Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bottle Manufacturing Process
2.2. Characterizing MRF-Recovered Post-Consumer PP EBM Bottles
2.2.1. Melt Flow Index
2.2.2. Infrared Spectroscopy
2.2.3. Differential Scanning Calorimetry
2.2.4. Oxygen Induction Time
2.2.5. Barrier Properties
2.2.6. Mechanical Properties
2.3. Suitability of Post-Consumer PP EBM Bottles for Direct Food Contact
2.3.1. Heavy Metal Analysis
2.3.2. CFR Analysis and Cramer Classification
2.4. Statistical Analysis
3. Results and Discussions
3.1. Physical Performance of MRF-Recovered PCR-PP EBM Bottles
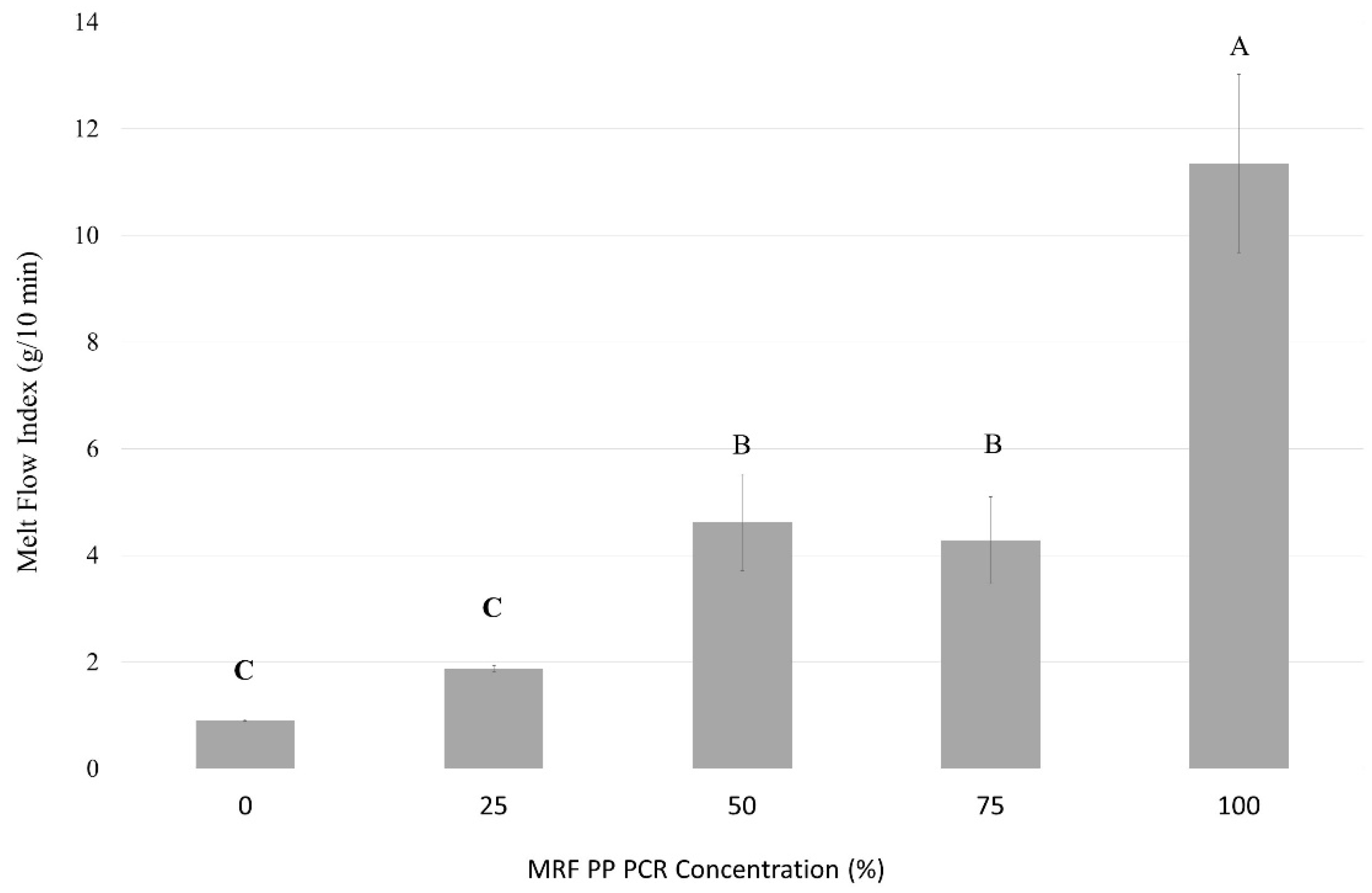
3.1.1. Polymer Viscosity
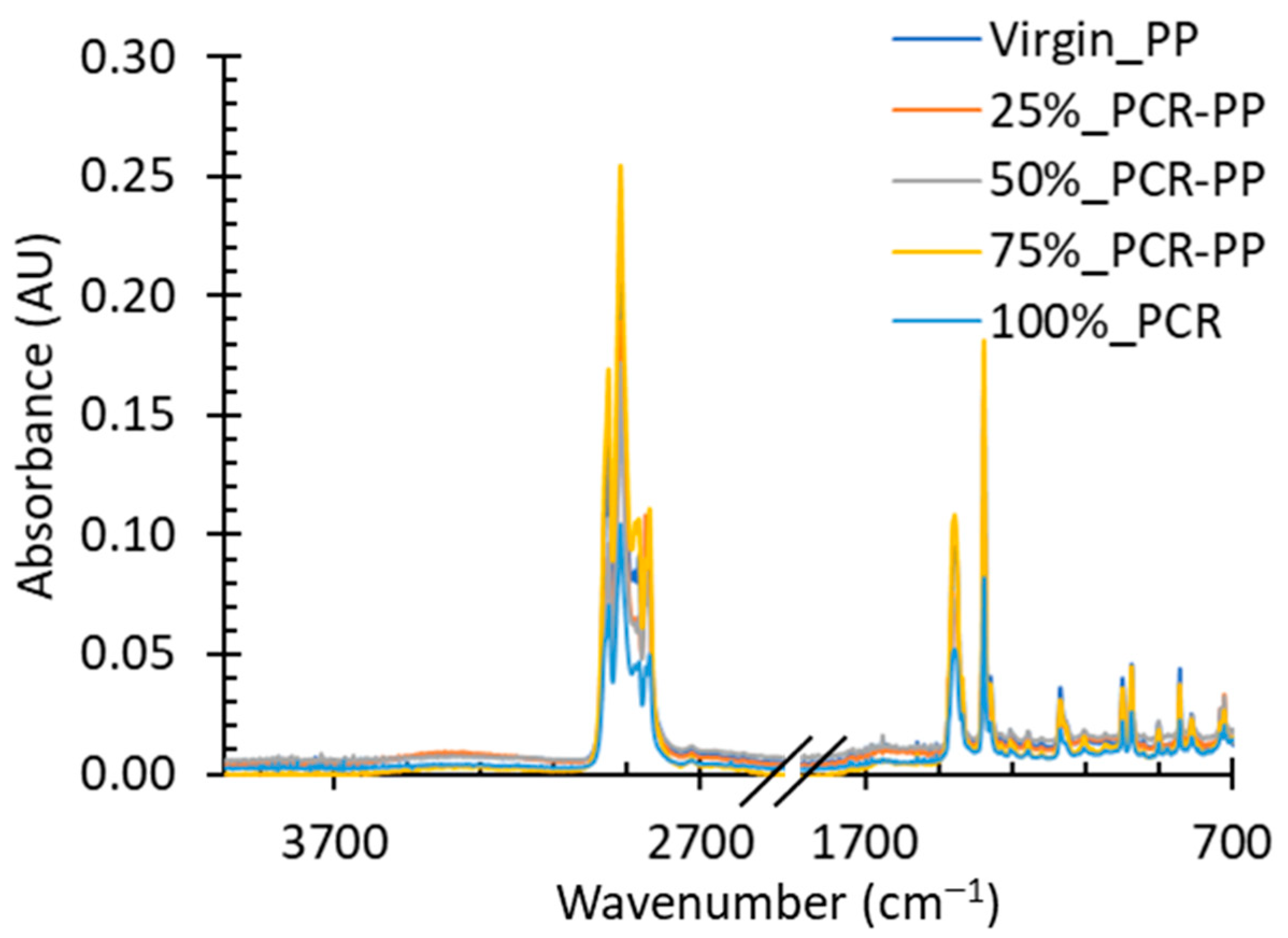
3.1.2. Polymer Molecular Structure
3.1.3. Thermal Properties
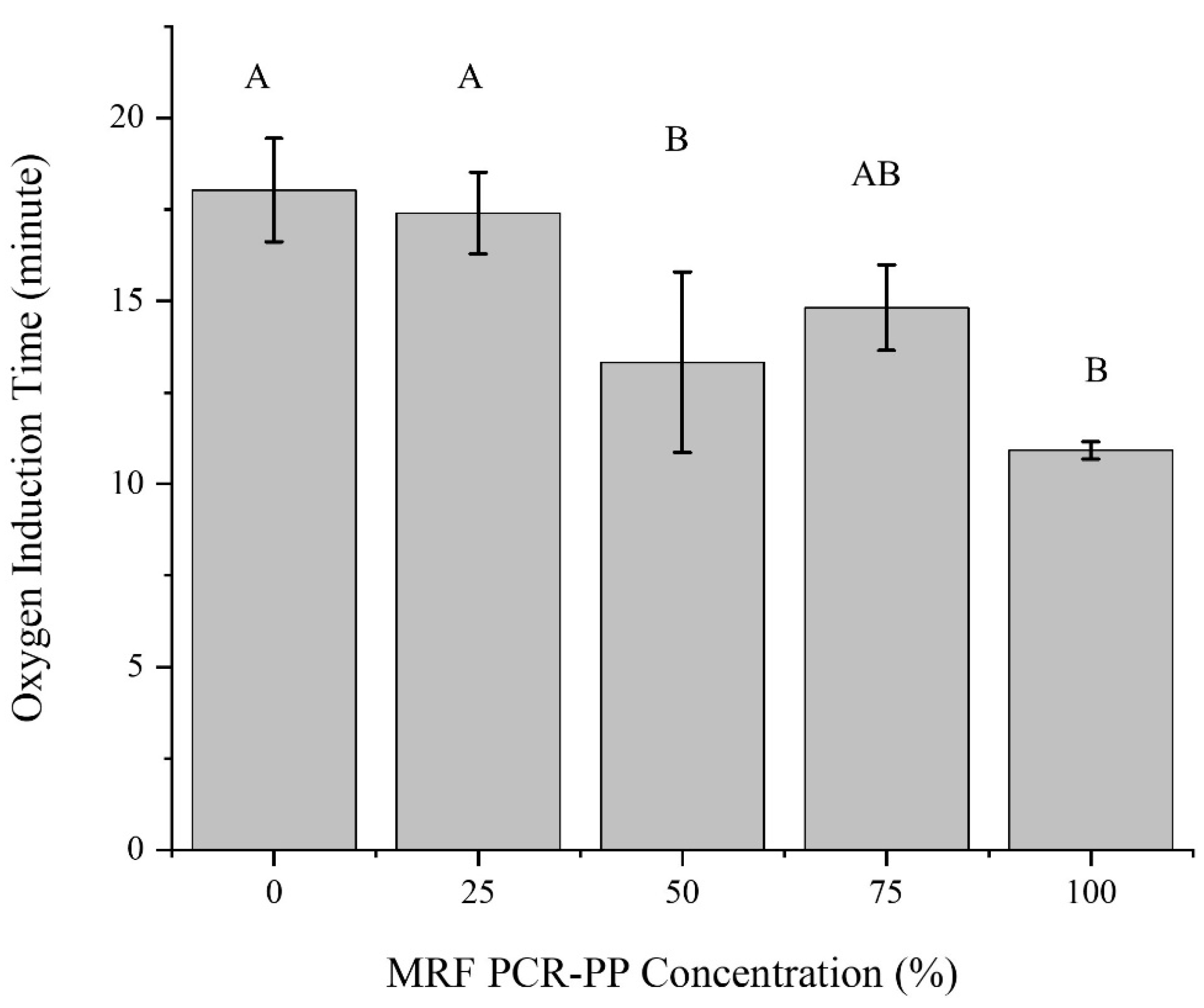
3.1.4. Thermooxidative Stability of Post-Consumer PP EBM Bottles
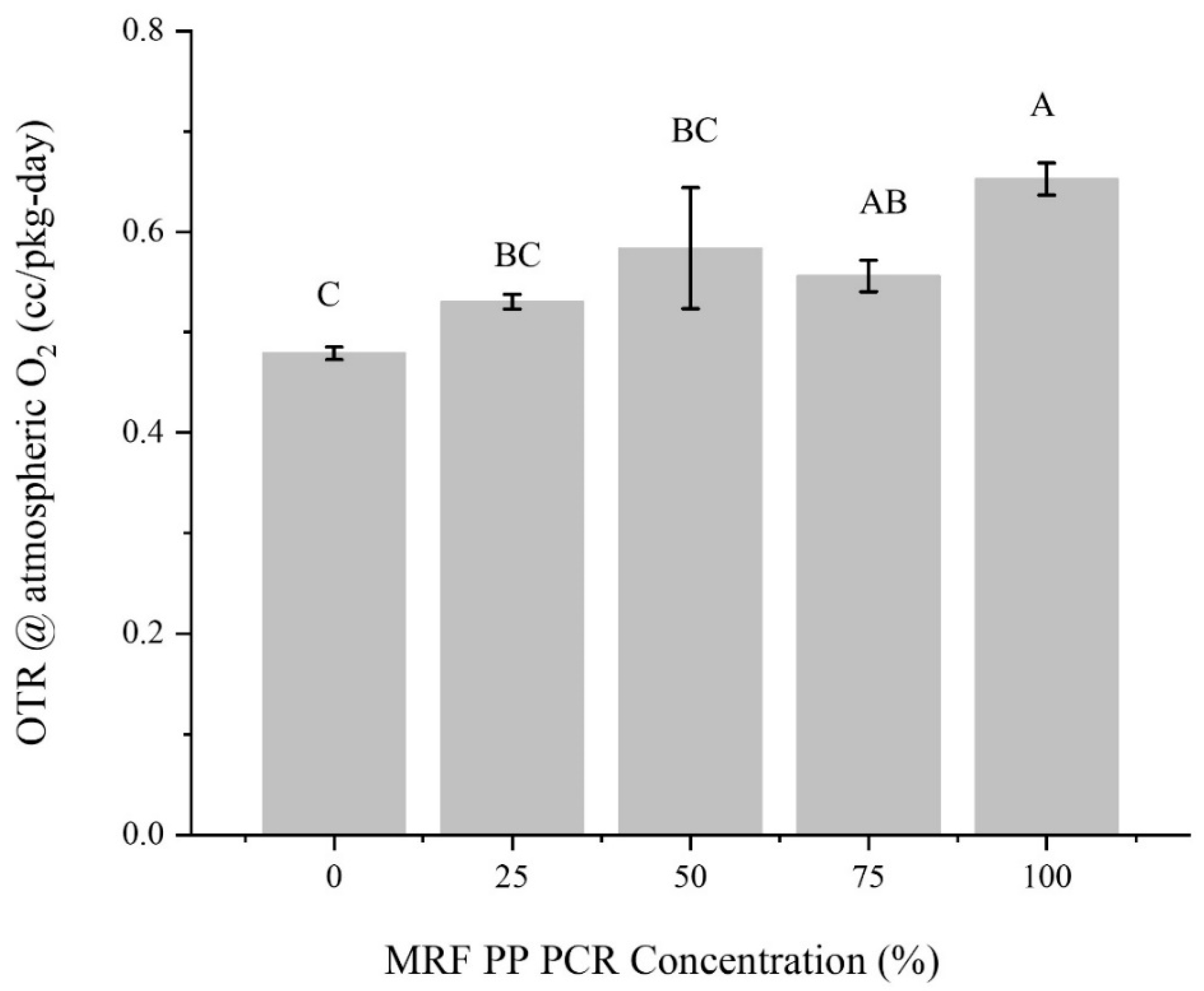
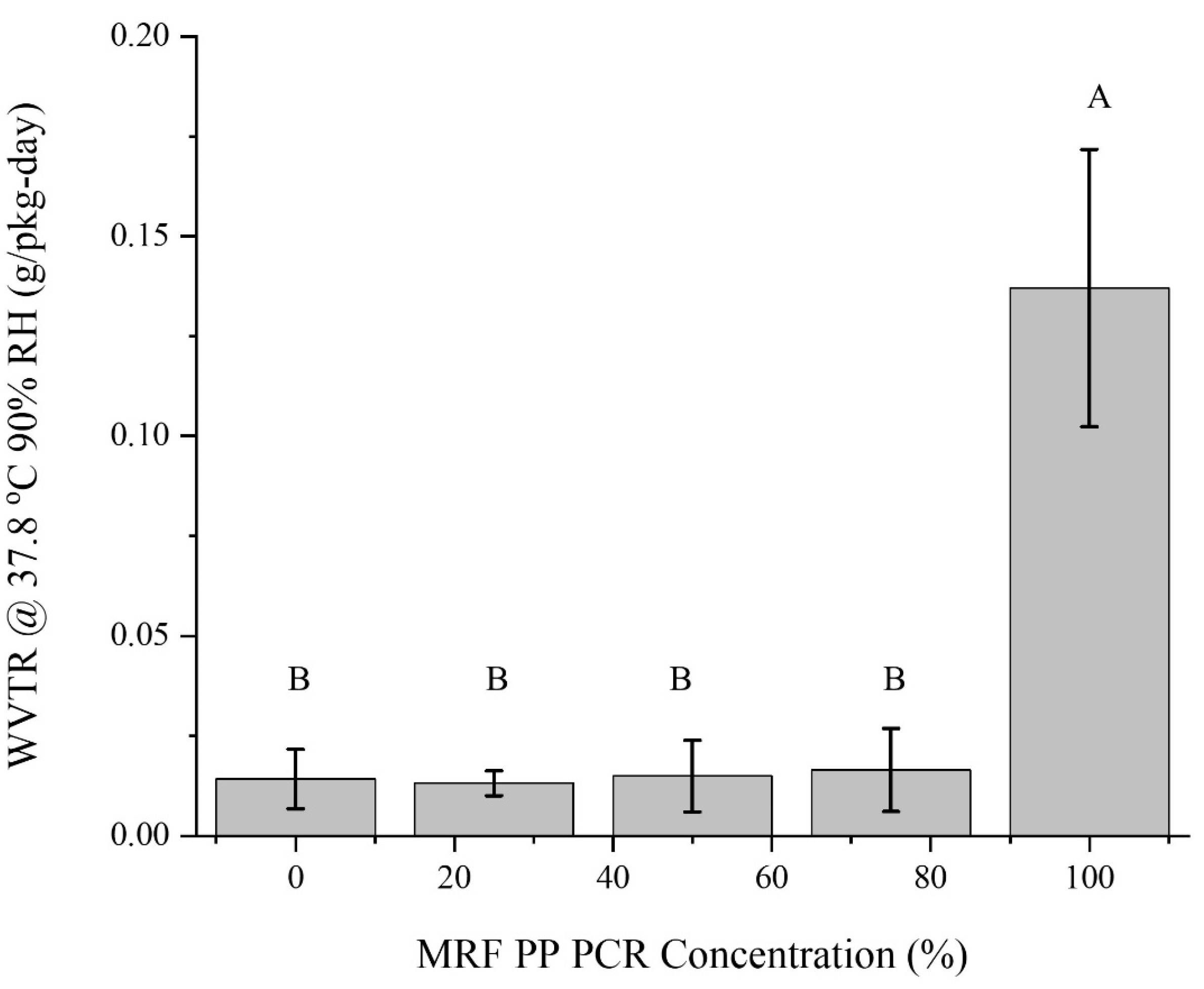
3.1.5. Barrier Properties
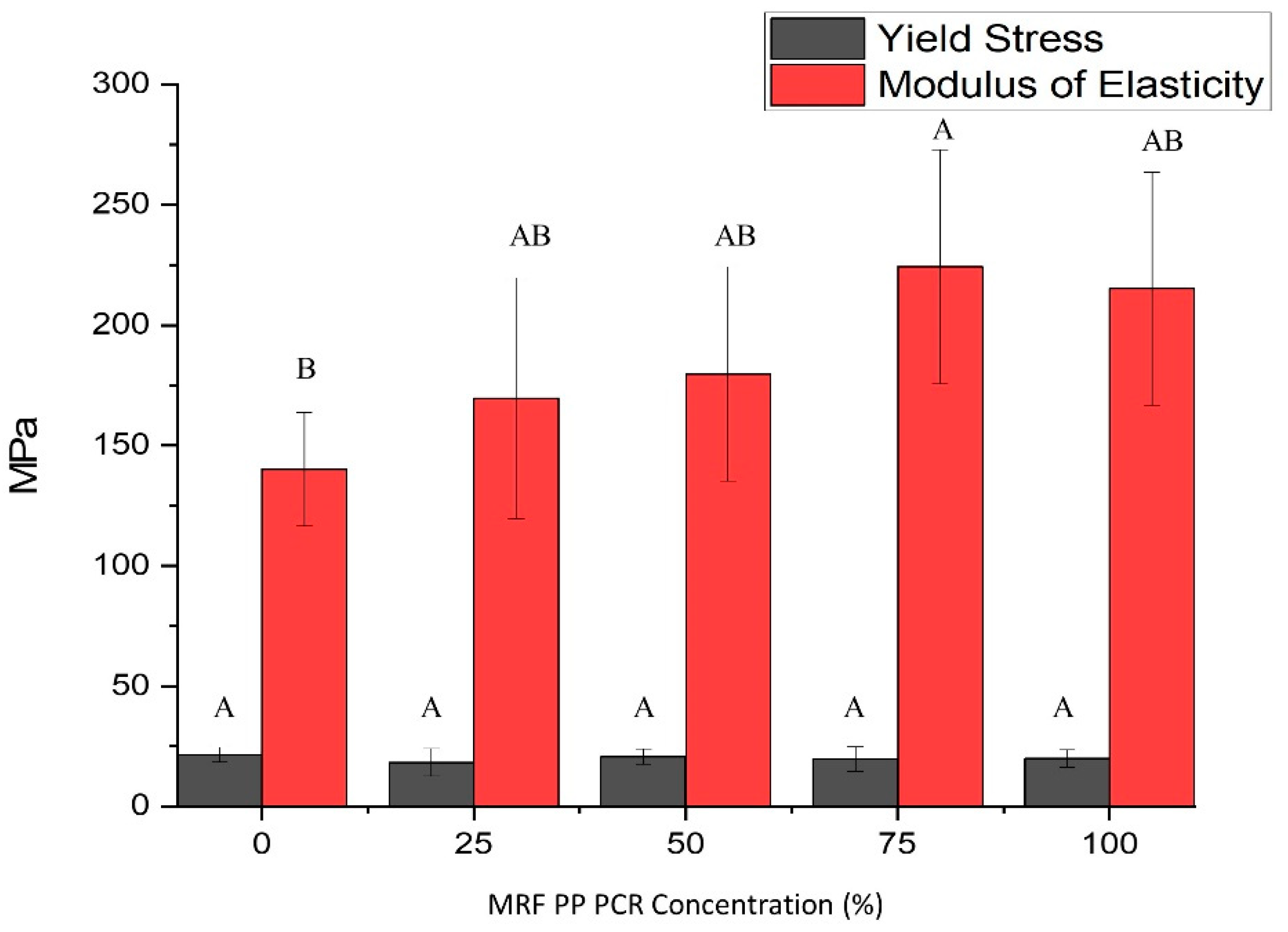
3.1.6. Tensile Properties
3.2. Compliance of MRF-Recovered Post-Consumer PP EBM Bottles
3.2.1. Metals’ ICP-OES
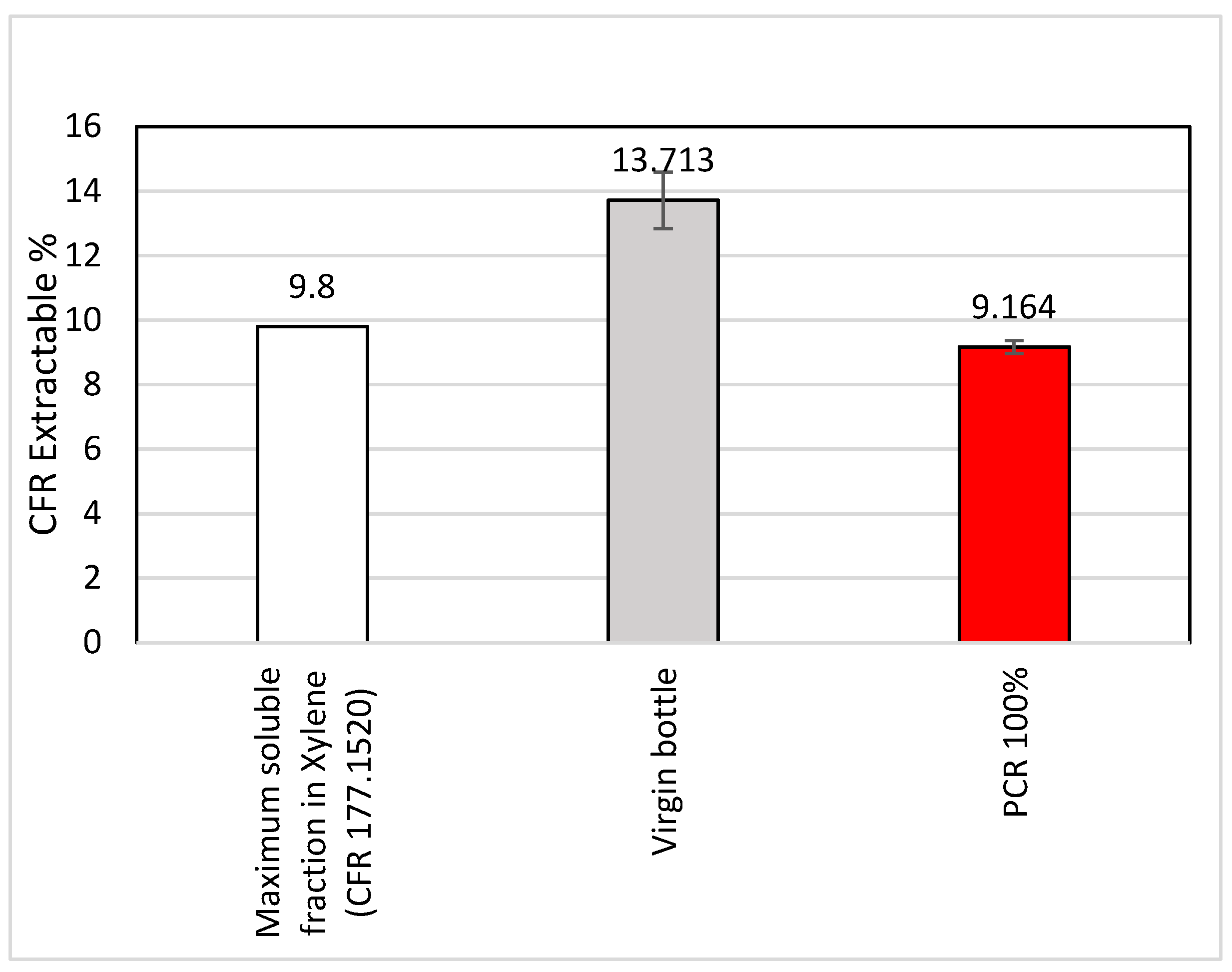
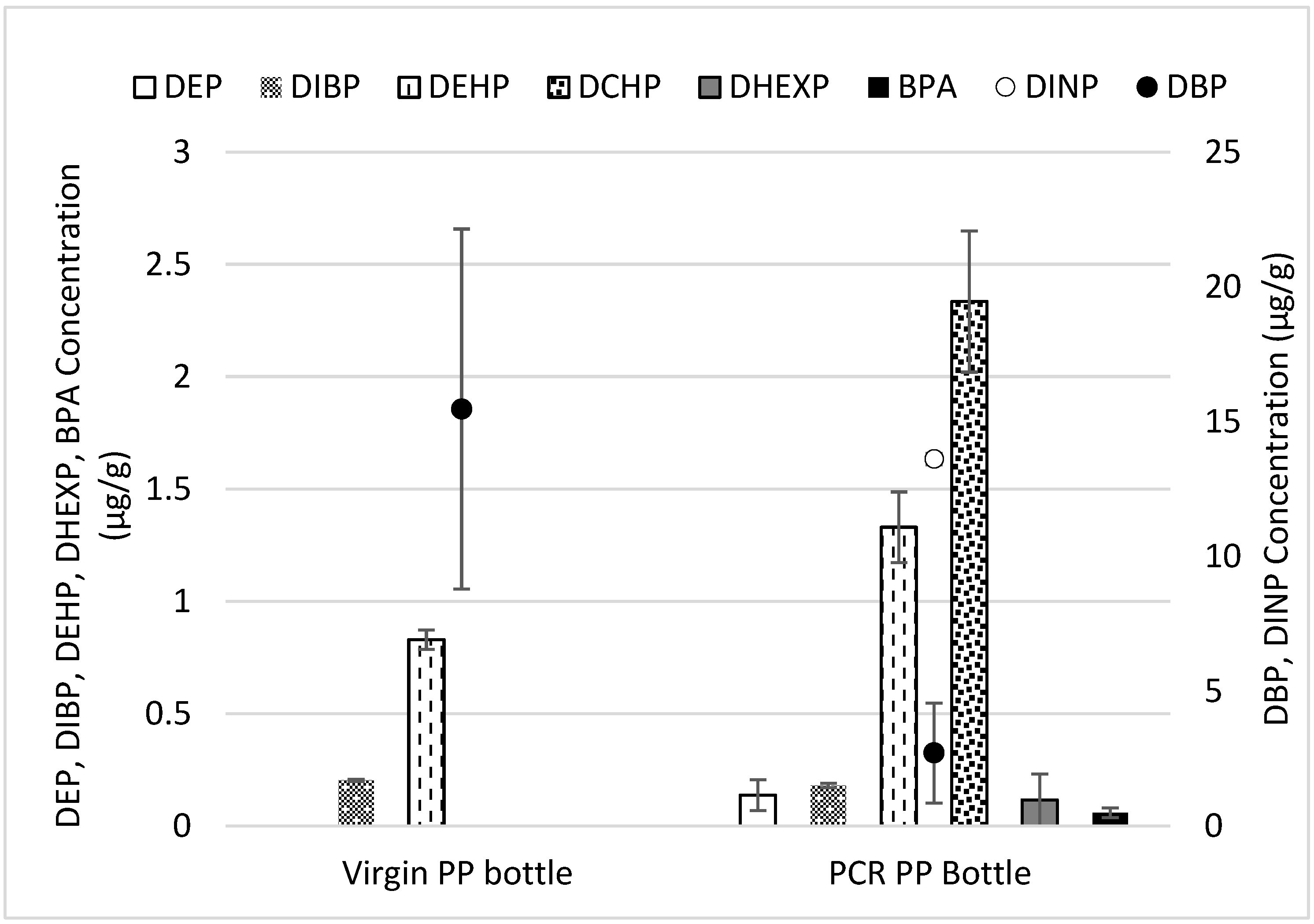
3.2.2. 21 CFR 177.1520 Extraction for Extractable and Cramer Classification
4. Conclusions
- Increased crystallization temperature when PCR is present in the blend;
- No practical difference in crystallinity as a function of PCR concentration as-molded (first heat curve);
- No other polymers are present in the thermograms of MRF-recovered PP materials, indicating high polymer purity (melting peaks);
- Oxygen and water-vapor barrier properties remained relatively constant unless the composition was 100% MRF material;
- MRF-recovered PCR-PP significantly (p-value < 0.05) improved the stiffness of virgin PP bottles. On the other hand, the measured yield stress for all treatments was significantly similar;
- A wider range of N/IAS was detected in PCR material compared to the virgin material, which can be attributed to plastic additives, food additives, and degradation byproducts;
- Regulatory compliance (limits of TPCH: 100 μg/g) and maintaining properties up to 75% MRF PCR demonstrates the increased value of MRF materials. Targeted phthalates did not exceed the limits of TPCH, and trace levels of BPA were detected in the MRF PCR-PP.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- PlasticsEurope. Plastics-The Facts 2022. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 17 May 2023).
- Bora, R.R.; Wang, R.; You, F. Waste Polypropylene Plastic Recycling toward Climate Change Mitigation and Circular Economy: Energy, Environmental, and Technoeconomic Perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Plastics. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Freudenthaler, P.J.; Fischer, J.; Liu, Y.; Lang, R.W. Polypropylene Post-Consumer Recyclate Compounds for Thermoforming Packaging Applications. Polymers 2023, 15, 345. [Google Scholar] [CrossRef]
- Fortune Business Insights. The Global Polypropylene Market Is Projected to Grow from USD 76.00 Billion in 2021 to USD 108.57 Billion in 2028 at a CAGR of 5.2% in Forecast Period, 2021–2028. Available online: https://www.fortunebusinessinsights.com/industry-reports/polypropylene-pp-market-101583 (accessed on 11 July 2023).
- EPA. National Recycling Strategy. Part One of a Series on Building a Circular Economy for all. 2021. U.S. EPA Office of Resource Conservation and Recovery 53f0-R-21-003. Available online: https://www.epa.gov/system/files/documents/2021-11/final-national-recycling-strategy.pdf (accessed on 17 May 2023).
- PlasticsEurope. PlasticsEurope-CircularityReport-2022_2804. 2022. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-overview-2/ (accessed on 17 May 2023).
- Cecon, V.S.; Da Silva, P.F.; Curtzwiler, G.W.; Vorst, K.L. The challenges in recycling post-consumer polyolefins for food contact applications: A review. Resour. Conserv. Recycl. 2021, 167, 105422. [Google Scholar] [CrossRef]
- Curtzwiler, G.W.; Schweitzer, M.; Li, Y.; Jiang, S.; Vorst, K.L. Mixed post-consumer recycled polyolefins as a property tuning material for virgin polypropylene. J. Clean. Prod. 2019, 239, 117978. [Google Scholar] [CrossRef]
- Greenpeace Reports. Circular Claims Fall Flat: Comprehensive U.S. SURVEY of Plastics Recyclability. 2022. Available online: Greenpeace.org/usa/plasticrecycling (accessed on 17 May 2023).
- Cecon, V.S.; Curtzwiler, G.W.; Vorst, K.L. A Study on Recycled Polymers Recovered from Multilayer Plastic Packaging Films by Solvent-Targeted Recovery and Precipitation (STRAP). Macromol. Mater. Eng. 2022, 307, 2200346. [Google Scholar] [CrossRef]
- Cecon, V.S.; Curtzwiler, G.W.; Vorst, K.L. Evaluation of Mixed #3-7 Plastic Waste from Material Recovery Facilities (MRFs) in the United States. SSRN 2023. [Google Scholar] [CrossRef]
- Möllnitz, S.; Feuchter, M.; Duretek, I.; Schmidt, G.; Pomberger, R.; Sarc, R. Processability of Different Polymer Fractions Recovered from Mixed Wastes and Determination of Material Properties for Recycling. Polymers 2021, 13, 457. [Google Scholar] [CrossRef]
- The Recycling Partnership; The Association of Plastic Recyclers. The Growing Market for Recycled Polypropylene; The Recycling Partnership: Washington, DC, USA, 2020. [Google Scholar]
- Recycling Today. The potential of Polypropylene. Available online: https://www.recyclingtoday.com/article/the-recycling-potential-of-polypropylene/ (accessed on 8 May 2023).
- The Recycling Partnership. Polypropylene Recycling Coalition; The Recycling Partnership: Washington, DC, USA, 2022. [Google Scholar]
- TPCH. Toxics in Packaging Clearinghouse—Fact Sheet; Toxic in Packaging Clearinghouse: Boston, MA, USA, 2021. [Google Scholar]
- TPCH. Model Toxics in Packaging Legislation; Toxic in Packaging Clearinghouse: Boston, MA, USA, 2022. [Google Scholar]
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.-X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef]
- APR. Polyolefin Standard Laboratory Processing Practices. The Association of Plastics Recyclers. 2020. Available online: https://plasticsrecycling.org/images/Design-Guidance-Tests/APR-O-P-00-olefin-practices.pdf (accessed on 22 June 2023).
- ASTM D1238-20; Standard Test Method for Melt Flow Rates of Thermo- plastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Mort, R.; Cecon, V.S.; Mort, P.; McInturff, K.; Jiang, S.; Vorst, K.; Curtzwiler, G. Sustainable Composites Using Landfill Bound Materials. Front. Mater. 2022, 9, 849955. [Google Scholar] [CrossRef]
- ASTM D3418-15; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM D3895-19; Standard Test Method for Oxidative-Induction Time of Polyolefins by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Kabir, A.S.; Yuan, Z.; Kuboki, T.; Xu, C.C. De-polymerization of industrial lignins to improve the thermo-oxidative stability of polyolefins. Ind. Crop. Prod. 2018, 120, 238–249. [Google Scholar] [CrossRef]
- ASTM D3985-17; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM F1249-20; Standard Test Method for Water Vapor Transmission Rate through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- FDA. 177.1520 Olefin Polymers. 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=177.1520 (accessed on 17 May 2023).
- NIST. NIST Standard Reference Database 1A. 2014. Available online: https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf (accessed on 3 March 2023).
- Cramer, G.; Ford, R.; Hall, R. Estimation of toxic hazard—A decision tree approach. Food Cosmet. Toxicol. 1976, 16, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Aptula, A.; Schultz, T.W.; Shen, J.; Api, A.M.; Bhatia, S.; Kromidas, L. A practical guidance for Cramer class determination. Regul. Toxicol. Pharmacol. 2015, 73, 971–984. [Google Scholar] [CrossRef]
- Engül, Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.H.; De Paoli, M.-A. Polypropylene compounding with post-consumer material: II. Reprocessing. Polym. Degrad. Stab. 2002, 78, 491–495. [Google Scholar] [CrossRef]
- Martínez-Jothar, L.; Montes-Zavala, I.; Rivera-García, N.; Díaz-Ceja, Y.; Pérez, E.; Waldo-Mendoza, M.A. Thermal degra-dation of polypropylene reprocessed in a co-rotating twin-screw extruder: Kinetic model and relationship between Melt Flow Index and Molecular weight. Rev. Mex. Ing. Química 2021, 20, 1079–1090. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the loop for PET, PE and PP waste from house-holds: Influence of material properties and product design for plastic recycling. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: West Sussex, UK, 2001. [Google Scholar]
- Mistry, B.D. Handbook of Spectroscopic Data: Chemistry; Oxford Book Company: Oxford, UK, 2009. [Google Scholar]
- Akhoundi, B.; Nabipour, M.; Hajami, F.; Shakoori, D. An Experimental Study of Nozzle Temperature and Heat Treatment (Annealing) Effects on Mechanical Properties of High-Temperature Polylactic Acid in Fused Deposition Modeling. Polym. Eng. Sci. 2020, 60, 979–987. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Singh, A.A.; Genovese, M.E.; Mancini, G.; Marini, L.; Athanassiou, A. Green Processing Route for Polylactic Acid–Cellulose Fiber Biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4128–4136. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E.; Gillan, M.; Lambeth, R.H. Recycled polyethylene terephthalate as a new FFF feedstock material. Addit. Manuf. 2018, 21, 174–182. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.-M.; Fu, X.-N.; Li, Y.-L.; Huang, P.-X.; Zhang, Q.; Li, W.-G.; Ma, L.; Lai, F.; Wang, P.-F. Thermal stability and safety of dimethoxymethane oxidation at low temperature. Fuel 2018, 234, 207–217. [Google Scholar] [CrossRef]
- Selke, S.; Cutler, E.M.; Hernandez, R.J. Plastics Packaging: Properties, Processing, Applications, and Regulations, 2nd ed.; Hanser Gardner Publications: Cincinnati, OH, USA, 2004. [Google Scholar]
- Perkins, W. Effect of molecular weight and annealing temperature on the oxygen barrier properties of oriented PET film. Polym. Bull. 1988, 19, 397–401. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- De Santis, F.; Pantani, R. Optical Properties of Polypropylene upon Recycling. Sci. World J. 2013, 2013, 354093. [Google Scholar] [CrossRef]
- Demets, R.; Van Kets, K.; Huysveld, S.; Dewulf, J.; De Meester, S.; Ragaert, K. Addressing the complex challenge of understanding and quantifying substitutability for recycled plastics. Resour. Conserv. Recycl. 2021, 174, 105826. [Google Scholar] [CrossRef]
- Nerín, C.; Bourdoux, S.; Faust, B.; Gude, T.; Lesueur, C.; Simat, T.; Stoermer, A.; Van Hoek, E.; Oldring, P. Guidance in selecting analytical techniques for identification and quantification of non-intentionally added substances (NIAS) in food contact materials (FCMS). Food Addit. Contam. Part A 2022, 39, 620–643. [Google Scholar] [CrossRef]
- Sander, M.M.; Nicolau, A.; Guzatto, R.; Samios, D. Plasticiser effect of oleic acid polyester on polyethylene and polypropylene. Polym. Test. 2012, 31, 1077–1082. [Google Scholar] [CrossRef]
- Patti, A.; Lecocq, H.; Serghei, A.; Acierno, D.; Cassagnau, P. The universal usefulness of stearic acid as surface modifier: Applications to the polymer formulations and composite processing. J. Ind. Eng. Chem. 2021, 96, 1–33. [Google Scholar] [CrossRef]
- Sivakumar, P. Extraction and Estimation of antistatic agent Glycerol Mono Stearate in Polypropylene by Gas Chromatography Coupled with Flame Ionisation Detector. Indian J. Chem. (IJC) 2023, 62, 153–157. [Google Scholar] [CrossRef]
- Han, J. What Is Butylated Hydroxytoluene (BHT, e321) in Food: Uses, Safety, Side Effects. 2020. Available online: https://foodadditives.net/antioxidant/butylated-hydroxytoluene-bht/ (accessed on 3 March 2023).
- ECHA. Substance identity. European Chemicals Agency. 2023. Available online: https://www.echa.europa.eu/registration-dossier/-/registered-dossier/15975 (accessed on 3 March 2023).
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Singh, R.; Jana, S. Physical, Thermal and Spectroscopic Characterization of m-Toluic Acid: An Impact of Biofield Treatment. Biochem. Pharmacol. Open Access 2015, 4, 1000178. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekil, A.; Akcay, M.; Onal, A.; Kavak, F. Polymer/palmitic acid blends as shape-stabilized phase change ma-terial for latent heat thermal energy storage. Asian J. Chem. 2006, 18, 439–446. [Google Scholar]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Evaluation of the Occurrence of Phthalates in Plastic Materials Used in Food Packaging. Appl. Sci. 2021, 11, 2130. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano-Cuadro, H.; Puello-Polo, E. Emission of Bisphenol A and Four New Analogs from Industrial Wastewater Treatment Plants in the Production Processes of Polypropylene and Polyethylene Terephthalate in South America. Sustainability 2022, 14, 10919. [Google Scholar] [CrossRef]
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1337–1359. [Google Scholar] [CrossRef]
- Alp, A.C.; Yerlikaya, P. Phthalate ester migration into food: Effect of packaging material and time. Eur. Food Res. Technol. Food Packag. Forum 2020, 246, 425–435. [Google Scholar] [CrossRef]
- Food-Packaging-Forum. Toxics in Packaging Legislation to Restrict PFAS, Ortho-Phthalates. 2020. Available online: https://www.foodpackagingforum.org/news/toxics-in-packaging-legislation-to-restrict-pfasortho-phthalate (accessed on 17 May 2023).
- WebMD. What to Know About the Toxicity of Polypropylene. 2021. Available online: https://www.webmd.com/a-to-z-guides/what-to-know-about-the-toxicity-of-polypropylene (accessed on 12 March 2023).





| Treatments | % (wt) MRF-Recovered Post-Consumer PP |
|---|---|
| 1 | 0 (virgin PP) |
| 2 | 25 |
| 3 | 50 |
| 4 | 75 |
| 5 | 100 |
| Treatment | Tm1 (°C) | Tm2 (°C) | Tc (°C) | Hc (J/g) | Hm1 (J/g) | Hm2 (J/g) | Crystal 1 (%) | Crystal 2 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 165.5 a | 162.1 a | 115.9 b | 66.2 ab | 49.1 ab | 49.7 a | 23.7 ab | 24 a |
| 2 | 165.9 a | 162.3 a | 122.8 a | 62.6 b | 48 b | 51.3 a | 23.2 b | 24.8 a |
| 3 | 165.3 a | 161.2 a | 123.2 a | 72.6 ab | 51.2 ab | 51.4 a | 24.7 ab | 24.9 a |
| 4 | 164.0 a | 161.6 a | 123.4 a | 76.2 a | 54.1 ab | 54.8 a | 26.1 ab | 26.5 a |
| 5 | 164.8 a | 159.3 a | 123.1 a | 73.6 ab | 54.8 a | 54.9 a | 26.5 a | 26.5 a |
| Element Concentration (µg/g or ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Al | Sb | Cd | Cr | Fe | Pb | Ti |
| PP virgin | 3.45 | * b | 0.13 | 0.17 | * b | 4.10 | * b |
| PP MRF | 84.2 | * b | * b | * b | 49.02 | * b | 68.26 |
| MLOD (ppm) | 0.124 | 0.011 | 0.002 | 0.003 | 0.010 | 0.689 | 0.002 |
| MLOQ (ppm) | 0.41 | 0.04 | 0.01 | 0.01 | 0.03 | 2.30 | 0.01 |
| Virgin Sample | 100% PCR Sample | ||
|---|---|---|---|
| Compound (CAS) | Cramer Class | Compound (CAS) | Cramer Class |
| M-Toluic acid, TMS derivative (959296-29-8) | Class I | m-Toluic acid, TMS derivative | Class I |
| Butylated Hydroxytoluene (BHT) (128-37-0) | Class II | 1-iodo- Decane (2050-77-3) | Class III |
| 4-cyano-3-fluorophenyl 4-(4-butylcyclohexyl)benzoate (92118-83-7) | Class III | 1,1′-(1,2-dimethyl-1,2-ethanediyl)bis- Benzene (4613-11-0) | - |
| 1,1′-(1,2-dimethyl-1,2-ethanediyl)bis Benzene (5789-35-5) | Class III | 1′-(1,2-ethanediyl)bis[4-methyl- Benzene | Class III |
| 1′-(1,2-ethanediyl)bis 4-methyl- Benzene (538-39-6) | Class III | Myristic acid, TBDMS derivative (104255-79-0) | Class I |
| 4-Allyl-2-methoxyphenyl benzoate | Class I | Tricyclo[4.4.0.0(2,7)]decan-3-one, 1-methoxy-2,6-dimethyl- (62648-63-9) | Class III |
| Palmitic Acid, TMS derivative (55520-89-3) | Class I | 1,2-Bis(3,5-dimethylphenyl)-diazene 1-oxide (64857-67-6) | Class III |
| Stearic acid, TMS derivative (18748-91-9) | Class I | Palmitic Acid, TMS derivative (55520-89-3) | Class I |
| - | - | Linoelaidic acid, tert.-butyldimerthylsilyl ester | - |
| - | - | Oleic Acid, (Z)-, TMS derivative (21556-26-3) | Class I |
| - | - | Stearic acid, TMS derivative (18748-91-9) | Class I |
| - | - | 1-Monopalmitin, 2TMS derivative (1188-74-5) | Class III |
| - | - | 2-Monostearin, 2TMS derivative (53336-13-3) | Class III |
| - | - | 4-tert-Octylphenol, TMS derivative (78721-87-6) | Class I |
| - | - | Glycerol monostearate (GMS), 2TMS derivative (1188-75-6) | Class I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignacio, M.C.C.D.; Tumu, K.N.; Munshi, M.; Vorst, K.L.; Curtzwiler, G.W. Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging. Polymers 2023, 15, 3471. https://doi.org/10.3390/polym15163471
Ignacio MCCD, Tumu KN, Munshi M, Vorst KL, Curtzwiler GW. Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging. Polymers. 2023; 15(16):3471. https://doi.org/10.3390/polym15163471
Chicago/Turabian StyleIgnacio, Ma. Cristine Concepcion D., Khairun N. Tumu, Mita Munshi, Keith L. Vorst, and Greg W. Curtzwiler. 2023. "Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging" Polymers 15, no. 16: 3471. https://doi.org/10.3390/polym15163471
APA StyleIgnacio, M. C. C. D., Tumu, K. N., Munshi, M., Vorst, K. L., & Curtzwiler, G. W. (2023). Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging. Polymers, 15(16), 3471. https://doi.org/10.3390/polym15163471








