A Novel Microwave Hot Pressing Machine for Production of Fixed Oils from Different Biopolymeric Structured Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Management Plan
- 1.
- Specifying the seeds amounts required for the extraction processes.
- 2.
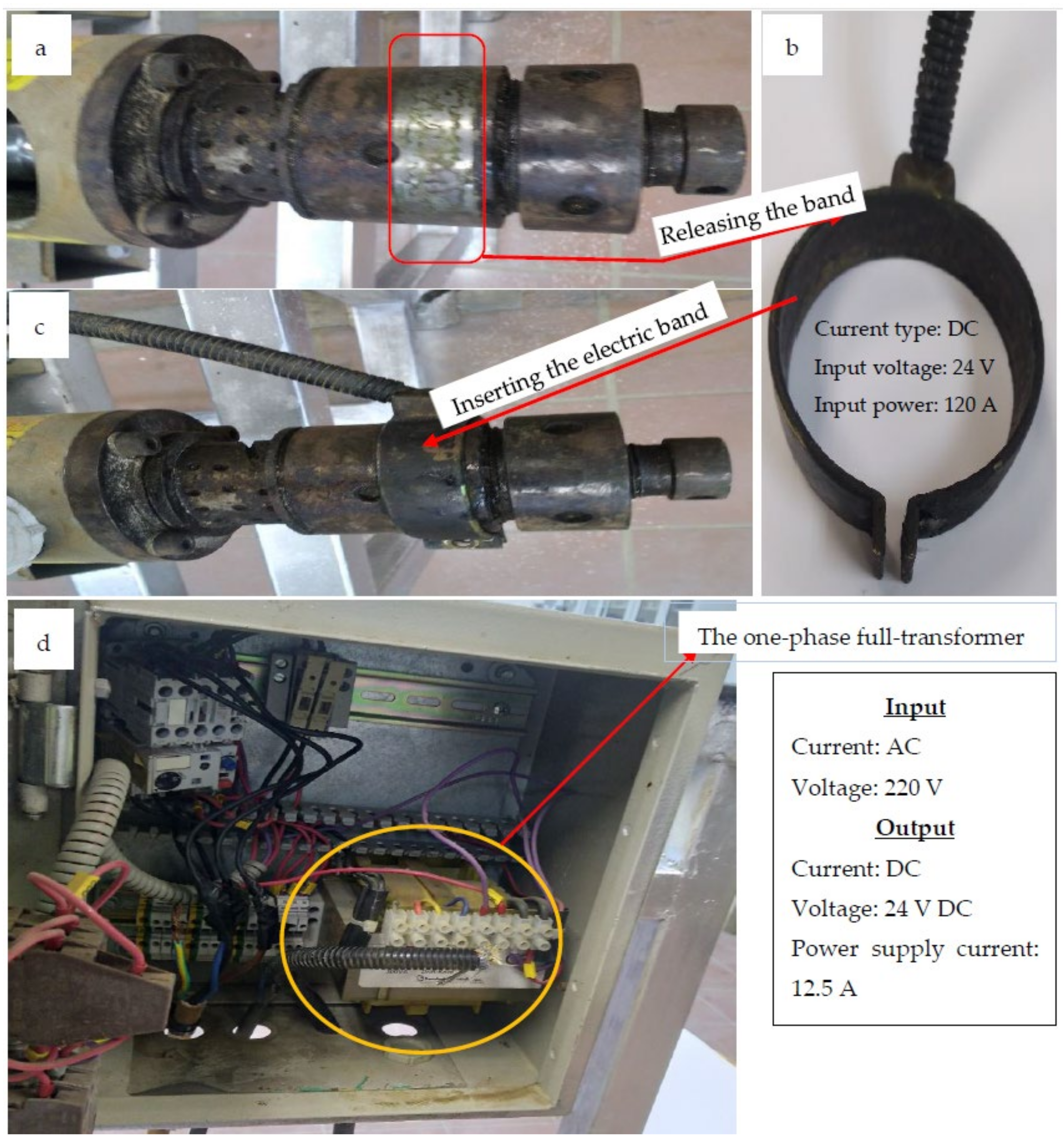
- Preparation of the electric hot pressing machine by inserting the electric heating coil around the extruder head of the machine.
- 3.
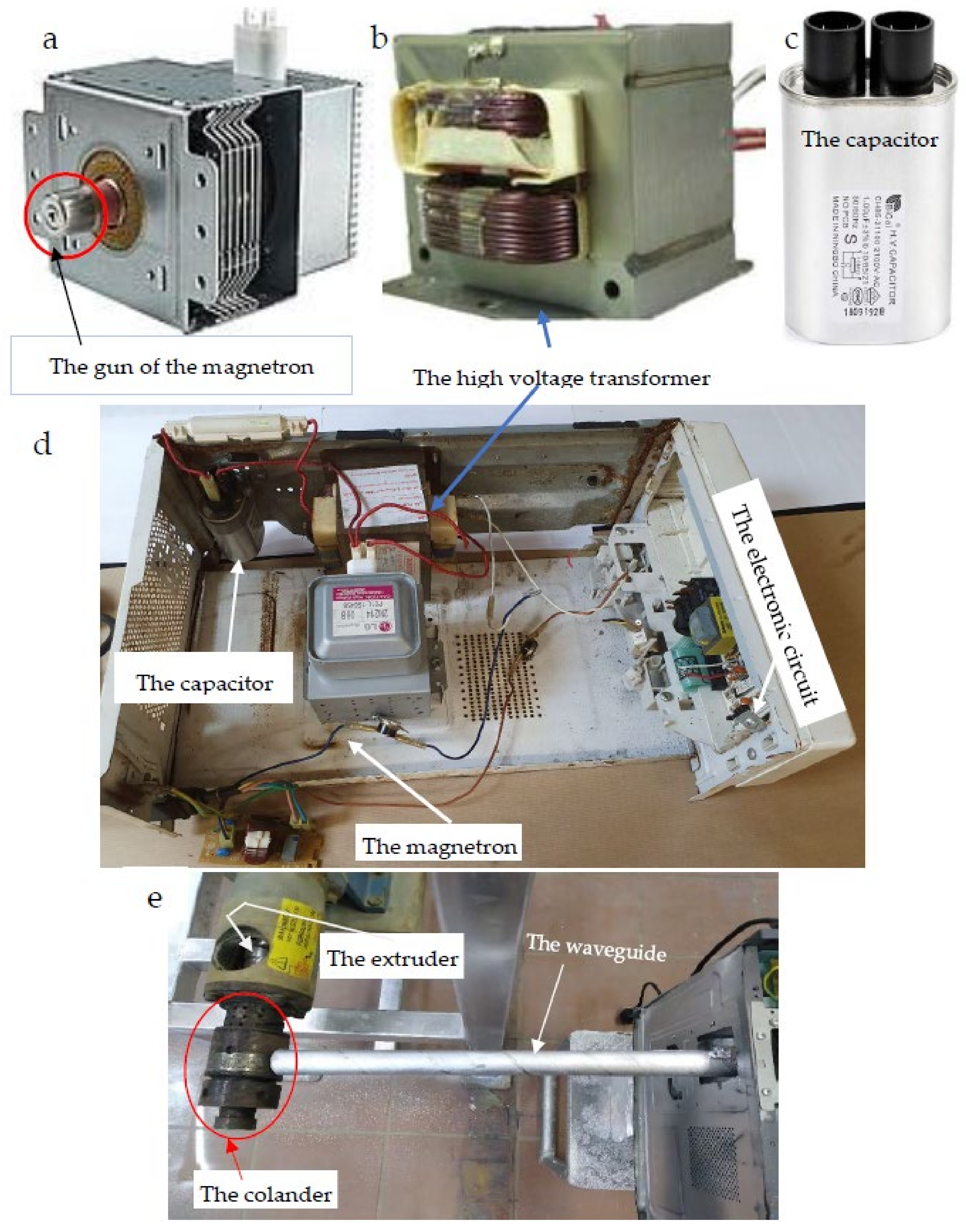
- Preparation of the microwave hot pressing machine by releasing the electric heating coil from the extruder head of the machine.
- 4.
- Pressing the seeds of each of the four oil crops (castor, sunflower, rapeseed, or moringa).
- 5.
- Dehydration, settlement, and filtration of the crude fixed oils.
- 6.
- Characterization of the fixed oils and comparing the oil extracted by microwave to that obtained by electric band heater.
2.2. Raw Materials
2.2.1. Preparation of the Seeds
2.2.2. The Mechanical Extraction of the Four Fixed Oils
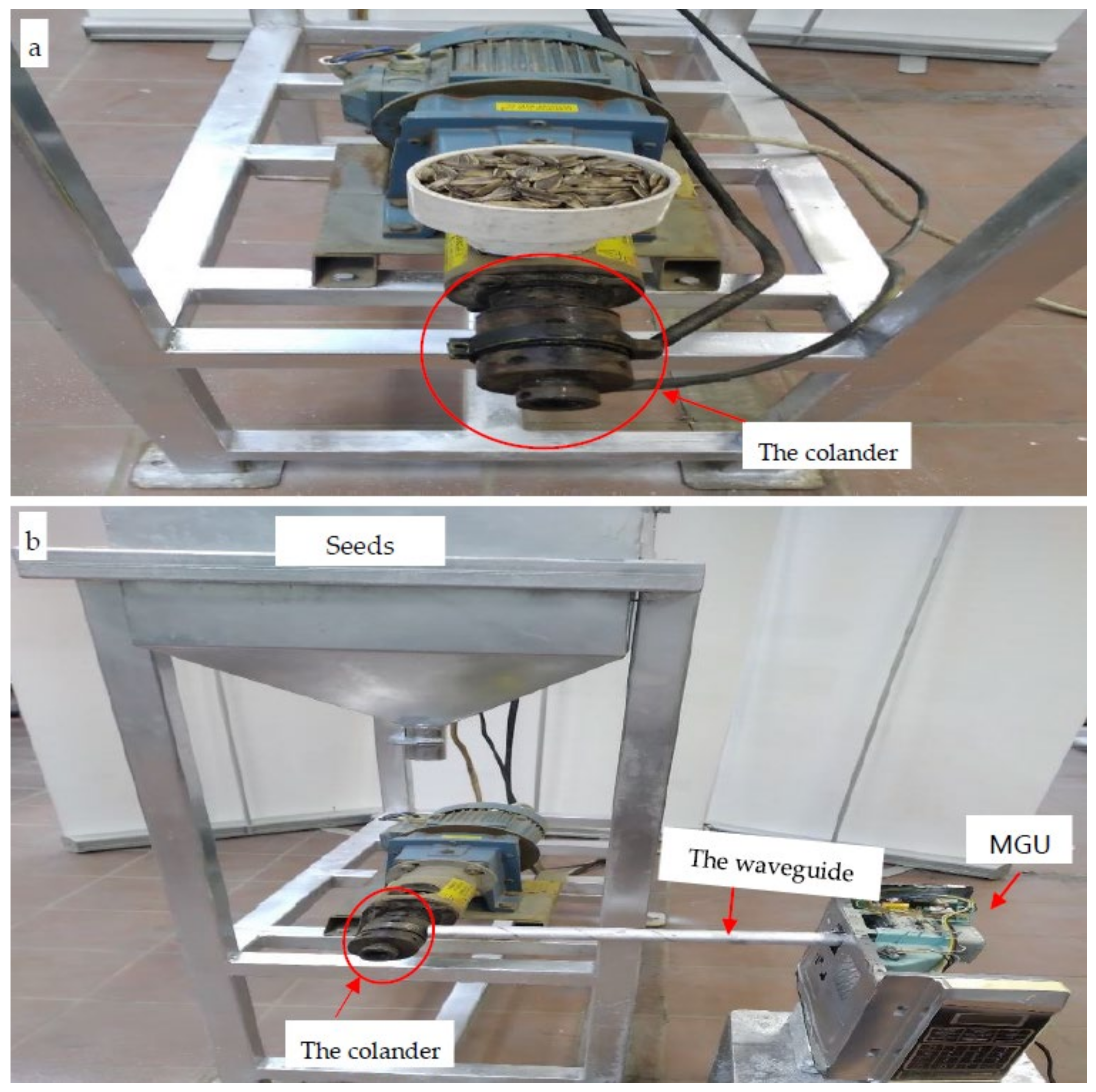
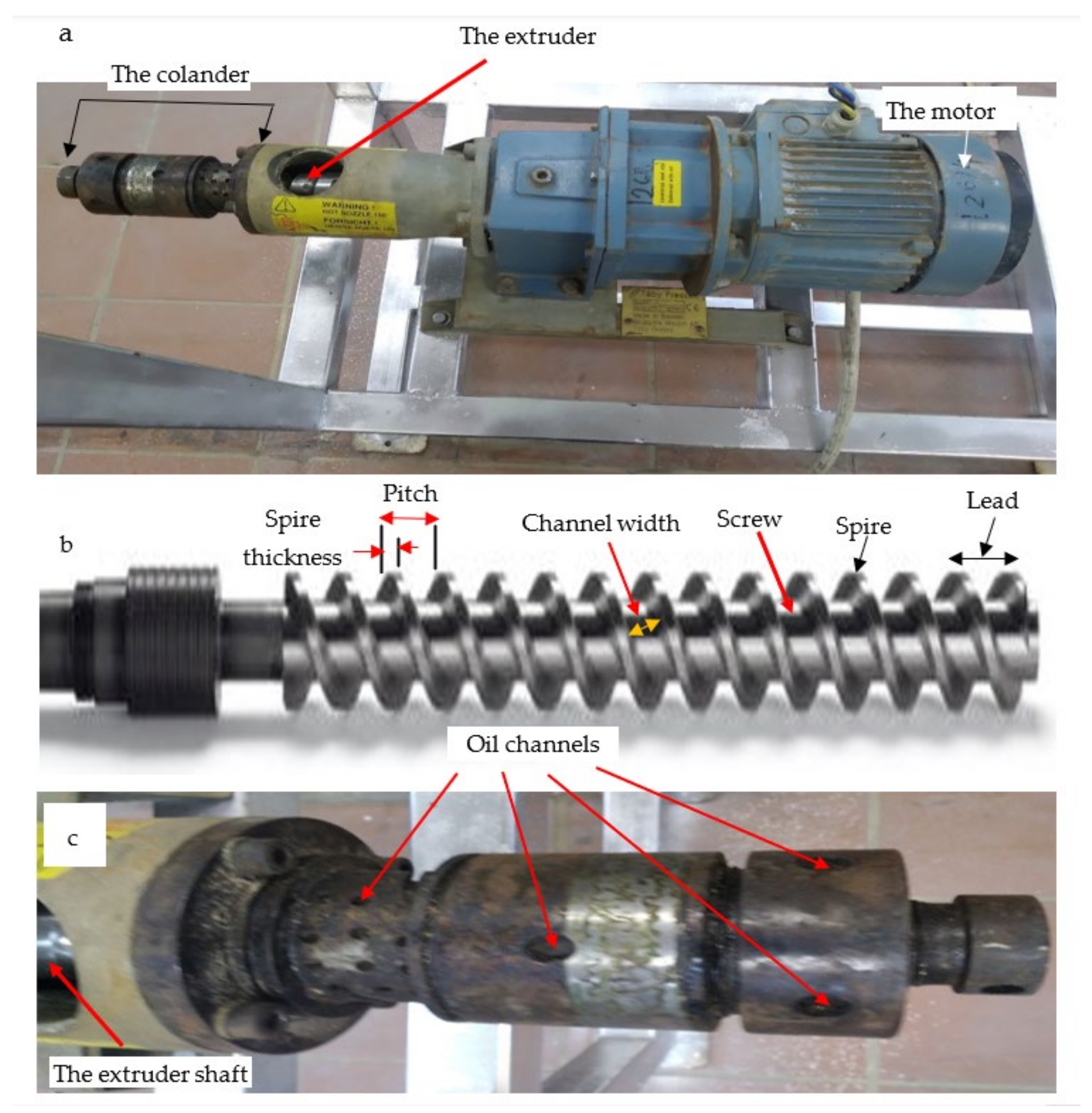
2.2.3. The Hot Pressing Machine (HPM)
2.3. Characterization of the Four Species
2.3.1. Physical Characterization of the Seeds and Oils
2.3.2. Chemical Characterization of the Fixed Oils
Determination of Iodine Number (IN)
Determination of Saponification Value (SV)
Determination of Acid Value (AV)
Determination of pH Value
Determination of the Fatty Acids of the Fixed Oils
- 1.
- Preparation of the Methyl Esters
- 2.
- Gas Chromatography-Mass spectrometer (GC–MS)
2.4. Scanning Electron Microscopy (SEM)
2.5. Statistical Design and Analysis
3. Results
3.1. Characterization of the Fixed Oils
3.1.1. Physical Properties of Seeds
3.1.2. Physical Properties of the Fixed Oils
Specific Gravity of Fixed Oil (SGfo)
3.1.3. Chemical Properties of the Fixed Oils
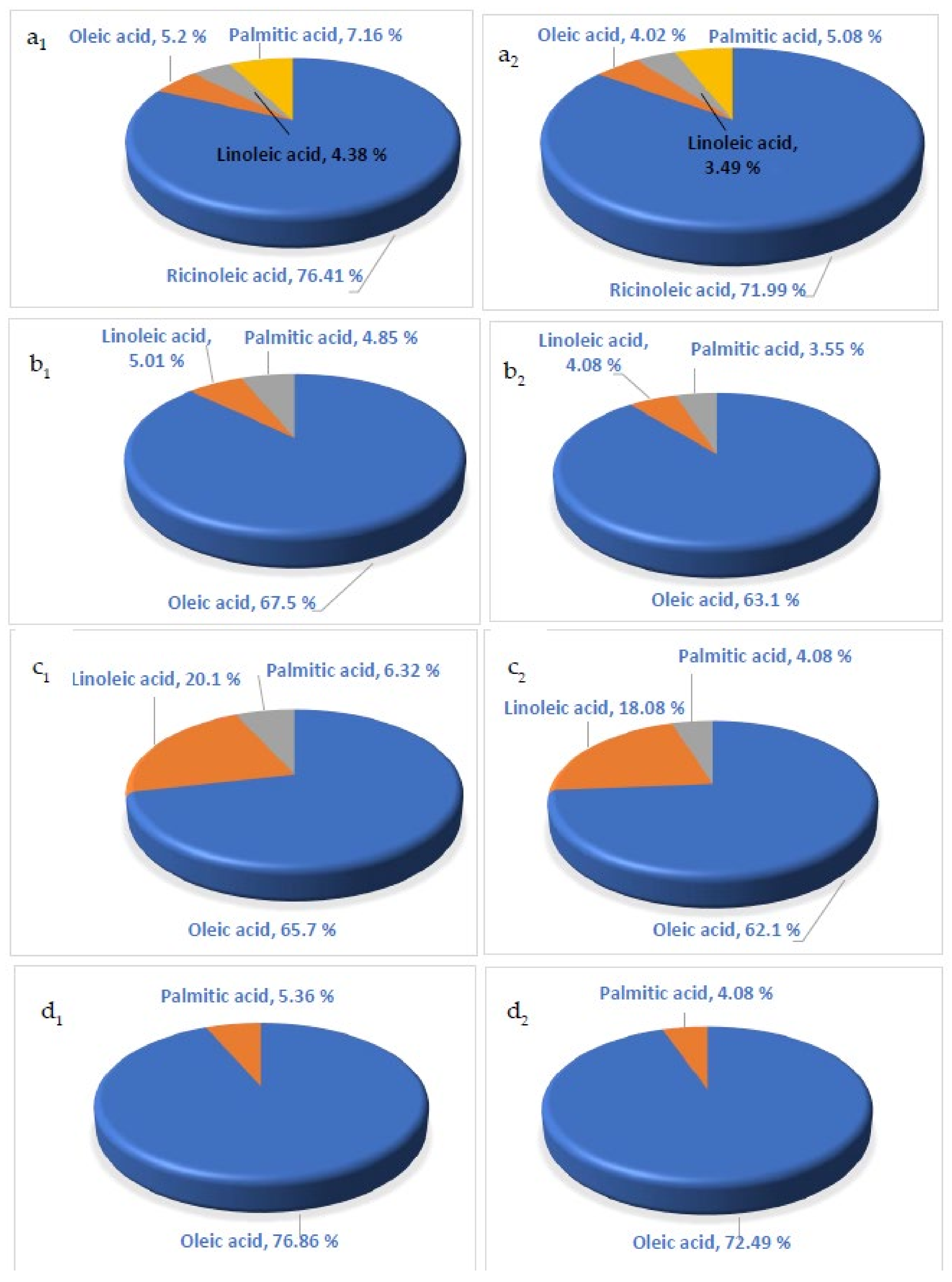
- 1.
- For castor oil (Figure 5a1,a2), ricinoleic acid was the most abundant fatty acid (76.41 and 71.99%) among the four fixed oils resulting from MHPM (Figure 5a1) and EHPM (Figure 5a2), respectively. However, castor oil had the lowest fatty acid content due to lower contributions from oleic acid (3.31%), linoleic acid (4.32%), and palmitic acid (4.32%).
- 2.
- For the sunflower fixed oil (Figure 5b1,b2), the oleic acid was the prominent fatty acid with high contents of 67.5% and 61.1% for the MHPM and EHPM, respectively. In addition, linoleic and palmitic acid had lower contents in the sunflower oil.
- 3.
- For the rapeseed fixed oil (Figure 5c1,c2), oleic acid, linoleic acid, and palmitic acid were the major fatty acids detected in the rapeseed fixed oil (65.7%, 20.1%, and 6.32%, respectively) produced by the MHPM, while their yields were 62.1%, 18.08%, and 4.08%, respectively, by using the EHPM.
- 4.
- For the moringa fixed oil (Figure 5d1,d2), oleic acid and palmitic acid were found in the moringa fixed oil in concentrations of 76.86% and 5.36%, respectively, using the MHPM, while their contents were 72.49 and 4.08% for the EHPM.
3.2. Effect of Microwave Irradiation on Biopolymeric Structured-Tissues (BST)
4. Discussion
4.1. Physical Characterization of Physical Characterization of the Fixed Oils
- 1.
- The moisture content of seed (MCs) values were found to be varied in seeds that ranged from 3.6% to about 7% [42,67]. The variation could be attributed to the difference in the nature of beans from different locations [42] and/or seed macro-structure, including hull-to-kernel weight ratio, hull thickness, and their oil content [66].
- 2.
- Focusing on the seed content of fixed oil (Scfo) showed that the recovery of the remained traces in the seeds’ cake by using solvent extraction procedure allowed the yield of recovered fixed oil (Yrfo) to be maximized as well as reducing the extraction loss (EL) for the fixed oils. Some remaining traces of fixed oils in the cakes can be attributed to incomplete cell lysis within the seeds which possibly trapped and retained some amount of oil [52].
4.2. Chemical Characterization of the Fixed Oils
4.3. Effect of Microwave Irradiation on Biopolymeric Structured-Seed Tissues
4.4. Effect of Ultrastructure of Seeds on Fixed Oils Extraction
5. Conclusions
- 1.
- A microwave beam was used to heat the extruder’s colander of a hot pressing machine instead of the ordinary electric one.
- 2.
- The invented microwave–hot pressing machine was used to produce, individually, four different fixed oils extracted from seeds of castor, sunflower, rapeseed, and moringa species and compared to those obtained using the ordinary electric–hot pressing machine.
- 3.
- The physical properties, namely moisture content, oil content, oil yield, oil extraction efficiency, specific gravity, and refractive index, were determined for the four fixed oils.
- 4.
- The chemical properties, namely iodine number, saponification value, acid value, pH, and chemical constituents, using gas chromatography coupled with a mass spectrometer of the four oils extracted by using both heating tools were evaluated based on those in the literature for the four fixed oils.
- 5.
- The higher oil extraction efficiency indicated that using microwave irradiation enhanced the oil yield with retaining the parent quality of the fixed oils that is very encouraging for candidating such an invention as a pivot of the industrial fixed oil projects.
- 6.
- Studying the histological features of the biopolymeric structured tissues revealed that castor bean species has the highest singular lipid body diameter (0.44 µm–0.94 µm) followed by those for moringa seeds. This explains the highest fixed oil yield obtained from such species.
- 7.
Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Definition | Symbol | Definition |
| AC | Alternate current | S | Sunflower (Helianthus annuus L.) |
| ACS | The American Chemical Society | MAEO | Microwave-assisted extracted oil |
| ADB | Air-dried membranes | MFT | Maximum final temperature |
| AFM | Atomic force microscopy | MGU | Microwave generator unit |
| ASTM | American Society for Testing and Materials | MHPM | Microwave hot pressing machine |
| AV | Acid value | NDB | Nanodehydrated-bioplastic membrane |
| BS | Basic extraction | NIST | The National Institute of Standards and Technology |
| BST | Biopolymeric Structured-Tissues | NPS | Nanometric particle Size |
| C | Castor bean (Ricinus communis L.) | PD | Pore diameter |
| CI | Crystallinity index | pH | The acidity or basicity number |
| CLB | Compound lipid bodies | PS | Particle size |
| CW | Cell walls | PubChem | An open chemistry database managedby the National Institutes of Health (NHI) |
| CY | Cytoplasm | R | Rapeseed (Brassica napus L.) |
| DC | Direct current | SD | Standard deviation |
| DSC | Differential scanning calorimetry | SE | Secondary extraction |
| DTA | Differential thermal analysis | RI | Refractive index |
| EC | endosperm cells | SEP | Self-electrostatic peeling |
| EHPO | Electric hot pressed-oil | SGfo | Specific gravity of fixed oil |
| EHPM | Electric-hot pressing machine | SLB | Singular lipid bodies |
| FEG | Field emission gun in the SEM | SP | Statistical parameters |
| FEI | Field electron and ion US-company | SV | Saponification value |
| EL | Extraction loss, % | SWC | Sinusoidal wave curve |
| Efoe | Efficiency of fixed oil extraction, % | TGA | Thermogravimetric analysis |
| FTIR | Fourier transform infrared spectroscopy | TR | Temperature range |
| GC-MS | Gas chromatography-mass spectrometer | VFHF | Vibrated-free horizontal flow |
| GHz | Frequency | VV | Void volume |
| HC | Heat change in µVs/mg | Wgvs | Weight of ground virgin seeds, g |
| HPM | Hot pressing machine | Wmfo | Weight of main fixed oil, g |
| HVT | High voltage transformer | Wrfo | Weight of recovered fixed oil, g |
| IN | Iodine number | XRD | X-ray diffraction |
| LSD | Least significant differencce | Yfa | Yield of fatty acids |
| M | Moringa (Moringa oleifera Lam.) | Ymfo | Yield of main fixed oil, % |
| RI | Refractive index | Yrfo | Yield of recovered fixed oil, % |
References
- Fernández-Luqueño, F.; López-Valdez, F.; Miranda-Arámbula, M.; Rosas-Morales, M.; Pariona, N.; Espinoza-Zapata, R. An introduction to the sunflower crop. In Sunflowers; Arribas, J.I., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Boesewinkel, F.D.; Bouman, F. The Seed: Structure. In Embryology of angiosperms; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Perea-Flores, M.J.; Chanona-Perez, J.J.; Garibay-Febles, V.; Calderon-Dominguez, G.; Terrés-Rojas, E.; Mendoza-Perez, J.A.; Herrera-Bucio, R. Microscopy techniques and image analysis for evaluation of some chemical and physical properties and morphological features for seeds of the castor oil plant (Ricinus communis). Ind. Crops. Prod. 2011, 34, 1057–1065. [Google Scholar] [CrossRef]
- Walters, C.; Pierre Landre, P.; Hill, L.; Corbineau, F.; Bailly, C. Organization of lipid reserves in cotyledons of primed and aged sunflower seeds. Planta 2005, 222, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-Y.; Hua, W.; Zhang, L.; Deng, L.-B.; Wang, X.-F.; Liu, G.H.; Hao, W.J.; Wang, H.Z. Seed structure characteristics to form ultrahigh oil content in rapeseed. PLoS ONE 2013, 8, e62099. [Google Scholar] [CrossRef] [PubMed]
- Fotouo, M.H.; du Toit, E.S.; Robbertse, P.J. Germination and ultrastructural studies of seeds produced by a fast-growing, drought-resistant tree: Implications for its domestication and seed storage. AoB PLANTS 2015, 7, plv016. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.S.; Dawoud, U.M. Characterization of hot pressed-fixed oil extracted from Ricinus communis L. seeds. Int. J. Innov. Res. Technol. Sci. Eng. 2019, 8, 10172–10191. [Google Scholar]
- Mascolo, N.; Izzo, A.A.; Autore, G.; Barbato, F.; Capasso, F. Nitric oxide and castor oil-induced diarrhea. J. Pharmacol. Exp. Ther. 1994, 268, 291–295. [Google Scholar]
- Trevino, A.S.; Trumbo, D.L. Acetoacetylated castor oil in coatings applications. Prog. Org. Coat. 2002, 44, 49–54. [Google Scholar] [CrossRef]
- Girard, P.; Pansart, Y.; Lorette, I.; Gillardin, J.M. Dose–response relationship and mechanism of action of Saccharomyces boulardii in castor oil-induced diarrhea in rats. Dig. Dis. Sci. 2003, 48, 770–774. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1089. [Google Scholar] [CrossRef]
- Saraf, S.; Sahu, S.; Kaur, C.D.; Saraf, S. Comparative measurement of hydration effects of herbal moisturizers. Pharmacognosy Res. 2010, 2, 146–151. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy. 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Salimon, J.; Noor, D.A.M.; Nazrizawati, A.T.; Firdaus, M.M.; Noraishah, A. Fatty acid composition and physicochemical properties of Malaysian castor bean Ricinus communis L. seed oil. Sains Malays. 2010, 39, 761–764. [Google Scholar]
- Nangbes, J.G.; Nvau, J.B.; Buba, W.M.; Zukdimma, A.N. Extraction and characterization of castor (Ricinus communis) seed oil. IJES 2013, 2, 105–109. [Google Scholar]
- Salihu, B.; Gana, A.K.; Apuyor, B.O. Castor oil plant (Ricinus communis L.): Botany, ecology and uses. IJSR 2014, 3, 1333–1341. [Google Scholar]
- Yeganeh, H.; Hojati-Talemi, P. Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly (ethylene glycol). Polym. Degrad. Stab. 2007, 92, 480–489. [Google Scholar] [CrossRef]
- Mukherjea, R.N.; Saha, K.K.; Sanya, S.K. Plasticizing effect of acetylated castor oil on castor oil-based, moisture-cured polyurethane film. J. Am. Oil. Chem. Soc. 1978, 55, 653–656. [Google Scholar] [CrossRef]
- Marc, R.L.; Furst, M.R.L.; Le Goff, R.; Quinzler, D.; Mecking, S.; Botting, C.H.; Cole-Hamilton, D.J.C. Polymer precursors from catalytic reactions of natural oils. Green. Chem. J. 2012, 14, 472–477. [Google Scholar]
- Imankulov, N. Preparation and research on properties of castor oil as a diesel fuel additive. Appl. Tech. Innov. J. 2012, 6, 30–37. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Dunford, N.T. Food and Industrial Bioproducts and Bioprocessing, 1st ed.; Dunford, N.T., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012; pp. 115–143. [Google Scholar]
- Alwaseem, H.; Donahue, C.J.; Marincean, S. Catalytic transfer hydrogenation of castor oil. J. Chem. Educ. 2014, 91, 575–578. [Google Scholar] [CrossRef]
- Marwat, S.K.; Rehman, F.; Khan, E.A.; Baloch, M.S.; Sadiq, M.; Ullah, I.; Javaria, S.; Shaheen, S. Ricinus communis: Ethnomedicinal uses and pharmacological activities. Pak. J. Pharm. Sci. 2017, 30, 1815–1827. [Google Scholar] [PubMed]
- Campos Flexa Ribeiro Filho, P.R.; Rocha do Nascimento, M.; Otaviano da Silva, S.S.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Loureiro Cavalcante, C., Jr. Synthesis and frictional characteristics of bio-based lubricants obtained from fatty acids of castor oil. Lubricants 2023, 11, 57. [Google Scholar] [CrossRef]
- Osorio, J.; Fernandez˗Martínez, J.M.; Mancha, M.; Garces, R. Mutant sunflower with high concentration of saturated fatty acids in the oil. Cropl. Sci. 1995, 35, 739–742. [Google Scholar] [CrossRef]
- Piva, G.; Bouniols, A.; Mondies, G. Effect of cultural conditions on yield, oil content and fatty acid composition of sunflower kernel. In Proceedings of the 15th International Sunflower Conference, Toulouse, France, 12–16 June 2000; pp. 61–66. [Google Scholar]
- Vrânceanu, A.V.; Soare, G.; Craiciu, D.S. Breeding sunflower for high oleic acid content. An. ICCPT-Fundulea 1995, LXII, 96–97. [Google Scholar]
- Meller, J.F.; Zimmerman, D.C. Inheritance of high oleic fatty acid content in sunflower. In Proc. Sunflower Research workshop, Fargo, N.D. 26 January; National Sunflower Association: Bismark, ND, USA, 1983; p. 10. [Google Scholar]
- Urie, L.A. Inheritance of high oleic acid in sunflower. Crop. Sci. 1985, 25, 986–989. [Google Scholar] [CrossRef]
- Fernandez-Martinez, J.; Munoz, J.; Jimenez-Ramirez, A.; Dominguez Jimenez, J.; Alcantara, A. Temperature effect on the oleic and linoleic acid of three genotypes in sunflower. Grasas Aceites. 1986, 37, 327–333. [Google Scholar]
- Woźniak, E.; Waszkowska, E.; Zimny, T.; Sowa, S.; Twardowski, T. The rapeseed potential in Poland and Germany in the context of production, legislation, and intellectual property rights. Front. Plant Sci. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M.; Al Juhaimi, F. Some rape/canola seed oils: Fatty acid composition and tocopherols. Z. Naturforsch C. J. Biosci. 2016, 71, 73–77. [Google Scholar] [CrossRef]
- Chew, S.C. Cold pressed rapeseed (Brassica napus) oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 65–80. [Google Scholar]
- Bhatnagar, A.S.; Gopala Krishna, A.G. Natural antioxidants of the Jaffna variety of Moringa oleífera seed oil of Indian origin as compared to other vegetable oils. Grasasy Aceites 2013, 64, 537–545. [Google Scholar]
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283. [Google Scholar] [CrossRef]
- Kayode, R.M.O.; Afolayan, A.J. Cytotoxicity and effect of extraction methods on the chemical composition of essential oils of Moringa oleifera seeds. J. Zhejiang Univ. Sci. B. 2015, 16, 680–689. [Google Scholar] [CrossRef]
- Anwar, F.; Ashraf, M.; Bhanger, M.I. Interprovenance variation in the composition of Moringa oleifera oil seeds from Pakistan. J. Am. Oil Chem. Soc. 2005, 82, 45–51. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Ogunsina, B.S.; Indira, T.N.; Bhatnagar, A.S.; Radha, C.; Debnath, S.; Gopala Krishna, A.G. Quality characteristics and stability of Moringa oleifera seed oil of Indian origin. J. Food Sci. Technol. 2014, 5, 503–510. [Google Scholar] [CrossRef]
- Dasari, S.R.; Goud, V.V. Comparative extraction of castor seed oil using polar and nonpolar solvents. IJCET 2013, 1, 121–123. [Google Scholar]
- Muzenda, E.; Member, I.; Kabuba, J.; Mdletye, P.; Belaid, M. Optimization of process parameters for castor oil production. In Proceedings of the World Congress on Engineering, London, UK, 3–5 July 2012. [Google Scholar]
- Kittiphoom, S.; Sutasinee, S. Effect of microwaves pretreatments on extraction yield and quality of mango seed kernel oil. Int. Food Res. J. 2015, 22, 960–964. [Google Scholar]
- Azadmard-Damirchi, S.; Alirezalu, K.; Fathi Achachlouei, B. Microwave pretreatment of seeds to extract high quality vegetable oil. World Acad. Sci. Eng. Technol. 2011, 57, 72–75. [Google Scholar]
- Watanabe, S.; Karakawa, M.; Hashimoto, O. Temperature of a heated material in a microwave oven considering change of complex relative permittivity. In Proceedings of the 2009 European Microwave Conference (EuMC), Rome, Italy, 29 September–1 October 2009; pp. 798–801. [Google Scholar]
- Hindi, S.S.; Dawoud, U.M.; Assiry, K. System and Method for Manufacturing Shellac Floss. U.S. Patent 11060208, 22 March 2021. [Google Scholar]
- Hindi, S.S.; Dawoud, U.M.; Asiry, K.A. Bioplastic floss of a novel microwave-thermospun shellac: Synthesis and bleaching for some dental applications. Polymers J. 2023, 15, 142. [Google Scholar] [CrossRef]
- Hindi, S.S.; Dawoud, U.M.; Ismail, I.M.; Asiry, K.A.; Ibrahim, O.H.; Alharthi, M.A.; Mirdad, Z.M.; Al-Qubaie, A.I.; Shiboob, M.H.; Alanazi, R.A.; et al. Microwave-Assisted Extraction of Fixed Oils from Seeds. U.S. Patent Application No. 63445512, 14 February 2023. [Google Scholar]
- Whelan, M.; Dehn, R.; Kornrumpf, W.; Microwave Ovens. Edison Tech. Center. 2012. Available online: https://edisontechcenter.org/Microwaves.html (accessed on 28 July 2022).
- Abitogun, A.S.; Alademeyin, O.J.; Oloye, D.A. Extraction and characterization of castor seed oil. Internet. J. Nutr. Wellness. 2009, 8, 1–8. [Google Scholar]
- Omari, A.; Mgani, Q.A.; Mubofu, E.B. Fatty acid profile and physico-chemical parameters of castor oils in Tanzania. Green Sustain. Chem. 2015, 16, 154–163. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Hussein, Y.A.; Adongo, T.A. Extraction yield, efficiency and loss of the traditional hot water floatation (HWF) method of oil extraction from the seeds of Allanblackia floribunda. Int. J. Sci. Technol. Res. 2015, 4, 92–95. [Google Scholar]
- Keneni, Y.G.; Bahiru, L.A.; Marchetti, J.M. Effects of different extraction solvents on oil extracted from jatropha seeds and the potential of seed residues as a heat Provider. Bio. Energy Res. 2021, 14, 1207–1222. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction methods of oils and phytochemicals from seeds and their environmental and economic impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Isah, A.G. Production of detergent from castor oil. Leonardo El J. Pract. Technol. 2006, 9, 153–160. [Google Scholar]
- Auta, M. Extraction and characterization of drilling oil from castor oil. Int. J. Innov. Appl. Stud. 2013, 3, 382–387. [Google Scholar]
- Akpan, U.G.A.; Jimoh, A.; Mohamed, A.D. Extraction, characterization and modification of castor seed oil. Leonardo. J. Sci. 2006, 5, 43–52. [Google Scholar]
- Warra, A.A.; Wawata, I.G.; Gunu, S.Y.; Aujara, K.M. Extraction and physicochemical analysis of some selected Northern Nigerian industrial oils. Arch. Appl. Sci. Res. 2011, 3, 536–554. [Google Scholar]
- Thomas, A.; Matthaus, B.; Fiebeg, H.-J. Fats and fatty oils. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: KgaA, NJ, USA, 2015. [Google Scholar]
- AL-Hamdany, A.J.; Jihad, T.W. Oxidation of some primary and secondary alcohols using pyridinium chlorochromate. Tikrit J. Pure Sci. 2012, 17, 72–76. [Google Scholar]
- Hansel, F.A.; Bull, I.D.; Evershed, R.P. Gas chromatographic mass spectrometric detection of dihydroxy fatty acids preserved in the ‘bound’ phase of organic residues of archaeological pottery vessels. Rapid Commun. Mass Spectrom. 2011, 25, 1893–1898. [Google Scholar] [CrossRef]
- Silva, R.A.C.D.; de Lamos, L.G.; Ferreira, D.A.; Monte, F.J.Q. Chemical study of the seeds of Ximenia americana: Analysis of methyl esters by gas chromatography coupled to mass spectrometry. J. Anal. Pharm. Res. 2018, 7, 79–83. [Google Scholar]
- Bhukya, G.; Kaki, S.S. Design and synthesis of sebacic acid from castor oil by new alternate route. Eur. J. Lipid Sci. Technol. 2022, 124, e2100244. [Google Scholar] [CrossRef]
- Gaul, J.A. Quantitative calculation of gas chromatographic peaks in pesticide residue analyses. J. Assoc. Off. Anal. Chem. 1966, 49, 389–399. [Google Scholar] [CrossRef]
- El-Nakhlawy, F.S. Experimental Design and Analysis in Scientific Research; Sci. Pub. Center, King Abdulaziz University: Jeddah, Saudi Arabia, 2009. [Google Scholar]
- Chapman, G.W.; Robertson, J.A. Moisture content/relative humidity equilibrium of high-oil and confectionery type sunflower seed. J. Stored Prod. Res. 1987, 23, 115–118. [Google Scholar] [CrossRef]
- Akaranta, O.; Anusiem, A.C.I. A boiresource solvent for extraction of castor oil. Ind. Crops. Prod. 1996, 5, 273–277. [Google Scholar] [CrossRef]
- Doan, L.G. Ricin: Mechanism of toxicity, clinical manifestations, and vaccine development. A Review. J. Toxicol. 2004, 42, 201–208. [Google Scholar] [CrossRef]
- Jans-Joachim, H. Handbook of GC/MS. In Fundamental and Applications, 2nd eds.; WILEY-VCH, Verlag Gbmh: Weinheim, Germany, 2009. [Google Scholar]
- Foglia, T.A.; Jones, K.C.; Sonnet, P.E. Selectivity of lipases: Isolation of fatty acids from castor, coriander and Meadowfoam oils. Eur. J. Lipid Sci. Techn. 2000, 102, 612–617. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.D. The Lipid Handbook; CRC Press: Cambridge, MA, USA, 2007; p. 1472. [Google Scholar]
- Anonymous. National Institute Standards and Technology, NIST Chemistry WebBook, SRD69. 2012. Available online: https://webbook.nist.gov/chemistry/ (accessed on 28 July 2022).
- Choi, G.H.; Kim, L.; Lee, D.Y.; Jin, C.L.; Lim, S.-J.; Park, B.J.; Cho, N.J.; Kim, J.-H. Quantitative analyses of ricinoleic acid and ricinine in Ricinus communis extracts and its biopesticides. J. Appl. Biol. Chem. 2016, 59, 165–169. [Google Scholar] [CrossRef]
- Omowanle, J.; Ayo, R.J.; Habila, J.; Ilekhaize, J.; Adegbe, E.A. Physico-chemical and GC/MS analysis of some selected plant seed oils; castor, neem and rubber seed oils. FUW Trends Sci. Tech. J. 2018, 3, 644–651. [Google Scholar]
- Odoom, W.; Edusei, V.O. Evaluation of saponification value, iodine value and insoluble impurities in coconut oils from Jomoro District in the western region of Ghana. J. Agric. Food Sci. 2015, 3, 494–499. [Google Scholar]
- Saribiyik, O.Y.; Ozcanli, M.; Serin, H.; Serin, S.; Aydin, K. Biodiesel production from Ricinus communis oil and its blends with soybean biodiesel. J. Mech. Eng. 2010, 56, 811–816. [Google Scholar]
- Uzoh, C.F.; Nwanbanne, J. Investigating the effect of catalyst type and concentration on the functional group conversion in castor seed oil alkyd resin production. Adv. Chem. Eng. Sci. 2016, 6, 190–200. [Google Scholar] [CrossRef]
- Farag, R.S.; Hewedi, F.M.; Abu-Raiia, S.H.; Elbaroty, G.S. Comparative study on the deterioration of oils by microwave and conventional heating. J. Food Prot. 1992, 55, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Ienaga, H.; Tsuchiya, C.; Yoshida, H. Microwave roasting effects on the composition of tocopherols and acyl lipids within each structural part and section of a soya bean. J. Sci. Food Agric. 1999, 79, 1155–1162. [Google Scholar] [CrossRef]
- Takagi, S.; Yoshida, H. Microwave heating influences on fatty acid distribution of triacylglycerols and phospholipids in hypocotyls of soybeans (Glycine max L.). Food Chem. 1999, 66, 345–351. [Google Scholar] [CrossRef]
- Ramanadhan, B. Microwave Extraction of Essential Oils (from Black Pepper and Coriander) at 2.46 Ghz. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2005. [Google Scholar]
- Uquiche, E.; Jerez, M.; Ortiz, J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol). J. Innov. Food Sci. Emerg. Technol. 2008, 9, 495–500. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirakawa, Y.; Tomiyama, Y.; Mizushina, Y. Effects of microwave treatment on the oxidative stability of peanut (Arachis hypogaea) oils and the molecular species of their triacylglycerols. Eur. J. Lipid Sci. Technol. 2003, 105, 351–358. [Google Scholar] [CrossRef]
- Anonymous. Microwave Assisted Drying. 1993. Available online: http://ecoursesonline.iasri.res.in/mod/page/view.php?id=882 (accessed on 4 April 2023).
- Rakesh, V.; Seo, Y.; Datta, A.K.; McCarthy, K.L.; McCarthy, M.J. Heat transfer during microwave combination heating: Computational modeling and MRI experiments. Bioeng. Food Nat. Prod. 2010, 56, 2468–2478. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J. Castor oil: Properties, uses, and optimization of processing parameters in commercial production. PMC J. 2016, 9, LPI-S40233. [Google Scholar] [CrossRef]
| Equation | Definitions |
|---|---|
| 1 MC, % = [(Wads − Wods)/Wods] × 100 2 Scfo, % = (Wmfo + Wrfo)/Wods 3 Ymfo, % = (Wmfo/Wods) × 100 4 Yrfo, % = (Wods − Wrc)/Wods] × 100 = (Wrfo/Wods) × 100 5 EL, % = [{Wods − (Wrfo + Wrc)}/Wods] × 100 6 Efoe, % = [Wrfo/(Scfo × Wods)] × 100 | Wads: Weight of air-dried seeds. Wods: Weight of oven-dried seeds, g. Wmfo: Weight of the main fixed oil (g). Wrfo: Weight of recovered fixed oil (from seed’cake), g Wrc = Weight of residual cake after extraction. Scfo: Seed content of fixed oil |
| 7 SGfo = (W1 − W2)/(W2 − W0) | W0: Weight of an empty bottle. W1: Weight of the bottle filled with fixed oil. W2: Weight of the bottle filled with deionized water. |
| 8 IN = 12.69 × C × (V1 − V2)/W | C, V1, V2: Parameters of sodium thiosulphate: C: Concentration. V1: The volume used for the blank test. V2: The volume used for the fixed oil. W: The fixed oil weight. |
| 9 SV = 56.1N × (V1 − V2)/W | V1: Volume of the solution used for the blank test. V2: Volume of the solution used for fixed oil. N: The actual normality of the HCl used. W: The fixed oil weight. |
| 10 AV = 5.61(V × N)/W | V: Volume of KOH, IN mL. N: Normality of KOH. W: Fixed oil weight. |
| 11 Yfa = (A1/A2) × 100 | A1: Peak area (mm2) of a certain fatty acid detected at a certain retention time (min.) A2: The overall peak’s areas (mm2) of all fatty acids in the fixed oil. |
| Properties | Extraction Method 3 | ASTM 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MHPM | EHPM | |||||||||
| C | S | R | M | C | S | R | M | |||
| MC, % | AD | ± | ± | ± | ± | ± | ± | ± | ± | |
| OD | 3.09 ± 0.15 | ± | ± | ± | 4.47 ± 0.18 | ± | ± | ± | N.d. 12 | |
| OY 5, % | 44.8 ± 1.03 | 42.5 ± 0.98 | 42.2 ± 1.02 | 39.6 ± 0.89 | 38.3 ± 1.09 | 36.4 ± 1.25 | 35.2 ± 1.33 | 34.6 ± 0.93 | N.d. 12 | |
| SG 6 | 0.973 ± 0.08 | 0.914 ± 0.07 | 0.906 ± 0.1 | 0.946 ± 0.093 | 0.968 ± 0.09 | 0.918 ± 0.07 | 0.914 ± 0.09 | 0.957 ± 0.088 | 0.96–0.97 | |
| RI 7 | 1.48 ± 0.092 | 1.473 ± 0.08 | 1.465 ± 0.069 | 1.464 ± 0.13 | 1.43 ± 0.07 | 1.469 ± 0.086 | 1.467 ± 0.102 | 1.563 ± 0.17 | 1.48–1.49 | |
| IN 8 (g I2/100 g oil) | 83.7 ± 1.09 | 126.3 ± 1.23 | 114.5 ± 1.48 | 69.7 ± 1.44 | 84.8 ± 1.05 | 127.1 ± 1.19 | 115.1 ± 1.29 | 71.4 ± 1.38 | 82–88 | |
| SV 9(mg KOH/g oil) | 181.5 ± 3.79 | 188.4 ± 2.34 | 168.3 ± 1.98 | 180.7 ± 2.18 | 182.1 ± 3.07 | 194.6 ± 2.33 | 181.2 ± 2.56 | 190.4 ± 1.97 | 175–187 | |
| AV 10(mg KOH/g oil) | 0.869 ± 0.004 | 0.914 ± 0.008 | 2.107 ± 0.08 | 1.433 ± 0.005 | 0.882 ± 0.008 | 1.139 ± 0.004 | 2.306 ± 0.006 | 1.344 ± 0.007 | 0.4–4.0 | |
| pH 11 | 6.48 ± 0.359 | 6.46 ± 0.45 | 6.43 ± 0.77 | 6.45 ± 0.69 | 6.53 ± 0.48 | 6.38 ± 0.39 | 6.49 ± 0.78 | 6.37 ± 0.59 | N.d. 12 | |
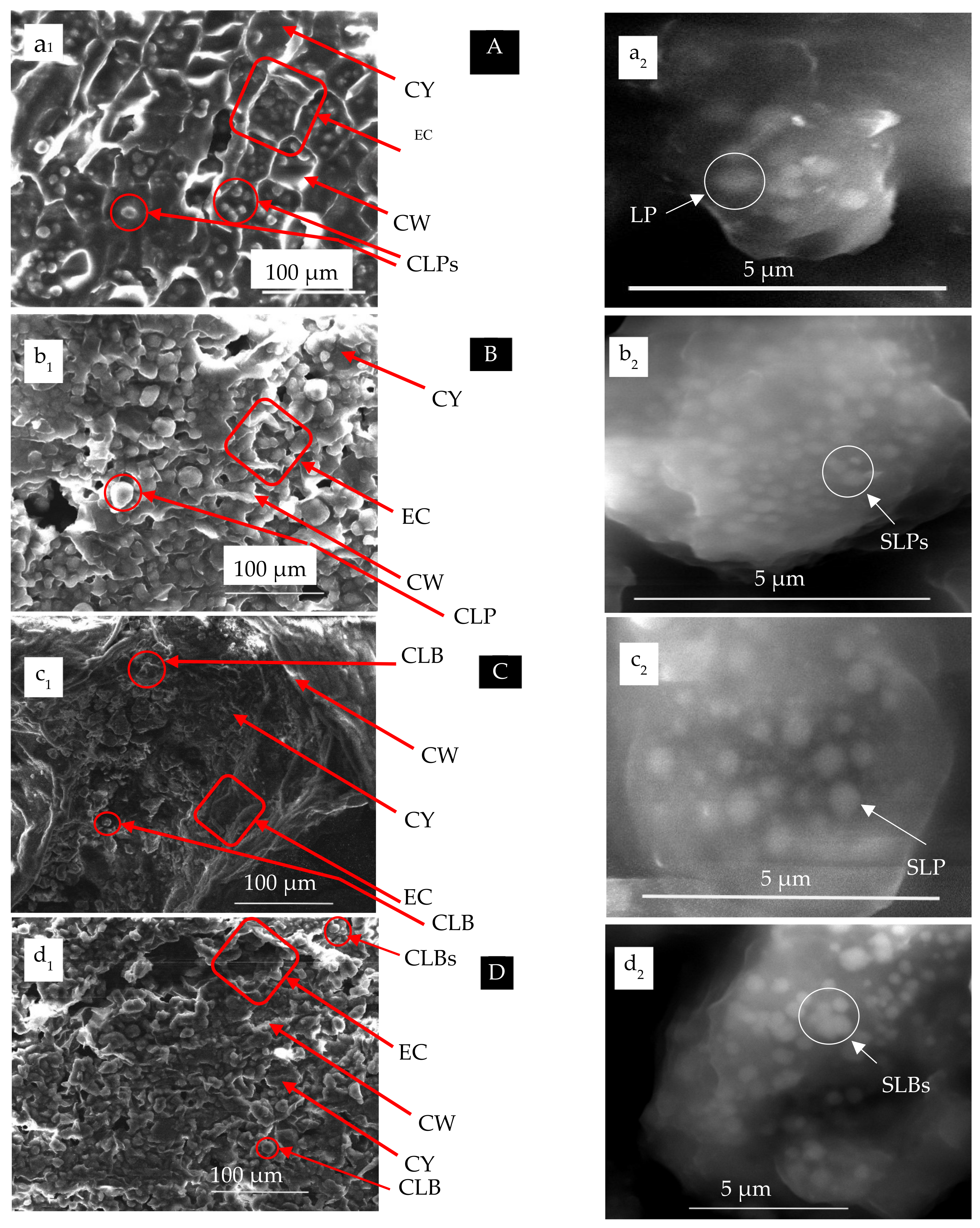
| Species | Body Diameter (µm) | |
|---|---|---|
| CLB | SLB | |
| Castor bean Common sunflower Rapeseed Moringa | 6.67 [0.58]–24.9 [3.25] 8.7 [0.48]–22.09 [2.74] 7.27 [0.68]–14.55 [2.06] 6.52 [0.69]–17.39 [1.83] | 0.44 [0.39]–0.94 [0.03] 0.19 [0.12]–0.47 [0.05] 0.97 [0.14]–0.59 [0.08] 0.24 [0.09]–0.71 [0.09] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hindi, S.S.; Dawoud, U.M.; Ismail, I.M.; Asiry, K.A.; Ibrahim, O.H.; Al-Harthi, M.A.; Mirdad, Z.M.; Al-Qubaie, A.I.; Shiboob, M.H.; Almasoudi, N.M.; et al. A Novel Microwave Hot Pressing Machine for Production of Fixed Oils from Different Biopolymeric Structured Tissues. Polymers 2023, 15, 2254. https://doi.org/10.3390/polym15102254
Hindi SS, Dawoud UM, Ismail IM, Asiry KA, Ibrahim OH, Al-Harthi MA, Mirdad ZM, Al-Qubaie AI, Shiboob MH, Almasoudi NM, et al. A Novel Microwave Hot Pressing Machine for Production of Fixed Oils from Different Biopolymeric Structured Tissues. Polymers. 2023; 15(10):2254. https://doi.org/10.3390/polym15102254
Chicago/Turabian StyleHindi, Sherif S., Uthman M. Dawoud, Iqbal M. Ismail, Khalid A. Asiry, Omer H. Ibrahim, Mohammed A. Al-Harthi, Zohair M. Mirdad, Ahmad I. Al-Qubaie, Mohamed H. Shiboob, Najeeb M. Almasoudi, and et al. 2023. "A Novel Microwave Hot Pressing Machine for Production of Fixed Oils from Different Biopolymeric Structured Tissues" Polymers 15, no. 10: 2254. https://doi.org/10.3390/polym15102254
APA StyleHindi, S. S., Dawoud, U. M., Ismail, I. M., Asiry, K. A., Ibrahim, O. H., Al-Harthi, M. A., Mirdad, Z. M., Al-Qubaie, A. I., Shiboob, M. H., Almasoudi, N. M., & Alanazi, R. A. (2023). A Novel Microwave Hot Pressing Machine for Production of Fixed Oils from Different Biopolymeric Structured Tissues. Polymers, 15(10), 2254. https://doi.org/10.3390/polym15102254







