RAFT Polymerisation and Hypercrosslinking Improve Crosslink Homogeneity and Surface Area of Styrene Based PolyHIPEs
Abstract
1. Introduction
2. Experimental
2.1. Materials
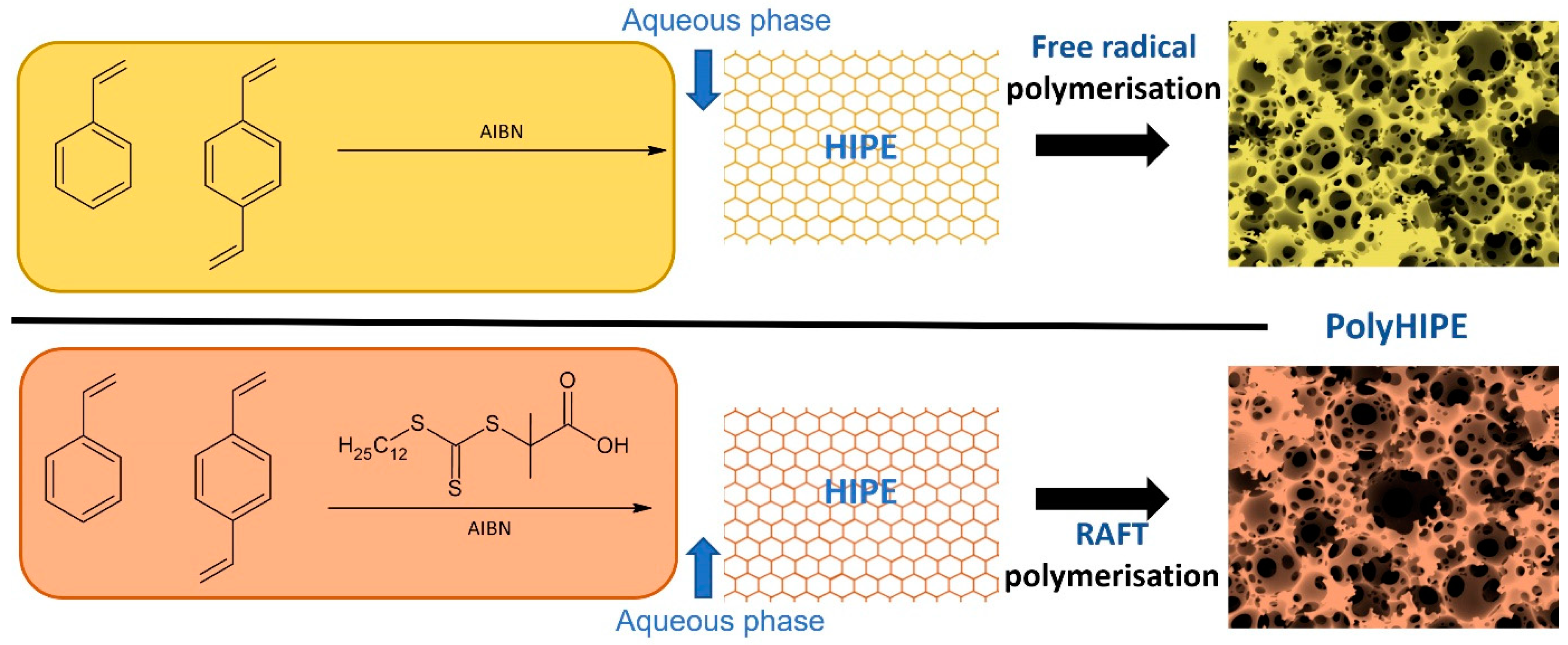
2.2. Synthesis of STY/DVB PolyHIPE Materials by Free Radical Polymerisation
2.3. Synthesis of STY/DVB PolyHIPE Materials by RAFT Polymerisation
2.4. Hypercrosslinking Procedure
2.5. Characterisation
3. Results and Discussion
3.1. PolyHIPE Preparation: FRP vs. RAFT
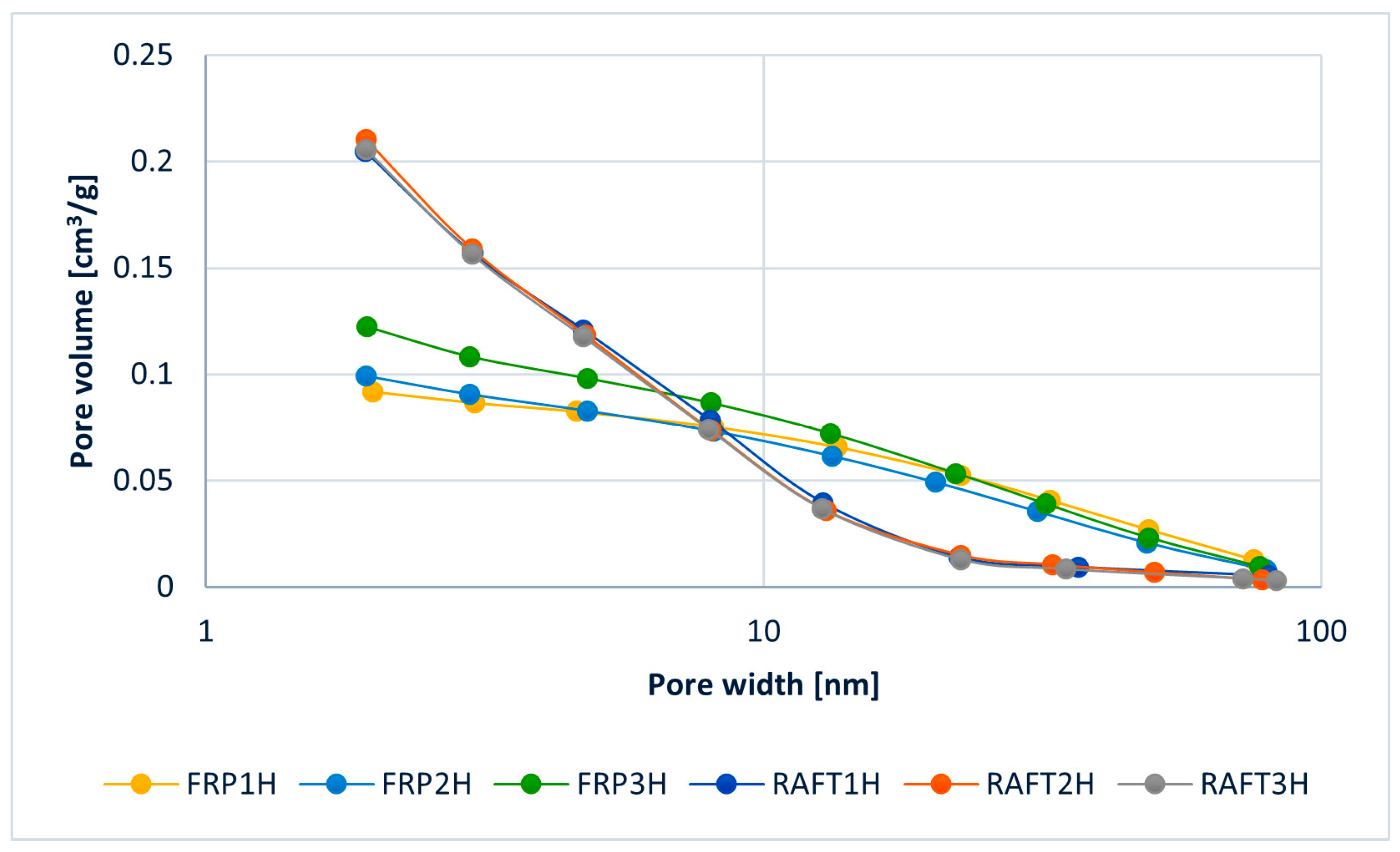
3.2. Post-Polymerisation Crosslinking (Hypercrosslinking)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Pulko, I.; Krajnc, P. High Internal Phase Emulsion Templating-A Path to Hierarchically Porous Functional Polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef]
- Cameron, N.R.; Sherrington, D.C. High Internal Phase Emulsions (HIPEs)—Structure, Properties and Use in Polymer Preparation. Adv. Polym. Sci. 1996, 126, 163–214. [Google Scholar] [CrossRef]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef]
- Kramer, S.; Cameron, N.R.; Krajnc, P. Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers 2021, 13, 1786. [Google Scholar] [CrossRef] [PubMed]
- Torquato, S.; Truskett, T.M.; Debenedetti, P.G. Is random close packing of spheres well defined? Phys. Rev. Lett. 2000, 84, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Davankov, V.A.; Tsyurupa, M.P. Structure and properties of hypercrosslinked polystyrene—The first representative of a new class of polymer networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- Koler, A.; Pulko, I.; Krajnc, P. Post Polymerisation Hypercrosslinking with Emulsion Templating for Hierarchical and Multi-Level Porous Polymers. Acta Chim. Slov. 2020, 67, 349–360. [Google Scholar] [CrossRef]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: Efficient nucleophilic catalyst supports. Chem. A Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, J.E.; Oh, C.G.; Ihm, S.K.; Cortez, J.; Sherrington, D.C. Rapid generation and control of microporosity, bimodal pore size distribution, and surface area in Davankov-type hyper-cross-linked resins. Macromolecules 2006, 39, 627–632. [Google Scholar] [CrossRef]
- Mezhoud, S.; Paljevac, M.; Koler, A.; Le Droumaguet, B.; Grande, D.; Krajnc, P. Novel hypercrosslinking approach toward high surface area functional 2-hydroxyethyl methacrylate-based polyHIPEs. React. Funct. Polym. 2018, 132, 51–59. [Google Scholar] [CrossRef]
- Koler, A.; Kolar, M.; Jeřábek, K.; Krajnc, P. Influence of Functional Group Concentration on Hypercrosslinking of Poly (vinylbenzyl chloride) PolyHIPEs: Upgrading Macroporosity with Nanoporosity. Polymers 2021, 13, 2721. [Google Scholar] [CrossRef] [PubMed]
- Pastukhov, A.V.; Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polystyrene: A polymer in a non-classical physical state. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2324–2333. [Google Scholar] [CrossRef]
- Veverka, P.; Jerabek, K. Mechanism of hypercrosslinking of chloromethylated styrene–divinylbenzene copolymers. React. Funct. Polym. 1999, 41, 21–25. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition-fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Corrigan, N.; Boyer, C. In the Limelight: 2D and 3D Materials via Photo-Controlled Radical Polymerization. Trends Chem. 2020, 2, 689–706. [Google Scholar] [CrossRef]
- Cuthbert, J.; Wanasinghe, S.V.; Matyjaszewski, K.; Konkolewicz, D. Are RAFT and ATRP Universally Interchangeable Polymerization Methods in Network Formation? Macromolecules 2021, 54, 8331–8340. [Google Scholar] [CrossRef]
- Wanasinghe, S.V.; Sun, M.; Yehl, K.; Cuthbert, J.; Matyjaszewski, K.; Konkolewicz, D. PET-RAFT Increases Uniformity in Polymer Networks. ACS Macro Lett. 2022, 11, 1156–1161. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, A.N.; Gao, X. One-pot interfacial polymerization to prepare PolyHIPEs with functional surface. Colloid Polym. Sci. 2015, 293, 1767–1779. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Dario Arrua, R.; Desire, C.T.; Rodemann, T.; Bon, S.A.F.; Thickett, S.C.; Hilder, E.F. Preparation of inverse polymerized high internal phase emulsions using an amphiphilic macro-RAFT agent as sole stabilizer. Polym. Chem. 2016, 7, 1803–1812. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Dario Arrua, R.; Mansour, F.R.; Thickett, S.C.; Hilder, E.F. PEO-based brush-type amphiphilic macro-RAFT agents and their assembled polyHIPE monolithic structures for applications in separation science. Sci. Rep. 2017, 7, 7847. [Google Scholar] [CrossRef]
- Audouin, F.; Heise, A. Surface-initiated RAFT polymerization of NIPAM from monolithic macroporous polyHIPE. Eur. Polym. J. 2013, 49, 1073–1079. [Google Scholar] [CrossRef]
- Koler, A.; Krajnc, P. Surface Modification of Hypercrosslinked Vinylbenzyl Chloride PolyHIPEs by Grafting via RAFT. Macromol. Chem. Phys. 2021, 222, 2000381. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, A.N.; Gao, X. Pushing the mechanical strength of PolyHIPEs up to the theoretical limit through living radical polymerization. Soft Matter 2012, 8, 1824–1830. [Google Scholar] [CrossRef]
- Benaddi, A.O.; Cohen, O.; Matyjaszewski, K.; Silverstein, M.S. RAFT polymerization within high internal phase emulsions: Porous structures, mechanical behaviors, and uptakes. Polymer 2021, 213, 123327. [Google Scholar] [CrossRef]
- Anderson, K.L.; Nazarov, W.; Musgrave, C.S.A.; Bazin, N.; Faith, D. Synthesis and characterisation of low density porous polymers by reversible addition-fragmentation chain transfer (RAFT). J. Radioanal. Nucl. Chem. 2014, 299, 969–975. [Google Scholar] [CrossRef]
- Moad, G. RAFT (Reversible addition-fragmentation chain transfer) crosslinking (co)polymerization of multi-olefinic monomers to form polymer networks. Polym. Int. 2015, 64, 15–24. [Google Scholar] [CrossRef]
- Carnachan, R.J.; Bokhari, M.; Przyborski, S.A.; Cameron, N.R. Tailoring the morphology of emulsion-templated porous polymers. Soft Matter 2006, 2, 608–616. [Google Scholar] [CrossRef]
- Langer, B.; Schnell, I.; Spiess, H.W.; Grimmer, A.-R. Temperature Calibration under Ultrafast MAS Conditions. J. Magn. Reson. 1999, 138, 182–186. [Google Scholar] [CrossRef]
- Brus, J. Heating of samples induced by fast magic-angle spinning. Solid State Nucl. Magn. Reson. 2000, 16, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Soukupová, K.; Sassi, A.; Jeřábek, K. Reinforcing of expanded polymer morphology using peroxy radical initiator. React. Funct. Polym. 2009, 69, 353–357. [Google Scholar] [CrossRef]
- Roa-Luna, M.; Jaramillo-Soto, G.; Castañeda-Flores, P.V.; Vivaldo-Lima, E. Copolymerization kinetics of styrene and divinylbenzene in the presence of S-thiobenzoyl thioglycolic acid as RAFT agent. Chem. Eng. Technol. 2010, 33, 1893–1899. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Li, B.G.; Zhu, S. Toward well-controlled ab initio RAFT emulsion polymerization of styrene mediated by 2-(((Dodecylsulfanyl)carbonothioyl)sulfanyl)propanoic acid. Macromolecules 2011, 44, 221–229. [Google Scholar] [CrossRef]
- Wan, W.M.; Pan, C.Y. One-pot synthesis of polymeric nanomaterials via RAFT dispersion polymerization induced self-assembly and re-organization. Polym. Chem. 2010, 1, 1475–1484. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E. Quantitative solid state 13C NMR studies of highly cross-linked poly(divinylbenzene) resins. Macromolecules 1997, 30, 2868–2875. [Google Scholar] [CrossRef]
- Sevšek, U.; Brus, J.; Jeřabek, K.; Krajnc, P. Post polymerisation hypercrosslinking of styrene/divinylbenzene poly(HIPE)s: Creating micropores within macroporous polymer. Polymer 2014, 55, 410–415. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
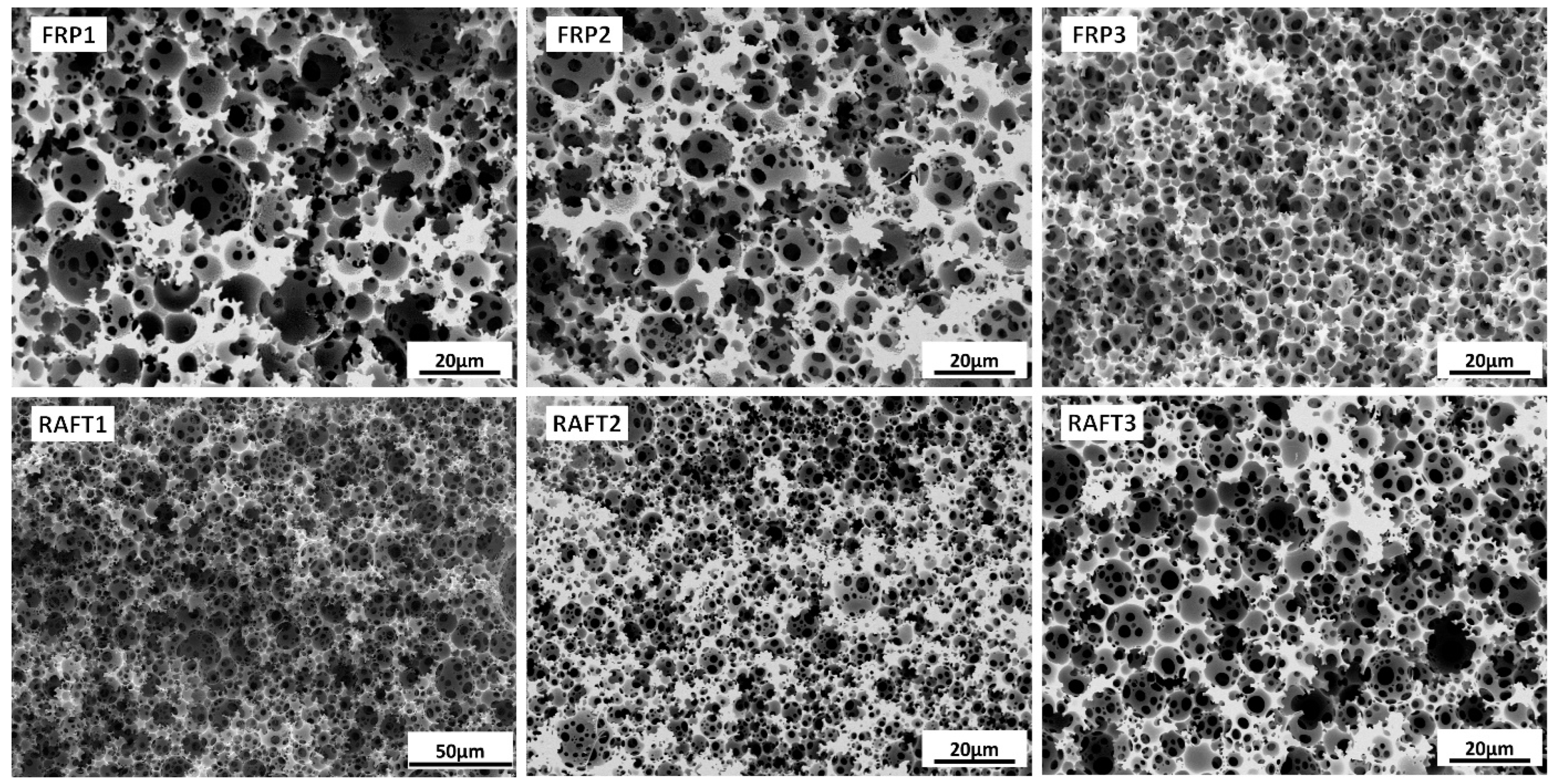
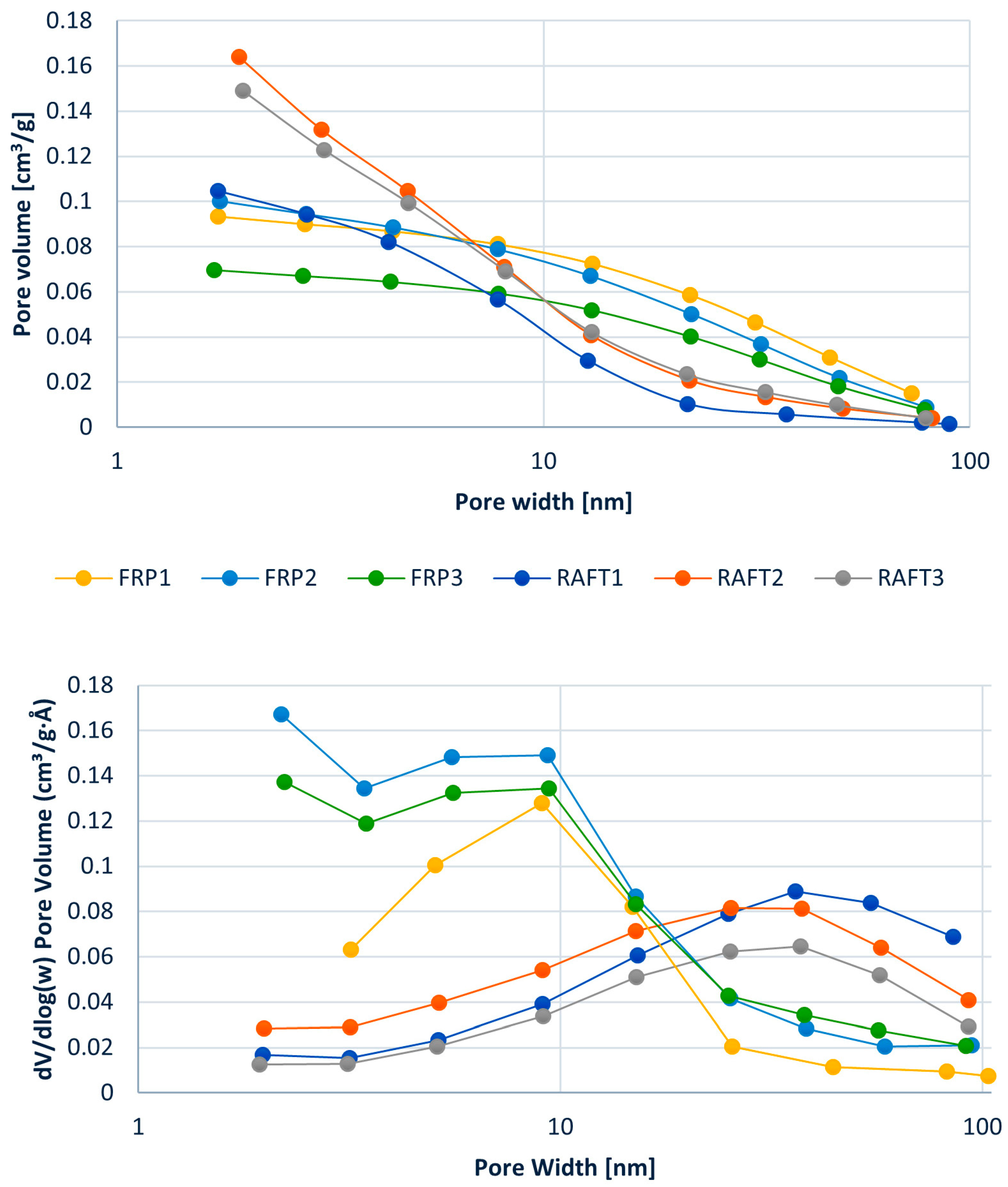

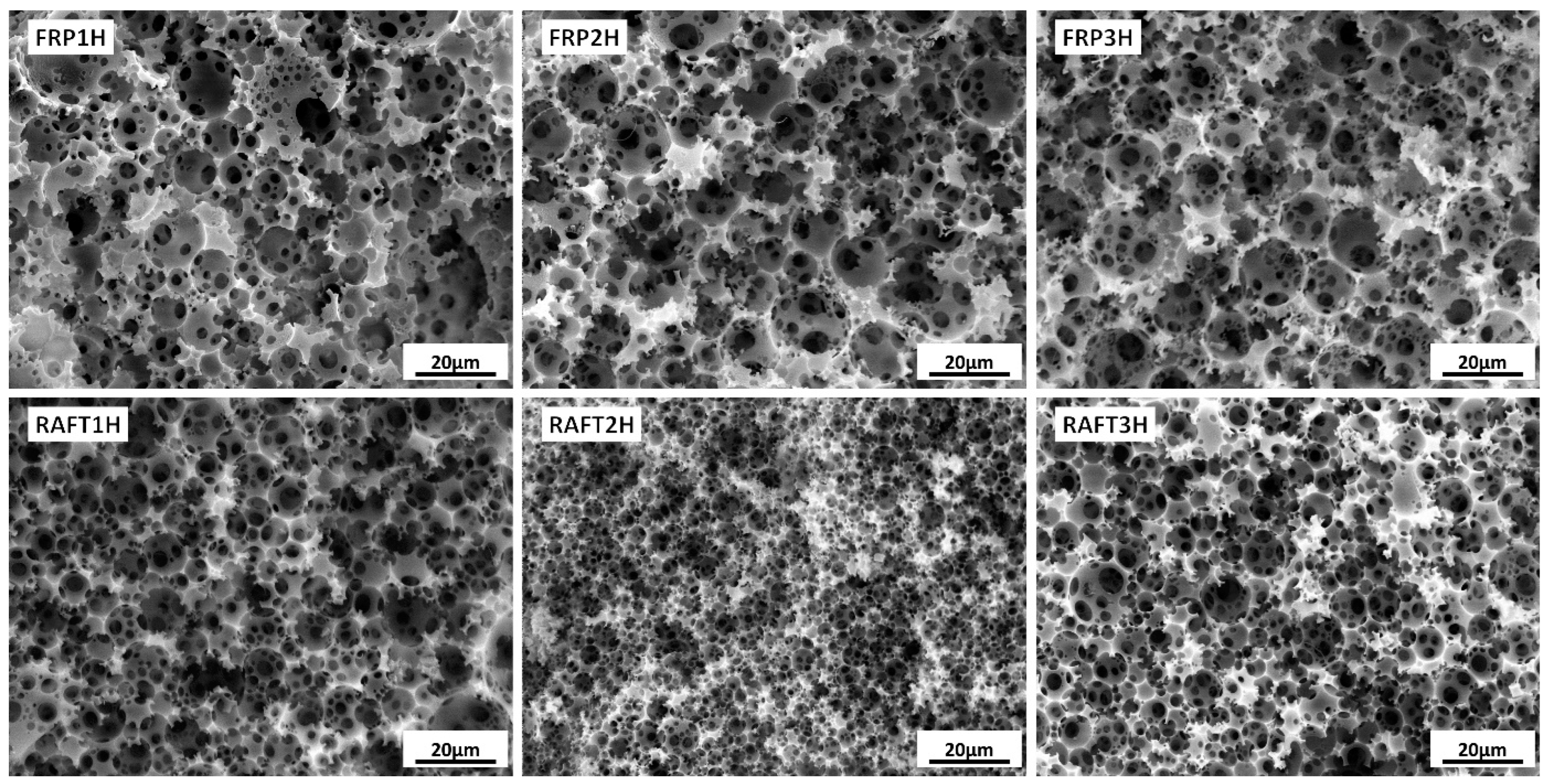
| Sample | m (STY) [g] | m (DVB) [g] | VWP [mL] | Xxlink |
|---|---|---|---|---|
| FRP1 | 2.114 | 4.479 | 40.0 | 50 |
| FRP2 | 1.098 | 3.956 | 30.7 | 60 |
| FRP3 | 0.761 | 6.114 | 42.0 | 70 |
| Sample | m (STY) [g] | m (DVB) [g] | m (RAFT) [g] | m (AIBN) [g] | VWP [mL] | Xxlink |
|---|---|---|---|---|---|---|
| RAFT1 | 2.061 | 4.396 | 0.051 | 0.064 | 40.0 | 50 |
| RAFT2 | 1.128 | 3.937 | 0.045 | 0.049 | 30.7 | 60 |
| RAFT3 | 0.707 | 6.128 | 0.054 | 0.068 | 42.0 | 70 |
| Sample | Crosslinker Content (mol%) | BET Surface Area [m2/g] | Vpore [cm3/g] | Vmicro * [cm3/g] | Micropore Area [m2/g] * | External Surface * [m2/g] |
|---|---|---|---|---|---|---|
| FRP1 | 50 | 24.6 | 0.089 | / | / | 30.3 |
| FRP2 | 60 | 35.4 | 0.095 | / | / | 43.1 |
| FRP3 | 70 | 19.4 | 0.066 | / | / | 24.3 |
| RAFT1 | 50 | 60.9 | 0.100 | / | / | 73.2 |
| RAFT2 | 60 | 156.5 | 0.166 | / | / | 152.3 |
| RAFT3 | 70 | 108.8 | 0.138 | / | / | 120.5 |
| Sample | Crosslinker Content (mol%) | mol. % (C=C) | mol. % (C=C) HP |
|---|---|---|---|
| FRP1 | 50 | 29.14 | 25.00 |
| FRP2 | 60 | 39.00 | 26.07 |
| FRP3 | 70 | 42.20 | 25.78 |
| RAFT1 | 50 | 33.60 | 24.67 |
| RAFT2 | 60 | 29.76 | 26.48 |
| RAFT3 | 70 | 45.25 | 30.65 |
| Sample | Initial Crosslinker Content (mol%) | BET Surface Area [m2/g] | Vpore [cm3/g] | Vmikro * [cm3/g] | Vmikro/Vpore | Vmeso * [cm3/g] | Micropore Area [m2/g] * | External Surface * [m2/g] |
|---|---|---|---|---|---|---|---|---|
| FRP1H | 50 | 40.8 | 0.095 | 0.0011 | 0.012 | 0.0939 | 3.3 | 37.5 |
| FRP2H | 60 | 90.3 | 0.124 | 0.0066 | 0.053 | 0.1174 | 16.2 | 74.1 |
| FRP3H | 70 | 94.2 | 0.126 | 0.0105 | 0.083 | 0.1155 | 24.6 | 69.6 |
| RAFT1H | 50 | 273.3 | 0.236 | 0.0205 | 0.087 | 0.2155 | 49.9 | 223.2 |
| RAFT2H | 60 | 289.7 | 0.244 | 0.0246 | 0.101 | 0.2194 | 60.1 | 229.7 |
| RAFT3H | 70 | 282.4 | 0.239 | 0.0220 | 0.092 | 0.2170 | 53.4 | 228.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koler, A.; Brus, J.; Krajnc, P. RAFT Polymerisation and Hypercrosslinking Improve Crosslink Homogeneity and Surface Area of Styrene Based PolyHIPEs. Polymers 2023, 15, 2255. https://doi.org/10.3390/polym15102255
Koler A, Brus J, Krajnc P. RAFT Polymerisation and Hypercrosslinking Improve Crosslink Homogeneity and Surface Area of Styrene Based PolyHIPEs. Polymers. 2023; 15(10):2255. https://doi.org/10.3390/polym15102255
Chicago/Turabian StyleKoler, Amadeja, Jiři Brus, and Peter Krajnc. 2023. "RAFT Polymerisation and Hypercrosslinking Improve Crosslink Homogeneity and Surface Area of Styrene Based PolyHIPEs" Polymers 15, no. 10: 2255. https://doi.org/10.3390/polym15102255
APA StyleKoler, A., Brus, J., & Krajnc, P. (2023). RAFT Polymerisation and Hypercrosslinking Improve Crosslink Homogeneity and Surface Area of Styrene Based PolyHIPEs. Polymers, 15(10), 2255. https://doi.org/10.3390/polym15102255







