A Study on Aqueous Dispersing of Carbon Black Nanoparticles Surface-Coated with Styrene Maleic Acid (SMA) Copolymer
Abstract
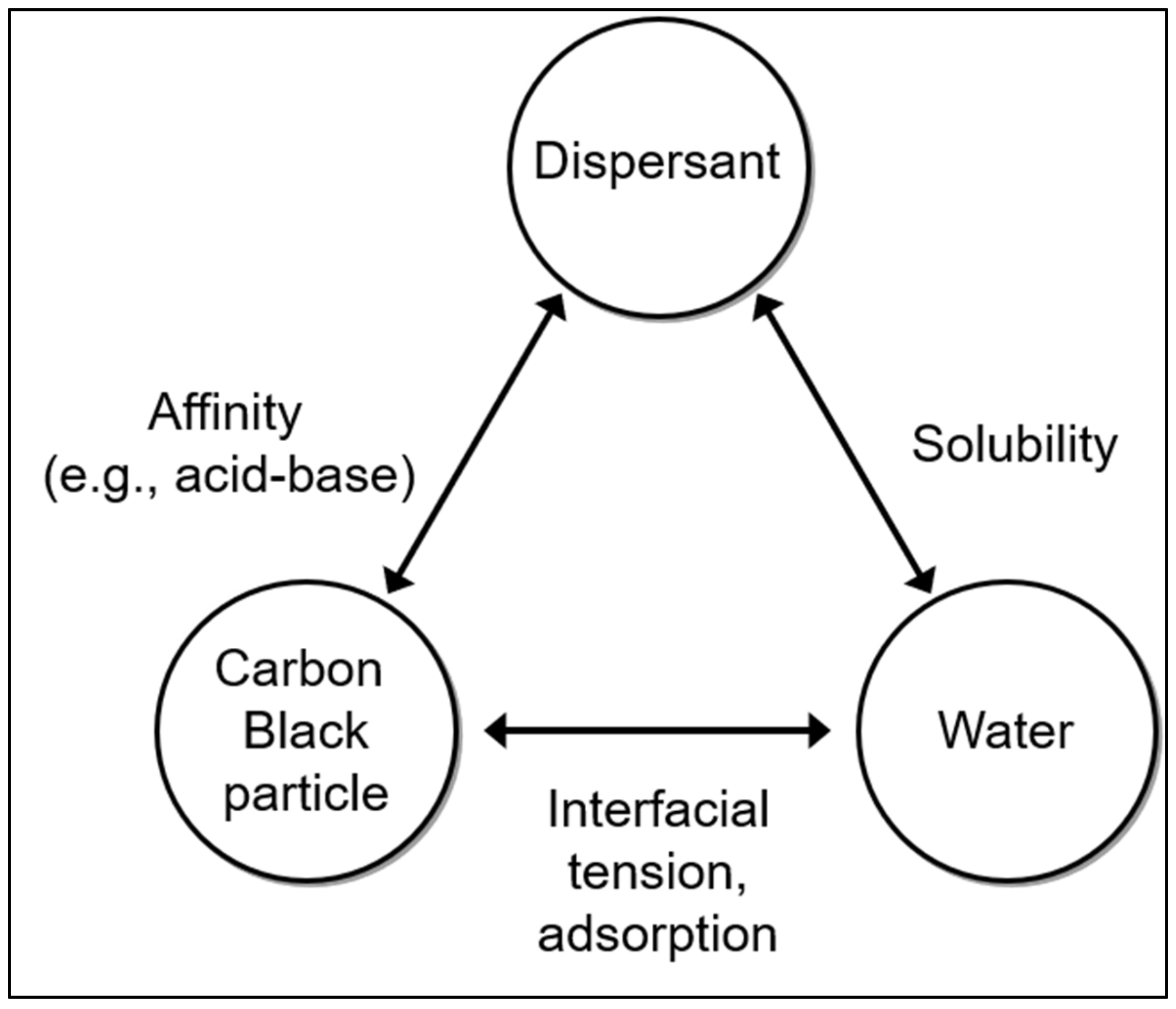
1. Introduction
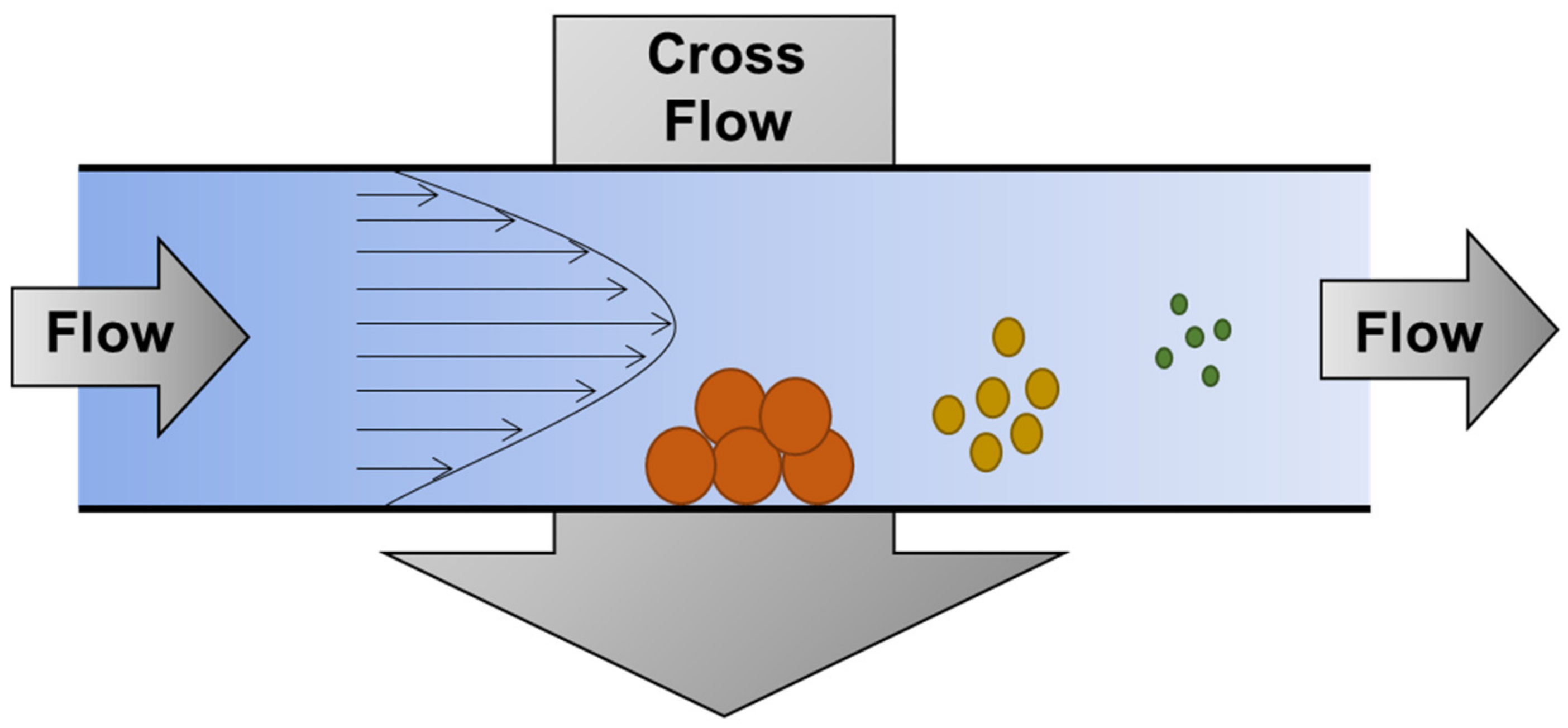
2. AsFlFFF Theory
3. Experimental
3.1. Materials
3.2. Instruments
3.3. Synthesis of Styrene Maleic Acid (SMA) Copolymer Dispersants
3.4. Preparation of CB Dispersions
4. Results and Discussion
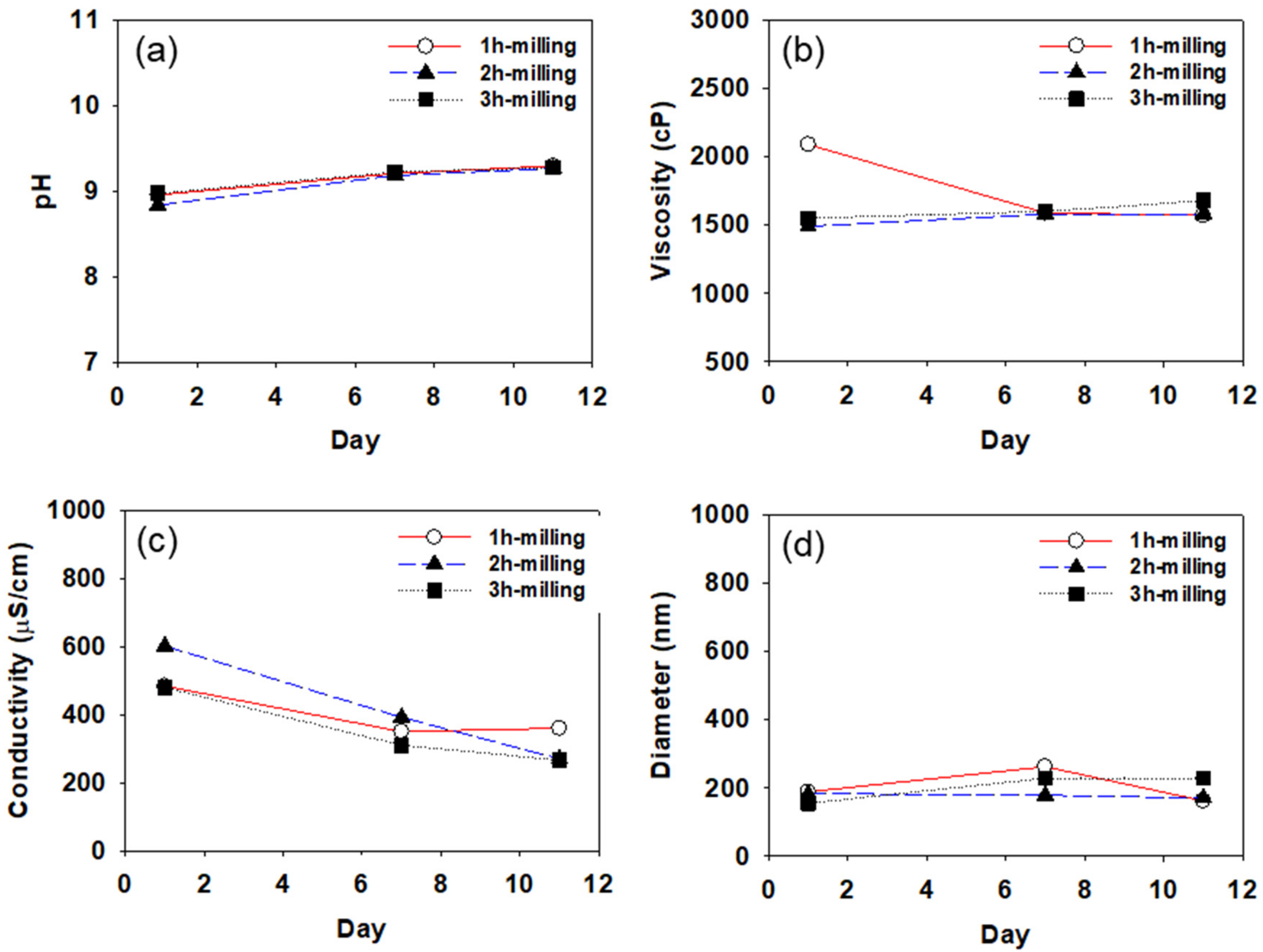
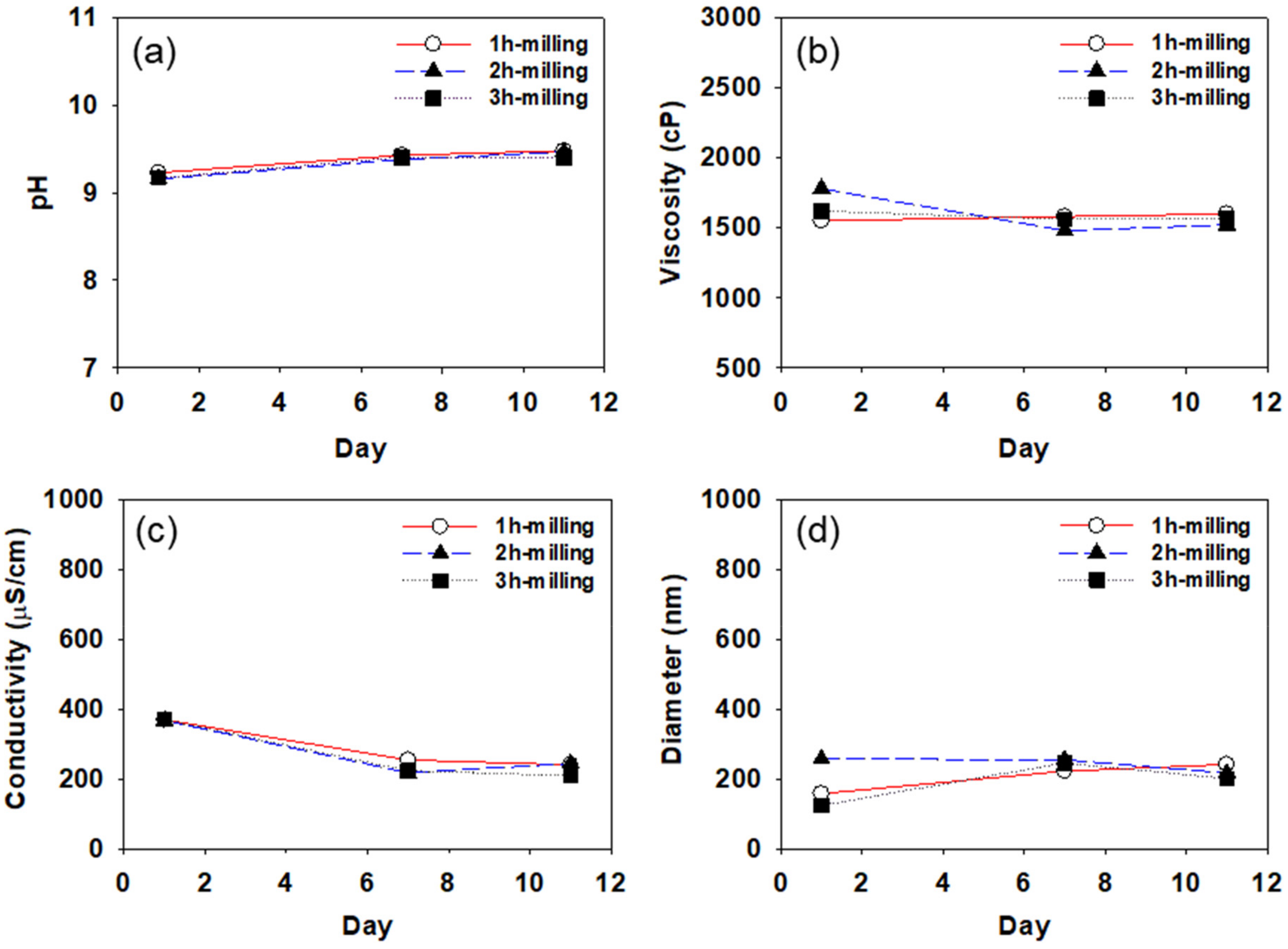
4.1. Determination of Storage Stability of CB Dispersions
4.2. Particle Size Analysis of CB Dispersions
4.3. Colorimetric Analysis of CB Dispersions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, S.; Ao, G.; Kim, M.S. Reinforcement of Rubbers by Carbon Black Fillers Modified by Hydrocarbon Decomposition. J. Ind. Eng. Chem. 2007, 13, 1162–1168. [Google Scholar]
- Boonstra, B.B.; Medalia, A.I. Effect of Carbon Black Dispersion on the Mechanical Properties of Rubber Vulcanizates. Rubber Chem. Technol. 1963, 36, 115–142. [Google Scholar] [CrossRef]
- Medalia, A.I. Electrical Conduction in Carbon Black Composites. Rubber Chem. Technol. 1986, 59, 432–454. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Alexandridis, P.; Yang, L. Micellization of Polyoxyalkylene Block Copolymers in Formamide. Macromolecules 2000, 33, 3382–3391. [Google Scholar] [CrossRef]
- Loganathan, K.; Bose, D.; Weinkauf, D. Surface modification of carbon black by nitrogen and allylamine plasma treatment for fuel cell electrocatalyst. Int. J. Hydrogen Energy 2014, 39, 15766–15771. [Google Scholar] [CrossRef]
- Iveson, S.M.; Litster, J.D.; Hapgood, K.; Ennis, B.J. Nucleation, Growth and Breakage Phenomena in Agitated Wet Gran-ulation Processes: A review. Powder Technol. 2001, 117, 3–39. [Google Scholar] [CrossRef]
- Tiarks, F.; Landfester, K.; Antonietti, M. Encapsulation of Carbon Black by Miniemulsion Polymerization. Macromol. Chem. Phys. 2001, 202, 51–60. [Google Scholar] [CrossRef]
- Lee, S.; Eum, C.H.; Kim, W.J. Surface Modification of Carbon Black Using Polymer Resin Synthesized by a Phenyl Radi-cal Reaction. J. Korean Chem. Soc. 2016, 60, 286–291. [Google Scholar] [CrossRef]
- Kim, W.; Bae, J.; Eum, C.H.; Jung, J.; Lee, S. Study on dispersibility of thermally stable carbon black particles in ink using asymmetric flow field-flow fractionation (AsFlFFF). Microchem. J. 2018, 142, 167–174. [Google Scholar] [CrossRef]
- Oyanagi, T.; Nakano, K. Pigment Dispersion, Ink Composition, Ink Set and Recording Device. U.S. Patent US20120098911A1, 26 April 2012. [Google Scholar]
- Tracon, A.A. Coatings Materials and Surface Coatings, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Malte, H. The DLVO Theory in Microbial Adhesion. Colloids Surf. B Biointerfaces 1999, 14, 105–119. [Google Scholar]
- Hartley, P.A.; Parfitt, G.D. Dispersion of powders in liquids. 1. The contribution of the van der Waals force to the cohesiveness of carbon black powders. Langmuir 1985, 1, 651–657. [Google Scholar] [CrossRef]
- Whitehouse, R.S. Carbon Black Compositions and Improved Polymer Compositions. U.S. Patent 5872177A, 16 February 1999. [Google Scholar]
- Morrison, I.D. Electrical charges in nonaqueous media. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 71, 1–37. [Google Scholar] [CrossRef]
- Belmont, J.A.; Johnson, J.E.; Adams, C.E. Inkjet Ink Formulations Containing Carbon Black Products. U.S. Patent 5US5571311A, 5 November 1996. [Google Scholar]
- Belmont, J.A.; Johnson, J.E. Modified Carbon Products and ink jet inks and coatings containing modified carbon products. U.S. Patent US5803959A, 8 September 1998. [Google Scholar]
- Lee, C.-F.; Yang, C.-C.; Wang, L.-Y.; Chiu, W.-Y. Novel amphiphilic carbon black composite nanoparticles from TEMPO-terminated polymer and TEMPO-terminated block copolymer grafted carbon black. Polymer 2005, 46, 5514–5523. [Google Scholar] [CrossRef]
- Dannenberg, E.M. Carbon Black Dispersion and Reinforcement. Rubber Chem. Technol. 1952, 25, 843–857. [Google Scholar] [CrossRef]
- Bae, J.; Jung, J.; Lee, S.; KiM, W. Characterization of Lycopene Pigments by Steric Effect of Polymer Adsorption Layer. Korean Oil Chem. Soc. 2017, 34, 357–366. [Google Scholar]
- Ranucci, E.; Spagnoli, G.; Sartore, L.; Bignotti, F.; Ferruti, P.; Schiavon, O.; Caliceti, P.; Veronese, F.M. Synthesis and Mo-lecular Weight Characterization of End-Functionalized N-vinyl-2-pyrrolidone Oligomers. Macromol. Chem. Phys. 1995, 196, 763–774. [Google Scholar] [CrossRef]
- Loffredo, F.; Mauro, A.D.G.D.; Burrasca, G.; La Ferrara, V.; Quercia, L.; Massera, E.; Di Francia, G.; Della Sala, D. Ink-jet printing technique in polymer/carbon black sensing device fabrication. Sens. Actuators B Chem. 2009, 143, 421–429. [Google Scholar] [CrossRef]
- Yoon, H.; Cho, S.; Park, S. Surface Properies and Dispersibility of Carbon Black with Different Surface Chemical Structure. Appl. Chem. 2007, 11, 501–504. [Google Scholar]
- Maiti, J.; Basfar, A.A. Encapsulation of carbon black by surfactant free emulsion polymerization process. Macromol. Res. 2017, 25, 120–127. [Google Scholar] [CrossRef]
- Kikuchi, T. Data transfer device and data transfer method. US. Patent US8799528B2, 5 August 2014. [Google Scholar]
- Bae, J.; Kim, W.; Rah, K.; Jung, E.C.; Lee, S. Application of Flow Field-Flow Fractionation (FlFFF) for Size Characteriza-tion of Carbon Black Particles in Ink. Microchem. J. 2012, 104, 44–48. [Google Scholar] [CrossRef]
- Eskelin, K.; Poranen, M.M.; Oksanen, H.M. Asymmetrical Flow Field-Flow Fractionation on Virus and Virus-Like Parti-cle Applications. Microorganisms 2019, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Jung, E.C.; Lee, S. Factors affecting measurement of channel thickness in asymmetrical flow field-flow fraction-ation. J. Chomatogr. A 2015, 1393, 115–121. [Google Scholar] [CrossRef]
- Wittgren, B.; Wahlund, K.-G.; Dérand, H.; Wesslén, B. Aggregation Behavior of an Amphiphilic Graft Copolymer in Aqueous Medium Studied by Asymmetrical Flow Field-Flow Fractionation. Macromolecules 1996, 29, 268–276. [Google Scholar] [CrossRef]
- Wahlund, G.; Giddings, J.C. Properties of an Asymmetrical Flow Field-Flow Fractionation Channel Having One Per-meable Wall. Anal. Chem. 1987, 59, 1332–1339. [Google Scholar] [CrossRef]
- Giddings, J.C. A New Separation Concept Based on a Coupling of Concentration and Flow Nonuniformities. Sep. Sci. 1996, 1, 123–125. [Google Scholar] [CrossRef]
- Litzen, A. Separation speed, retention, and dispersion in asymmetrical flow field-flow fractionation as functions of channel dimensions and flow rates. Anal. Chem. 1993, 65, 461–470. [Google Scholar] [CrossRef]
- Litzen, A.; Wahlund, K.G. Zone broadening and dilution in rectangular and trapezoidal asymmetrical flow field-flow fractionation channels. Anal. Chem. 1991, 63, 1001–1007. [Google Scholar] [CrossRef]
- Braun, D.; Sauerwein, R.; Hellmann, G.P. Polymeric surfactants from styrene-co-maleic-anhydride copolymers. Macromol. Symp. 2001, 163, 59–66. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Cao, K.; Huang, Y.; Yao, Z.; Li, B.-G.; Hu, G.-H. Kinetics and simulation of the imidization of poly(styrene-co-maleic anhydride) with amines. J. Appl. Polym. Sci. 2006, 100, 2744–2749. [Google Scholar] [CrossRef]
- French, L.; Veverka, J.; Thomas, P. Brighter material on Deimos: A particle size effect in a carbonaceous material? Icarus 1998, 75, 127–132. [Google Scholar] [CrossRef]



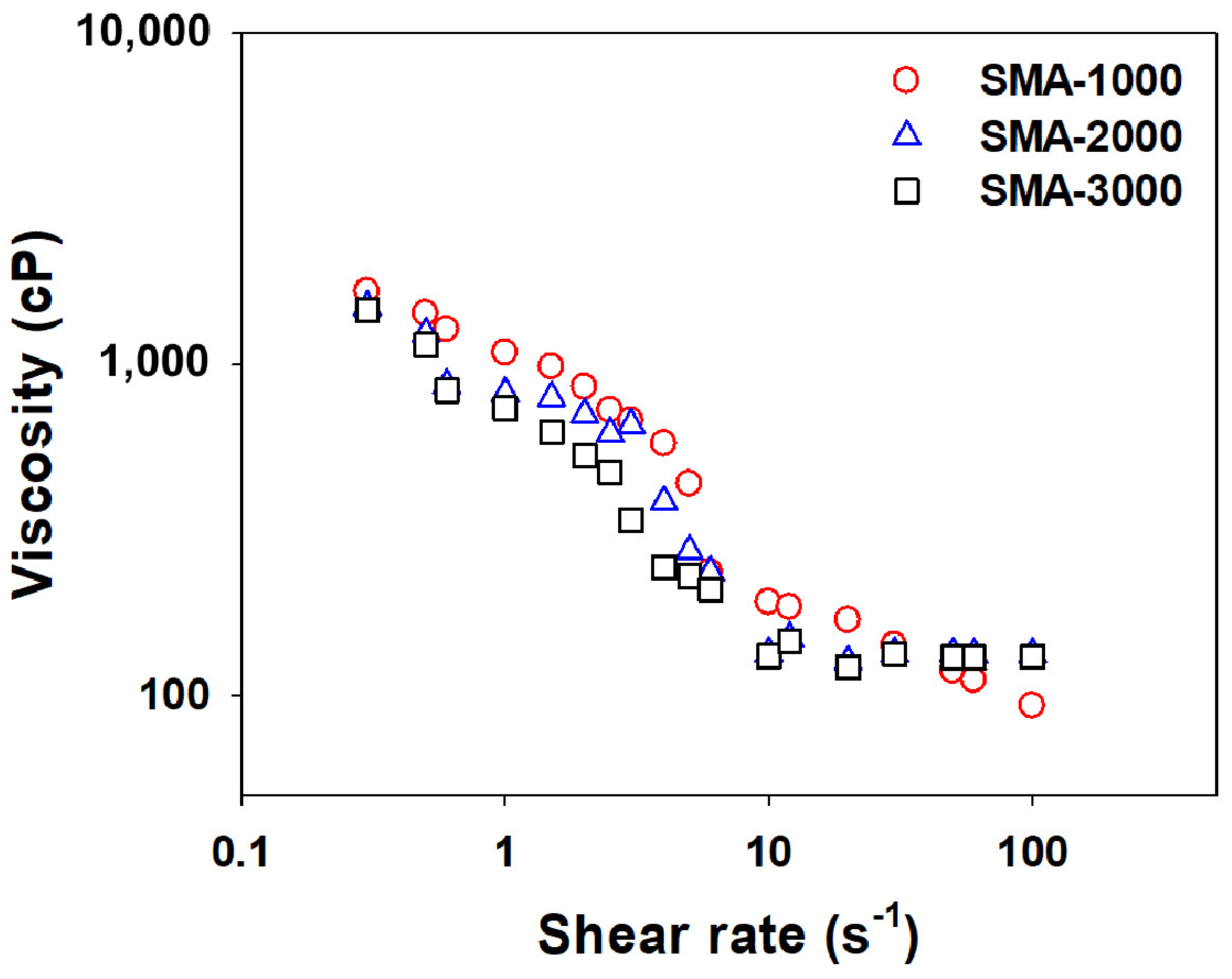




| Milling Time (h) | pH | Conductivity (μs/cm) | Viscosity (cP) | Diameter (nm) |
|---|---|---|---|---|
| 1 | 8.46 ± 0.39 | 573 ± 118 | 1520 ± 199 | 238 ± 21.7 |
| 2 | 8.43 ± 0.42 | 593 ± 128 | 1510 ± 200 | 215 ± 34.7 |
| 3 | 8.45 ± 0.39 | 577 ± 112 | 1660 ± 232 | 274 ± 91.6 |
| Milling Time (h) | pH | Conductivity (μs/cm) | Viscosity (cP) | Diameter (nm) |
|---|---|---|---|---|
| 1 | 9.14 ± 0.15 | 413 ± 168 | 1620 ± 358 | 204 ± 51.9 |
| 2 | 9.08 ± 0.19 | 462 ± 159 | 1450 ± 214 | 178 ± 65.6 |
| 3 | 9.12 ± 0.15 | 385 ± 111 | 1490 ± 241 | 204 ± 42.4 |
| Milling Time (h) | pH | Conductivity (μs/cm) | Viscosity (cP) | Diameter (nm) |
|---|---|---|---|---|
| 1 | 9.14 ± 0.15 | 304 ± 66.6 | 1450 ± 259 | 207 ± 43.8 |
| 2 | 9.08 ± 0.19 | 294 ± 74.8 | 1470 ± 280 | 243 ± 22.6 |
| 3 | 9.12 ± 0.15 | 289 ± 84.5 | 1460 ± 258 | 190 ± 61.5 |
| Milling Time (h) | Particle Diameter (nm) of CB Dispersions Prepared with | |||||
|---|---|---|---|---|---|---|
| SMA-1000 | SMA-2000 | SMA-3000 | ||||
| Mean ± SD | RSD | Mean ± SD | RSD | Mean ± SD | RSD | |
| 1 | 472 ± 292 | 61.7 | 257 ± 661,070 ± 335 | 25.731.3 | 390 ± 111 | 28.5 |
| 2 | 340 ± 168 | 49.4 | 252 ± 62 | 24.4 | 292 ± 121 | 41.4 |
| 3 | 359 ± 109 | 30.4 | 307 ± 146 | 47.5 | 240 ± 58.2 | 24.3 |
| Milling Time (h) | Particle Diameter (nm) of CB Dispersions Prepared with | |||||
|---|---|---|---|---|---|---|
| SMA-1000 | SMA-2000 | SMA-3000 | ||||
| Mean ± SD | RSD | Mean ± SD | RSD | Mean ± SD | RSD | |
| 1 | 291 ± 79.1 | 27.2 | 314 ± 90.0 | 28.7 | 274 ± 22.9 | 8.4 |
| 2 | 295 ± 77.1 | 26.1 | 279 ± 87.2 | 31.2 | 275 ± 25.3 | 9.2 |
| 3 | 286 ± 85.4 | 29.9 | 267 ± 46.0 | 17.2 | 295 ± 70.3 | 23.8 |
| Milling Time (h) | |||
|---|---|---|---|
| 1 | 24.3 ± 0.01 | 0.34 ± 0.03 | 0.08 ± 0.02 |
| 2 | 24.3 ± 0.01 | 0.34 ± 0.02 | 0.33 ± 0.04 |
| 3 | 23.9 ± 0.01 | 0.33 ± 0.04 | 0.28 ± 0.02 |
| Milling Time (h) | |||
|---|---|---|---|
| 1 | 23.3 ± 0.01 | 0.38 ± 0.06 | 0.23 ± 0.02 |
| 2 | 24.6 ± 0.01 | 0.29 ± 0.04 | 0.12 ± 0.02 |
| 3 | 24.1 ± 0.01 | 0.31 ± 0.03 | 0.25 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Bae, J.; Kim, W.; Lee, S. A Study on Aqueous Dispersing of Carbon Black Nanoparticles Surface-Coated with Styrene Maleic Acid (SMA) Copolymer. Polymers 2022, 14, 5455. https://doi.org/10.3390/polym14245455
Lee J, Bae J, Kim W, Lee S. A Study on Aqueous Dispersing of Carbon Black Nanoparticles Surface-Coated with Styrene Maleic Acid (SMA) Copolymer. Polymers. 2022; 14(24):5455. https://doi.org/10.3390/polym14245455
Chicago/Turabian StyleLee, Jaeseon, Jihyun Bae, Woonjung Kim, and Seungho Lee. 2022. "A Study on Aqueous Dispersing of Carbon Black Nanoparticles Surface-Coated with Styrene Maleic Acid (SMA) Copolymer" Polymers 14, no. 24: 5455. https://doi.org/10.3390/polym14245455
APA StyleLee, J., Bae, J., Kim, W., & Lee, S. (2022). A Study on Aqueous Dispersing of Carbon Black Nanoparticles Surface-Coated with Styrene Maleic Acid (SMA) Copolymer. Polymers, 14(24), 5455. https://doi.org/10.3390/polym14245455







