Composite Polymer Electrolytes Based on (PEO)4CF3COOLi and Multi-Walled Carbon Nanotube (MWCNT)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Techniques
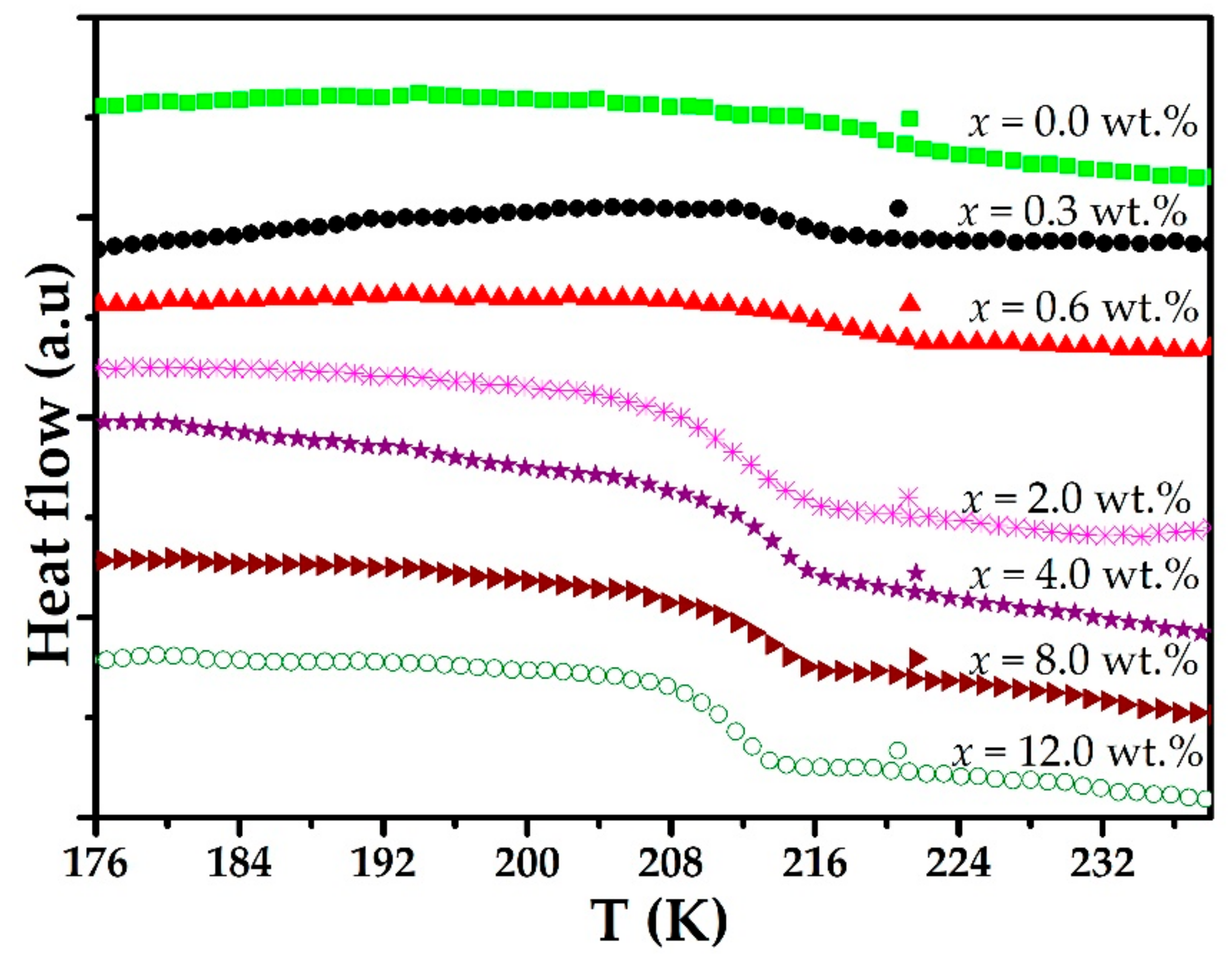
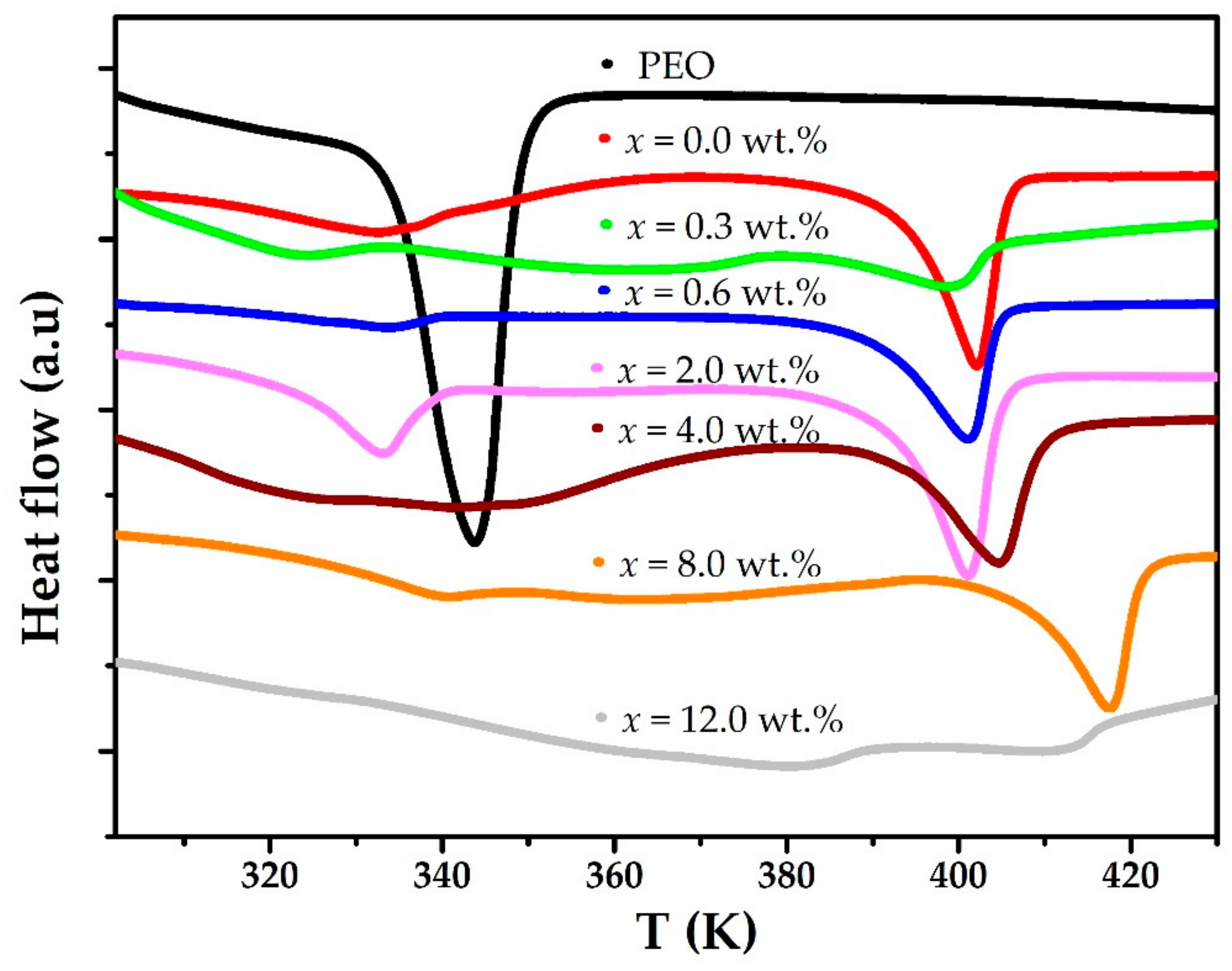
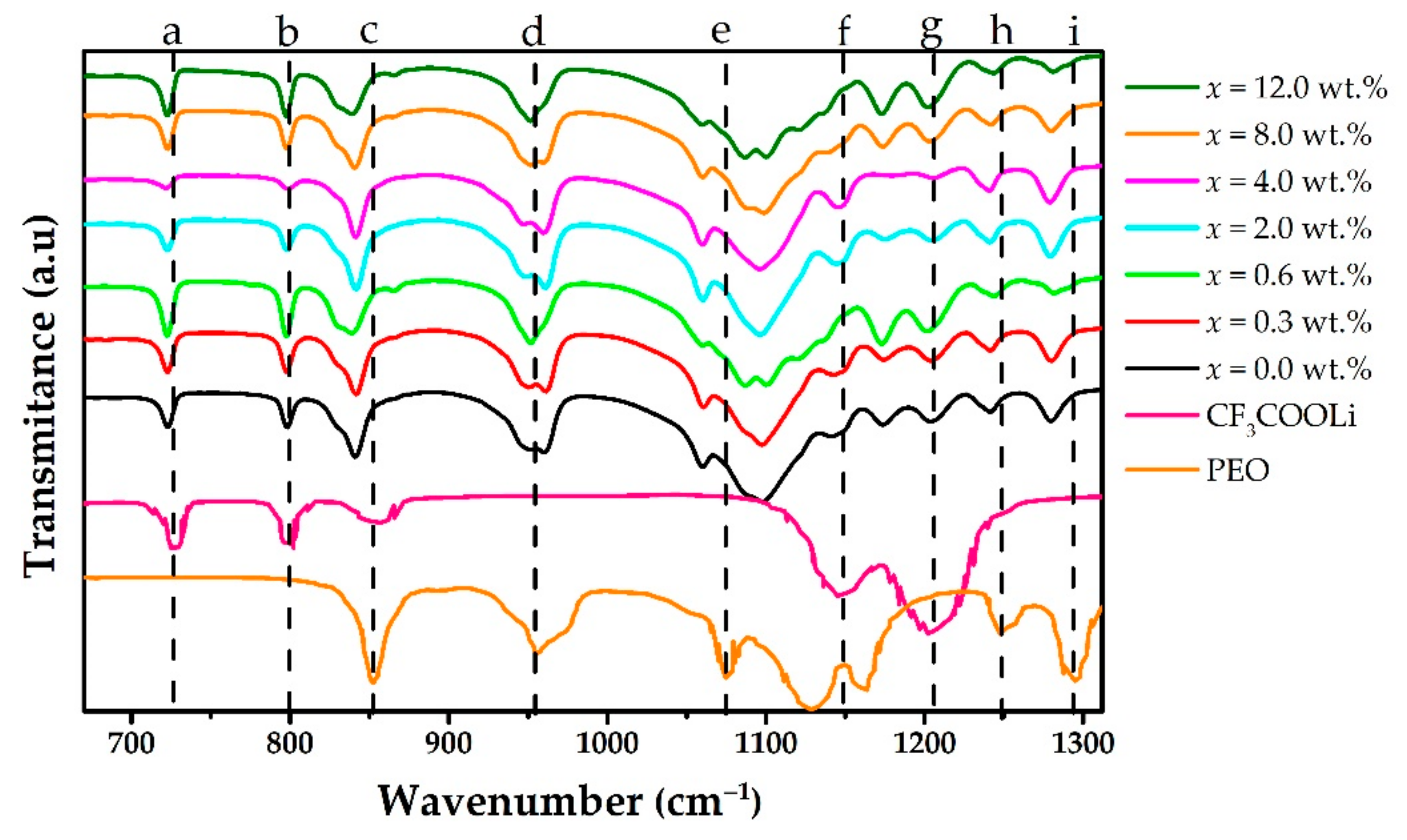
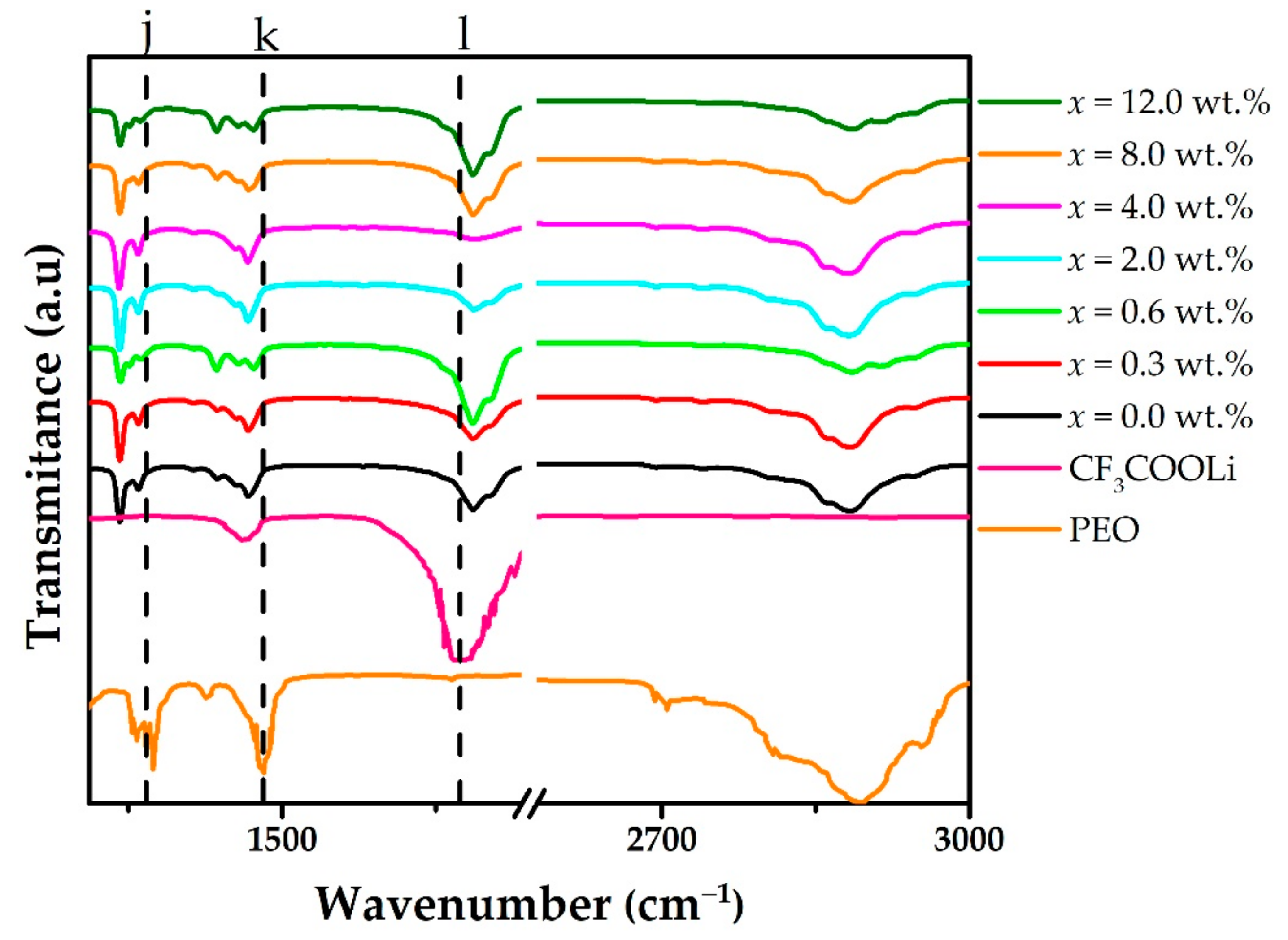
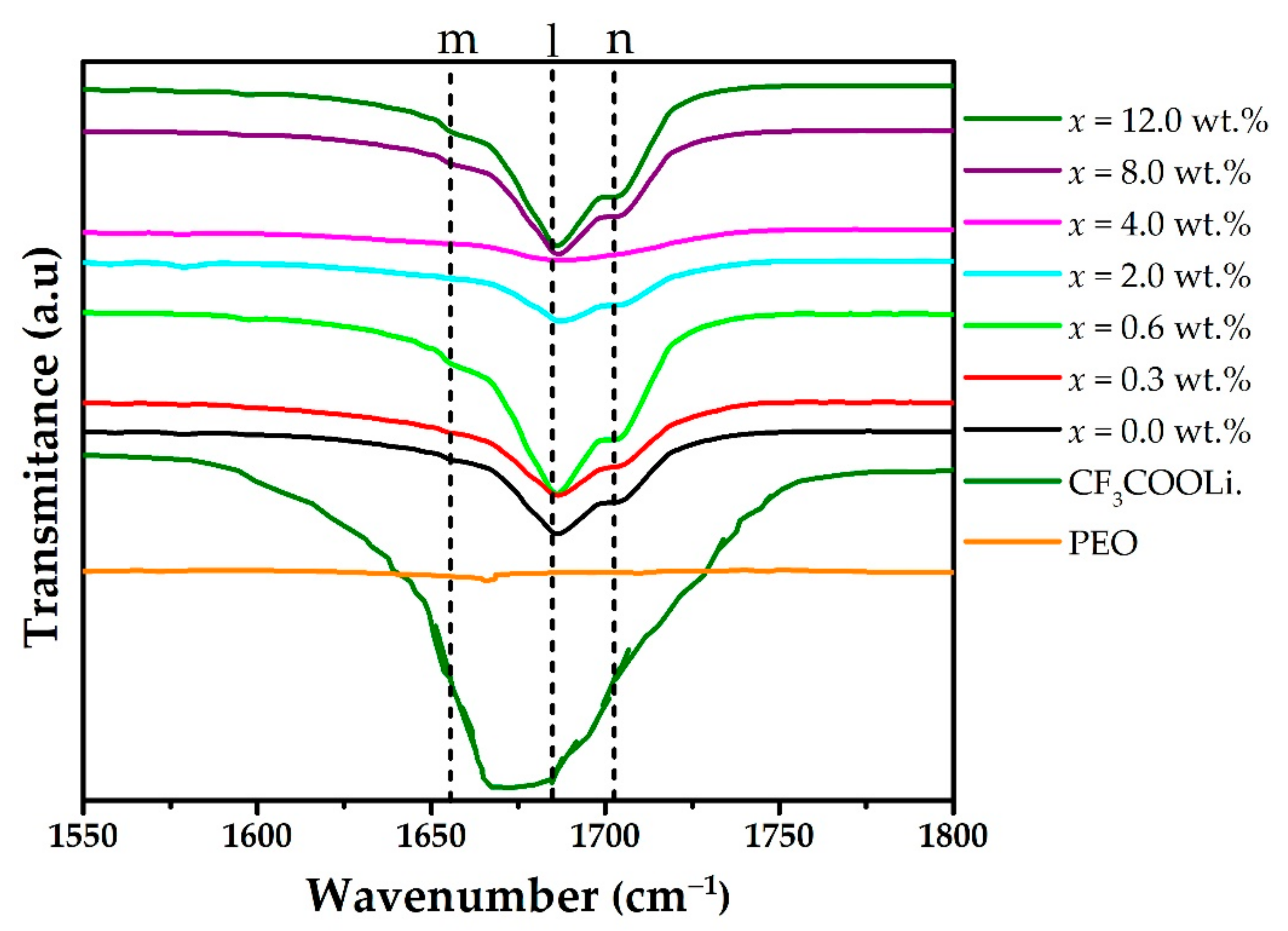
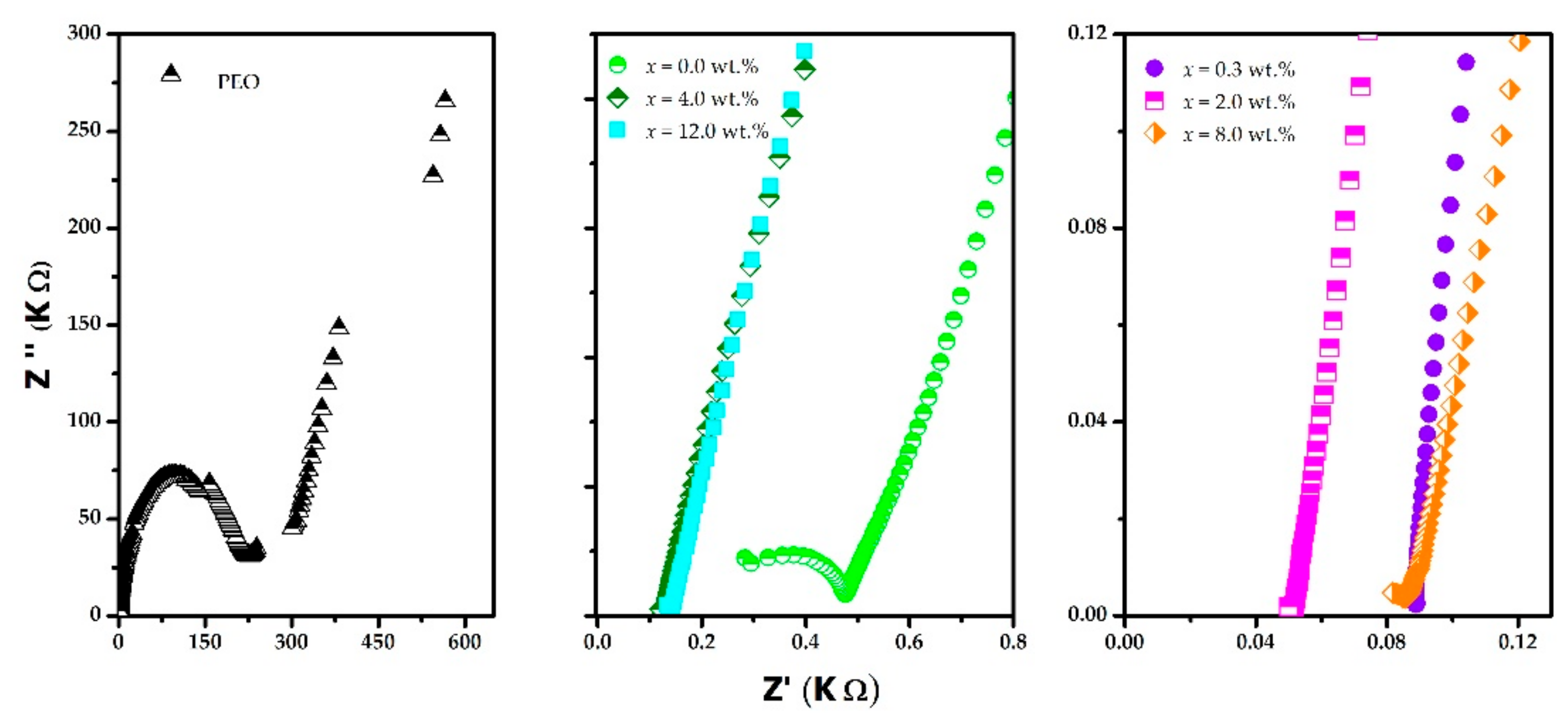
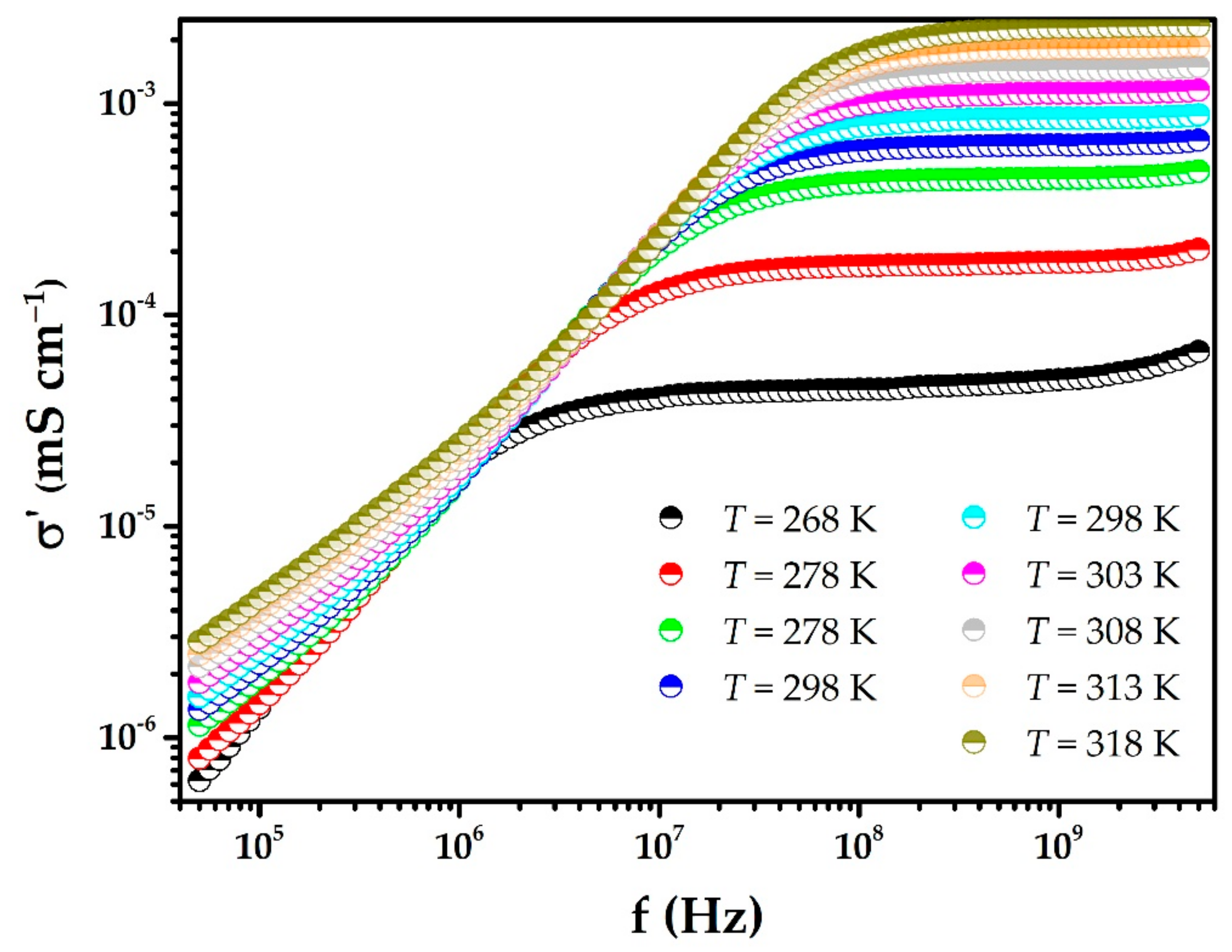
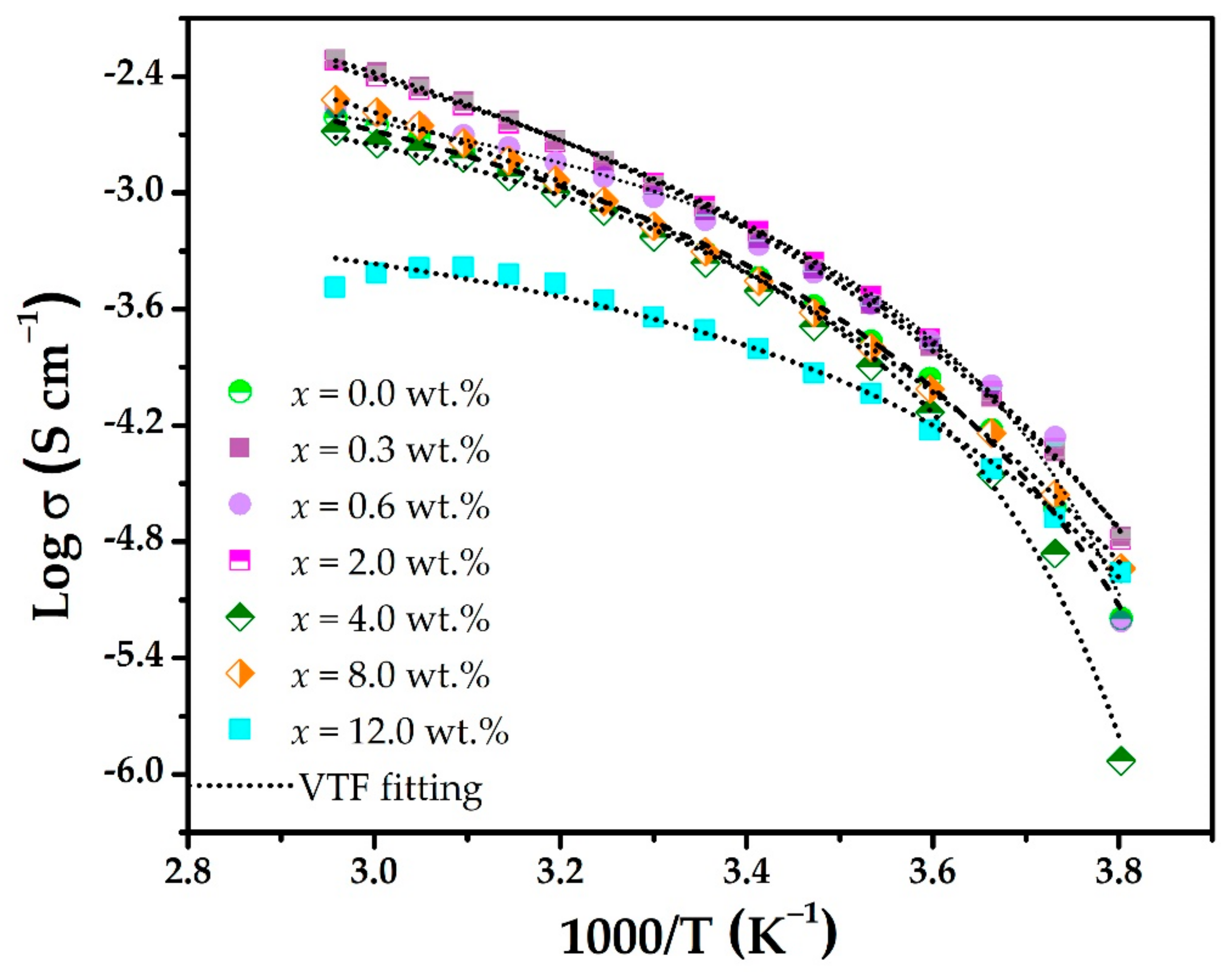
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.; Mouritz, A.P. Energy Storage Structural Composites with Integrated Lithium-Ion Batteries: A Review. Adv. Mater. Technol. 2021, 6, 2001059. [Google Scholar] [CrossRef]
- Meng, N.; Zhu, X.; Lian, F. Particles in composite polymer electrolyte for solid-state lithium batteries: A review. Particuology 2022, 60, 14–36. [Google Scholar] [CrossRef]
- Jurado Meneses, N.M.; Delgado Rosero, M.I.; Meléndez-Llira, M.A. Structural and vibrational studies on composites polymer electrolytes (PEO)10 CF3COONa + x wt.% Al2O3. Rev. Fac. Ing. Univ. Antioquia 2017, 83, 43–49. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Zhu, X.; Liu, S.; Wang, Y.; Xu, Y.; Zhou, S.; He, X.; Xue, Z. Composite polymer electrolytes reinforced by hollow silica nanotubes for lithium metal batteries. J. Memb. Sci. 2021, 618, 118697. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.M. Mechanical properties of linear low-density polyethylene fire-retarded with melamine polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano fibers as a Manufacturing Technology for corrosion protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and modeling analysis of mechanical-electrical behaviors of polypropylene composites filled with graphite and MWCNT fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Jang, D.; Yoon, H.N.; Nam, I.W.; Lee, H.K. Effect of carbonyl iron powder incorporation on the piezoresistive sensing characteristics of CNT-based polymeric sensor. Compos. Struct. 2020, 244, 112260. [Google Scholar] [CrossRef]
- Marcos, M.A.; Lugo, L.; Ageev, S.V.; Podolsky, N.E.; Cabaleiro, D.; Postnov, V.N.; Semenov, K.N. Influence of molecular mass of PEG on rheological behaviour of MWCNT-based nano fluids for thermal energy storage. J. Mol. Liq. 2020, 318, 113965. [Google Scholar] [CrossRef]
- Fabrication, N.; Matori, K.A.; Samikannu, K.; Sa, U. Biodegradable Poly (lactic acid)/Poly (ethylene glycol) Reinforced Multi-Walled Carbon Nanotube Nanocomposite Fabrication, Characterization, Properties, and Applications. Polymers 2020, 12, 427. [Google Scholar]
- Ahn, J.; Kim, Y.; Wang, G.X. Electrochemical Properties of Carbon Nanotube-Dispersed PEO-LiX Electrolytes. Met. Mater. Int. 2006, 12, 69–73. [Google Scholar] [CrossRef]
- Khalid, H.R.; Choudhry, I.; Jang, D.; Abbas, N.; Salman Haider, M.; Lee, H.K. Facile synthesis of sprayed cnts layer-embedded stretchable sensors with controllable sensitivity. Polymers 2021, 13, 311. [Google Scholar] [CrossRef]
- Khalid, H.R.; Jang, D.; Abbas, N.; Haider, M.S.; Bukhari, S.N.A.; Mirza, C.R.; Elboughdiri, N.; Ahmad, F. Electrical Stability and Piezoresistive Sensing Performance of High Strain-Range Ultra-Stretchable CNT-Embedded Sensors. Polymers 2022, 14, 1366. [Google Scholar] [CrossRef]
- Delgado Rosero, M.I.; Jurado Meneses, N.M.; Uribe Kaffure, R. Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa+x wt.% Al2O3. Materials 2019, 12, 1464. [Google Scholar] [CrossRef]
- Zygadło-Monikowska, E.; Florjańczyk, Z.; Ostrowska, J.; Tomaszewska, A.; Bołtromiuk, P.; Langwald, N.; Golodnitsky, D.; Peled, E. Synthesis and characterization of lithium-salt complexes with di fluoroalkoxyborates for application as lithium electrolytes. Electrochim. Acta 2015, 175, 104–112. [Google Scholar] [CrossRef]
- Ghorbanzade, P.; Loaiza, L.C.; Johansson, P. Plasticized and salt-doped single-ion conducting polymer electrolytes for lithium batteries. RSC Adv. 2022, 12, 18164–18167. [Google Scholar] [CrossRef]
- Salehan, S.S.; Nadirah, B.N.; Saheed, M.S.M.; Yahya, W.Z.N.; Shukur, M.F. Conductivity, structural and thermal properties of corn starch-lithium iodide nanocomposite polymer electrolyte incorporated with Al2O3. J. Polym. Res. 2021, 28, 222. [Google Scholar] [CrossRef]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Study of microplastics with semicrystalline and amorphous structure identification by TGA and DSC. J. Environ. Chem. Eng. 2022, 10, 106886. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Aït Hocine, N. Properties of poly(lactic acid)/montmorillonite/carbon nanotubes nanocomposites: Determination of percolation threshold. J. Mater. Sci. 2021, 56, 16887–16901. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, M.; Peng, Y.; Guan, S. Organic ionic plastic crystal enhanced interface compatibility of PEO-based solid polymer electrolytes for lithium-metal batteries. Solid State Ion. 2021, 373, 115806. [Google Scholar] [CrossRef]
- Ganta, K.K.; Jeedi, V.R.; Kumar, K.V.; Laxmi, E. Preparation, characterization and impedance spectroscopic studies of Na+ ion conducting PEO + PVDF-blended polymer electrolytes. Int. J. Polym. Anal. Charact. 2021, 26, 130–144. [Google Scholar] [CrossRef]
- Delgado, I.; Chacón, M.; Vargas, R.A. Thermal and transport properties of the polymer electrolyte based on poly (vinyl alcohol)-LiOH-H2O. Phys. Status Solidi. 2005, 3805, 3802–3805. [Google Scholar] [CrossRef]
- Delgado, M.I.; Jurado, N.M.; Vargas, R.A. Phase diagram of polymer electrolyte: (x)(PEO)–(1-x)CF3COOLi. Rev. Fac. Ing. Univ. Antioquia 2012, 62, 77–82. [Google Scholar]
- Christe, K.O.; Naumann, D. Vibrational spectra of trifluoroacetates, Spectrochim. Acta Part A Mol. Spectrosc. 1973, 29, 2017–2024. [Google Scholar] [CrossRef]
- Beckers, H.; Bürger, H.; Eujen, R.; Rempfer, B.; Oberhammer, H. Vibrational spectra, normal coordinate analysis and electron diffraction investigation of CF3SiH3 and its deuterated varieties. J. Mol. Struct. 1986, 140, 281–301. [Google Scholar] [CrossRef]
- Boudreau, P.A.; Hooper, R.J. An Ir spectroscopic study of metal chelates of Dl-Isovaline. J. Inorg. Nucl. Chem. 1977, 39, 1247–1251. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Li, W. Evolution in electrochemical performance of the solid blend polymer electrolyte (PEO/PVDF) with the content of ZnO nanofiller, Colloids Surfaces A Physicochem. Eng. Asp. 2022, 632, 127773. [Google Scholar] [CrossRef]
- Regis, A.; Corset, J. IR study of solutions of lithium trifluoroacetate in non-aqueus solvents. Structure of ions pairs and triple ions. Chem. Phys. Lett. 1975, 32, 462–465. [Google Scholar] [CrossRef]
- Bucheli, W.; Jiménez, R.; Sanz, J.; Várez, A. The log(σ) vs. log(ω) derivative plot used to analyze the ac conductivity. Application to fast Li + ion conductors with perovskite structure. Solid State Ion. 2012, 227, 113–118. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, N.; Mishra, K.; Shahid, R.; Arif, T.; Kanchan, D.K. Sodium ion conducting flame-retardant gel polymer electrolyte for sodium batteries and electric double layer capacitors (EDLCs). J. Energy Storage 2022, 46, 103899. [Google Scholar] [CrossRef]
| Property | Value |
|---|---|
| Molecular weight average | 5,000,000 |
| Melting point | 65 °C |
| Glass transition | −65 °C |
| Density | 1.21 g/cm3 |
| Property | Value |
|---|---|
| Molecular weight | 119.96 g/mol |
| Purity | 95% |
| Melting point | 250 °C |
| Density | 1.743 g/cm 3 |
| Property | Value |
|---|---|
| Density | 2.1 g/cm3 |
| Outer diameter | 7–15 nm |
| Length | 0.5–10 μm |
| Melting point | 3652–3697 °C |
| Concentration (wt.%) | Glass Transition Temperature Tg (K) |
|---|---|
| 0.0 | 219.5 |
| 0.3 | 215.2 |
| 0.6 | 214.4 |
| 2.0 | 210.7 |
| 4.0 | 212.7 |
| 8.0 | 213.5 |
| 12.0 | 211.3 |
| x% MWCNT | 1st Anomaly | 2nd Anomaly | ||
|---|---|---|---|---|
| ∆H (J/g) | Tm (K) | ∆H (J/g) | Tm (K) | |
| PEO | 134.9 | 344.0 | - | - |
| 0.0 | 44.8 | 336.2 | 84.5 | 402.1 |
| 0.3 | 25.9 | 334.0 | 16.8 | 399.0 |
| 0.6 | 20.6 | 333.6 | 67.2 | 401.1 |
| 2.0 | 18.2 | 332.9 | 64.7 | 401.1 |
| 4.0 | 92.6 | 341.1 | 49.4 | 404.7 |
| 8.0 | 4.8 | 350.2 | 48.0 | 403.4 |
| 12.0 | 10.4 | 367.2 | 4.6 | 397.6 |
| PEO4CF3COOLi + x wt.% | σ0 (S cm−1) | EA (eV) | T0 (K) |
|---|---|---|---|
| x = 0.0% | 2.68 × 10−2 | 2.24 × 10−2 | 231.41 |
| x = 0.3% | 1.91 × 10−1 | 3.94 × 10−2 | 213.56 |
| x = 0.6% | 1.17 × 10−2 | 1.27 × 10−2 | 242.88 |
| x = 2.0% | 1.02 × 10−1 | 3.17 × 10−2 | 220.57 |
| x = 4.0% | 1.25 × 10−2 | 1.52 × 10−2 | 243.58 |
| x = 8.0% | 1.57 × 10−1 | 4.38 × 10−2 | 209.30 |
| x = 12.0% | 1.82 × 10−3 | 1.21 × 10−2 | 235.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Rosero, M.I.; Jurado-Meneses, N.M.; Uribe-Kaffure, R. Composite Polymer Electrolytes Based on (PEO)4CF3COOLi and Multi-Walled Carbon Nanotube (MWCNT). Polymers 2023, 15, 49. https://doi.org/10.3390/polym15010049
Delgado-Rosero MI, Jurado-Meneses NM, Uribe-Kaffure R. Composite Polymer Electrolytes Based on (PEO)4CF3COOLi and Multi-Walled Carbon Nanotube (MWCNT). Polymers. 2023; 15(1):49. https://doi.org/10.3390/polym15010049
Chicago/Turabian StyleDelgado-Rosero, Miguel I., Nori M. Jurado-Meneses, and Ramiro Uribe-Kaffure. 2023. "Composite Polymer Electrolytes Based on (PEO)4CF3COOLi and Multi-Walled Carbon Nanotube (MWCNT)" Polymers 15, no. 1: 49. https://doi.org/10.3390/polym15010049
APA StyleDelgado-Rosero, M. I., Jurado-Meneses, N. M., & Uribe-Kaffure, R. (2023). Composite Polymer Electrolytes Based on (PEO)4CF3COOLi and Multi-Walled Carbon Nanotube (MWCNT). Polymers, 15(1), 49. https://doi.org/10.3390/polym15010049











