Research Progress of a Pesticide Polymer-Controlled Release System Based on Polysaccharides
Abstract
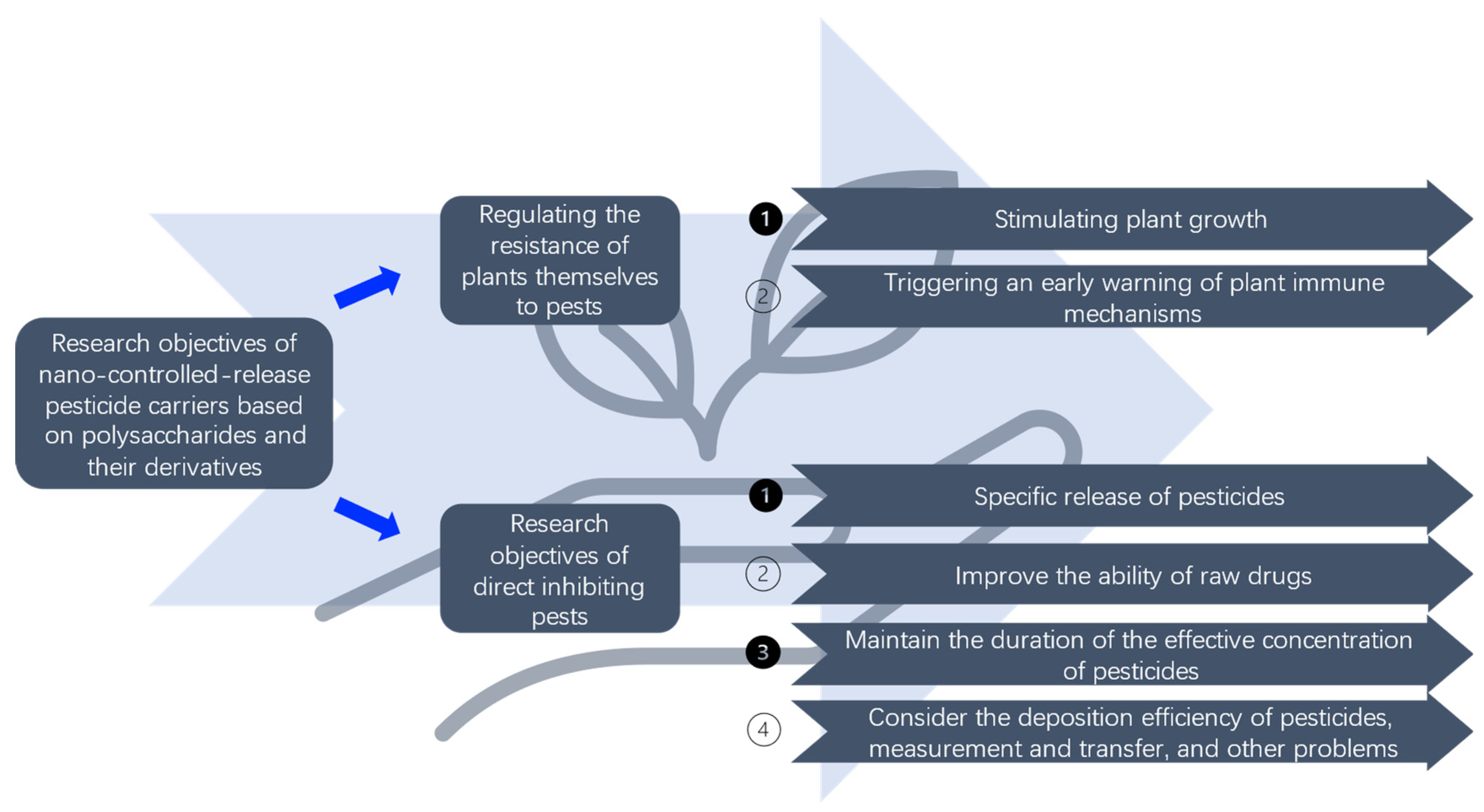
1. Introduction
2. Research Progress of Natural Polysaccharide Nano-Formulations of Pesticides
2.1. Research Progress of Chitosan and Its Derivatives as Nanoscale Sustained and Controlled Release Carriers
2.2. Research Progress of Cellulose and Its Derivatives as Nanoscale Sustained and Controlled Release Carriers
2.3. Research Progress of Starch, Dextrin and Their Derivatives as Nanoscale Sustained and Controlled Release Carriers
2.4. Research Progress of Alginic Acid and Its Derivatives as Nanoscale Sustained and Controlled Release Carriers
2.5. Research Progress of Other Polysaccharide Materials and Their Derivatives as Nanoscale Sustained and Controlled Release Carriers
3. Outlook
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira, J., Jr.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Sayed, A.M.M.; Kim, S.; Behle, R.W. Behle, Characterisation of silver nanoparticles synthesised by Bacillus thuringiensis as a nanobiopesticide for insect pest control. Biocontrol Sci. Technol. 2017, 27, 1308–1326. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects–An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Forest, V. Combined effects of nanoparticles and other environmental contaminants on human health—An issue often overlooked. NanoImpact 2021, 23, 100344. [Google Scholar] [CrossRef]
- Stoffolano, J.; Wong, R.; Lo, T.; Ford, B.; Geden, C.J. Effect of chitosan on adult longevity when fed, in no-choice experiments, to Musca domestica L., Tabanus nigrovittatus Macquart, and Phormia regina (Meigen) adults and its consumption in adult Musca domestica L. Pest Manag. Sci. 2020, 76, 4293–4300. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Muthukumar, S. Chitosan guar nanoparticle preparation and its in vitro antimicrobial activity towards phytopathogens of rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef]
- Silva, M.D.S.; Cocenza, D.S.; Grillo, R.; de Melo, N.F.S.; Tonello, P.; de Oliveira, L.C.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef]
- Li, M.; Huang, Q.; Wu, Y. A novel chitosan-poly (lactide) copolymer and its submicron particles as imidacloprid carriers. Pest Manag. Sci. 2011, 67, 831–836. [Google Scholar] [CrossRef]
- Brunel, F.; El Gueddari, N.E.; Moerschbacher, B.M. Complexation of copper(II) with chitosan nanogels: Toward control of microbial growth. Carbohydr. Polym. 2013, 92, 1348–1356. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, S.; Chang, D.; Zheng, H.; Li, J.; Liu, X.; Xu, P.; Xiong, F. One-pot synthesis of biopolymeric hollow nanospheres by photocrosslinking. Chem. Commun. 2009, 46, 8222–8224. [Google Scholar] [CrossRef]
- Sun, C.; Shu, K.; Wang, W.; Ye, Z.; Liu, T.; Gao, Y.; Zheng, H.; He, G.; Yin, Y. Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int. J. Pharm. 2014, 463, 108–114. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Meng, X.; Chen, X.; Ren, G. The preparation and characterization of a novel amphiphilic oleoyl-carboxymethyl chitosan self-assembled nanoparticles. Carbohydr. Polym. 2011, 83, 130–136. [Google Scholar] [CrossRef]
- Kamari, A.; Aljafree, N.; Yusoff, S. N,N-dimethylhexadecyl carboxymethyl chitosan as a potential carrier agent for rotenone. Int. J. Biol. Macromol. 2016, 88, 263–272. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.; Garamus, V.M.; Li, N.; Zou, A. Preparation of an environmentally friendly formulation of the insecticide nicotine hydrochloride through encapsulation in chitosan/tripolyphosphate nanoparticles. J. Agric. Food Chem. 2018, 66, 1067–1074. [Google Scholar] [CrossRef]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Jia, J.; Xu, H. Chitosan-based nanoparticles of avermectin to control pine wood nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263. [Google Scholar] [CrossRef]
- Xuan, T.-T.; Liu, J.-Q.; Xie, R.-J.; Li, H.-L.; Sun, Z. Microwave-Assisted Synthesis of CdS/ZnS:Cu Quantum Dots for White Light-Emitting Diodes with High Color Rendition. Chem. Mater. 2015, 27, 1187–1193. [Google Scholar] [CrossRef]
- Midya, L.; Patra, A.S.; Banerjee, C.; Panda, A.B.; Pal, S. Novel nanocomposite derived from ZnO/CdS QDs embedded crosslinked chitosan: An efficient photocatalyst and effective antibacterial agent. J. Hazard. Mater. 2019, 369, 398–407. [Google Scholar] [CrossRef]
- Rashidipour, M.; Maleki, A.; Kordi, S.; Birjandi, M.; Pajouhi, N.; Mohammadi, E.; Heydari, R.; Rezaee, R.; Rasoulian, B.; Davari, B. Pectin/chitosan/tripolyphosphate nanoparticles: Efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity. J. Agric. Food Chem. 2019, 67, 5736–5745. [Google Scholar] [CrossRef]
- Rashidipour, M.; Rasoulian, B.; Maleki, A.; Davari, B.; Pajouhi, N.; Mohammadi, E. Mohammadi, Pectin/chitosan/tripolyphosphate encapsulation protects the rat lung from fibrosis and apoptosis induced by paraquat inhalation. Pestic. Biochem. Physiol. 2021, 178, 104919. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Preparation of chitosan–hexaconazole nanoparticles as fungicide nanodelivery system for combating Ganoderma disease in oil palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef]
- Fu, Y.; He, H.; Liu, R.; Zhu, L.; Xia, Y.; Qiu, J. Preparation and performance of a BTDA-modified polyurea microcapsule for encapsulating avermectin. Colloids Surf. B Biointerfaces 2019, 183, 110400. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, Q.; Zeng, X.; Ma, S.; Zhong, B.; Chen, Y.; Zhang, Q.; Jia, Z.; Jia, D. Electrostatic wrapping of eupatorium-based botanical herbicide with chitosan derivatives for controlled release. Carbohydr. Polym. 2020, 247, 116700. [Google Scholar] [CrossRef]
- Xiao, N.-Y.; Zhang, X.-Q.; Ma, X.-Y.; Luo, W.-H.; Li, H.-Q.; Zeng, Q.-Y.; Zhong, L.; Zhao, W.-H. Construction of EVA/chitosan based PEG-PCL micelles nanocomposite films with controlled release of iprodione and its application in pre-harvest treatment of grapes. Food Chem. 2020, 331, 127277. [Google Scholar] [CrossRef]
- Li, G.-B.; Wang, J.; Kong, X.-P. Coprecipitation-based synchronous pesticide encapsulation with chitosan for controlled spinosad release. Carbohydr. Polym. 2020, 249, 116865. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Lu, Z.; Xing, R.; Yao, X.; Jin, Z.; Wang, Y.; Yu, F. Citral-loaded chitosan/carboxymethyl cellulose copolymer hydrogel microspheres with improved antimicrobial effects for plant protection. Int. J. Biol. Macromol. 2020, 164, 986–993. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Jiang, W.; Li, F.; Zhang, Y.; Liang, X.-Y.; Wang, M.; Zhou, X.; Wu, S.-Y.; Zhang, C.-H. Controlled release of spirotetramat using starch–chitosan–alginate-encapsulation. Bull. Environ. Contam. Toxicol. 2020, 104, 149–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Y.; Yu, M.; Ren, L.; Guo, Y.; Li, Q.; Yin, M.; Li, X.; Chen, F. Controlled release of dinotefuran with temperature/pH-responsive chitosan-gelatin microspheres to reduce leaching risk during application. Carbohydr. Polym. 2022, 277, 118880. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Bharani, R.A.; Karunamoorthy, K. Insecticidal fungal metabolites fabricated chitosan nanocomposite (IM-CNC) preparation for the enhanced larvicidal activity—An effective strategy for green pesticide against economic important insect pests. Int. J. Biol. Macromol. 2018, 120, 921–944. [Google Scholar] [CrossRef]
- Dai, R.Y.; You, S.Y.; Lu, L.M.; Liu, Q.; Li, Z.X.; Wei, L.; Huang, X.G.; Yang, Z.Y. High blades spreadability of chlorpyrifos microcapsules prepared with polysiloxane sodium carboxylate/sodium carboxymethylcellulose/gelatin via complex coacervation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 13–19. [Google Scholar] [CrossRef]
- Zhuang, C.; Shi, C.; Tao, F.; Cui, Y. Honeycomb structural composite polymer network of gelatin and functional cellulose ester for controlled release of omeprazole. Int. J. Biol. Macromol. 2017, 105, 1644–1653. [Google Scholar] [CrossRef]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-Chitosan Nanoparticle Mediated Sustainable Approach To Enhance Seedling Growth in Maize by Mobilizing Reserved Food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef]
- Hao, L.; Lin, G.; Lian, J.; Chen, L.; Zhou, H.; Chen, H.; Xu, H.; Zhou, X. Carboxymethyl cellulose capsulated zein as pesticide nano-delivery system for improving adhesion and anti-UV properties. Carbohydr. Polym. 2020, 231, 115725. [Google Scholar] [CrossRef]
- Beckers, S.J.; Wetherbee, L.; Fischer, J.; Wurm, F.R. Fungicide-loaded and biodegradable xylan-based nanocarriers. Biopolymers 2020, 111, e23413. [Google Scholar] [CrossRef]
- Viscusi, G.; Gorrasi, G. Gelatin Beads/Hemp Hurd as pH Sensitive Devices for Delivery of Eugenol as Green Pesticide. J. Polym. Environ. 2021, 29, 3756–3769. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. Natural rosin modified carboxymethyl cellulose delivery system with lowered toxicity for long-term pest control. Carbohydr. Polym. 2021, 259, 117749. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Qin, X.; Guo, Z.; Li, D.; Li, C.; Wan, H.; Zhu, F.; Li, J.; Zhang, Z.; et al. Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. J. Hazard. Mater. 2021, 414, 125513. [Google Scholar] [CrossRef]
- Denardin, C.C.; Silva, L.P.D. Starch granules structure and its regards with physicochemical properties. Ciência Rural 2009, 39, 945–954. [Google Scholar] [CrossRef]
- Laycock, B.G.; Halley, P.J. Starch applications: State of market and new trends. Starch Polym. 2014, 381–419. [Google Scholar] [CrossRef]
- Zhong, K.; Lin, Z.-T.; Zheng, X.-L.; Jiang, G.-B.; Fang, Y.-S.; Mao, X.-Y.; Liao, Z.-W. Starch derivative-based superabsorbent with integration of water-retaining and controlled-release fertilizers. Carbohydr. Polym. 2013, 92, 1367–1376. [Google Scholar] [CrossRef]
- Zhong, B.; Wang, S.; Dong, H.; Luo, Y.; Jia, Z.; Zhou, X.; Chen, M.; Xie, D.; Jia, D. Halloysite tubes as nanocontainers for herbicide and its controlled release in biodegradable poly (vinyl alcohol)/starch film. J. Agric. Food Chem. 2017, 65, 10445–10451. [Google Scholar] [CrossRef]
- Fernandes, C.; Encarnação, I.; Gaspar, A.; Garrido, J.; Borges, F.; Garrido, E.M. Influence of hydroxypropyl--cyclodextrin on the photostability of fungicide pyrimethanil. Int. J. Photoenergy 2014, 2014, 489873. [Google Scholar] [CrossRef]
- Spychaj, T.; Zdanowicz, M.; Kujawa, J.; Schmidt, B. Carboxymethyl starch with high degree of substitution: Synthesis, properties and application. Polimery 2013, 58, 503–511. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Spychaj, T.; Paździoch, W. Carboxymethyl starch/montmorillonite composite microparticles: Properties and controlled release of isoproturon. Carbohydr. Polym. 2016, 136, 101–106. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Melville, C.C.; Della Vechia, J.F.; de Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Caldera, F.; Dhakar, N.K.; Vidotto, F.; Trotta, F.; Scariot, V. Functionalized dextrin-based nanosponges as effective carriers for the herbicide ailanthone. Ind. Crops Prod. 2021, 164, 113346. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.I.; Morillo, E.; Ginés, J. β-CD effect on 2, 4-D soil adsorption. Chemosphere 1999, 39, 2047–2056. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Veglia, A.V. Determination of poorly fluorescent carbamate pesticides in water, bendiocarb and promecarb, using cyclodextrin nanocavities and related media. Anal. Chim. Acta 2007, 583, 63–71. [Google Scholar] [CrossRef]
- Szente, L. Stable, controlled-release organophosphorous pesticides entrapped in β-cyclodextrin. J. Therm. Anal. Calorim. 1998, 51, 957–963. [Google Scholar] [CrossRef]
- Yosha, I.; Shani, A.; Magdassi, S. Slow release of pheromones to the atmosphere from gelatin−alginate beads. J. Agric. Food Chem. 2008, 56, 8045–8049. [Google Scholar] [CrossRef]
- Roy, A.; Bajpai, A.K.; Bajpai, J. Designing swellable beads of alginate and gelatin for controlled release of pesticide (cypermethrin). J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 847–859. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Feng, Y.; Yu, G.; Zhou, Q.; He, F.; Xiao, D.; Chen, K.; Zhang, L. Self-aggregation behavior of hydrophobic sodium alginate derivatives in aqueous solution and their application in the nanoencapsulation of acetamiprid. Int. J. Biol. Macromol. 2018, 106, 418–424. [Google Scholar] [CrossRef]
- Xiang, S.; Lv, X.; He, L.; Shi, H.; Liao, S.; Liu, C.; Huang, Q.; Li, X.; He, X.; Chen, H. Dual-action pesticide carrier that continuously induces plant resistance, enhances plant anti-tobacco mosaic virus activity, and promotes plant growth. J. Agric. Food Chem. 2019, 67, 10000–10009. [Google Scholar] [CrossRef]
- Ma, X.; Xiang, S.; Xie, H.; He, L.; Sun, X.; Zhang, Y.; Huang, J. Fabrication of pH-sensitive tetramycin releasing gel and its antibacterial bioactivity against ralstonia solanacearum. Molecules 2019, 24, 3606. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, M.; Xi, J.; Cai, W.; Yi, Y.; He, G.; Dai, Y.; Zhou, T.; Jiang, M. A sodium alginate-based nano-pesticide delivery system for enhanced in vitro photostability and insecticidal efficacy of phloxine B. Carbohydr. Polym. 2020, 247, 116677. [Google Scholar] [CrossRef]
- Xing, J.; Dang, W.; Li, J.; Huang, J. Photo/thermal response of polypyrrole-modified calcium alginate/gelatin microspheres based on helix-coil structural transition and the controlled release of agrochemicals. Colloids Surf. B Biointerfaces 2021, 204, 111776. [Google Scholar] [CrossRef]
- Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes 2021, 9, 1641. [Google Scholar] [CrossRef]
- da Costa, M.P.M.; Rabelo, K.; Ferreira, I.L.D.M.; Cruz, M.T.D.M. Sodium alginate/chitosan/glyphosate superabsorbent bio-foam as a release system for herbicide. J. Appl. Polym. Sci. 2022, 139, 51776. [Google Scholar] [CrossRef]
- Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2014, 35, 47–66. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Jia, H.; Yao, Y.; Song, J.; Dong, H.; Cao, Y.; Zhu, F.; Huo, Z. Pectin functionalized metal-organic frameworks as dual-stimuli-responsive carriers to improve the pesticide targeting and reduce environmental risks. Colloids Surf. B Biointerfaces 2022, 219, 112796. [Google Scholar] [CrossRef]
- Kala, S.; Sogan, N.; Naik, S.N.; Agarwal, A.; Kumar, J. Impregnation of pectin-cedarwood essential oil nanocapsules onto mini cotton bag improves larvicidal performances. Sci. Rep. 2020, 10, 14107. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef]
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Svitel, J.; Surugiu, I.; Dzgoev, A.; Ramanathan, K.; Danielsson, B. Functionalized surfaces for optical biosensors: Applications to in vitro pesticide residual analysis. J. Mater. Sci. Mater. Med. 2001, 12, 1075–1078. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Doong, R.-A. Optimization of sol-gel based fibre-optic cholinesterase biosensor for the determination of organophosphorus pesticides. Water Sci. Technol. 2000, 42, 283–290. [Google Scholar] [CrossRef]
- Karnakar, R.R.; Gite, V.V. Eco-friendly slow release of ZnSO4 as a micronutrient from poly (acrylic acid: Acrylamide) and guar gum based crosslinked biodegradable hydrogels. Polym. Plast. Technol. Mater. 2022, 61, 691–708. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Prakash, O.; Tiwari, S.; Maiti, P.; Ghosh, S.; Singh, R.K.; Maiti, P. Efficient Herbicide Delivery through a Conjugate Gel Formulation for the Mortality of Broad Leaf Weeds. ACS Omega 2022, 7, 19964–19978. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, A.; Das, T.K.; Sarkar, D.J.; Singh, S.B.; Dhaka, R.; Kumar, A. Release behavior and bioefficacy of imazethapyr formulations based on biopolymeric hydrogels. J. Environ. Sci. Health Part B 2017, 52, 402–409. [Google Scholar] [CrossRef]
- Harper, P.; Chisholm, B.; Chandrasena, N. Herbicide Trials for the control of submerged aquatic weeds. In Proceedings of the 14th Biennial NSW Weeds Conference, Wollongong, Australia, 25–27 September 2007. [Google Scholar]
- Gao, X.; Hu, X.; Mo, F.; Ding, Y.; Li, M.; Li, R. Repellency Mechanism of Natural Guar Gum-Based Film Incorporated with Citral against Brown Planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Int. J. Mol. Sci. 2022, 23, 758. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar]
- Fu, J.; Zhang, S.; Wu, J.; Chen, Y.; Zhong, Y.; Zhou, Y.; Wang, J.; Chen, S. Structural characterization of a polysaccharide from dry mycelium of Penicillium chrysogenum that induces resistance to Tobacco mosaic virus in tobacco plants. Int. J. Biol. Macromol. 2020, 156, 67–79. [Google Scholar] [CrossRef]
- Dhiman, A.; Sharma, A.K.; Bhardwaj, D.; Agrawal, G. Biodegradable dual stimuli responsive alginate based microgels for controlled agrochemicals release and soil remediation. Int. J. Biol. Macromol. 2023, 228, 323–332. [Google Scholar] [CrossRef]
- Singh, J.; Singh, B. Designing Starch-Alginate Hydrogels for Controlled Delivery of Fungicide for the Alleviation of Environmental Pollution. ACS Agric. Sci. Technol. 2022, 2, 1239–1250. [Google Scholar] [CrossRef]
| Methods | Key Features | pH Sensitivity | Temperature Sensitivity | Publish Date | Results | References |
|---|---|---|---|---|---|---|
| Ionotropic pre-gelation of polyanion with f chloride followed by polycationic crosslinking | Reduce the concentration of the release of Paraquat in the soil | NULL | NULL | 15 June 2011 | The herbicide showed good association with the alginate/chitosan nanoparticles, which altered its release profile. Sorption tests showed that the soil sorption profile was reduced when paraquat was associated with the nanoparticles, hence improving the herbicidal action. | Silva et al. [7] |
| 86 °C with magnetic stirring in a nitrogen atmosphere | Sustained release, equipped with lipophilus | NULL | NULL | July 2011 | Control of the submicron particle size, the imidacloprid loading content and the imidacloprid release behaviors can be achieved by optimizing the copolymer to imidacloprid ratio. The submicron particles can prolong the pesticide release time owing to the amphiphilic structure of the copolymer. | Li et al. [8] |
| Physical gelation in reverse emulsion | pH sensitivity and enzyme sensitivity (chitosanase). | The cumulative release increased with the decreased pH from 5.5 | NULL | 15 February 2013 | The adsorption capacity of the pure chitosan nanogels was comparable to that of chitosan in solution and strongly depended on pH. The maximum adsorption capacity for copper(II) was obtained at pH 5 where 300 mg of copper(II) were bound to 1 g of chitosan. | Brunel et al. [9] |
| Methods reports by Yin et al. [10] | Oil-water amphiphilic carrier | NULL | NULL | 10 March 2014 | The release of methomyl from the various methomyl-loaded samples into an aqueous medium at pH 6.0 has been shown to be diffusion controlled. The diffusion rate or release rate was controlled by the shell cross-linking and its degree of cross-linking. | Sun et al. [11] |
| Methods reports by Li et al. [12] | Oil-water amphiphilic carrier | NULL | NULL | July 2016 | DCMC which consists of hydrophilic segment of carboxymethyl group and hydrophobic region of hexadecyl chain was successfully synthesized and characterized. | Kamari et al. [13] |
| Ionic gelification method | Increase the stability of the original drug and prevent its non-target diffusion | NULL | NULL | 7 February 2018 | The release of pesticide nicotine was significantly underdeveloped in NPs, especially in NPs with NaCl. | Yang et al. [14] |
| Poly-electrolyte self-assembly method | Reduce photolysis, alkaline control interpretation | The cumulative release increased with the increasing pH from 5.5–8.5 | NULL | June 2018 | The AVM in the CS/γ-PGA nanoparticles was rapidly released under alkaline conditions and had good ability to resist photolysis. | Liang et al. [15] |
| Methods reports by Xuan et al. [16] | Prevent light degradation and antibacterial activity, by changing pH reuse use | NULL | NULL | 5 May 2019 | The synthesized nanocomposite has high effectivity for adsorption as well as degradation under solar light irradiation and is worth to be considered as an excellent antibacterial agent towards E. coli and B. subtilis. | Midya et al. [17] |
| Ionic gelification technique | The delay release of the Paraquat | NULL | NULL | 22 May 2019 | There was a kind of delayed release of paraquat from PEC/CS/TPP nanoparticles. There was almost no release until 30 min. | Rashidipour et al. [18,19] |
| Ionic gelification method | Sustained release, improve antibacterial activity | NULL | NULL | 8 July 2019 | Chitosan–hexaconazole nanoparticles at 5 mg/mL of TPP presented the highest loading content of 16.7% as well as the sustained release of 99.91% with a prolonged release time of hexaconazole up to 86 h. The fungicide nanodelivery system provided longer efficient time, low toxicity, and high antifungal activity towards G. boninense as well as low EC50 value. | Maluin et al. [20] |
| Grafting reaction | Sustained release, prevent the original drug degradation, and no residual pollution | NULL | NULL | 1 November 2019 | A BTDA-modified polyurea microcapsule was successfully prepared by first grafting BTDA to CO followed by emulsion interfacial polymerization between CO and MDI. The prepared microcapsules showed a sustained release of AVM for a longer period and protected the encapsulated AVM from sunlight. | Fu et al. [21] |
| Electrostatic attraction, self-assembly | Sustained release, water -soluble control conveying system | NULL | NULL | 1 November 2020 | The use of chitosan derivatives for the electrostatic encapsulation of herbicides on plants has achieved the release of herbicides. The use of chitosan as a carrier for the release of herbicides has been well controlled. The herbicides released from the chitosan carrier show good weed control effects on herbaceous plants. | Xie et al. [22] |
| Nano-precipitation method | Improved antifungal ability and temperature sensitive drug release | NULL | The release of pesticide increased with the increasing temperature from 4 to 40 °C | 30 November 2020 | By incorporating CS-reinforced EVA with ID-encapsulated PEG-PCL micelles to fabricate nanocomposite films with good permeability and controlled drug release capability. The introduction of the IPP micelles into the films obtained an improved antifungal ability (10 ± 0.5 mm) and the temperature-sensitive drug release ability (71.24%). | Xiao et al. [23] |
| Coprecipitation-based synchronous encapsulation | pH and temperature sensitivity and excellent ultraviolet shielding ability | The cumulative release decreased with the increasing pH from 1.2–9.0 | the release of pesticide increased with the increasing temperature from 20 to 50 °C | 1 December 2020 | The in vitro release tests showed good pH and temperature sensitivity, high CRR (>80%) and long sustained-release time (>18 d), with the release dynamics obeying the Fickian diffusion mechanism of Ritger-Peppas model. | Li et al. [24] |
| the CS-citral emulsion was slowly dripped into the CMC solution under slow stirring to form microspheres. | Increase the stability of the original drug, prevent its non-target diffusion and extend the effect period | NULL | NULL | 1 December 2020 | Polymeric CS/CMC-based bio-composite scaffolds were successfully prepared, and CS/CMC hydrogel microspheres were loaded with citral in situ. The bioactive matrix systems serving dual functions may not only improve the antibacterial activity of CS under neutral pH conditions, but at the same time has the advantages of reducing the loss of volatile organic compounds and oxidation of citral. | Ma et al. [25] |
| Extrusion–exogenous gelation method | Slow release, increase the loading rate | NULL | NULL | January 2022 | SCCA formulation exhibited the highest EE and DL and the highest capacity of controlled release of spirotetramat. The degradation rate of spirotetramat in the SCCA formulation was obviously slower than that of spirotetramat in the commercial formulation. | Xie et al. [26] |
| Spray-drying technology | pH and temperature sensitivity, reduce the loss of original drug immersion | The cumulative release increased with the increasing pH from 5.0–10.0 | The release of pesticide increased with the increasing temperature from 10 to 30 °C | 1 February 2022 | DIN@CS-GEL enabled marked pH and temperature responsiveness, a feature suitable for the intelligent controlled release of DIN. The biodegradable composite carriers (CS-GEL) immobilized and protected the DIN in soil, thereby significantly reducing pesticide leaching. | Zhang et al. [27] |
| Methods | Key Features | pH Sensitivity | Temperature Sensitivity | Publish Date | Results | References |
|---|---|---|---|---|---|---|
| Complex coacervation method | Sustained release, Improve the foliar spread ability of the drug | The cumulative release increased with the increasing pH from 5.0–9.0 | NULL | 5 October 2017 | The chlorpyrifos-loaded microcapsules are of remarkable sustainable-release property. The chlorpyrifos accumulative release rate from PSiSC/NaCMC/GE microcapsules increases with increasing pH. | Dai et al. [29] |
| Crosslinking, electrostatic interactions and hydrogen bonding between active ester groups | Sustained release | NULL | NULL | December 2017 | The drug release mechanism was dominated by a combined effect of drug molecule diffusion and polymer matrix degradation to achieve controlled release. And the release rates decreased with cross-linking degree increased. The drug-loaded composites degraded faster at initial 6 h then slowed down. | Chen et al. [30] |
| Methods reports by Hao et al. [31] | Good dispersibility and excellent UV resistance | The cumulative release decreased with the increasing pH from 3.0–9.0 | NULL | 1 March 2020 | The thickness of CMC-g-PDMDAAC increased after encapsulated on AVM@P-Zein, meanwhile the anti-UV light performance increased by almost 10% and the adhesion ability increased by approximately 20%. CMC-g-PDMDAAC encapsulated on AVM@P-Zein could lower the sustained release rate of pesticides, in 300 h the release rate slowed down by 10%. | Li et al. [32] |
| the CS-citral emulsion was slowly dripped into the CMC solution under slow stirring to form microspheres. | Improved fiber adhesion and UV resistance for optimized pesticide loading and intelligent controlled release | NULL | NULL | 1 December 2020 | The bioactive matrix systems serving dual functions may not only improve the antibacterial activity of CS under neutral pH conditions, but at the same time has the advantages of reducing the loss of volatile organic compounds and oxidation of citral. The best formulation in terms of the ratios of CS, CMC and citral was evaluated, and the highest citral loading ratio reached 68%. | Ma et al. [25] |
| Interfacial polyaddition in an inverse miniemulsion using toluene diisocyanate (TDI) as a crosslinking agent | degraded selectively by fungal enzymes to release encapsulated agrochemicals | NULL | NULL | 11 December 2020 | Pyraclostrobin-loaded nanocarriers proved to be active against several pathogenic fungi responsible for devastating plant diseases in horticulture or agriculture. The xylan-based nanocarriers could be degraded by fungi. | Beckers et al. [33] |
| The hemp hurd, Gelatin, Eugenol and HH were mixed with 15 mL of water and stirred at 45 °C for 4 h. | pH controlled release | The cumulative release decreased with the increasing pH from 3.0–12.0 | NULL | 22 April 2021 | Swelling phenomenon is reduced in acidic conditions while gelatin dissolution rate was slower at basic pH compared to neutral and acidic ones. | Viscusi et al. [34] |
| Self-assembly | pH controlled release, enhancing the stability of the original drug | The cumulative release increased with the increasing pH from 3.0–9.0 | NULL | 1 May 2021 | AVM encapsulated in AVM@CMC-g-PRSG nanoparticles can be released slowly and last for more than 160 h. AVM@CMC-g-PRSG improve the dispersion and stability of typical insoluble AVM in water and can control the release of drugs by adjusting pH value. | Zhao et al. [35] |
| HMS-NH2 were synthesized by a one-step method, and then the HMS-COOH, HMS-HPC were synthesized by chemical reaction | pH and Cellulase controlled release | The cumulative release decreased with the increasing pH from 3.0–7.0 | NULL | 15 July 2021 | The PYR-HMS-HPC had a PYR loading capacity of approximately 12.1 wt%, quickly released the encapsulated cargo molecule in response to cellulase or a low-pH environment. | Gao et al. [36] |
| Methods | Key Features | pH Sensitivity | Temperature Sensitivity | Publish Date | Results | References |
|---|---|---|---|---|---|---|
| Kneading procedure | Protect the photodegradation of the original drug and sustained release | NULL | NULL | 11 March 2014 | The formation of pyrimethanil/HP-β-CD inclusion complex increased significantly the photostability of this fungicide in aqueous solutions, presenting a special significance in natural water. Cyclodextrin-encapsulated pyrimethanil was more slowly photodegraded in all the waters. In river water, the fungicide half-lives were increased approximately by a factor of four. | Fernandes et al. [41] |
| Preparing CMS by method reported elsewhere [42] | Sustained release | NULL | NULL | 20 January 2016 | The herbicide release rate from CMS/MMT composites in water was significantly reduced when compared to commercial isoproturon—95% released after ca. 700 h and ca. 24 h, respectively. | Wilpiszewska et al. [43] |
| Casting method | Sustained release | NULL | NULL | 6 December 2017 | Herbicide loaded in nanotubes displayed much slower release from biodegradable polymer film in water than did free herbicide. | Zhong et al. [40] |
| Kneading method described by Santos et al. [44] | Protect the photodegradation of the original drug and increase the solubility of the original drug | NULL | NULL | 1 February 2018 | The nanoformulations presented good colloidal characteristics (size, polydispersity, and zeta potential) and high encapsulation efficiencies for both active agents. The nanocarrier systems may be good candidates for improving the lifetimes of carvacrol and linalool when exposed to the environment. | Campos et al. [45] |
| Carbonate NSs were prepared by heating a solution of dextrin and CDI in anhydrous DMF | Sustained release | NULL | NULL | June 2021 | Dextrin-based nanosponges and γNS-CDI can be suggested as suitable carriers in the formulation of Ail-based herbicide, being able to improve its phytotoxicity and persistence in laboratory under controlled conditions. | Sonia et al. [46] |
| Extrusion–exogenous gelation method | Sustained release and increase the encapsulation rate | NULL | NULL | January 2022 | SCCA formulation exhibited the highest EE and DL and the highest capacity of controlled release of spirotetramat. The degradation rate of spirotetramat in the SCCA formulation was obviously slower than that of spirotetramat in the commercial formulation. | Xie et al. [26] |
| Methods | Key Features | pH Sensitivity | Temperature Sensitivity | Publish Date | Results | References |
|---|---|---|---|---|---|---|
| mixing a solution containing gelatin, pheromone, and sodium alginate, and dripping it into a CaCl2 solution using an injection needle. | Sustained release, extended drug holding period | NULL | NULL | 9 August 2008 | The release of dodecyl acetate from the beads may be controlled by changes in bead porosity by varying the alginate and gelatin concentrations. | Yosha et al. [50] |
| Constant stirring | Prevent the original drug light and microbial degradation, sustained release | The cumulative release decreased with the increasing pH from 5.8–11.2 | The release of pesticide increased with the increasing temperature from 12 to 27 °C and decreased with temperature from 27 to 35 °C | 3 August 2009 | The fractional release of the cypermethrin is found to increase with greater percent loading of the pesticide. The extent of release is found to be dependent on the chemical architecture of the bead. | Roy et al. [51] |
| Ionotropic pre-gelation of polyanion with calcium chloride followed by polycationic crosslinking | Reduce the concentration of the release of Paraquat in the soil | NULL | NULL | 15 June 2011 | The herbicide showed good association with the alginate/chitosan nanoparticles, which altered its release profile. Sorption tests showed that the soil sorption profile was reduced when paraquat was associated with the nanoparticles, hence improving the herbicidal action. | Silva et al. [7] |
| Esterification reaction, self-assembled | Controlled release, high encapsulation rate | NULL | NULL | January 2018 | A high encapsulation efficiency of acetamiprid in the H-CSAD-CaCl2 nanoparticles was achieved, reaching up to 90.8%. The release rate of acetamiprid from the nanoparticles could be controlled by changing the MW of CSAD. | Zhao et al. [52] |
| Electrostatic action | The resistance and mild release of the plant to the tobacco and lobe virus, sustained release | The cumulative release increased with the increasing pH from 3.0–9.0 | The release of pesticide increased with the increasing temperature from 10 to 40 °C | 23 August 2019 | The AL-hydrogel guaranteed that the antiviral effect of LNT was maintained in the soil for a long time and achieved the sustained release of LNT to induce plant resistance. The formation of the amino-oligosaccharide film further enhanced the slow-release ability of the AL-hydrogel, prolonged the sustained release time, and achieved long-term induction of plant resistance. | Xiang et al. [53] |
| Stir and mix under temperature control | pH controlled release, sustained release | The cumulative release increased with the increasing pH from 3.0–5.0 and decreased with the increasing pH from 5.0–8.0 | NULL | 7 October 2019 | The Schiff base crosslinking point can provide a gel pH-dependent pesticide releasing ability, whereas the Ca2+ crosslinking point can avoid the rapid releasing of the pesticide during environmental pH change. The gel could obviously increase its pesticide releasing rate when the environmental pH was decreased from 7 to 5. | Ma et al. [54] |
| Electrostatic attraction, self-assembly | Sustained release, water -soluble control conveying system | The cumulative release increased with the increasing pH from 5.0–7.4 | NULL | 1 November 2020 | The propesticide could self-assemble into core-shell structure NPs in aqueous solution. The NPs were capable of releasing pesticide in response to esterase and GSH stimulation and showing high photoactivity. | Xie et al. [55] |
| Constant stirring, crosslinking | Light controlled release, sustained release | NULL | The release of pesticide increased with the increasing temperature from 25 to 45 °C | August 2021 | Based on the volume change caused by the helix-coil transition of gelatin and the photothermal effect of PPy, PPy/Ca-alginate/Gel is sensitive to both temperature and light. It can effectively release the agrochemical contained in it through external photothermal stimulation when using CBZ as a template molecule. | Xing et al. [56] |
| An inverse miniemulsion template in sunflower oil | Sustained release, extended drug holding period | NULL | NULL | 11 September 2021 | Alginate nanohydrogels promoted the controlled and sustained release of dicamba over more than two weeks. The nanoformulation of the pesticide slowed down the release rate and exerted a protective action toward hydrophilic active substances. | Artusio et al. [57] |
| Methods | Key Features | pH Sensitivity | Temperature Sensitivity | Publish Date | Results | References |
|---|---|---|---|---|---|---|
| Stir and mix under temperature control | Sustained release | NULL | NULL | 8 March 2017 | The controlled release study and the kinetics suggest that based on the network properties of the test carriers, the release rate can be manipulated, thus leading to the formation of controlled release systems. | Kumar et al. [68] |
| Free polymerization under certain temperature and pH conditions | Sustained release and prevents degradation | NULL | NULL | 5 January 2022 | Higher thermal stability was seen to the loaded PAA-PAAm hydrogels over pure GG and GG-g-PAA-PAAm systems. The GG-g-PAA-PAAm hydrogel system was excellent water holding material in neutral and basic mediums and salt solution. | Karnakar et al. [66] |
| A solution route abbreviated as “P + H” method | Sustained release | NULL | NULL | 3 June 2022 | Utilizing the control release system, the polymer–herbicide conjugate gel formulation was prepared and sprayed in the weed nursery of the broad leaf weeds, which increased the retention period of herbicide over the weed leaf surface. | Bhardwaj et al. [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, N.; Yu, J.; Jin, S.; Shen, G.; Chen, H.; Yuzhen, N.; Xiang, D.; Qian, K. Research Progress of a Pesticide Polymer-Controlled Release System Based on Polysaccharides. Polymers 2023, 15, 2810. https://doi.org/10.3390/polym15132810
Zhang Z, Yang N, Yu J, Jin S, Shen G, Chen H, Yuzhen N, Xiang D, Qian K. Research Progress of a Pesticide Polymer-Controlled Release System Based on Polysaccharides. Polymers. 2023; 15(13):2810. https://doi.org/10.3390/polym15132810
Chicago/Turabian StyleZhang, Zan, Ni Yang, Jie Yu, Shuo Jin, Guangmao Shen, Hanqiu Chen, Nima Yuzhen, Dong Xiang, and Kun Qian. 2023. "Research Progress of a Pesticide Polymer-Controlled Release System Based on Polysaccharides" Polymers 15, no. 13: 2810. https://doi.org/10.3390/polym15132810
APA StyleZhang, Z., Yang, N., Yu, J., Jin, S., Shen, G., Chen, H., Yuzhen, N., Xiang, D., & Qian, K. (2023). Research Progress of a Pesticide Polymer-Controlled Release System Based on Polysaccharides. Polymers, 15(13), 2810. https://doi.org/10.3390/polym15132810






