Effect of Extraction Ingredients on the Conformation and Stability of Silk Sericin (SS)
Abstract
1. Introduction
2. Experimental Section
2.1. Material and Methods
2.2. SS Extraction in an Autoclave Using Pure Water
2.3. Preparation of EtOH Non-Precipitated SS
2.4. Preparation of Glycine Non-Precipitated SS
2.5. Characterization of SS Filtrate
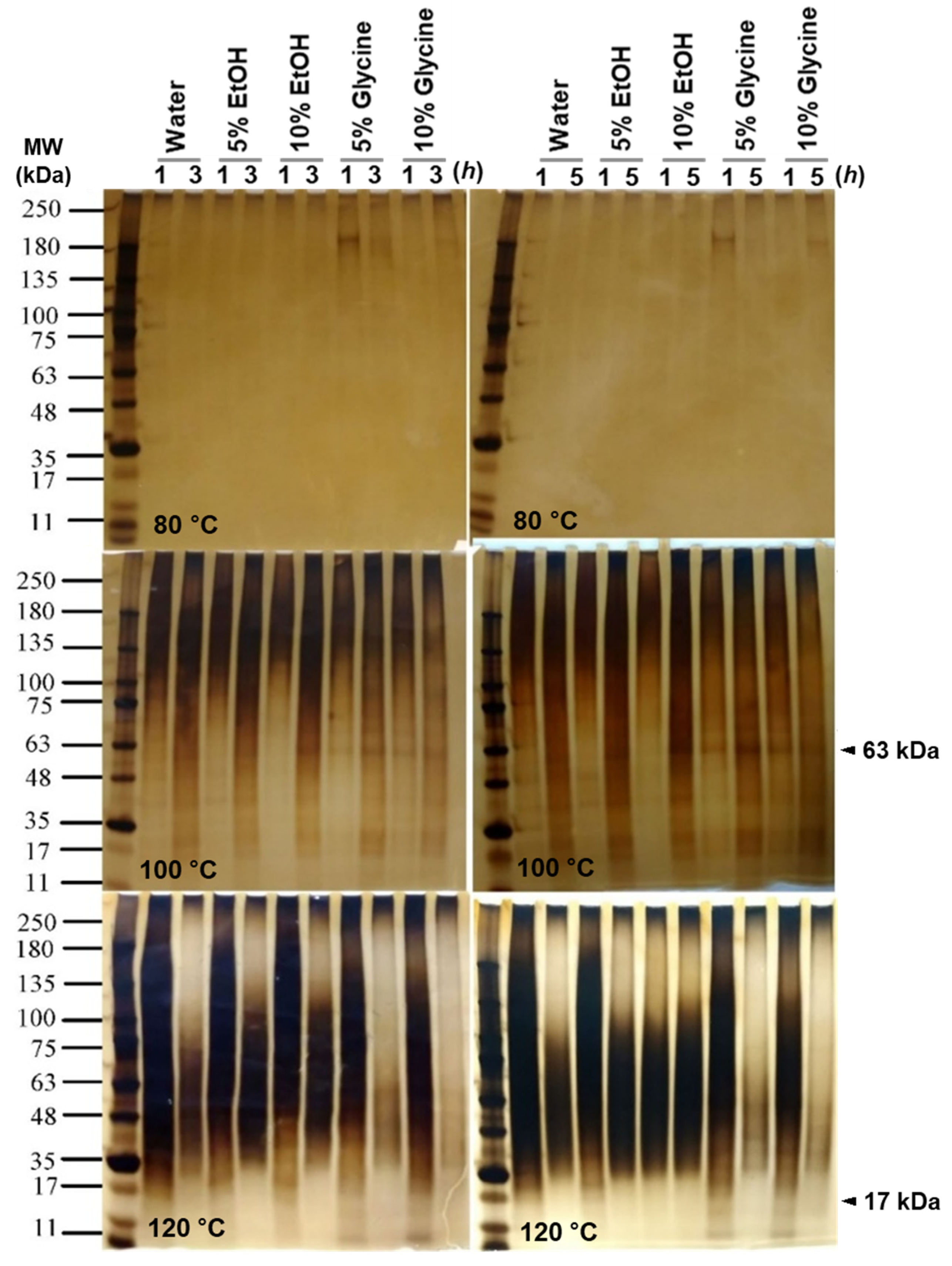
2.5.1. Molecular Weight Estimation of SS Filtrate with SDS-PAGE
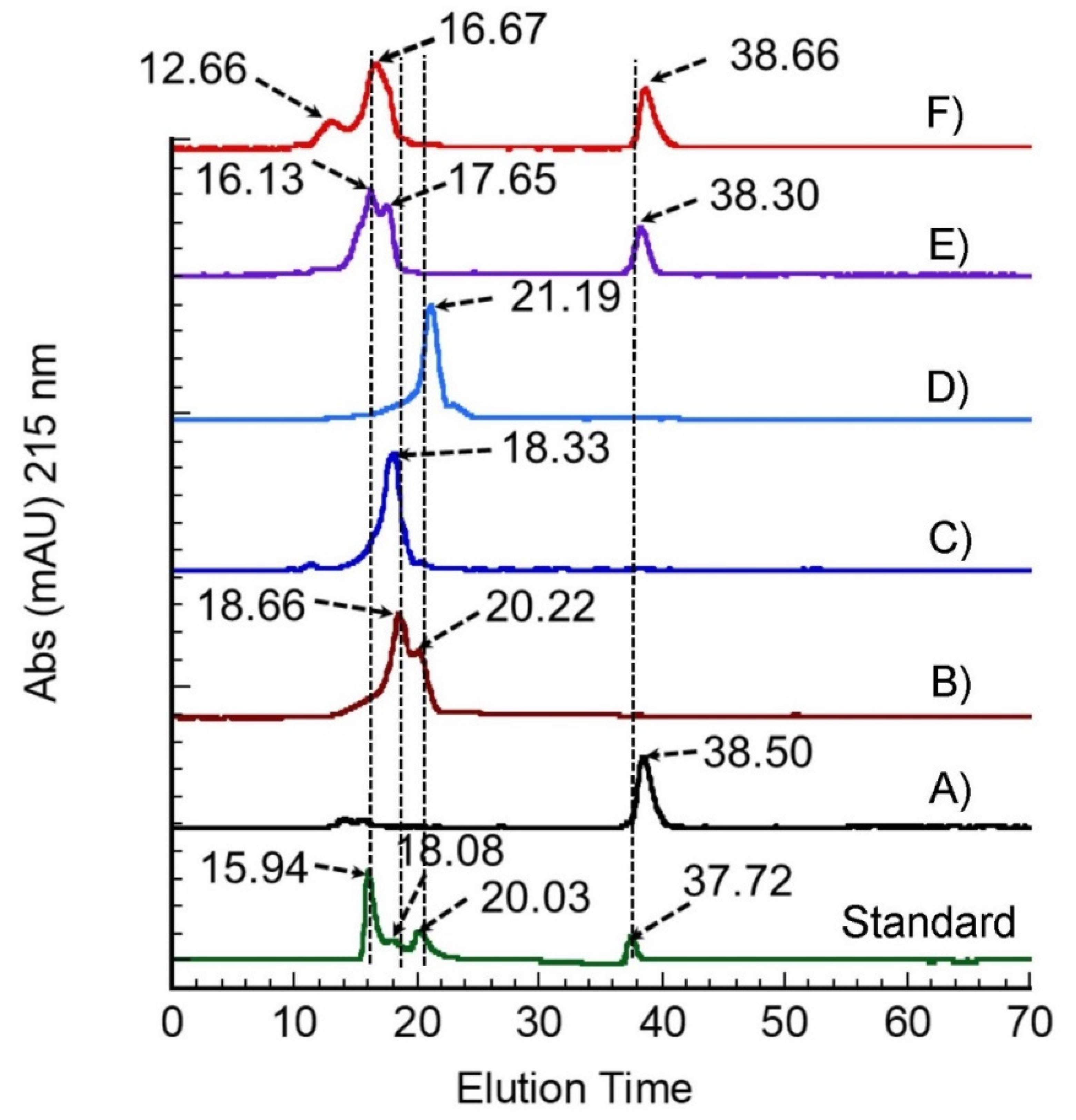
2.5.2. Fast Performance Liquid Chromatography (FPLC)
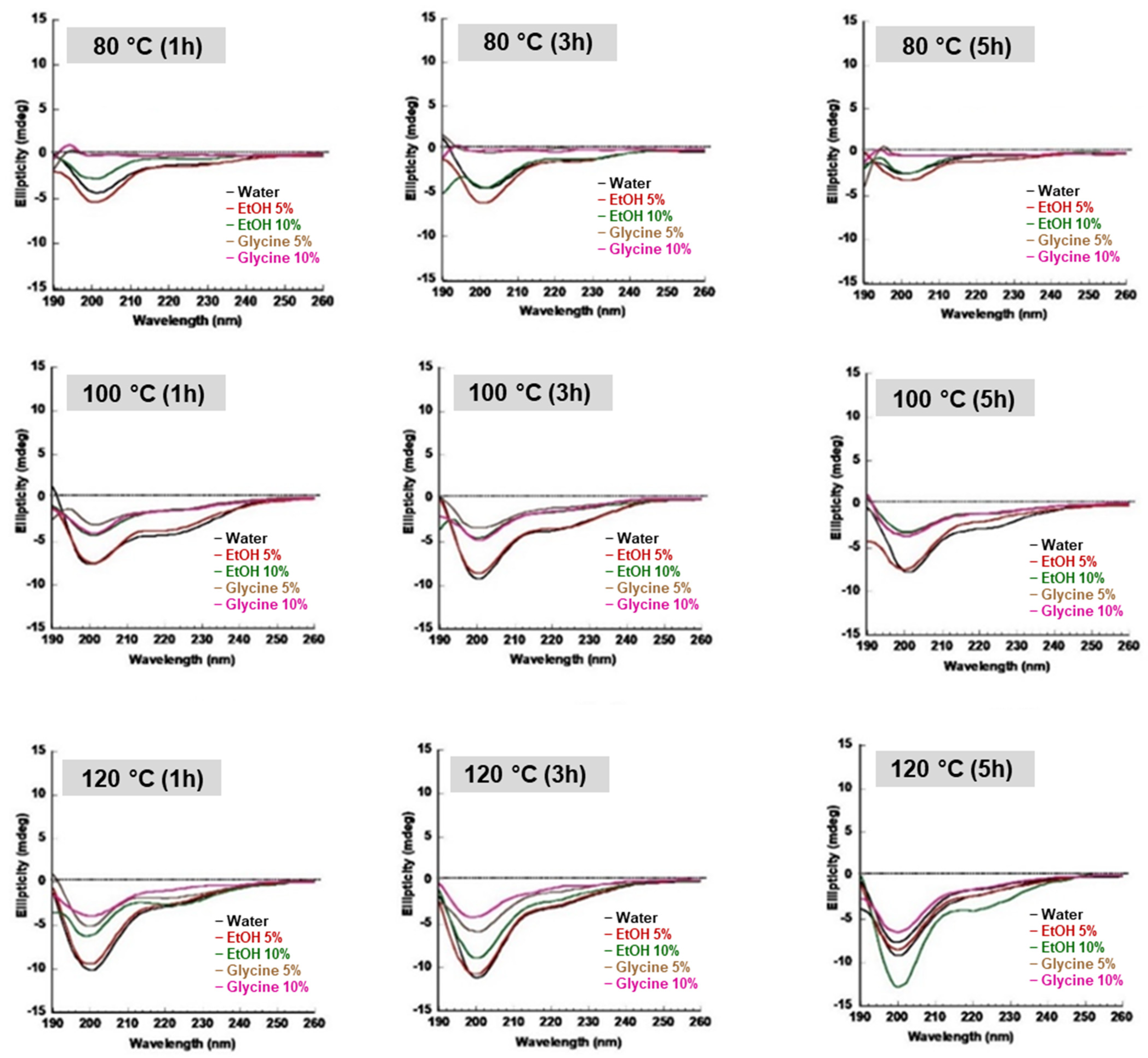
2.5.3. Circular Dichroism (CD) of SS Solution
2.5.4. SS Gelation Monitoring
2.5.5. Fourier Transformed Infrared (FT-IR) Analysis
2.5.6. Cytotoxicity Assay of SS Solution
2.5.7. Raw 264.7 Cell Proliferation after SS Solution and Gel Treatment
3. Results and Discussion
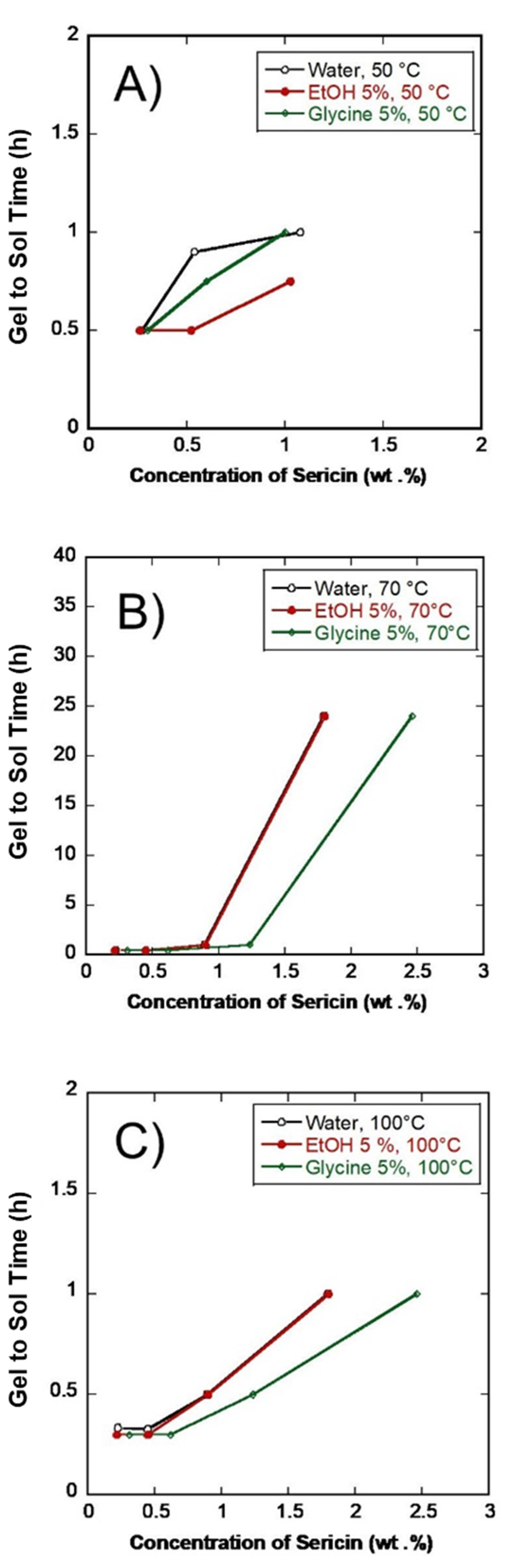
3.1. SS Concentration (wt. %)
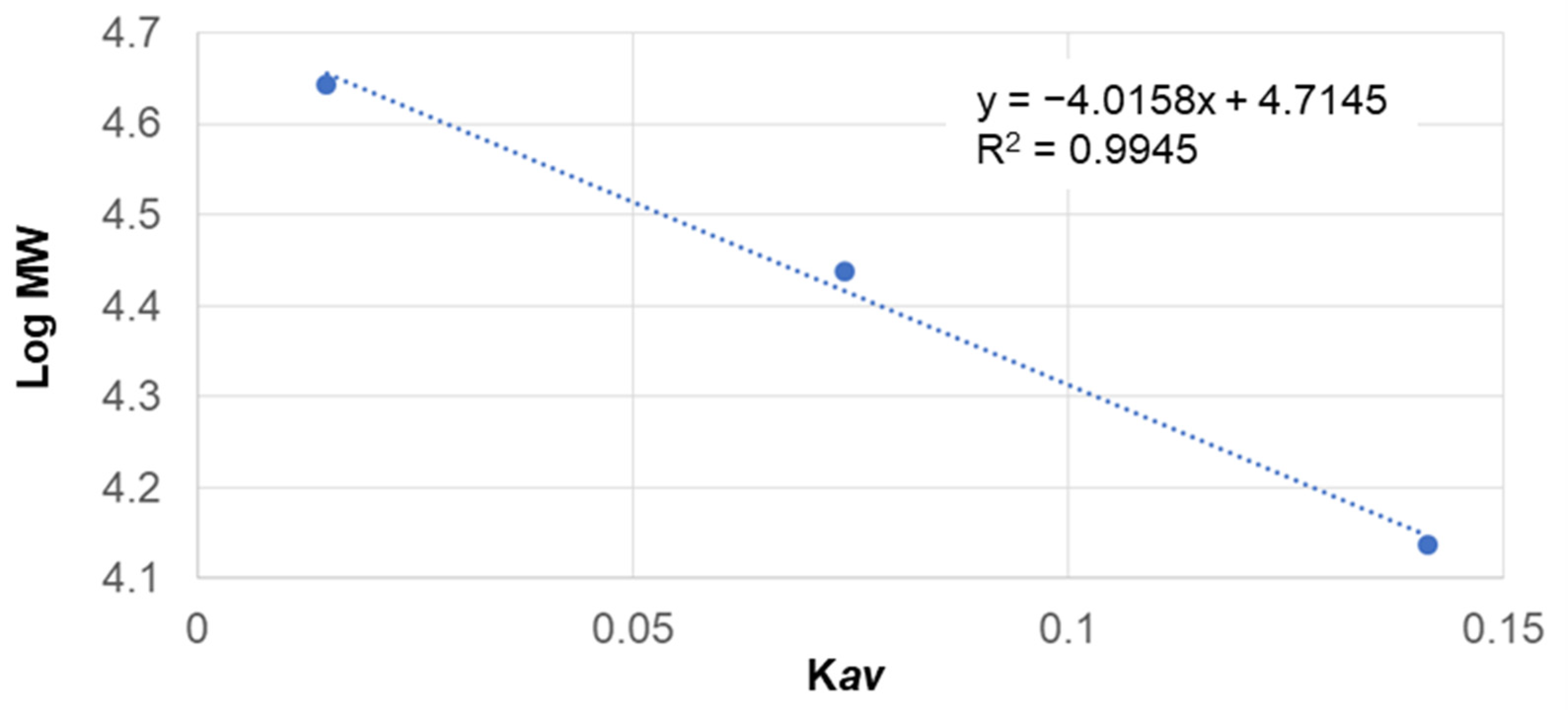
3.2. The Molecular Weight Distribution of Degummed SS Protein
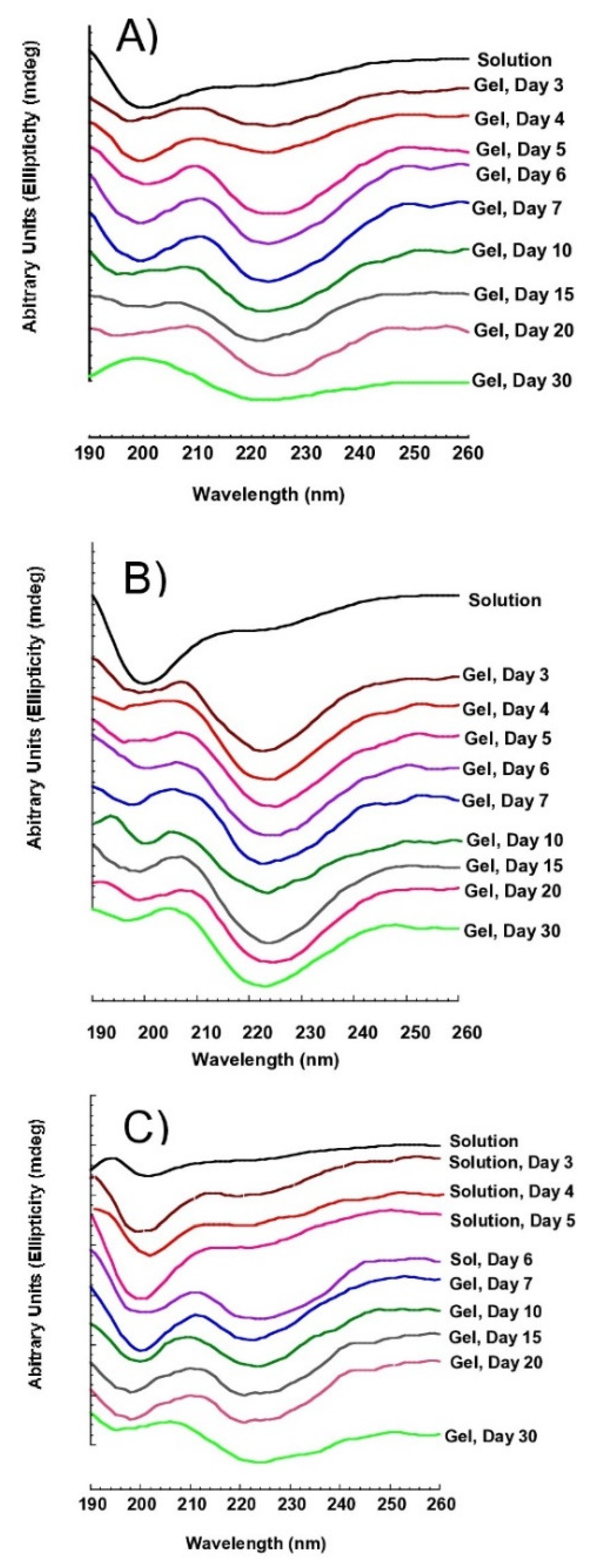
3.3. Secondary Structure of SS Solution and Gel
3.4. Stability Monitoring of SS Aqueous at 4 °C
3.5. SS Gel Thermal-Reversibility
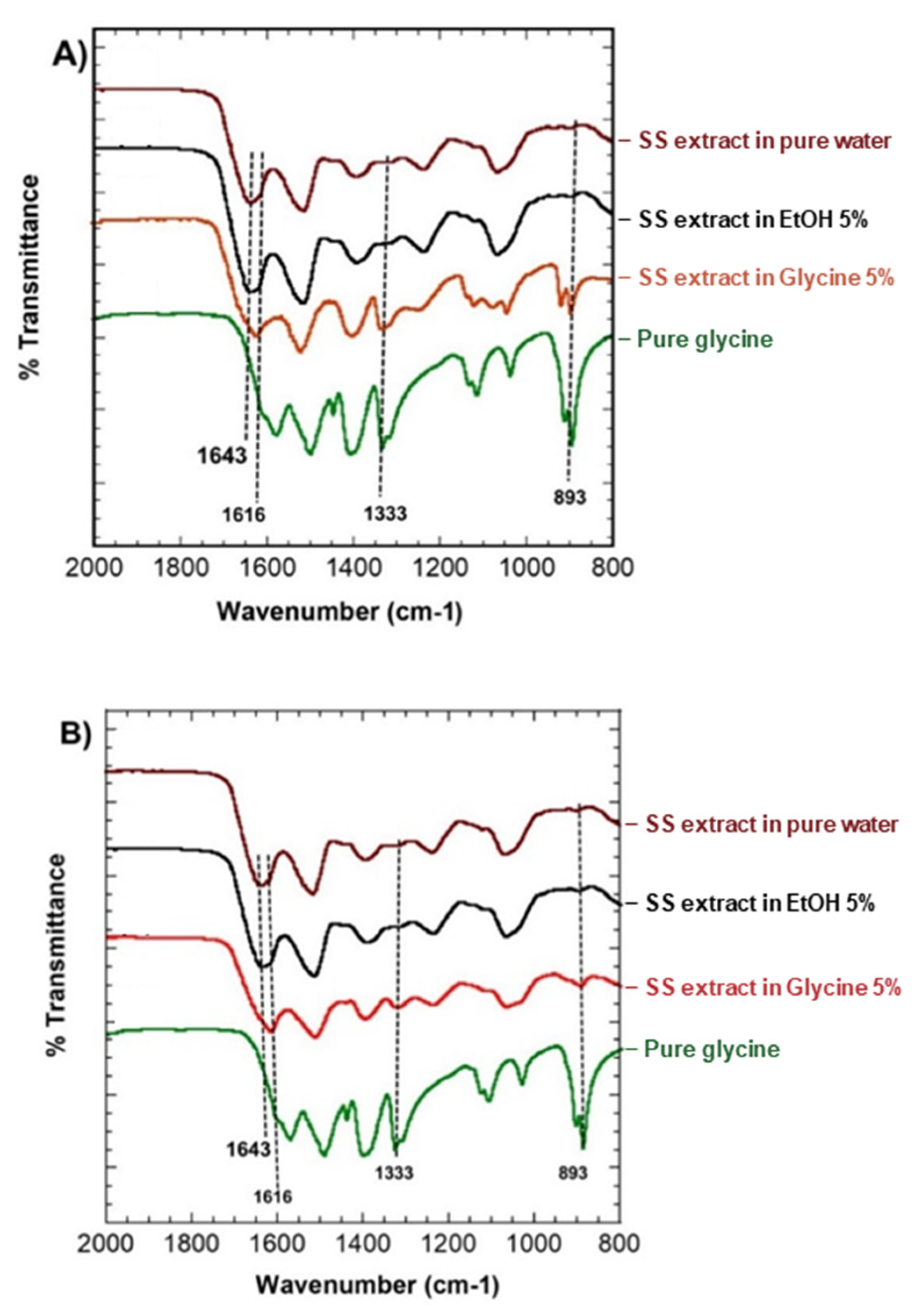
3.6. IR Spectroscopy of SS Solution and Gel
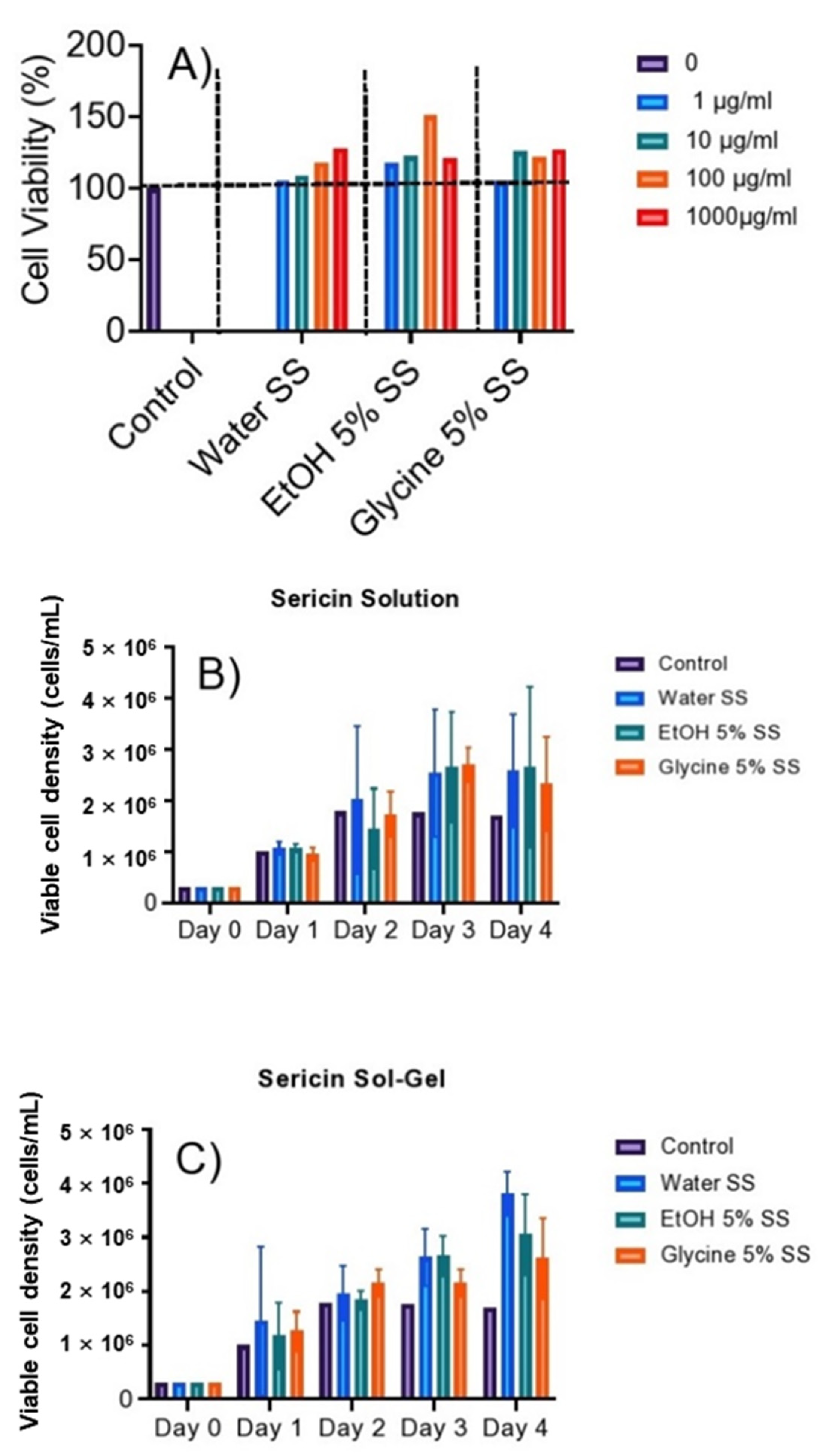
3.7. Cell Viability and Cell Proliferation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.G.; Kweon, H.; Jo, Y.Y. Toll-like receptor and silk sericin for tissue engineering. Int. J. Ind. Entomol. 2021, 42, 1–6. [Google Scholar] [CrossRef]
- Lee, K.G.; Yeo, J.H.; Lee, Y.W.; Kweon, H.Y.; Kim, J.H. Bioactive and skin-compatible properties of silk sericin. Korean J. Seric. Sci. 2001, 43, 109–115. [Google Scholar]
- Kweon, H.Y.; Yeo, J.H.; Kim, K.Y.; Kim, Y.S.; Song, H.S.; Kim, S.J.; Woo, S.O.; Han, S.M.; Lee, K.G. Characteristics of Silk Sericin Extracted from Sericinjam. Int. J. Ind. Entomol. 2009, 18, 121–124. [Google Scholar]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Use of Silk Protein, Sericin, as a Sustained-Release Material in the Form of a Gel, Sponge and Film. Chem. Pharm. Bull. 2010, 58, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Vaithanomsat, P.; Kitpreechavanich, V. Sericin separation from silk degumming wastewater. Sep. Purif. Technol. 2008, 59, 129–133. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Lee, K.H. Effect of Salts on Gelation Time of Silk Sericin Solution. Int. J. Ind. Exntomol. 2013, 27, 326–328. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, A.; Ki, C.S.; Kim, J.W.; Park, Y.H.; Lee, K.H. Preparation of Silk Sericin Beads Using LiCl/DMSO Solvent and Their Potential as a Drug Carrier for Oral Administration. Fibers Polym. 2007, 8, 470–476. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Chaudhary, H.; Gulrajani, M.; Gupta, C. Cleaner process for extraction of sericin using infrared. J. Clean. Prod. 2013, 52, 488–494. [Google Scholar] [CrossRef]
- Silva, V.R.; Ribani, M.; Gimenes, M.L.; Scheer, A.P. High Molecular Weight Sericin Obtained by High Temperature and Ultrafiltration Process. Procedia Eng. 2012, 42, 833–841. [Google Scholar] [CrossRef]
- Teramoto, H.; Kakazu, A.; Yamauchi, K.; Asakura, T. Role of Hydroxyl Side Chains in Bombyx mori Silk Sericin in Stabilizing Its Solid Structure. Macromolecules 2007, 40, 1562–1569. [Google Scholar] [CrossRef]
- Dash, R.; Mukherjee, S.; Kundu, S. Isolation, purification and characterization of silk protein sericin from cocoon peduncles of tropical tasar silkworm, Antheraea mylitta. Int. J. Biol. Macromol. 2006, 38, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Yeo, J.H.; Lee, K.G.; Lee, Y.W.; Park, Y.H.; Nahm, J.H.; Cho, C.S. Effects of poloxamer on the gelation of silk sericin. Macromol. Rapid Commun. 2000, 21, 1302–1305. [Google Scholar] [CrossRef]
- Wu, J.-H.; Wang, Z.; Xu, S.-Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Towell, J.F.; Manning, M.C. Analysis of protein structure by circular dichroism spectroscopy. Technol. Instrum. Anal. Chem. 1994, 14, 175–205. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, M.K.; Um, I.C.; Lee, K.H. Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 2011, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Kim, Y.A.; Barbeau, W.E. Evaluation of SDS-PAGE Method for Estimating Protein Digestibility. J. Food Sci. 1991, 56, 1082–1086. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of Three Main Sericin Components from the Cocoon of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef]
- Sprague, K.U. Bombyx mori silk proteins. Characterization of large polypeptides. Biochemistry 1975, 14, 925–931. [Google Scholar] [CrossRef]
- Deller, M.C.; Kong, L.; Rupp, B. Protein stability: A crystallographer’s perspective. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 72–95. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Mitaku, S. Mechanisms of secondary structure breakers in soluble proteins. BIOPHYSICS 2005, 1, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Saiani, A.; Mohammed, A.; Frielinghaus, H.; Collins, R.; Hodson, N.; Kielty, C.M.; Sherratt, M.J.; Miller, A.F. Self-assembly and gelation proper-ties of α-helix versus β-sheet forming peptides. Soft Matter. 2009, 5, 326–328. [Google Scholar] [CrossRef]
- Padamwar, M.N.; Pawar, A.P. Silk sericin and its applications: A review. J. Sci. Ind. Res. 2004, 63, 323–329. [Google Scholar]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- DeFlores, L.P.; Ganim, Z.; Nicodemus, R.A.; Tokmakoff, A. Amide I′−II′ 2D IR Spectroscopy Provides Enhanced Protein Secondary Structural Sensitivity. J. Am. Chem. Soc. 2009, 131, 3385–3391. [Google Scholar] [CrossRef]
- Jo, Y.N.; Park, B.-D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941. [Google Scholar] [CrossRef]
- Arango, M.C.; Álvarez-López, C. Effect of freezing temperature on the properties of lyophilized silk sericin scaffold. Mater. Res. Express 2019, 6, 095414. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Singh, V.; Rai, S. Vibrational spectrum of glycine molecule. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2004, 61, 2741–2746. [Google Scholar] [CrossRef]



| Extraction Ingredients | Time (h) | Temperature (°C) | ||
|---|---|---|---|---|
| 80 °C | 100 °C | 120 °C | ||
| Water | 1 | 0.06 | 0.40 | 1.63 |
| 3 | 0.09 | 0.64 | 1.88 | |
| 5 | 0.09 | 1.00 | 1.98 | |
| EtOH 5% | 1 | 0.08 | 0.20 | 1.32 |
| 3 | 0.09 | 0.70 | 1.70 | |
| 5 | 0.09 | 0.74 | 1.86 | |
| EtOH 10% | 1 | 0.07 | 0.90 | 1.23 |
| 3 | 0.16 | 1.00 | 1.74 | |
| 5 | 0.22 | 1.23 | 1.86 | |
| Glycine 5% | 1 | 0.03 | 0.75 | 1.60 |
| 3 | 0.04 | 1.03 | 1.70 | |
| 5 | 0.04 | 1.60 | 2.10 | |
| Glycine 10% | 1 | 0.03 | 0.80 | 1.61 |
| 3 | 0.04 | 1.03 | 1.86 | |
| 5 | 0.05 | 1.61 | 2.01 | |
| Parameter | Retention Time (min) | Kav | Log MW | MW (kDa) |
|---|---|---|---|---|
| Pure glycine | 38.50 | - | - | - |
| Water extract | 18.66 | 0.000681 | 1.173818 | 69.5 |
| 20.22 | 0.005014 | 1.172739 | 69 | |
| EtOH 5% extract | 18.33 | 0.000872 | 1.173771 | 69.5 |
| EtOH 10% extract | 21.19 | 0.004086 | 1.172970 | 69.2 |
| Glycine 5% extract | 16.13 | 0.102703 | 1.148413 | 60 |
| 17.65 | 0.033747 | 1.165584 | 65.65 | |
| 38.30 | - | - | - | |
| Glycine 10% extract | 12.66 | 0.006551 | 1.172356 | 69 |
| 16.67 | 0.001366 | 1.17348 | 69.5 | |
| 38.66 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muindi, M.P.; Lee, J.H.; Kweon, H.; Kasina, M. Effect of Extraction Ingredients on the Conformation and Stability of Silk Sericin (SS). Polymers 2022, 14, 4118. https://doi.org/10.3390/polym14194118
Muindi MP, Lee JH, Kweon H, Kasina M. Effect of Extraction Ingredients on the Conformation and Stability of Silk Sericin (SS). Polymers. 2022; 14(19):4118. https://doi.org/10.3390/polym14194118
Chicago/Turabian StyleMuindi, Munguti Peter, Ji Hae Lee, HaeYong Kweon, and Muo Kasina. 2022. "Effect of Extraction Ingredients on the Conformation and Stability of Silk Sericin (SS)" Polymers 14, no. 19: 4118. https://doi.org/10.3390/polym14194118
APA StyleMuindi, M. P., Lee, J. H., Kweon, H., & Kasina, M. (2022). Effect of Extraction Ingredients on the Conformation and Stability of Silk Sericin (SS). Polymers, 14(19), 4118. https://doi.org/10.3390/polym14194118






