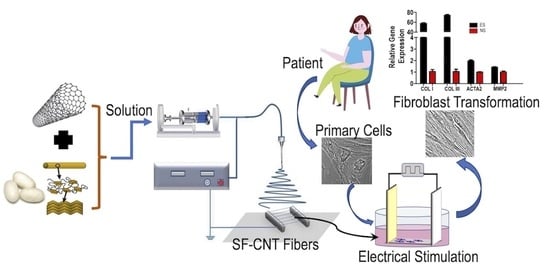
Electrospun Silk Fibroin-CNT Composite Fibers: Characterization and Application in Mediating Fibroblast Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Silk Fibroin Regeneration
2.2. Preparation of E-Spun SF-CNT Fibers
2.3. Aqueous Stability of Fibers and Biodegradation Assays
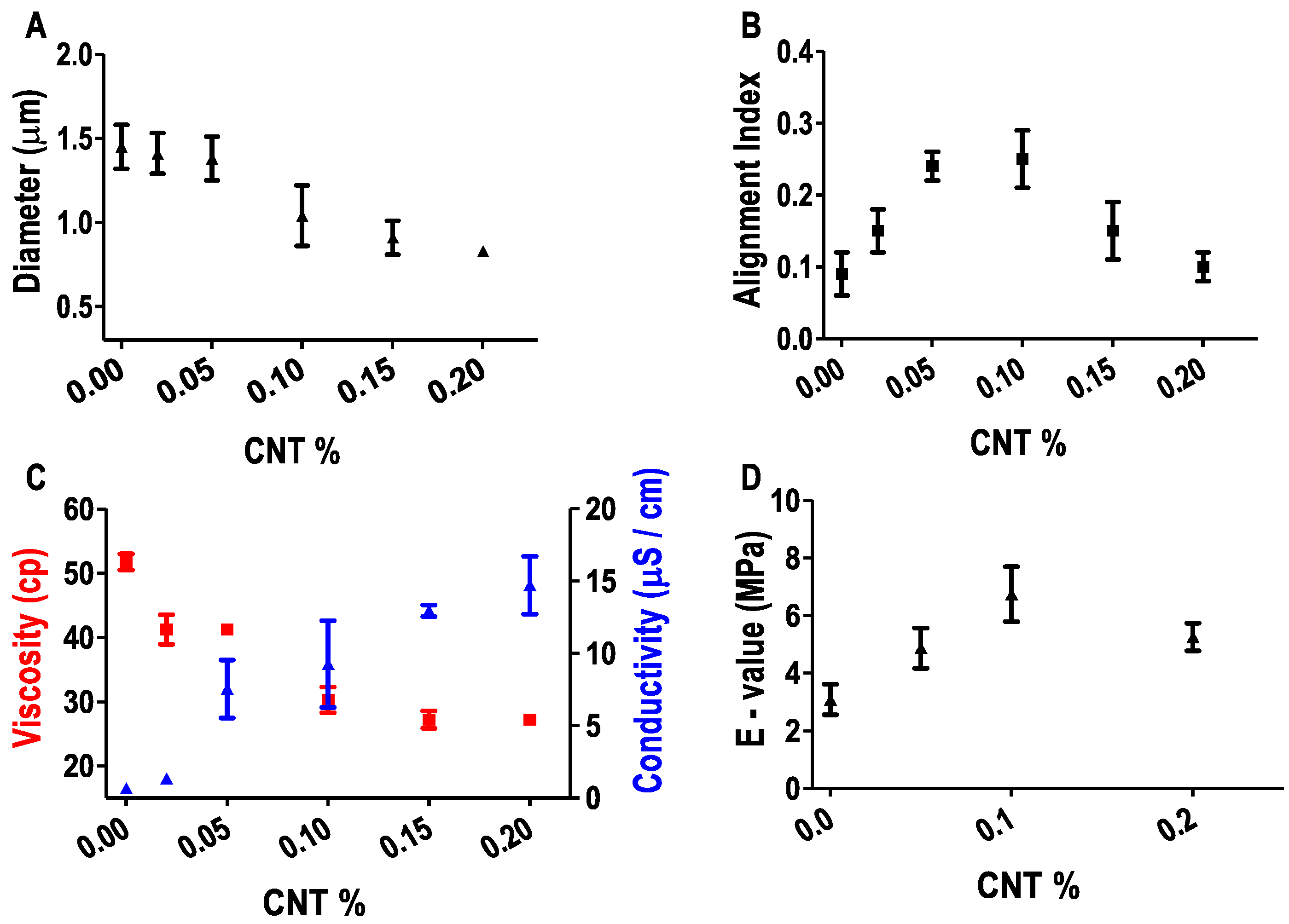
2.4. Characterization of Fibers’ Physical Properties
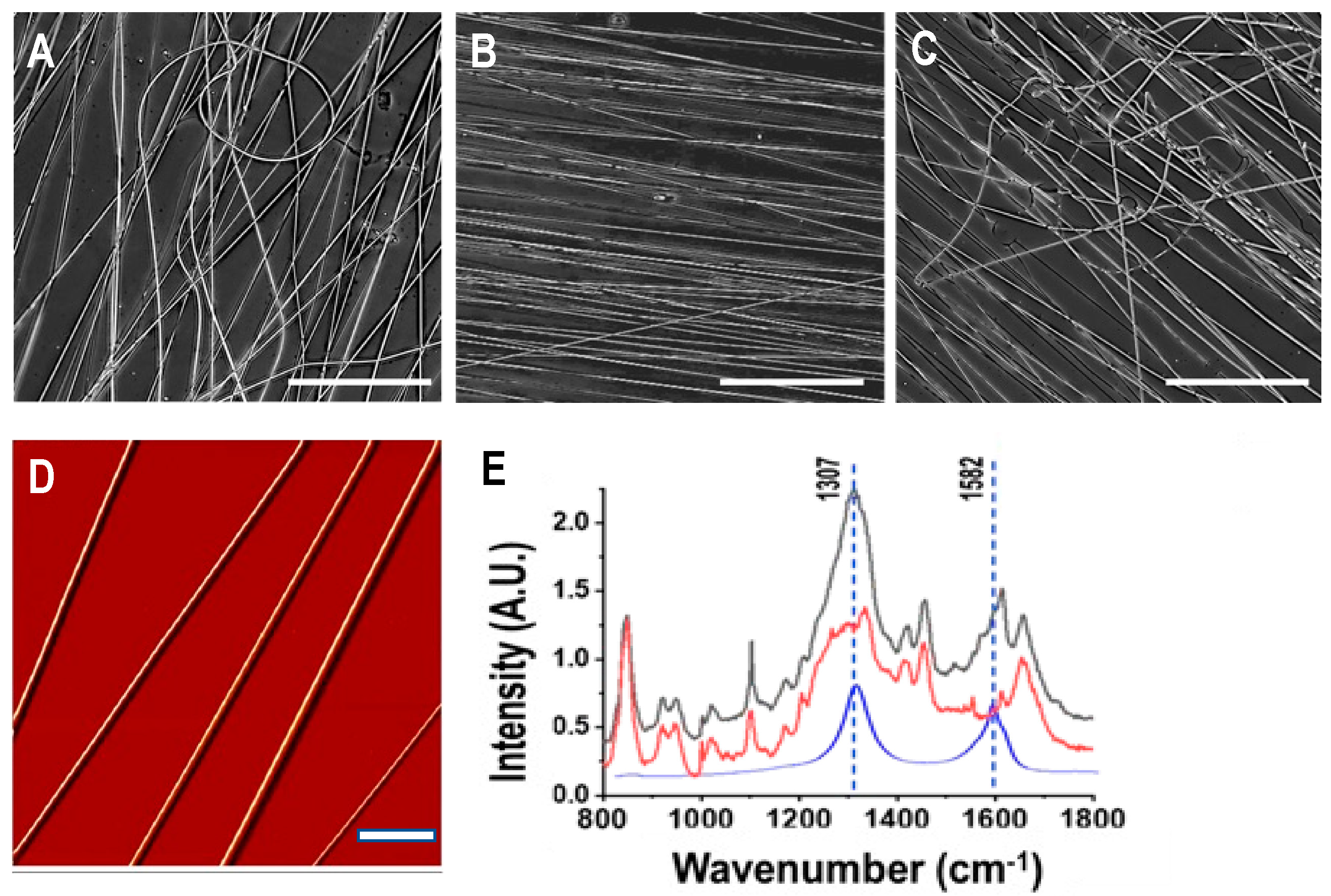
2.5. Spectroscopic Analysis
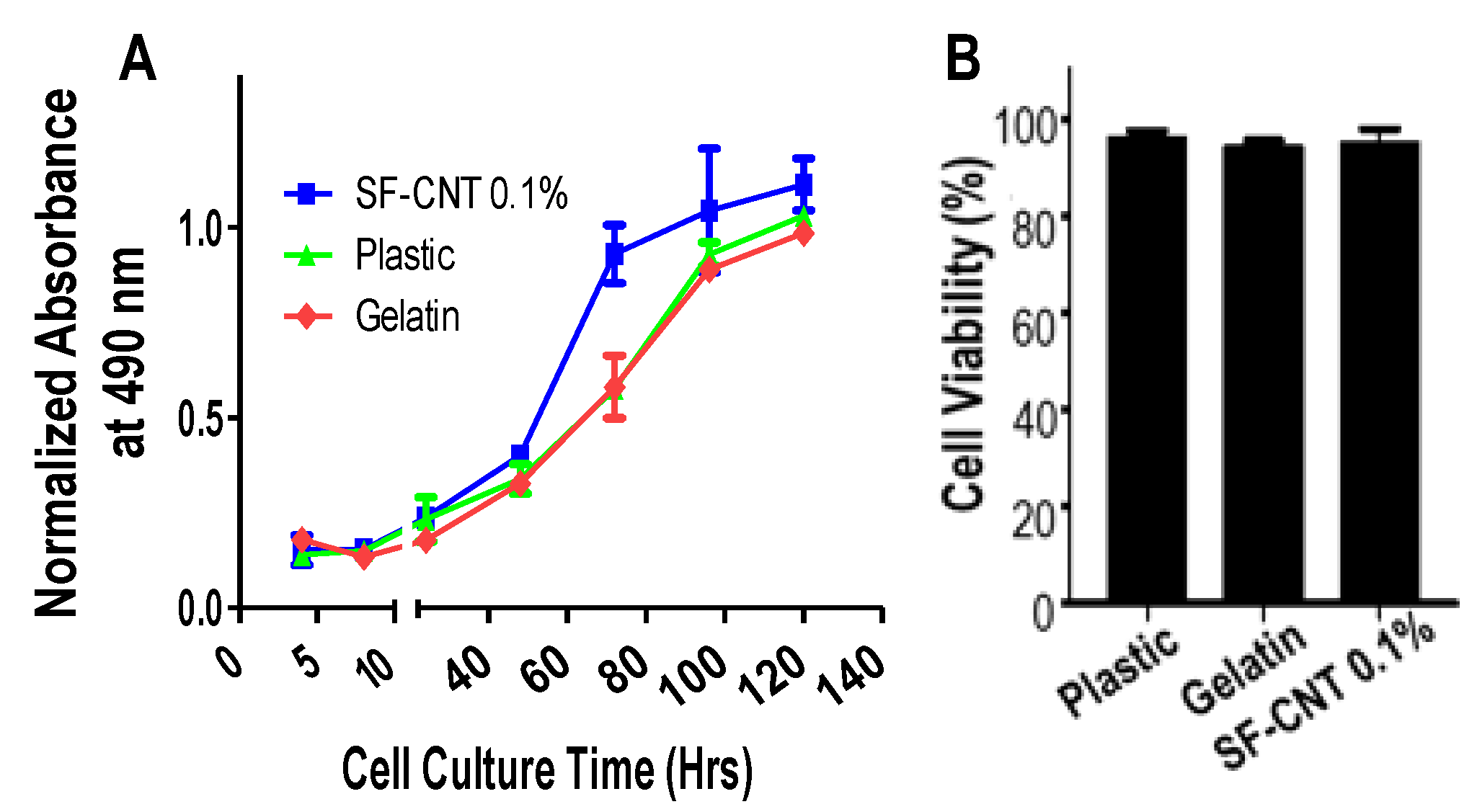
2.6. Cell Extraction, Culture, and Testing
2.7. Electrical Stimulation of Cells
2.8. Optical Imaging
2.9. Total RNA Extraction and RT-qPCR Analysis
3. Results
3.1. Characterization of As-Spun Silk-CNT Fibers
3.2. Effect of Ethanol Post-Treatment
3.3. Effect of CNT on Fiber Properties
3.4. Biocompatibility of SF-CNT Fibers
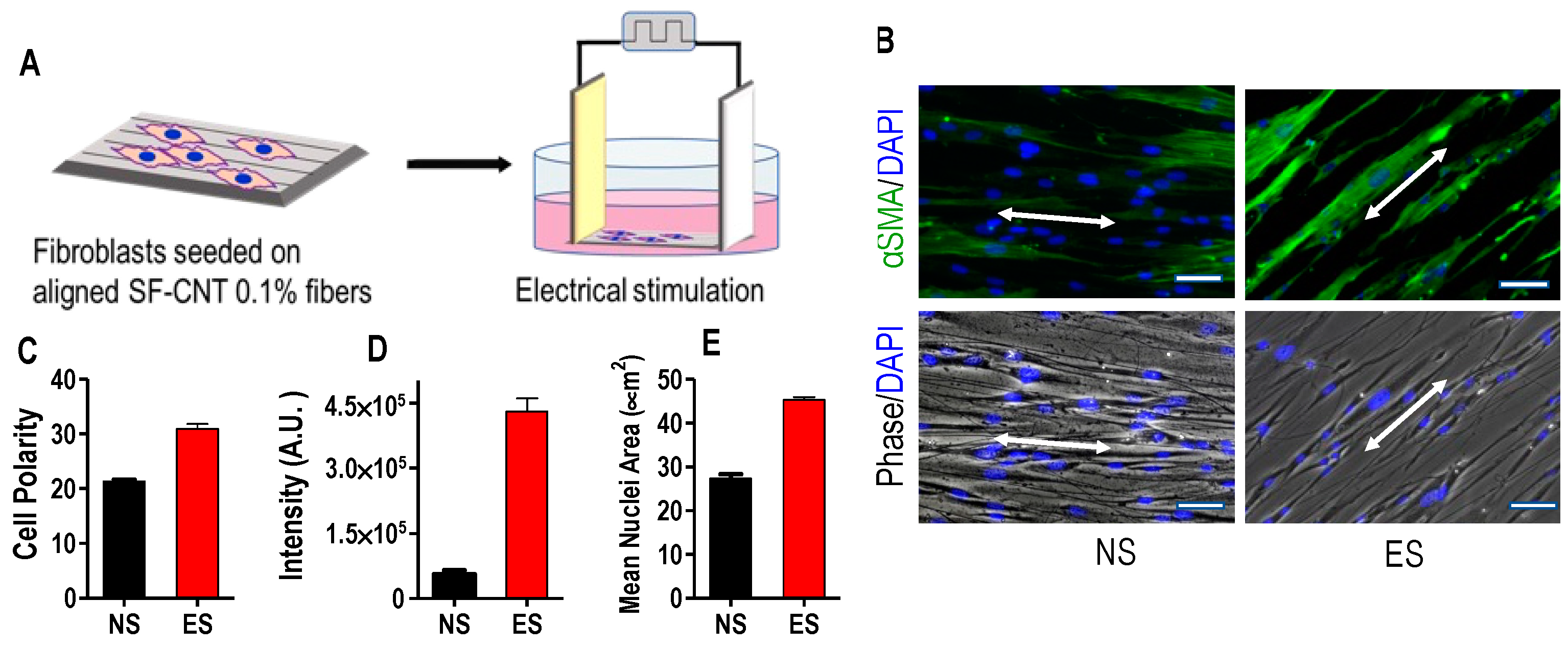
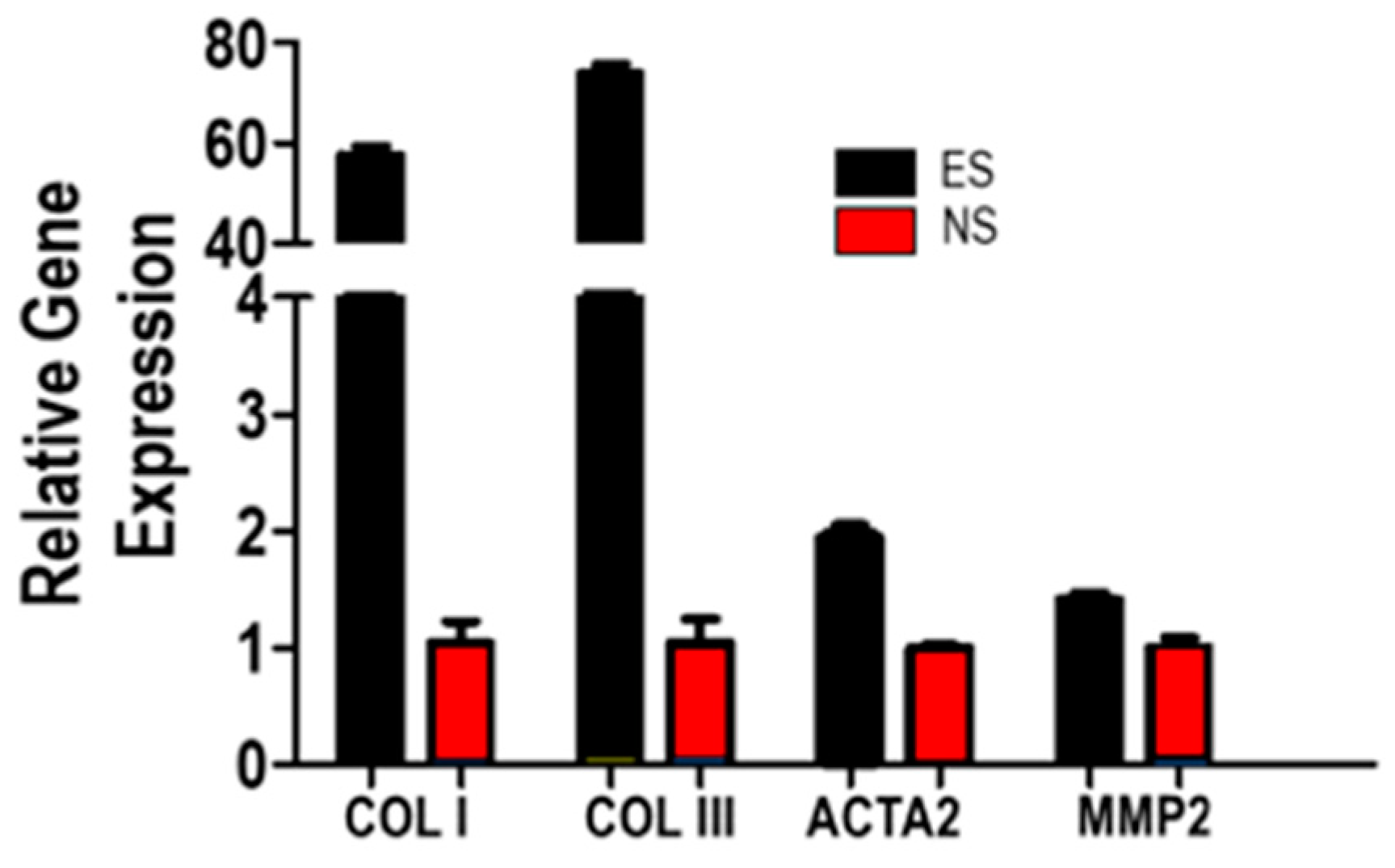
3.5. Fibroblast Response to SF-CNT Mediated Electrical Stimulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Zhu, B.; Li, W.; Lewis, R.V.; Segre, C.U.; Wang, R. E-spun composite fibers of collagen and dragline silk protein: Fiber mechanics, biocompatibility, and application in stem cell differentiation. Biomacromolecules 2015, 16, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, W.; Chi, N.; Lewis, R.V.; Osamor, J.; Wang, R. Optimization of Glutaraldehyde Vapor Treatment for Electrospun Collagen/Silk Tissue Engineering Scaffolds. ACS Omega 2017, 2, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Sridharan, I.; Zhu, B.; Orgel, J.; Wang, R. Effect of CNT on collagen fiber structure, stiffness assembly kinetics and stem cell differentiation. Mater. Sci. Eng. C 2015, 49, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, I.; Kim, T.; Strakova, Z.; Wang, R. Matrix-specified differentiation of human decidua parietalis placental stem cells. Biochem. Biophys. Res. Commun. 2013, 437, 489–495. [Google Scholar] [CrossRef][Green Version]
- Chi, N.; Wang, R. Electrospun protein-CNT composite fibers and the application in fibroblast stimulation. Biochem. Biophys. Res. Commun. 2018, 504, 211–217. [Google Scholar] [CrossRef]
- Chi, N.; Zheng, S.; Clutter, E.; Wang, R. Silk-CNT Mediated Fibroblast Stimulation toward Chronic Wound Repair. Recent Prog. Mater. 2019, 1, 16. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Muralidhar, S.S.; Gangaraju, V.; Shastri, M.; Marilingaiah, N.R.; Singh, S.K.; Rangappa, D. Silk Fiber Multiwalled Carbon Nanotube-Based Micro-/Nanofiber Composite as a Conductive Fiber and a Force Sensor. ACS Omega 2022, 7, 20809–20818. [Google Scholar] [CrossRef]
- Li, W.; Chi, N.; Rathnayake, R.A.C.; Wang, R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: A nanoscopic study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Zuluaga-Vélez, A.; Quintero-Martinez, A.; Orozco, L.M.; Sepúlveda-Arias, J.C. Silk fibroin nanocomposites as tissue engineering scaffolds—A systematic review. Biomed. Pharmacother. 2021, 141, 111924. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Ghazanfari, S.; Zandieh-Doulabi, B.; Stoop, R.; Smit, T.H.; Helder, M.N. Vaginal Fibroblastic Cells from Women with Pelvic Organ Prolapse Produce Matrices with Increased Stiffness and Collagen Content. Sci. Rep. 2016, 6, 22971. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, Z.; Lin, T.; Qin, M. Transforming Growth Factor Beta 1 and p44/42 Expression in Cardinal Ligament Tissues of Patients with Pelvic Organ Prolapse. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930433. [Google Scholar] [CrossRef]
- Guler, Z.; Roovers, J.P. Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse. Biomolecules 2022, 12, 94. [Google Scholar] [CrossRef]
- Kim, T.; Sridharan, I.; Ma, Y.; Zhu, B.; Chi, N.; Kobak, W.; Rotmensch, J.; Schieber, J.D.; Wang, R. Identifying distinct nanoscopic features of native collagen fibrils towards early diagnosis of pelvic organ prolapse. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 667–675. [Google Scholar] [CrossRef]
- Sridharan, I.; Ma, Y.; Kim, T.; Kobak, W.; Rotmensch, J.; Wang, R. Structural and mechanical profiles of native collagen fibers in vaginal wall connective tissues. Biomaterials 2012, 33, 1520–1527. [Google Scholar] [CrossRef]
- Chi, N.; Lozo, S.; Rathnayake, R.A.C.; Botros, S.; Ma, Y.; Damaser, M.; Wang, R.R. Distinctive structure, composition and biomechanics of collagen fibrils in vaginal wall connective tissues associated with pelvic organ prolapse. Acta Biomater. 2022, 152, 335–344. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Ayres, C.E.; Jha, B.S.; Meredith, H.; Bowman, J.R.; Bowlin, G.L.; Henderson, S.C.; Simpson, D.G. Measuring fiber alignment in electrospun scaffolds: A user’s guide to the 2D fast Fourier transform approach. J. Biomater. Sci. Polym. Ed. 2008, 19, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Heim, A.J.; Matthews, W.G.; Koob, T.J. Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl. Phys. Lett. 2006, 89, 181902. [Google Scholar] [CrossRef]
- Frushour, B.G.; Koenig, J.L. Raman scattering of collagen, gelatin, and elastin. Biopolymers 1975, 14, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Kreplak, L.; Doucet, J.; Dumas, P.; Briki, F. New aspects of the alpha-helix to beta-sheet transition in stretched hard alpha-keratin fibers. Biophys. J. 2004, 87, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Zandieh-Doulabi, B.; Brölmann, H.A.M.; Smit, T.H.; Helder, M.N. Fibroblasts from women with pelvic organ prolapse show differential mechanoresponses depending on surface substrates. Int. Urogynecol. J. 2013, 24, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Freddi, G.; Bertoluzza, A.; Kasai, N.; Tsukada, M. Raman spectroscopic studies of silk fibroin from Bombyx mori. J. Raman Spectrosc. 1998, 29, 297–304. [Google Scholar] [CrossRef]
- Puerta, M.; Arango, M.C.; Jaramillo-Quiceno, N.; Álvarez-López, C.; Restrepo-Osorio, A. Influence of ethanol post-treatments on the properties of silk protein materials. SN Appl. Sci. 2019, 1, 1443. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A review on advances in the applications of spider silk in biomedical issues. Int. J. Biol. Macromol. 2021, 192, 258–271. [Google Scholar] [CrossRef]
- Belton, D.J.; Plowright, R.; Kaplan, D.L.; Perry, C.C. A robust spectroscopic method for the determination of protein conformational composition—Application to the annealing of silk. Acta Biomater. 2018, 73, 355–364. [Google Scholar] [CrossRef]
- Wu, J.J.; Weis, M.A.; Kim, L.S.; Eyre, D.R. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010, 285, 18537–18544. [Google Scholar] [CrossRef]
- Volkov, V.; Ferreira, A.V.; Cavaco-Paulo, A. On the Routines of Wild-Type Silk Fibroin Processing Toward Silk-Inspired Materials: A Review. Macromol. Mater. Eng. 2015, 300, 1199–1216. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins. 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.; Vo, D.-V.N.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Silk Fibroin Nanofiber. Electrospinning, Properties, and Structure. Polym. J. 2003, 35, 185–190. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Dynamic Protein-Water Relationships during β-Sheet Formation. Macromolecules 2008, 41, 3939–3948. [Google Scholar] [CrossRef]
- Fang, G.; Zheng, Z.; Yao, J.; Chen, M.; Tang, Y.; Zhong, J.; Qi, Z.; Li, Z.; Shao, Z.; Chen, X. Tough protein-carbon nanotube hybrid fibers comparable to natural spider silks. J. Mater. Chem. B 2015, 3, 3940–3947. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Li, W.; Zhu, B.; Strakova, Z.; Wang, R. Two-way regulation between cells and aligned collagen fibrils: Local 3D matrix formation and accelerated neural differentiation of human decidua parietalis placental stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1377–1382. [Google Scholar] [CrossRef]
- Carlson, M.A.; Longaker, M.T. The fibroblast-populated collagen matrix as a model of wound healing: A review of the evidence. Wound Repair Regen. 2004, 12, 134–147. [Google Scholar] [CrossRef]
- Kapeller, B.; Mueller, J.; Losert, U.; Podesser, B.K.; Macfelda, K. Microcurrent stimulation promotes reverse remodelling in cardiomyocytes. ESC Heart Fail. 2016, 3, 122–130. [Google Scholar] [CrossRef]
- Howard, E.W.; Crider, B.J.; Updike, D.L.; Bullen, E.C.; Parks, E.E.; Haaksma, C.J.; Sherry, D.M.; Tomasek, J.J. MMP-2 expression by fibroblasts is suppressed by the myofibroblast phenotype. Exp. Cell Res. 2012, 318, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.H.; Ruiz-Zapata, A.M.; Bril, H.; Bleeker, M.C.; Belien, J.A.; Stoop, R.; Helder, M.N. Changes in tissue composition of the vaginal wall of premenopausal women with prolapse. Am. J. Obstet. Gynecol. 2014, 210, 168.e1–168.e9. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Edwards, S.L.; Su, K.; Tan, K.S.; White, J.F.; Ramshaw, J.A.; Lo, C.; Rosamilia, A.; Werkmeister, J.A.; Gargett, C.E. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng. Part A 2014, 20, 785–798. [Google Scholar] [CrossRef]
- Ruiz-Zapata, A.M.; Heinz, A.; Kerkhof, M.H.; van de Westerlo-van Rijt, C.; Schmelzer, C.E.H.; Stoop, R.; Kluivers, K.B.; Oosterwijk, E. Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 4762. [Google Scholar] [CrossRef]
- Stephens, J.S.; Fahnestock, S.R.; Farmer, R.S.; Kiick, K.L.; Chase, D.B.; Rabolt, J.F. Effects of Electrospinning and Solution Casting Protocols on the Secondary Structure of a Genetically Engineered Dragline Spider Silk Analogue Investigated via Fourier Transform Raman Spectroscopy. Biomacromolecules 2005, 6, 1405–1413. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Beaulieu, L.; Lefèvre, T.; Paradis, J.; Asakura, T.; Pézolet, M. Characterization by Raman Microspectroscopy of the Strain-Induced Conformational Transition in Fibroin Fibers from the Silkworm Samia cynthia ricini. Biomacromolecules 2006, 7, 2512–2521. [Google Scholar] [CrossRef]
- Zheng, S.; Li, G.; Yao, W.; Yu, T. Raman Spectroscopic Investigation of the Denaturation Process of Silk Fibroin. Appl. Spectrosc. 1989, 43, 1269–1272. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Lefèvre, T.; Beaulieu, L.; Asakura, T.; Pézolet, M. Study of Protein Conformation and Orientation in Silkworm and Spider Silk Fibers Using Raman Microspectroscopy. Biomacromolecules 2004, 5, 2247–2257. [Google Scholar] [CrossRef]
- Asakura, T.; Ashida, J.; Yamane, T.; Kameda, T.; Nakazawa, Y.; Ohgo, K.; Komatsu, K. A repeated β-turn structure in Poly(Ala-Gly) as a model for silk I of Bombyx mori silk fibroin studied with two-dimensional spin-diffusion NMR under off magic angle spinning and rotational echo double resonance11Edited by M. F. Summers. J. Mol. Biol. 2001, 306, 291–305. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy of Biological Applications. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathnayake, R.A.C.; Yoon, S.; Zheng, S.; Clutter, E.D.; Wang, R.R. Electrospun Silk Fibroin-CNT Composite Fibers: Characterization and Application in Mediating Fibroblast Stimulation. Polymers 2023, 15, 91. https://doi.org/10.3390/polym15010091
Rathnayake RAC, Yoon S, Zheng S, Clutter ED, Wang RR. Electrospun Silk Fibroin-CNT Composite Fibers: Characterization and Application in Mediating Fibroblast Stimulation. Polymers. 2023; 15(1):91. https://doi.org/10.3390/polym15010091
Chicago/Turabian StyleRathnayake, Rathnayake A. C., Shinhae Yoon, Shuyao Zheng, Elwin D. Clutter, and Rong R. Wang. 2023. "Electrospun Silk Fibroin-CNT Composite Fibers: Characterization and Application in Mediating Fibroblast Stimulation" Polymers 15, no. 1: 91. https://doi.org/10.3390/polym15010091
APA StyleRathnayake, R. A. C., Yoon, S., Zheng, S., Clutter, E. D., & Wang, R. R. (2023). Electrospun Silk Fibroin-CNT Composite Fibers: Characterization and Application in Mediating Fibroblast Stimulation. Polymers, 15(1), 91. https://doi.org/10.3390/polym15010091