exp. Ther. Med. 2021, 22, 9. [Google Scholar] [CrossRef]
- Bai, C.; Wang, S.Z.; Yang, J.F.; Li, Y. The comparison of the effect of anti-tumor with G-lucidum and T-mastutake co-fermentation exopolysaccharide. Microbiol. China 2004, 31, 76–80. [Google Scholar]
- Li, T.T. The Investigation of Tricholoma matsutake and Cordyceps militaris Co-Cultivating and the Activities of Polysaccharides; Jilin University: Changchun, China, 2008. [Google Scholar]
- Wen, Z.S.; Xiang, X.W.; Jin, H.X.; Guo, X.Y.; Liu, L.J.; Huang, Y.N.; OuYang, X.K.; Qu, Y.L. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int. J. Biol. Macromol. 2016, 88, 403–413. [Google Scholar] [CrossRef]
- Bai, R.R.; Yao, C.S.; Zhong, Z.C.; Ge, J.M.; Bai, Z.Q.; Ye, X.Y.; Xie, T.; Xie, Y.Y. Discovery of natural anti-inflammatory alkaloids: Potential leads for the drug discovery for the treatment of inflammation. Eur. J. Med. Chem. 2021, 213, 22. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 21. [Google Scholar] [CrossRef]
- Cui, C.; Chen, S.; Wang, X.Y.; Yuan, G.W.; Jiang, F.; Chen, X.Y.; Wang, L. Characterization of Moringa oleifera roots polysaccharide MRP-1 with anti-inflammatory effect. Int. J. Biol. Macromol. 2019, 132, 844–851. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).