Active Loading of Pectin Hydrogels for Targeted Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
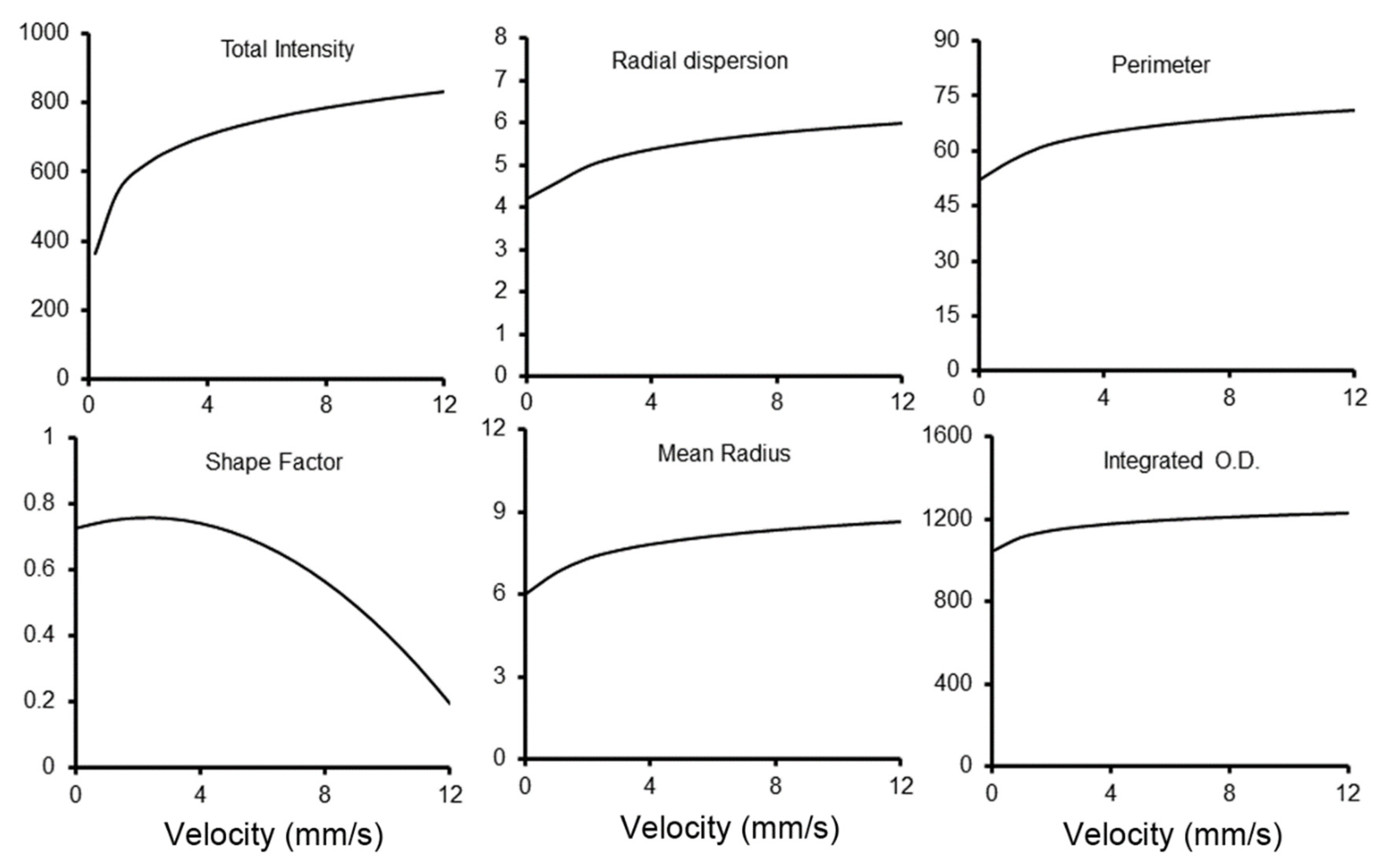
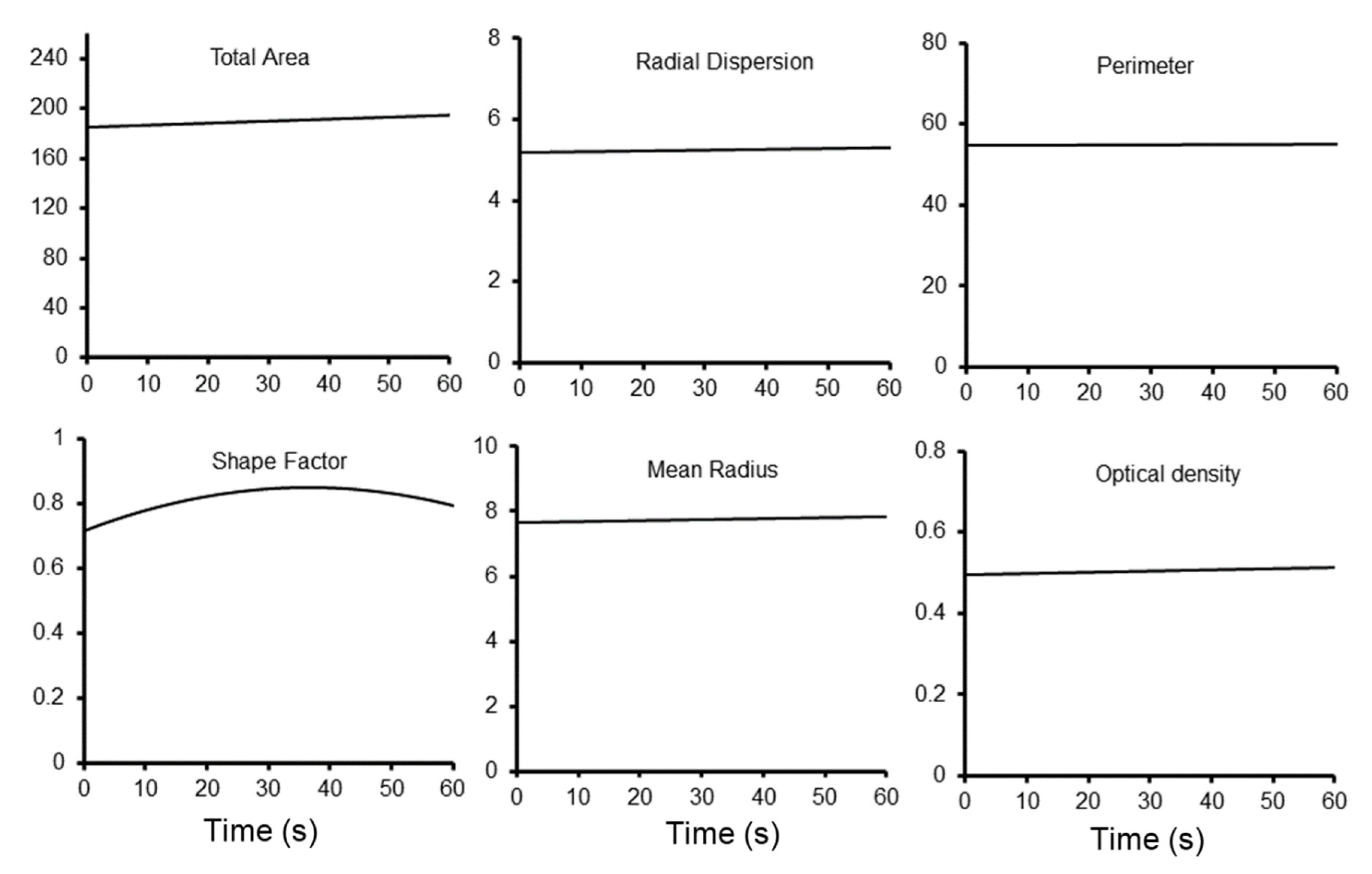
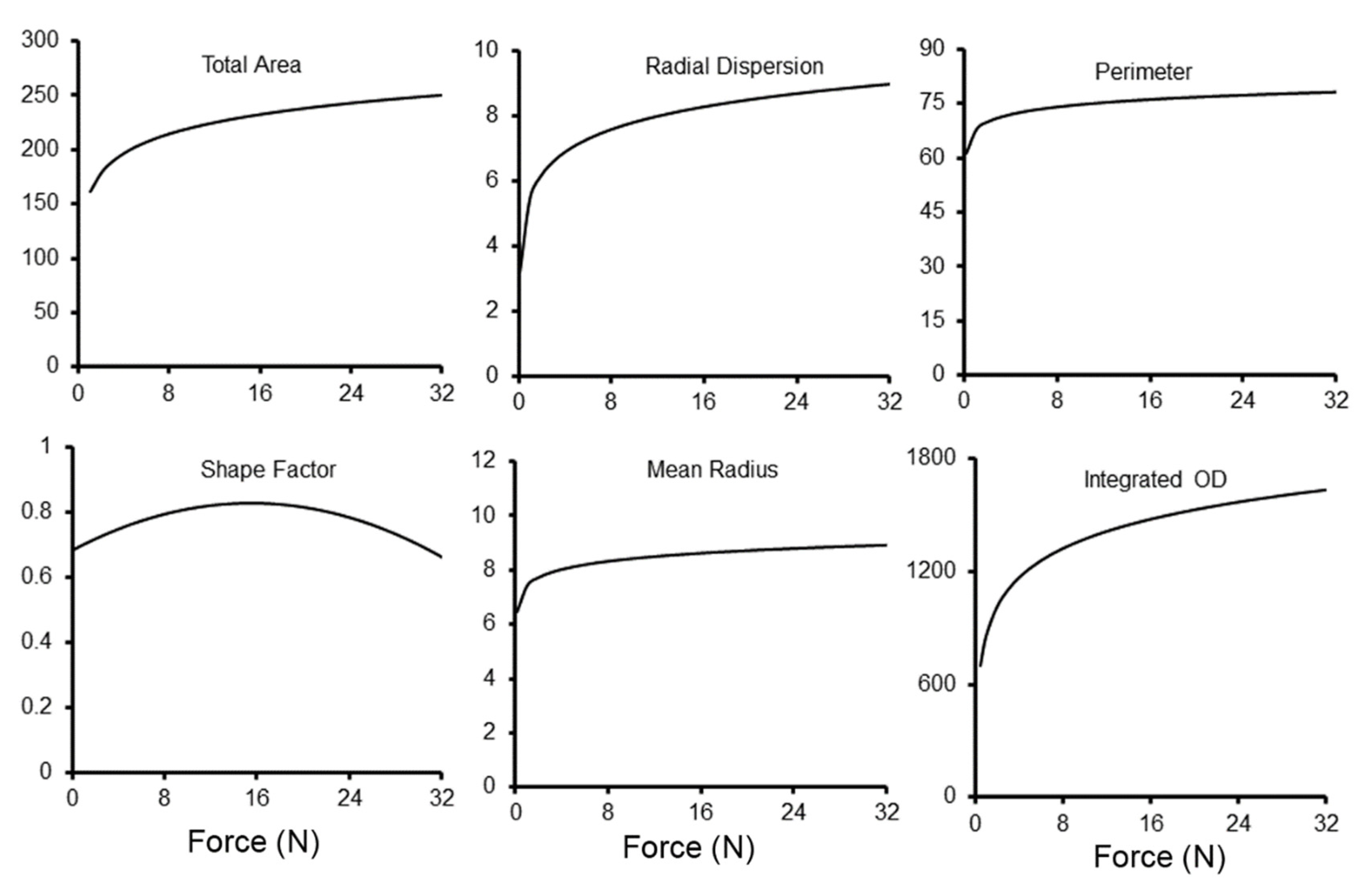
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Menz, B.D.; Wiese, M.D.; Kichenadasse, G.; Gurney, H.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Nuances to precision dosing strategies of targeted cancer medicines. Pharm. Res. Perspect. 2020, 8, e00625. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart hydrogels for advanced drug delivery systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Zarrintaj, P.; Khodadadi, A.; Arefi, A.; Seidi, F.; Shokrani, H.; Saeb, M.R.; Mozafari, M. Polysaccharide-based electroconductive hydrogels: Structure, properties and biomedical applications. Carbohydr. Polym. 2022, 278, 118998. [Google Scholar] [CrossRef]
- Vazquez-Gonzalez, M.; Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef]
- Deng, L.; Lu, H.; Tu, C.; Zhou, T.; Cao, W.; Gao, C. A tough synthetic hydrogel with excellent post-loading of drugs for promoting the healing of infected wounds in vivo. Biomater. Adv. 2022, 134, 112577. [Google Scholar] [CrossRef]
- Scheiner, K.C.; Maas-Bakker, R.F.; van Steenbergen, M.J.; Schwendeman, S.P.; Hennink, W.E.; Kok, R.J. Post-loading of proangiogenic growth factors in PLGA microspheres. Eur. J. Pharm. Biopharm. 2021, 158, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, B.S.; Sutlive, J.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Ackermann, M.; Mentzer, S.J. Kinetics of pectin biopolymer facial erosion characterized by fluorescent tracer microfluidics. Polymers 2022, 14, 3911. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D.; Doong, R.L.; Liljebjelke, K.; Fralish, G.; Chan, J. Cell free synthesis of the pectic polysaccharide homogalacturonan. In Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 109–126. [Google Scholar]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Visualizing pectin polymer-polymer entanglement produced by interfacial water movement. Carbohydr. Polym. 2020, 246, 116618. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pierce, A.; Wagner, W.L.; Khalil, H.; Chen, Z.; Servais, A.B.; Ackermann, M.; Mentzer, S.J. Functional adhesion of pectin biopolymers to the lung visceral pleura. Polymers 2021, 13, 2976. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.B.; Valenzuela, C.D.; Kienzle, A.; Ysasi, A.B.; Wagner, W.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Functional mechanics of a pectin-based pleural sealant after lung injury. Tissue Eng. Part A 2018, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.B.; Kienzle, A.; Ysasi, A.B.; Valenzuela, C.D.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharides as air-tight sealants of the human pleura. J. Biol. Mat. Res. 2018, 107, 799–806. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Popper, H.; Volk, B.W.; Meyer, K.A.; Kozoll, D.D.; Steigmann, F. Evaluation of gelatin and pectin solutions as substitutes for plasma in the treatment of shock—Histologic changes produced in human beings. Arch. Surg. 1945, 50, 34–45. [Google Scholar] [CrossRef]
- World Health Organization. Evaluation of Certain Food Additives; Technical Report Series 669; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 1981. [Google Scholar]
- U.S. Food & Drug Administration. Code of Federal Regulation, Title 21, Food for Human Consumption, Paragraph 184.1588, Listing of Specific Substances Affirmed as GRAS, Pectins; U.S. Food & Drug Administration: Silver Spring, MD, USA, 1986. [Google Scholar]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Panchev, I.N.; Slavov, A.; Nikolova, K.; Kovacheva, D. On the water-sorption properties of pectin. Food Hydrocoll. 2010, 24, 763–769. [Google Scholar] [CrossRef]
- Kienzle, A.; Servais, A.B.; Ysasi, A.B.; Gibney, B.C.; Valenzuela, C.D.; Ackermann, M.; Mentzer, S.J. Free-floating mesothelial cells in pleural fluid after lung surgery. Front. Med. 2018, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.S.; Kinasewitz, G.T. Pathophysiology of the Pleural Space. Am. J. Med. Sci. 1995, 309, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of biopolymer coatings: Contact angle kinetics and image analysis investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef]
- Palacio, L.; Calvo, J.I.; Pradanos, P.; Hernandez, A.; Vaisanen, P.; Nystrom, M. Contact angles and external protein adsorption onto UF membranes. J. Membr. Sci. 1999, 152, 189–201. [Google Scholar] [CrossRef]
- Brutin, D.; Starov, V. Recent advances in droplet wetting and evaporation. Chem. Soc. Rev. 2018, 47, 558–585. [Google Scholar] [CrossRef] [PubMed]
- Saffman, P.G.; Taylor, G. The penetration of a fluid into a porous medium or hele-shaw cell containing a more viscous liquid. Proc. R. Soc. Lond. A Math. Phys. Sci. 1958, 245, 312–329. [Google Scholar] [CrossRef]
- Saffman, P.G. Viscous fingering in Hele-Shaw cells. J. Fluid Mech. 1986, 173, 73–94. [Google Scholar] [CrossRef]
- Vandenbroeck, J.M. Fingers in a Hele-Shaw cell with surface-tension. Phys. Fluids 1983, 26, 2033–2034. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Sahn, S.A.; Willcox, M.L.; Good, J.T.; Potts, D.E.; Filley, G.F. Characteristics of normal rabbit pleural fluid—Physiologic and biochemical implications. Lung 1979, 156, 63–69. [Google Scholar] [CrossRef]
- Agostoni, E. Mechanics of the pleural space. Physiol. Rev. 1972, 52, 57–128. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.L.; Zheng, Y.; Pierce, A.; Ackermann, M.; Horstmann, H.; Kuner, T.; Ronchi, P.; Schwab, Y.; Konietzke, P.; Wunnemann, F.; et al. Mesopolysaccharides: The extracellular surface layer of visceral organs. PLoS ONE 2020, 15, e0238798. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L. A new preparation for rapidly fixing and staining blood. Lancet 1899, 153, 370–371. [Google Scholar] [CrossRef][Green Version]
- Sherlock, D.J.; Holl-Allen, R.T. Intravital methylene blue staining of parathyroid glands and tumours. Ann. R. Coll. Surg. Engl. 1984, 66, 396–398. [Google Scholar]
- Hirsch, J.I.; Bosch, H.A.; Horsley, J.S., 3rd. Methylene blue may be a preferable breast tissue marker to isosulfan blue. AJR Am. J. Roentgenol. 1983, 140, 1038–1039. [Google Scholar] [CrossRef]
- Bernaudin, J.-F.; Fleury, J. Anatomy of the blood and lymphatic circulation of the pleural serosa. In The Pleura in Health and Disease; Chrétien, J., Bignon, J., Hirsch, A., Eds.; Dekker: New York, NY, USA, 1985; pp. 101–124. [Google Scholar]
- Bennecke, M.; Kriegl, L.; Bajboubj, M.; Retzlaff, K.; Robine, S.; Jung, A.; Arkan, M.C.; Kirchner, T.; Greten, F.R. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 2010, 18, 135–146. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J. Biol. Mat. Res. Part A 2020, 108, 246–253. [Google Scholar] [CrossRef]
- Pierce, A.F.; Liu, B.S.; Liao, M.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Ackermann, M.; Mentzer, S.J. Optical and mechanical properties of self-repairing pectin biopolymers. Polymers 2022, 14, 1345. [Google Scholar] [CrossRef]
- Servais, A.B.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Kienzle, A.; Loring, S.H.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pressure-decay testing of pleural air leaks in intact murine lungs: Evidence for peripheral airway regulation. Physiol. Rep. 2018, 6, e13712. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.; Wagner, W.L.; Khalil, H.; Chen, Z.; Funaya, C.; Ackermann, M.; Mentzer, S.J. Biomaterial-assisted anastomotic healing: Serosal adhesion of pectin films. Polymers 2021, 13, 2811. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesvoranan, O.; Liu, B.S.; Zheng, Y.; Wagner, W.L.; Sutlive, J.; Chen, Z.; Khalil, H.A.; Ackermann, M.; Mentzer, S.J. Active Loading of Pectin Hydrogels for Targeted Drug Delivery. Polymers 2023, 15, 92. https://doi.org/10.3390/polym15010092
Vesvoranan O, Liu BS, Zheng Y, Wagner WL, Sutlive J, Chen Z, Khalil HA, Ackermann M, Mentzer SJ. Active Loading of Pectin Hydrogels for Targeted Drug Delivery. Polymers. 2023; 15(1):92. https://doi.org/10.3390/polym15010092
Chicago/Turabian StyleVesvoranan, Oraya, Betty S. Liu, Yifan Zheng, Willi L. Wagner, Joseph Sutlive, Zi Chen, Hassan A. Khalil, Maximilian Ackermann, and Steven J. Mentzer. 2023. "Active Loading of Pectin Hydrogels for Targeted Drug Delivery" Polymers 15, no. 1: 92. https://doi.org/10.3390/polym15010092
APA StyleVesvoranan, O., Liu, B. S., Zheng, Y., Wagner, W. L., Sutlive, J., Chen, Z., Khalil, H. A., Ackermann, M., & Mentzer, S. J. (2023). Active Loading of Pectin Hydrogels for Targeted Drug Delivery. Polymers, 15(1), 92. https://doi.org/10.3390/polym15010092






