Abstract
Glass fiber reinforced plastic (GFRP) composites have great potential to replace metal components in vehicles by maintaining their mechanical properties and improving fire resistance. Ease of form, anti-corrosion, lightweight, fast production cycle, durability and high strength-to-weight ratio are the advantages of GFRP compared to conventional materials. The transition to the use of plastic materials can be performed by increasing their mechanical, thermal and fire resistance properties. This research aims to improve the fire resistance of GFRP composite and maintain its strength by a combination of pumice-based active nano filler and commercial active filler. The nano active filler of pumice particle (nAFPP) was obtained by the sol–gel method. Aluminum trihydroxide (ATH), sodium silicate (SS) and boric acid (BA) were commercial active fillers that were used in this study. The GFRP composite was prepared by a combination of woven roving (WR) and chopped strand mat (CSM) glass fibers with an unsaturated polyester matrix. The composite specimens were produced using a press mold method for controlling the thickness of specimens. Composites were tested with a burning test apparatus, flexural bending machine and Izod impact tester. Composites were also analyzed by SEM, TGA, DSC, FT-IR spectroscopy and macro photographs. The addition of nAFPP and reducing the amount of ATH increased ignition time significantly and decreased the burning rate of specimens. The higher content of nAFPP significantly increased the flexural and impact strength. TGA analysis shows that higher ATH content had a good contribution to reducing specimen weight loss. It is also strengthened by the lower exothermic of the specimen with higher ATH content. The use of SS and BA inhibited combustion by forming charcoal or protective film; however, excessive use of them produced porosity and lowered mechanical properties.
1. Introduction
Recently, transportation industries have replaced metals with plastic materials. Plastics have a faster production cycle than that metal processing, so they save costs and increase product quality [1]. The limitation of weight is the main consideration in this material due to its high efficiency and economic value. The use of plastics in various modes of transportation is much better [2]. Plastics are commonly used in industry in the form of composites.
In producing composite material, it is necessary to add fiber as reinforcement. Glass fibers improve the mechanical properties and fire resistance of composites [3,4]. They have good insulation properties, high melting point, resistance to chemical and water environments, and fire resistance up to 1050 °C. It cannot be ignited if the heat flux is below 100 to 125 kW/m2 [5,6]. Glass fiber reinforced plastic (GFRP) composite with unsaturated polyester resins (UPRs) matrix has a big trend in the transportation industry.
UPRs are widely used as matrix composite materials in construction, transportation and other industries. This matrix has many advantages, such as good mechanical properties, corrosion resistance, good processability, low viscosity and density, high strength-to-weight ratio and fast production cycle [7,8,9]. However, the polymer matrix has low flame retardant properties and reaction to fire [10]. The flammability of polymers can be reduced by adding flame retardants [11]. The development of halogen-free flame retardants must be fabricated to meet the regulations and safety standards [12,13] of the government’s program towards green products [14].
An active inorganic flame retardant, aluminum trihydroxide (ATH), is the most popular flame retardant that is used in polyester composites [15]. ATH is relatively cheap, easy to apply in polymers and environmentally friendly in smoke density and low toxicity [16]. The presence of ATH produces good flame retardant properties and thermal stability of composite [17,18]. At a temperature of 80–220 °C, ATH decomposes into alumina and water vapor and forms thermal insulating charcoal [19]. Alumina forms a protective layer and moisture to lower the temperature of the system and acts as a diluent agent in the gas phase [15]. The addition of sodium silicate (SS) contributes to the formation of polysilicate during composite manufacturing [20]. Char forms larger because the polysilicate forms a protective layer and insulates the composite surface after exposure to heat, resulting in good product and fire-retardant features in the composite [21,22]. Boric acid (BA) has a good flame retardant and active insulating effect on polyester composites. During the combustion process, BA reduces heat because it produces wet B2O3 and volatile H2O. B2O3 forms charcoal between the combustion process and oxygen [23,24]. Smoke density also decreased with the addition of BA into the UPRs [25].
In addition to being influenced by the glass transition temperature (Tg) and decomposition temperature (Td) [7], composite properties are also influenced by the shape and size of the particles [26]. Nano-sized materials are very promising in improving the properties of polymer composites [10]. The addition of nanosilica can form hydrogen bonds between the silanol group on the nano surface and the carbonyl ester group on the soft segment, thereby improving the thermal properties and adhesion of polyester [27]. Certain compositions improve the mechanical properties of composites [28]. Nanosilica or silicon dioxide (SiO2) is commonly found in the pumice [29,30], kaolin [31] and clay [32]. Pumice is formed as a result of the rapid cooling of gases and volcanic material from volcanic eruptions [33]. Indonesia has active volcanoes, which are about 30% of the world’s active volcanoes in the world [34]. The pumice contains major elements of 58.3% SiO2, 12.4% Fe2O3 and 12% Al2O3 [35]. The characteristics of silica are influenced by the method and parameters of the synthesis process, which can be performed by acid or alkaline treatment [29].
The flame retardant properties of composites increased with the addition of ATH into polyester, which was marked by a decrease in the burning rate [36]. There is an increase in flame retardant, thermal stability and decomposition temperature of the composite by adding BA to the composite [37]. The addition of SS in the composite can result in better flame retardant properties and thermal stability [38]. Likewise, the incorporation of ATH, BA and SS into the polyester is able to overcome the flammability of the composite. However, the mechanical properties of the composites decreased along with the increase in the filler content of ATH, BA and SS [39]. Mechanical properties increased with the addition of polyester nanosilica composites [40]. The data of both properties can also be used to design some components or products according to the operating conditions.
In previous studies, many researchers synthesized pumice [29,30,35] and combined it with other flame retardants, such as phosphorus-based flame retardant, ammonium polyphosphate, zinc hydroxystannate, magnesium hydrate, ATH, nanosilica, nanoalumina and nanoclay [41]. However, at present, there are still few, and no one has even discussed the combination of four flame retardants at the same time. A combination of nano active filler particle pumice (nAFPP), ATH, SS and BA is researched to meet the fire resistance and mechanical properties of GFRP composites. The physical properties (TGA, DSC, SEM, FTIR, macro photo) were observed to give analysis contribution of both properties. All flame retardants (nAFPP, ATH, SS and BA) are analyzed and discussed to explore their contribution to fire resistance and mechanical properties. The purpose of this study was to expand knowledge about the synthesis of pumice by developing the sol–gel method and the performance of GFRP composites on fire resistance and mechanical properties. For this purpose, SEM morphological analysis was to determine the particle size of the synthesis results and fuel resistance test to determine the ignition time and the rate of combustion of the composite. Then, the analysis of flexural strength and impact strength to determine the mechanical properties of the composite.
2. Materials and Methods
2.1. Materials
Pumice was obtained from Rinjani Mountain, Lombok Island, West Nusa Tenggara, Indonesia. Chemicals were supplied by e-Merck. Unsaturated polyester resin 268 BQTN and methyl ethyl ketone peroxide catalyst were supplied by Singapore Highpolymer Chemical Products (SHCP). The chopped strand mat (EMC200) and woven rowing of E-glass fibers were supplied by PT. Makmur Fantawijaya Chemical Industries, Jakarta, Indonesia.
2.2. Methods
2.2.1. Synthesis of Nano Active Filler Particle Pumice (nAFPP)
Pumice contains major elements of 58.3% SiO2, 12.4% Fe2O3, 12% Al2O3 [42]. The nAFPP was successfully synthesized using the sol–gel precipitation method. The pumice was washed with water and dried at 100 °C for 12 h. Pumice particles were produced by crushing and sieving with a size of 200 mesh (≤74 µm). They were washed and thermally activated at 680 °C for 1 h. Activated particles (100 g) were dissolved in 1000 mL of 2.5 M HCl and stirred at 300 rpm and 95 °C for 2 h. The mixture was filtered to obtain silica-rich pumice particles. Particles were washed with distilled water to reach pH 7. Sodium silicate solution was prepared by dissolving 10% wt of silica-rich pumice particles in 2 M NaOH at 95 °C for 2 h and stirrer at 300 rpm. The solution was filtered to separate residues and impurities [32]. The filtered solution was precipitated using 10 mL ethanol dispersant and 5 M HNO3 at 65 °C and pH 7. The solution was further filtered to obtain silica gel and washed with hot distilled water to remove impurities. Finally, the silica gel was dried at 80 °C for 4 h (Figure 1) [43].
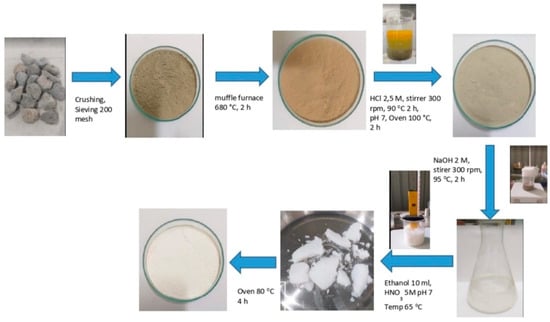
Figure 1.
Synthesis process of nano active filler pumice particle.
2.2.2. Preparation of Composites
Glass fiber reinforced plastic (GFRP) composites were made by a combination of hand layup press mold methods. Firstly, UPRs were mixed with fillers according to Table 1, and the mixture was stirred at 3000 rpm for 5 min, then it was allowed to stand so that the air bubbles disappeared [44]. The catalyst was added to the mixture at 1% by weight of the UPRs and stirred again so that the catalyst was well mixed. The fiber was cut to the size of the molding, and then the resin–filler mixture was poured into the molding according to the number of layers made. After 24 h, the composite was removed from the molding and then post-cured in an oven at 100 °C for 60 min [41]. The composites were cut to produce the test samples according to ASTM standards, i.e., ASTM D 635 for burning testing, ASTM D 790 for flexural testing and ASTM D 5941 for Izod impact testing [45,46].

Table 1.
Composite composition of GFRP with nAFPP, ATH, SS and BA.
2.2.3. Testing of Composites
Testing of fire resistance refers to ASTM D635-03 with standard bar specimens of 125 ± 5 mm in length, 13.0 ± 0.5 mm in width, and 3.0 (−0.0 to +0.2) mm in thickness [47]. The specimen was clamped in a horizontal direction with 5 mm of clamping length. The specimen was marked in lengths of 25 mm and 75 mm, as shown in Figure 2 [47].

Figure 2.
Burning test. Reprinted with permission from Ref. [41]. 2015, Diharjo, K.
In this study, the flexural test was carried out with the ASTM D-790 standard using the Universal Testing Machine JTM for a three-point bending method at a crosshead speed of 2 mm/min with a load cell of 200 kg. The load was placed in the center of the specimen, which was supported by two supports. This testing resulted in the curve of load–deflection, and then the flexural strength was obtained [46]. In the test of three-point bending, the failure occurred when the specimen changed shape due to the loading of the specimen at 200 kg. The impact strength of Izod was determined using an impact testing machine following the ASTM D-256 standard.
SEM observations were performed using a scanning electron microscope JEOL JCM-7000. Observations were made on the fracture side of the composite specimen as a result of impact testing. Thermogravimetric analysis (TGA) was carried out on STA PT 1600 (TG-DSC) equipment. Samples were loaded in alumina pans and heated at a rate of 20 °C/min from 30 to 600 °C under a dry air atmosphere. The Fourier transform infrared (FTIR) spectra were recorded using an IR Prestige-21 Shimadzu at 400–4000 cm−1.
3. Results and Discussion
Figure 3 shows the morphology and particle size of the synthesis of pumice particles. SEM observations show that the silica particles have an average size of 30 ± 16 nm. This indicates that the dispersant will be adsorbed onto the surface of the silica particles and form a protective layer of macromolecules. This macromolecular protective layer will inhibit the growth of particles to obtain a smaller particle size with better dispersibility in the sol–gel deposition process [48,49,50].

Figure 3.
The particle size distribution of nAFPP was measured by SEM.
Figure 4 shows the burning test results of the composites. The ignition time and burning rate of the C81, C82, C83 and C84 composites have similar properties. The composites containing nAFPP (reduction in ATH) have higher fire resistance compared to that without nAFPP, and the higher content of ATH until 4% gives a significant contribution to the increase in ignition time and reduced burning rate. The ATH releases water vapor, which functions as a diluent for volatile gases due to endothermic reactions when burned. ATH also forms an alumina layer (Al2O3), which functions as a protective layer from heat. This ATH reacts at a temperature of 180–200 °C into aluminum oxide through an endothermic reaction, which causes the UPRs to cool down, thereby reducing the pyrolysis product [39,51]. The cross-linking of UPRs also keeps the polymer chains from breaking under the influence of heat [52,53].
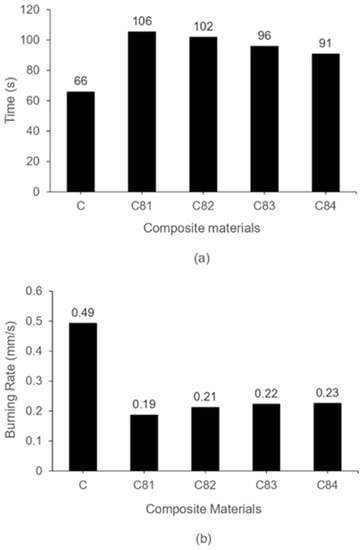
Figure 4.
Burning test of composites (a) ignition time (b) burning rate.
Both ignition time and burning rate show the best properties on the C81 for the composite containing 4% ATH and 1% nAFPP. Otherwise, the composite C84 with 4% nAFPP and 1% ATH has a higher burning rate and lower ignition time. This shows that the ATH gives a better contribution to the increase in fire resistance. All these fire retardants (nAFPP, ATH, SS and BA) are effective in increasing the fire resistance of the GFRP composite.
With the addition of BA, the protective layer is also stronger because BA melts at a temperature of 236 °C, and it dehydrates when heated above 300 °C [54]. If the heating continues, the boron oxide forms a protective film to inhibit the flame. Similarly, the presence of SS filler containing silicates can also increase the inhibition of flame. When SS mixes with UPRs, the silicates produce composites that have high thermal and mechanical properties [38,55] because SS forms an intumescent layer during the combustion process, so the temperature drops [56].
The nAFPP, which has a high silica content, causes the formation of a barrier preventing volatile evolution during degradation and increases the amount of char produced. In addition, nAFPP, which has a nano size, can reduce defects occurring on the GFRP so that it increases the mechanical strength of the composite [26].
Figure 5 shows the results of macro photos of the burnt composite sample surface. White spots on the surface of the composite are the remnants of unburned filler. Samples C81 and C82 show more spots compared to the other samples. The nAFPP content in this sample reached 4% by weight. This indicates that the filler is not well dispersed, caused by the stirring process during the manufacturing process of the composite. This could also be caused by agglomeration in the composites [17].
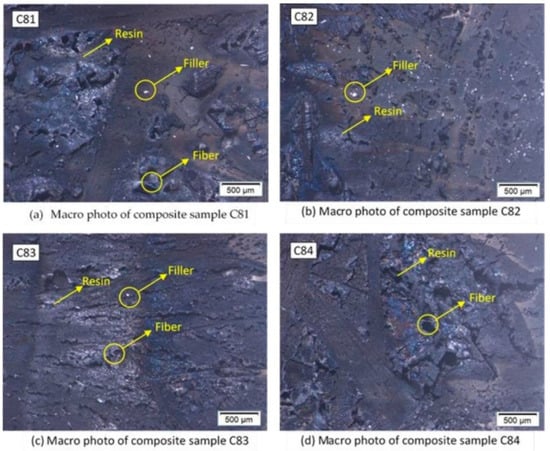
Figure 5.
Macro photo on the burnt surface of composites.
Figure 6 shows that the specimen experiences a deflection during loading. The specimen fractured at peak loading up to 103 N within 35 s, and the sample completely fractured within 63 s. Thus, specimen failure occurs in less than 60 s [57].
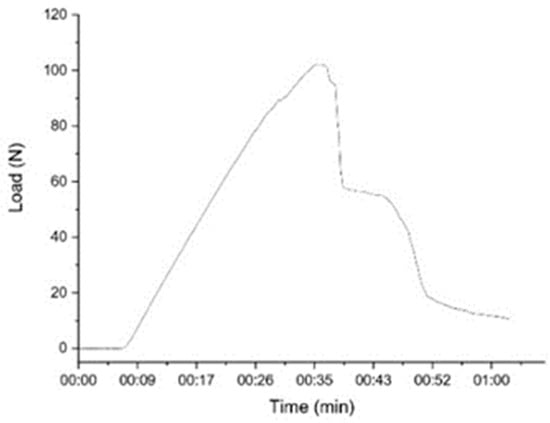
Figure 6.
Flexural test according to fracture time.
The flexural properties of GFRP composite specimens with various fillers nAFPP, ATH, SS and BA were tested, and the results are shown in Figure 7. It can be seen that increasing the nAFPP content up to 2% (wt) and reducing the ATH content up to 3% (wt) decreases the strength of the composite compared to the composite without filler (C). A further increase in the nAFPP filler content by up to 4% (reducing the ATH content up to 1%) could increase the composite strength compared to the unfilled composite. Specimen of C84 specimen has the highest flexural strength value of 58.5 MPa. It can be concluded that the addition of 4% of nAFPP increases the load-bearing capacity of the composite [58].
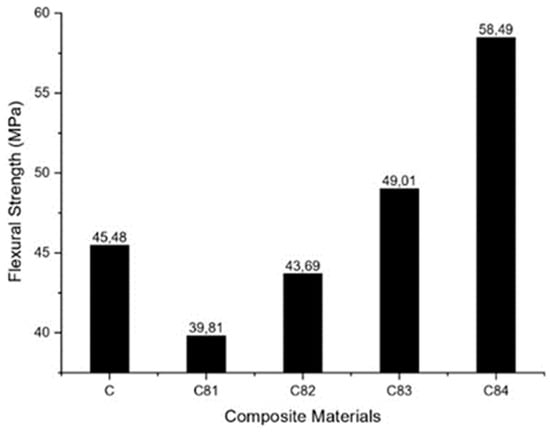
Figure 7.
Flexural strength of composites.
The relationship between nAFPP and commercial active fillers (ATH, BA, SS) with the impact strength of the composite is shown in Figure 8. The GFRP composite without filler has an impact strength of 69.22 KJ/m2. When the nAFPP content increases from 1 to 4% in weight, the impact strength of the composite also increases to 79.86, 85.99, 89.18 and 93.38 KJ/m2. The increase in impact strength was accompanied by an increase in nAFPP content up to 4% wt. The presence of nAFPP disperses in the matrix and easy to makes plastic deformation. Therefore, during the fracture of the composite in which nAFPP is well dispersed, the stress must be greater to initiate microcracking in the UPRs matrix. The impact energy will be mostly absorbed by the plastic deformation and more easily occurs in the vicinity of the nAFPP. Good nAFPP dispersion resulted in less agglomeration leading to better impact strength of the composite [59].
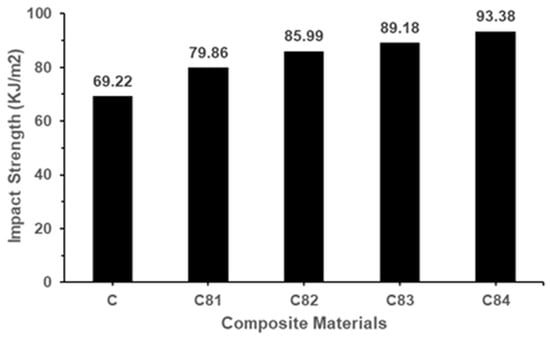
Figure 8.
Impact strength of composites.
Figure 9 shows the result of SEM observations on the fracture area of impact test specimens. The control sample (C.a and C.b) shows that the composite without filler produces a lower interfacial bond, thereby reducing the impact strength is occurred of the composite. Meanwhile, the C84.a and C84.b samples show a strong interfacial bond between the fiber and the matrix due to the high amount of nAFPP can reduce the occurrence of voids. The composition produces a solid material that can support more loads [60] so that it has a high impact strength.
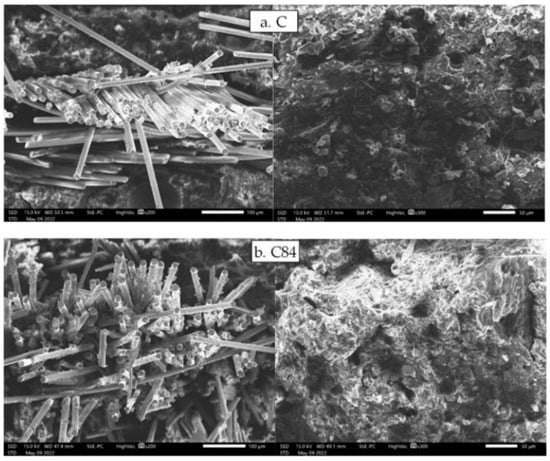
Figure 9.
SEM observation on impact testing specimens on impact testing specimens: (a) GFRP composite without filler and (b) GFRP composite with filler.
The TGA result in Figure 10 shows that the oxidative thermal degradation composites take place in two main stages. The first stage of degradation is occurred at about 200–300 °C, and the other takes place at or above 300 °C. The second stage comes to pass depolymerization, and the breakdown of the UPRs chain takes place at or above 300 °C [55]. Sample C81 has a lower initial temperature and a higher rate of weight loss from thermal decomposition compared to the others. Both BA and ATH have lower dehydration temperatures [61]. The results obtained in this study, the materials tested used a constant heating rate without any variation in the heating rate.
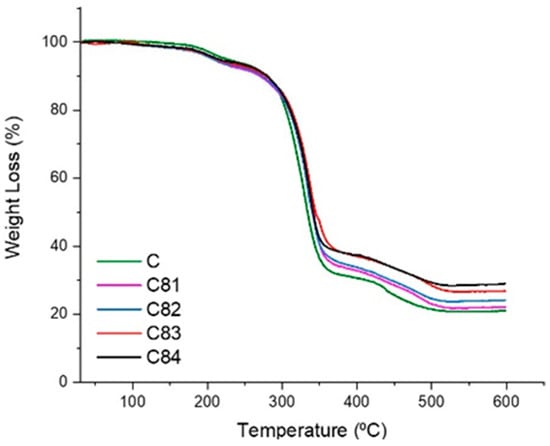
Figure 10.
TGA Thermograms analysis of composites.
In Figure 11, the initial test curve shows a slow endothermic reaction depicted by a gentle descending curve. At temperatures less than 200 °C, the absorbed heat is used to evaporate water vapor and evaporation due to the initial decomposition process [62]. When the specimen is above 200 °C, an endothermic peak appeared at 282 °C for the C81 and C82 composites. This provides information that the composite with filler requires more heat than the composite without filler. The results of the combustion test also show that the C81 and C82 composites have a longer initial ignition time than the composites without filler (C). This endothermic reaction describes the beginning of the thermal decomposition of the composite, which produces volatiles. Substances that are volatile will be broken down into smaller fractions so that they are more easily oxidized [63].
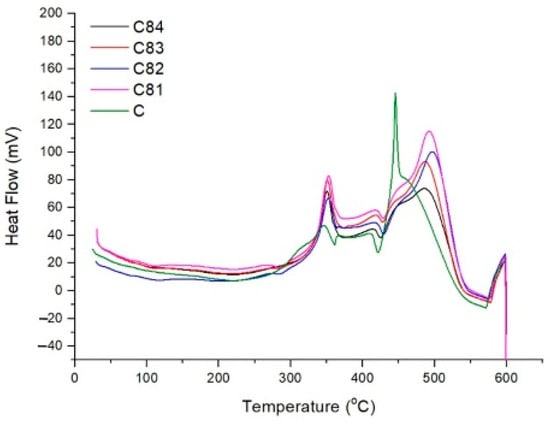
Figure 11.
Differential scanning calorimetry (DSC) analysis of composites.
In Figure 11, the curve starts to rise at 320 °C, and exothermic peaks occur at 346 °C for the C composite, 352 °C for the C81 and C82 composite, and 350 °C for the C83 and C84 composites. This exothermic reaction describes the thermal oxidation of volatiles with oxygen that produces heat (exothermic). The exothermic peaks of the three composites were at a higher temperature when compared to the C composite (without filler). This is because the filler prevents oxidation and is followed by an endothermic reaction during the decomposition of the filler. Filler decomposition produces water vapor, which plays a role in reducing the burning rate. The water vapor escapes in the flame and dissolves the concentration of combustible gases from the polymer matrix. This condition causes oxygen access on the composite surface to be limited [64].
Figure 12 shows the FTIR spectra curve in the range of 400–4000 cm−1. Absorption -H (3476 cm−1), aromatic = strain C-H (3028 cm−1), aliphatic vibration C-H (2942 cm−1), aromatic ring (699–857 cm−1) and O = C-O (1732 cm−1) was detected for composite C (without filler), which is a product of UPR gas decomposition [65]. In the other composites, peaks at 1067–1063 cm−1 were detected, which defined the strain asymmetry of Si-O-Si [66]. This indicates the presence of silicate compounds derived from AFPP and SS fillers. The intense band at 633 cm−1 for the C81 composite, 616 cm−1 for the C82 composite and 616 cm−1 for the C83 composite is the vibrational strain of Al-O. This is because the four composites contain ATH filler containing Al, except for the C84 composite because the ATH filler content is very low [67].
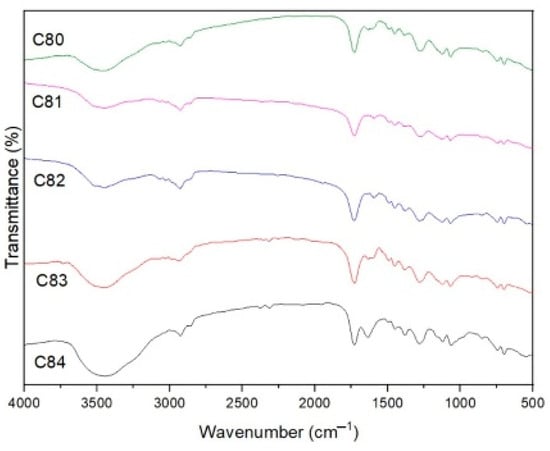
Figure 12.
FTIR spectra of composites.
The observed bands at 1279 and 1124 cm−1 for C81, 1280 and 1123 cm−1 for C82, 1280 and 1121 cm−1 for C83, and 1282–1121 cm−1 for C84 were designated as vibrations of B-O-B. In this vibration, the peak value is not much different because the composite composition contains the same BA filler. In the four composites that were added with filler, OH peaks also appeared at 3453 cm−1, 3512 cm−1, 3449 cm−1 and 3446 cm−1, which would dilute the volatiles so that oxidation was reduced.
4. Conclusions
The fire resistance of the GFRP composites increases along with the addition of ATH (reduction nAFPP), shown by the increase in ignition time and the decrease in the burning rate. Otherwise, the composites with higher nAFPP (lower ATH) have higher bending and impact strength. The ATH and nAFPP are more effective in inhibiting the flame and increasing the strength, respectively. BA is a good insulator to inhibit the flame by producing a protective layer in different temperature stages of dehydration, while SS produces a protective layer that does not melt easily. Both BA and SS in higher content can decrease the GFRP composite. The composite can be optimized by arranging the optimum combination content of each filler according to its operating conditions. The fracture of the composite shows a strong interfacial bond between fiber and matrix due to the high amount of nAFPP that can reduce the occurrence of voids. The composite with filler needs more heat for the decomposition process compared to those without filler.
Author Contributions
Conceptualization, A.R., K.D., W.W.R. and V.S.; methodology, A.R., K.D., W.W.R. and V.S.; software, A.R. and K.D.; validation, K.D., W.W.R., V.S. and S.K.; formal analysis, A.R., K.D. and S.K.; investigation, A.R., K.D. and S.K.; resources, A.R., K.D. and S.K.; data curation, A.R. and K.D.; writing—original draft preparation, A.R. and K.D.; writing—review and editing, A.R., K.D. and W.W.R.; visualization, A.R. and K.D.; supervision, K.D., W.W.R. and V.S.; project administration, A.R. and K.D.; funding acquisition, KD., W.W.R. and V.S.; A.R., K.D. and W.W.R. are the main contributors of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study has supported by the Institute for Research and Community Service of Sebelas Maret University, Indonesia, with number program of 254/UN.27.22/PT.01.03/2022.
Data Availability Statement
The data presented in this article are available from the corresponding author.
Acknowledgments
The authors acknowledge the facilities, scientific and technical support from Deputy For Infrastructure Research And Innovation in National Research and Innovation Agency.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarfraz, M.S.; Hong, H.; Kim, S.S. Recent developments in the manufacturing technologies of composite components and their cost-effectiveness in the automotive industry: A review study. Compos. Struct. 2021, 266, 113864. [Google Scholar] [CrossRef]
- Chandgude, S.; Salunkhe, S. In state of art: Mechanical behavior of natural fiber-based hybrid polymeric composites for application of automobile components. Polym. Compos. 2021, 42, 2678–2703. [Google Scholar] [CrossRef]
- Diharjo, K.; Sutrisno, T.; Afandi, R.; Himawanto, D.A. Enhancing of fire resistance on CFRP using Sokka-clay particle for lightweight car body panel. In Proceedings of the 2014 International Conference on Electrical Engineering and Computer Science (ICEECS), Bali, Indonesia, 24–25 November 2014; pp. 188–192. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Qin, Z.; Wu, Y.; Zhang, W.; Yang, R. High-transparency polysilsesquioxane/glycidyl-azide-polymer resin and its fiberglass-reinforced composites with excellent fire resistance, mechanical properties, and water resistance. Compos. Part B Eng. 2021, 219, 108913. [Google Scholar] [CrossRef]
- Raton, M. (Ed.) Fiber-Reinforced Composites: Materials, Manufacturing, and Design; Department of Mechanical Engineering, University of Michigan-Dearborn: Dearborn, MI, USA; CRC Press Taylor & Francis Group 6000 Broken Sound: Parkway, NW, USA, 2007. [Google Scholar]
- Cevahir, A. Glass fibers. In Fiber Technology for Fiber-Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–121. [Google Scholar]
- Rosa, I.; Firmo, J.; Correia, J.; Mazzuca, P. Influence of elevated temperatures on the bond behaviour of GFRP bars to concrete—Pull-out tests. Struct. Eng. Int. 2019, 29, 481–483. [Google Scholar]
- Rosa, I.C.; Firmo, J.P.; Correia, J.R.; Mazzuca, P. Influence of elevated temperatures on the bond behaviour of ribbed Gfrp bars in concrete. Cem. Concr. Compos. 2021, 122, 104119. [Google Scholar] [CrossRef]
- Mazzuca, P.; Firmo, J.P.; Correia, J.R.; Castilho, E. Influence of elevated temperatures on the mechanical properties of glass fibre reinforced polymer laminates produced by vacuum infusion. Constr. Build. Mater. 2022, 345, 128340. [Google Scholar] [CrossRef]
- Kandola, B.K.; Ebdon, J.R. Flammability and Thermal Stability of Unsaturated Polyester Resin-Based Blends and Composites. In Unsaturated Polyester Resins; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jiang, S.; Gui, Z.; Shi, Y.; Zhou, K.; Yuan, B.; Bao, C.; Lo, S.; Hu, Y. Bismuth subcarbonate nanoplates for thermal stability, fire retardancy and smoke suppression applications in polymers: A new strategy. Polym. Degrad. Stab. 2014, 107, 1–9. [Google Scholar] [CrossRef]
- Troitzsch, J.H. Fires, statistics, ignition sources, and passive fire protection measures. J. Fire Sci. 2016, 34, 171–198. [Google Scholar] [CrossRef]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef]
- Farag, M.M. Quantitative methods of materials substitution: Application to automotive components. Mater. Des. 2008, 29, 374–380. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Elbasuney, S. Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 2017, 305, 538–545. [Google Scholar] [CrossRef]
- Halim, Z.A.A.; Yajid, M.A.M.; Nurhadi, F.A.; Ahmad, N.; Hamdan, H. Effect of silica aerogel—Aluminium trihydroxide hybrid filler on the physio-mechanical and thermal decomposition behaviour of unsaturated polyester resin composite. Polym. Degrad. Stab. 2020, 182, 109377. [Google Scholar] [CrossRef]
- Uddin, M.; Alabbad, M.; Li, L.; Orell, O.; Sarlin, E.; Haapala, A. Novel Micronized Mica Modified Casein–Aluminum Hydroxide as Fire Retardant Coatings for Wood Products. Coatings 2022, 12, 673. [Google Scholar] [CrossRef]
- Suharty, N.; Ismail, H.; Wibowo, F.; Handayani, D.; Firdaus, M.; Lathifah, L. Effect of Bentonite and Zinc Borate (ZB) Addition on Recycled Polypropylene Composites against Tensile and Burning Rate Properties. Adv. Mater. Res. 2015, 1105, 56–61. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Rasyid, M.F.A.; Salim, M.S.; Akil, H.M.; Karger-Kocsis, J.; Ishak, Z.A.M. Non-woven flax fibre reinforced acrylic based polyester composites: The effect of sodium silicate on mechanical, flammability and acoustic properties. Express Polym. Lett. 2019, 13, 553–564. [Google Scholar] [CrossRef]
- Chen, S.-N.; Li, P.-K.; Hsieh, T.-H.; Ho, K.-S.; Hong, Y.-M. Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials. Materials 2021, 14, 6628. [Google Scholar] [CrossRef]
- Alizadeh, A.; Geraei, M.; Mahoodi, M.R. In situ fabrication of Al-Al2O3-TiB2 hybrid nanocomposite; evaluating the effect of TiO2 and B2O3 mechanical milling time on properties of composite created through vortex casting. Mater. Res. Express 2019, 6, 045037. [Google Scholar] [CrossRef]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Macsim, A.M.; Lisa, G.; Anghel, I.; Şofran, I.E. Effects of phosphorus and boron compounds on thermal stability and flame retardancy properties of epoxy composites. Polymers 2022, 14, 4005. [Google Scholar] [CrossRef]
- Demirel, M.; Pamuk, V.; Dilsiz, N. Investigation of flame retardancy and physical–mechanical properties of zinc borate/boric acid polyester composites. J. Appl. Polym. Sci. 2010, 115, 2550–2555. [Google Scholar] [CrossRef]
- NKandola, B.K.; Nazaré, S.H.O.N.A.L.I.; Horrocks, A.R. Thermal degradation behaviour of flame-retardant unsaturated polyester resins incorporating functionalised nanoclays. In Fire Retardancy of Polymers: The Use of Mineral Fillers in Micro-and Nanocomposites; Le Bras, M., Ed.; Royal Chemical Society: Cambridge, UK, 2015; pp. 147–160. [Google Scholar]
- Vega-Baudrit, J.; Navarro-Banon, V.; Vazquez, P.; Martín-Martínez, J.M. Addition of nanosilicas with different silanol content to thermoplastic polyurethane adhesives. Int. J. Adhes. Adhes. 2006, 26, 378–387. [Google Scholar] [CrossRef]
- Su, C.; Wang, X.; Ding, L.; Wu, Z. Enhancement of mechanical behavior of FRP composites modified by silica nanoparticles. Constr. Build. Mater. 2020, 262, 120769. [Google Scholar] [CrossRef]
- Mourhly, A.; Khachani, M.; Hamidi, A.E.; Kacimi, M.; Halim, M.; Arsalane, S. The synthesis and characterization of low-cost mesoporous silica SiO2 from local pumice rock. Nanomater. Nanotechnol. 2015, 5, 35. [Google Scholar] [CrossRef]
- Mourhly, A.; Jhilal, F.; el Hamidi, A.; Halim, M.; Arsalane, S. Highly efficient production of mesoporous nano-silica from unconventional resource: Process optimization using a central composite design. Microchem. J. 2019, 145, 139–145. [Google Scholar] [CrossRef]
- Shu, Z.; Li, T.; Zhou, J.; Chen, Y.; Sheng, Z.; Wang, Y.; Yuan, X. Mesoporous silica derived from kaolin: Specific surface area enlargement via a new zeolite-involved template-free strategy. Appl. Clay Sci. 2016, 123, 76–82. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Towards tunable size of silica particles from rice husk. J. Non-Cryst. Solids 2015, 429, 61–69. [Google Scholar] [CrossRef]
- İlter, O. Use of Pumice in Mortar and Rendering for Lightweight Building Blocks. Ph.D. Thesis, Eastern Mediterranean University (EMU), Famagusta, North Cyprus, 2010. [Google Scholar]
- Pratomo, I. Classification of Indonesian Active Volcanoes, Case Studies of Several Volcanic Eruptions in History. Indones. J. Geosci. 2014, 1, 209–227. [Google Scholar]
- Putri, M.; Darminto, D. Synthesis of Zeolites from Lombok Pumice As Silica Source for Ion Exchanger. In Proceedings of the International Basic Science Conference, Jember, Indonesia, 26–27 September 2016. [Google Scholar]
- Kaleg, S.; Ariawan, D.; Diharjo, K. The flexural strength of glass fiber reinforced polyester filled with aluminum tri-hydroxide and montmorillonite. Key Eng. Mater. 2018, 772, 28–32. [Google Scholar] [CrossRef]
- Demirhan, Y.; Yurtseven, R.; Usta, N. The effect of boric acid on flame retardancy of intumescent flame retardant polypropylene composites including nanoclay. J. Thermoplast. Compos. Mater. 2021. [Google Scholar] [CrossRef]
- Hossain, M.; Uddin, M.B.; Khan, R.A.; Chowdhury, A. Preparation and characterization of sodium silicate–treated jute-cotton blended polymer–reinforced UPR-based composite: Effect of γ-radiation. Adv. Compos. Hybrid Mater. 2021, 4, 257–264. [Google Scholar] [CrossRef]
- Susilo, M.; Raharjo, W.W.; Diharjo, K. Inflammability of GFRP composite with the addition of aluminum tri-hydroxide, boric acid, and sodium silicate. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2021; Volume 2338, p. 040009. [Google Scholar]
- Chowdary, M.S.; Raghavendra, G.; Kumar, M.; Ojha, S.; Boggarapu, V. Influence of nano-silica on enhancing the mechanical properties of sisal/kevlar fiber reinforced polyester hybrid composites. Silicon 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Diharjo, K.; Suharty, N.; Nusantara, A.; Afandi, R. The effect of sokka clay on the tensile and burning properties of rPP/clay composite. Adv. Mater. Res. 2015, 1123, 338–342. [Google Scholar] [CrossRef]
- Mega Putri, K.; Regina, G.L.D.; Ade, L.N.F.; Haiyina, H.A.; Nura, H.H.; Darminto, D. Synthesis Of Zeolites From Lombok Pumice As Silica Source For Ion Exchanger. UNEJ E-Proceeding, 8 August 2017. Available online: https://jurnal.unej.ac.id/index.php/prosiding/article/view/4232 (accessed on 26 March 2021).
- Utama, P.; Yamsaengsung, R.; Sangwichien, C. Production And Characterization Of Precipitated Silica From Palm Oil Mill Fly Ash Using Co2 Impregnation And Mechanical Fragmentation. Braz. J. Chem. Eng. 2019, 36, 523–530. [Google Scholar] [CrossRef]
- Hapuarachchi, T. Aluminium trihydroxide in combination with ammonium polyphosphate as flame retardants for unsaturated polyester resin. Express Polym. Lett. Express Polym. Lett. 2009, 3, 743–751. [Google Scholar] [CrossRef]
- ASTM D 635; Standard Test Method for Rate of Burning and/or Extent and Time of Burning of Plastics in a Horizontal Position. ASTM International: Conshohocken, PA, USA, 1998.
- ASTM D 790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: Conshohocken, PA, USA, 2020.
- Underwriters’ Laboratories. UL-94 Test for Flammability of Plastic Materials for Parts in Devices and Appliances; Underwriters’ Laboratories: Northbrook, IL, USA, 2003. [Google Scholar]
- Alam, M.S.; Chowdhury, M.A. Characterization of epoxy composites reinforced with CaCO3-Al2O3-MgO-TiO2/CuO filler materials. Alex. Eng. J. 2020, 59, 4121–4137. [Google Scholar] [CrossRef]
- Cai, X.; Hong, R.Y.; Wang, L.S.; Wang, X.Y.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis of silica powders by pressured carbonation. Chem. Eng. J. 2009, 151, 380–386. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, T.; Kim, K.; Kim, B.; Kwag, G.; Kim, J.Y.; Paik, H.J. Poly(styrene-r-butadiene)-b-poly(poly(ethylene glycol) methyl ether methacrylate) as a silica dispersant in rubber compounds. Polym. Int. 2014, 63, 908–914. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Ding, X. Research of the properties of flame-retardant flexible PVC. Am. J. Mater. Res. 2014, 1, 20–25. [Google Scholar]
- Pantic, O.; Spasojevic, M.; Dzunuzovic, E.; Nikolic, M.S.; Savic, S.; Markovic, M.; Spasojevic, P. The Effect of Glycol Derivatives on the Properties of Bio-Based Unsaturated Polyesters. Polymers 2022, 14, 2970. [Google Scholar] [CrossRef]
- Aurer. Unsaturated Polyester Resins Polymers with Unlimited Possibilities; Verlag Moderne Industrie: München, Germany, 2003. [Google Scholar]
- Hou, X.; Li, Z.; Zhang, Z. Selectively Producing Acetic Acid via Boric Acid-Catalyzed Fast Pyrolysis of Woody Biomass. Catalysts 2021, 11, 494. [Google Scholar] [CrossRef]
- Eryani, E.; Aprilia, S.; Mulana, F. Characterization of Bionanofillers from Rice Waste as an Alternative for Reinforcement in Composite Polymers. J. Serambi Eng. 2018, 3. [Google Scholar]
- Chukwunwike, S.A. Flammability properties of flame retarded natural fibre reinforced polymer composites: An overview. J. Mater. Environ. Sci. 2019, 10, 647–656. [Google Scholar]
- Korniejenko, K.; Figiela, B.; Miernik, K.; Ziejewska, C.; Marczyk, J.; Hebda, M.; Lin, W.T. Mechanical and Fracture Properties of Long Fiber Reinforced Geopolymer Composites. Materials 2021, 14, 5183. [Google Scholar] [CrossRef]
- Hiremath, P.; Arunkumar, H.S.; Shettar, M. Investigation on Effect of Aluminium Hydroxide on Mechanical and Fire Retardant Properties of GFRP- Hybrid Composites. Mater. Today Proc. 2017, 4, 10952–10956. [Google Scholar] [CrossRef]
- Baskaran, R.; Sarojadevi, M.; Vijayakumar, C.T. Unsaturated polyester nanocomposites filled with nano alumina. J. Mater. Sci. 2011, 46, 4864–4871. [Google Scholar] [CrossRef]
- Prasad, G.V.; Nagappa, S.; Kanth, Y.R.; Lakshmi, I.G.; Rao, J.B. Effect of brachyura shell particles on glass fibre reinforced epoxy polymer composite. Mater. Today Proc. 2021, 42, 555–562. [Google Scholar] [CrossRef]
- Laoubi, K.; Hamadi, Z.; Benyahia, A.A.; Serier, A.; Azari, Z. Thermal behavior of E-glass fiber-reinforced unsaturated polyester composites. Compos. Part B Eng. 2014, 56, 520–526. [Google Scholar] [CrossRef]
- Dwynda, I.; Zainul, R. Boric Acid (H3(BO3): Recognize The Molecular Interactions in Solutions; INA-Rxiv: Bandung, Indonesia, 2018. [Google Scholar]
- Salasinska, K.; Celiński, M.; Barczewski, M.; Leszczyński, M.K.; Borucka, M.; Kozikowski, P. Fire behavior of flame retarded unsaturated polyester resin with high nitrogen content additives. Polym. Test. 2020, 84, 106379. [Google Scholar] [CrossRef]
- Pawelec, W. New Families of Highly Efficient, Halogen-Free Flame Retardants for Polypropylene (PP). Ph.D. Thesis, Laboratory of Polymer Technology Center of Excellence for Functional Materials Department of Chemical Engineering Åbo Akademi University, Turku, Finland, 2014. [Google Scholar]
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials. ACI Struct. J. 2006, 105, 60–67. [Google Scholar]
- Aguiar, A.E.; da Silva, L.G.; Barbosa, H.F.d.; Glória, R.F.; Espanhol-Soares, M.; Gimenes, R. Synthesis of Al2O3-0.5B2O3-SiO2 fillers by sol-gel method for dental resin composites. J. Non-Cryst. Solids 2017, 458, 86–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Berns, A.E.; Willbold, S.; Buitenhuis, J. Synthesis of poly(ethylene glycol) (PEG)-grafted colloidal silica particles with improved stability in aqueous solvents. J. Colloid Interface Sci. 2007, 310, 446–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).