Phthalocyanines and Porphyrins/Polyaniline Composites (PANI/CuPctBu and PANI/TPPH2) as Sensing Materials for Ammonia Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Solvents
2.2. Materials Preparation
2.2.1. PANI Synthesis
2.2.2. PANI/MCs Nanostructured Composite Preparation
2.3. Characterization Methods
2.3.1. Sensor Devices and Sensor Preparation
2.3.2. Gas Sensing Experiments
3. Results
3.1. Materials Characterization
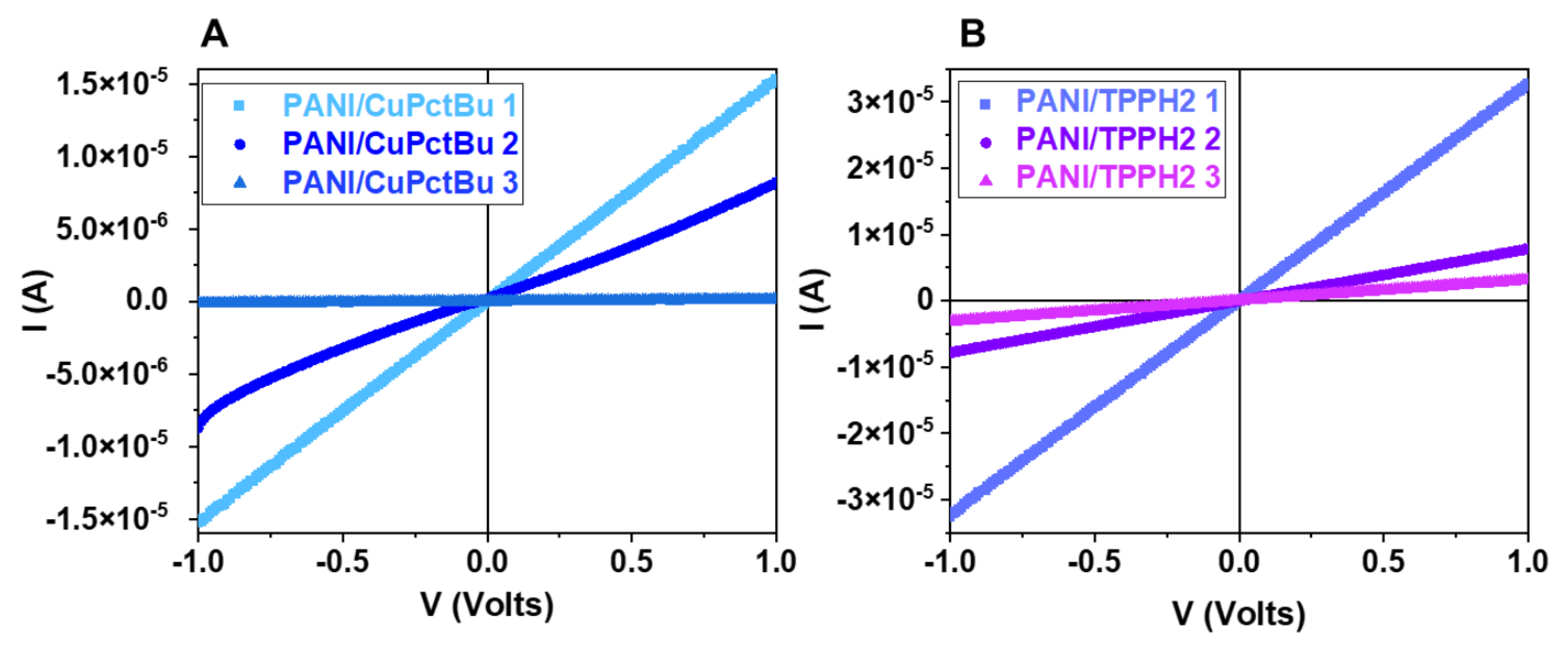
3.1.1. Effect of the Functionalization on the Electrical Characteristics
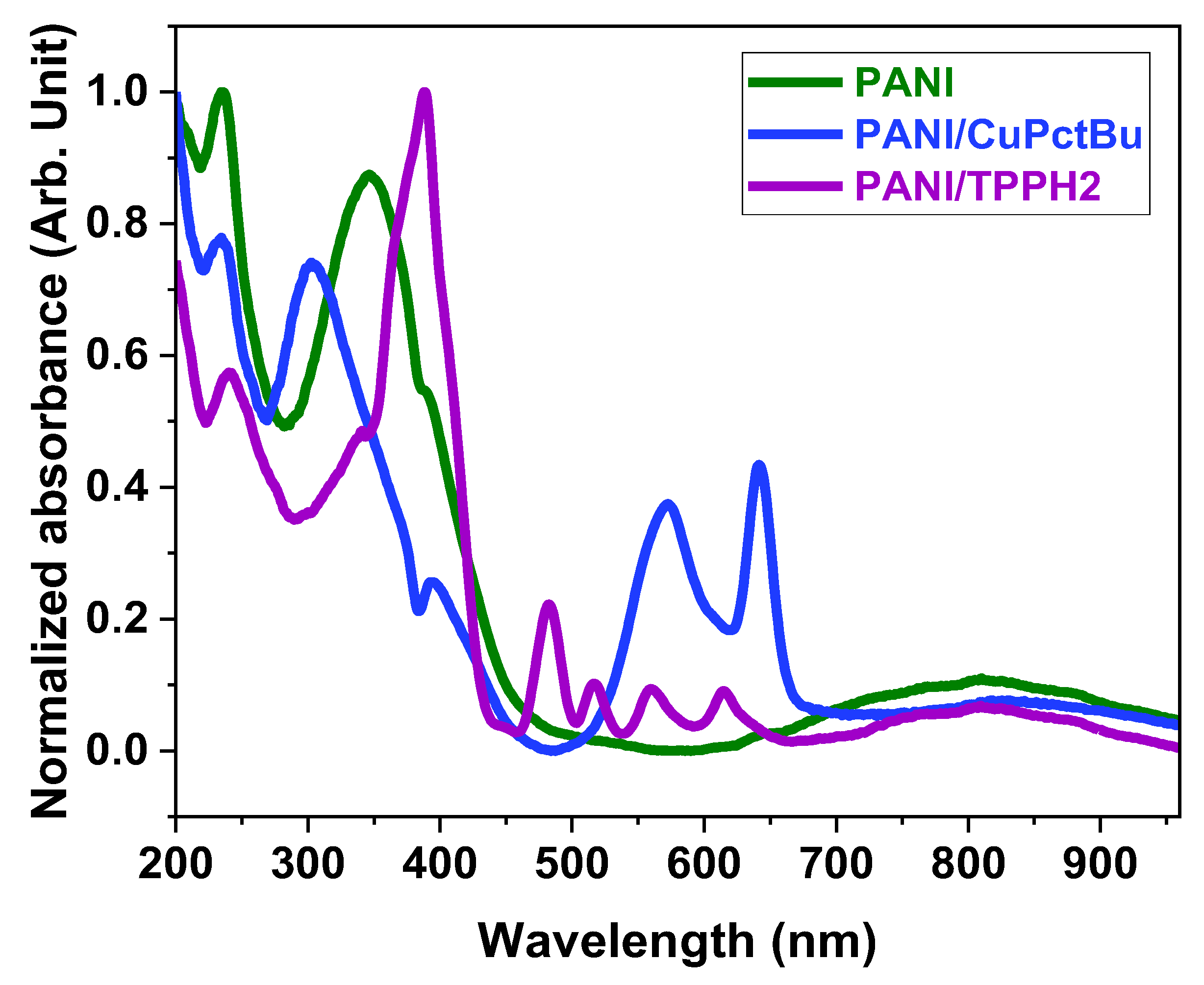
3.1.2. UV-Vis Spectroscopy Characterization
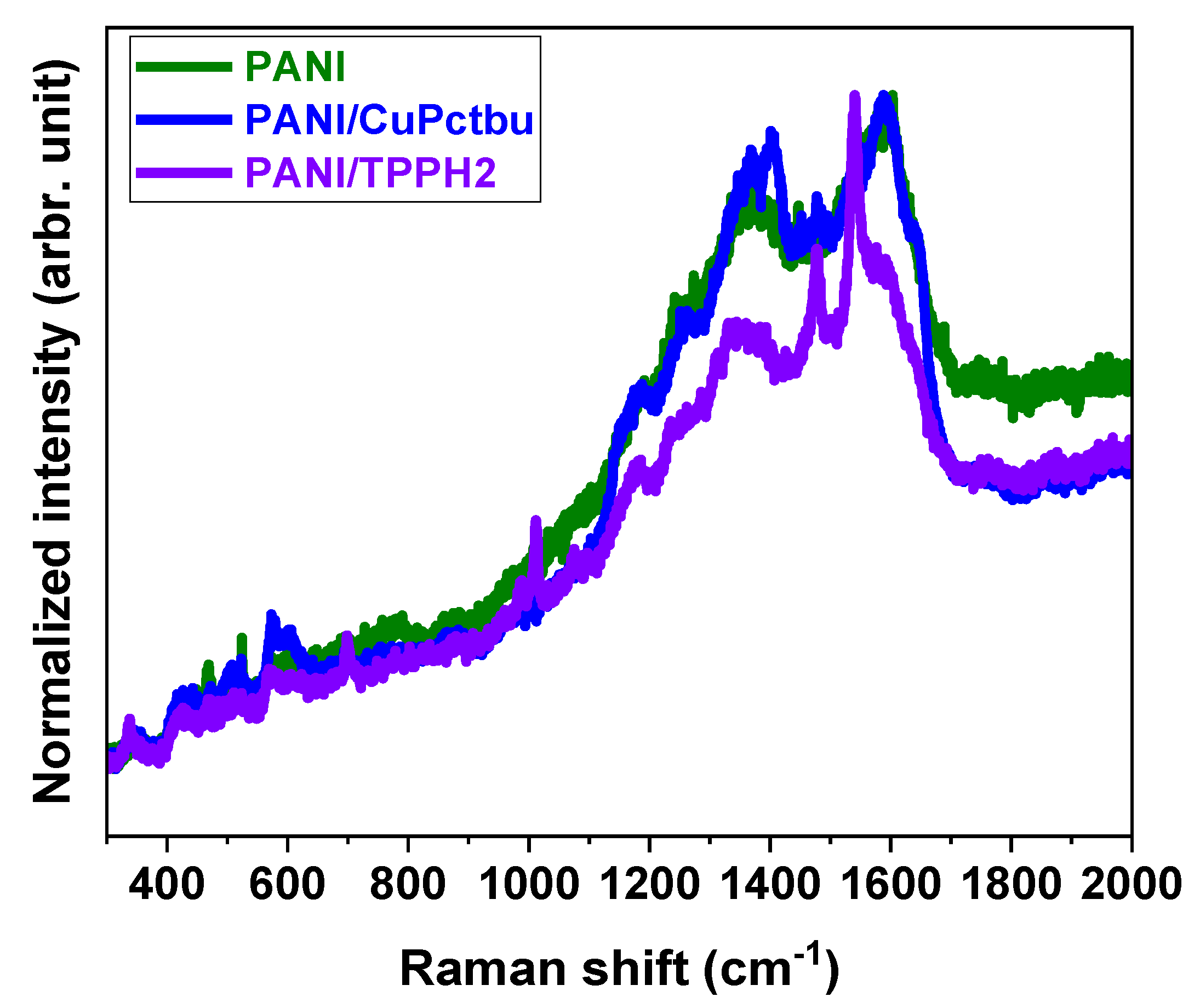
3.1.3. Raman Characterization
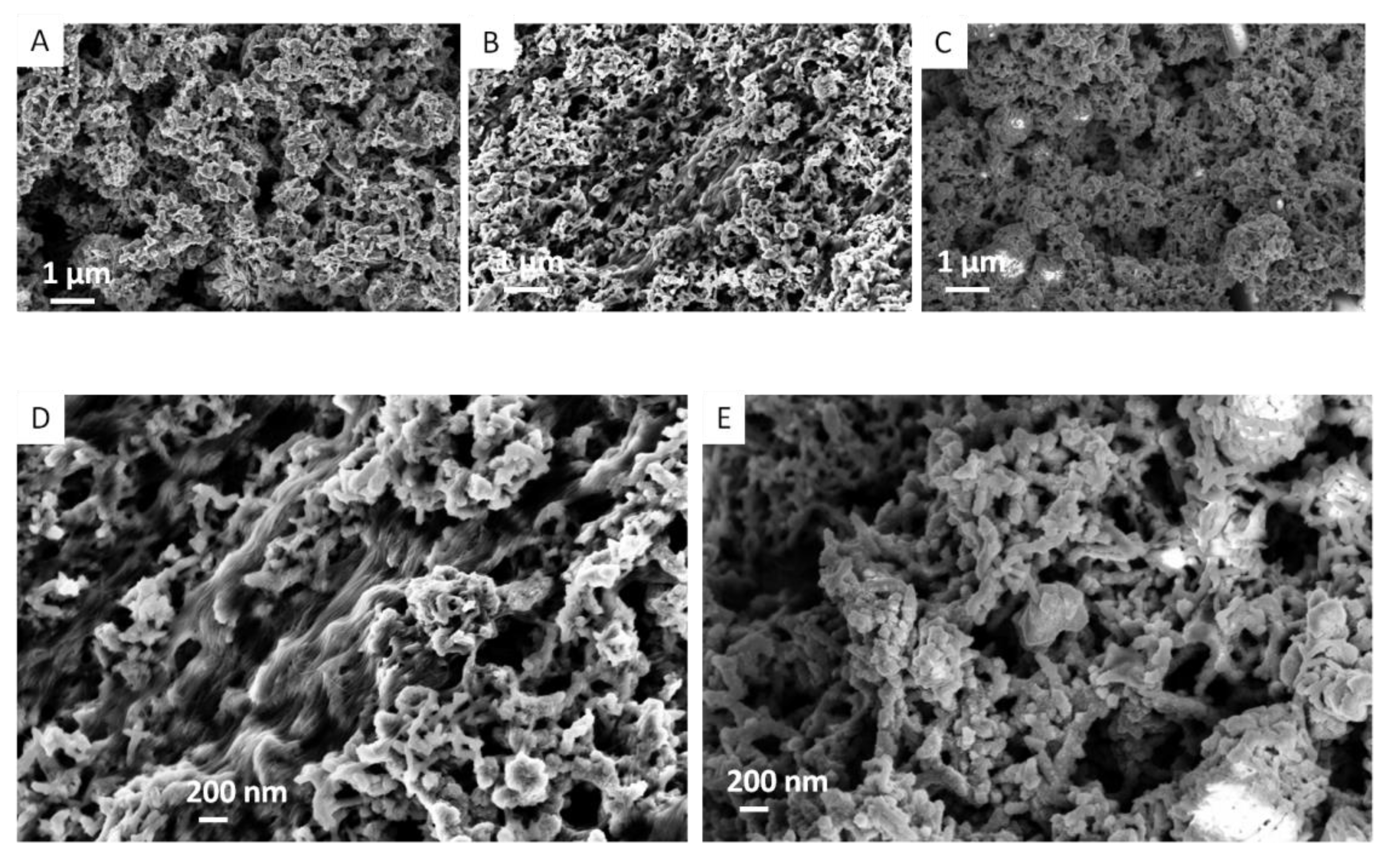
3.1.4. Scanning Electron Microscopy (SEM) Characterization
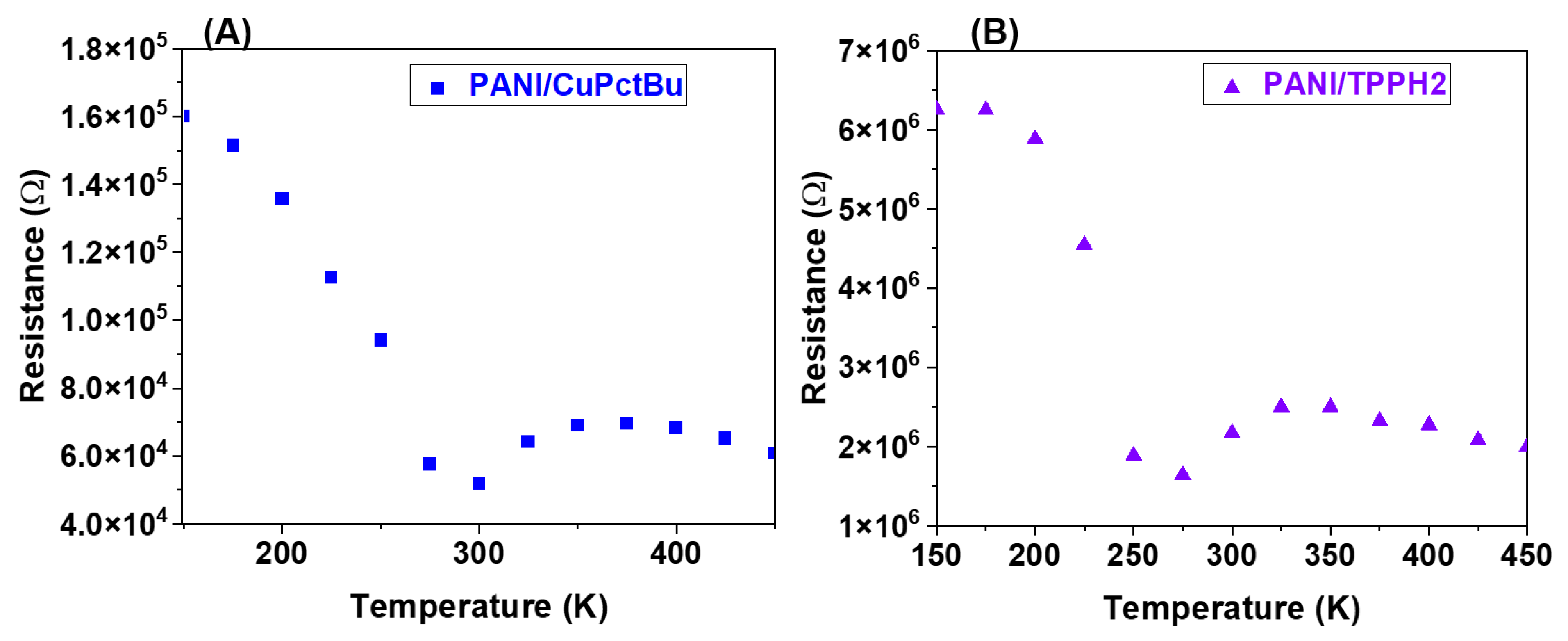
3.1.5. Electrical Characterization
3.2. Gas Sensing Performance
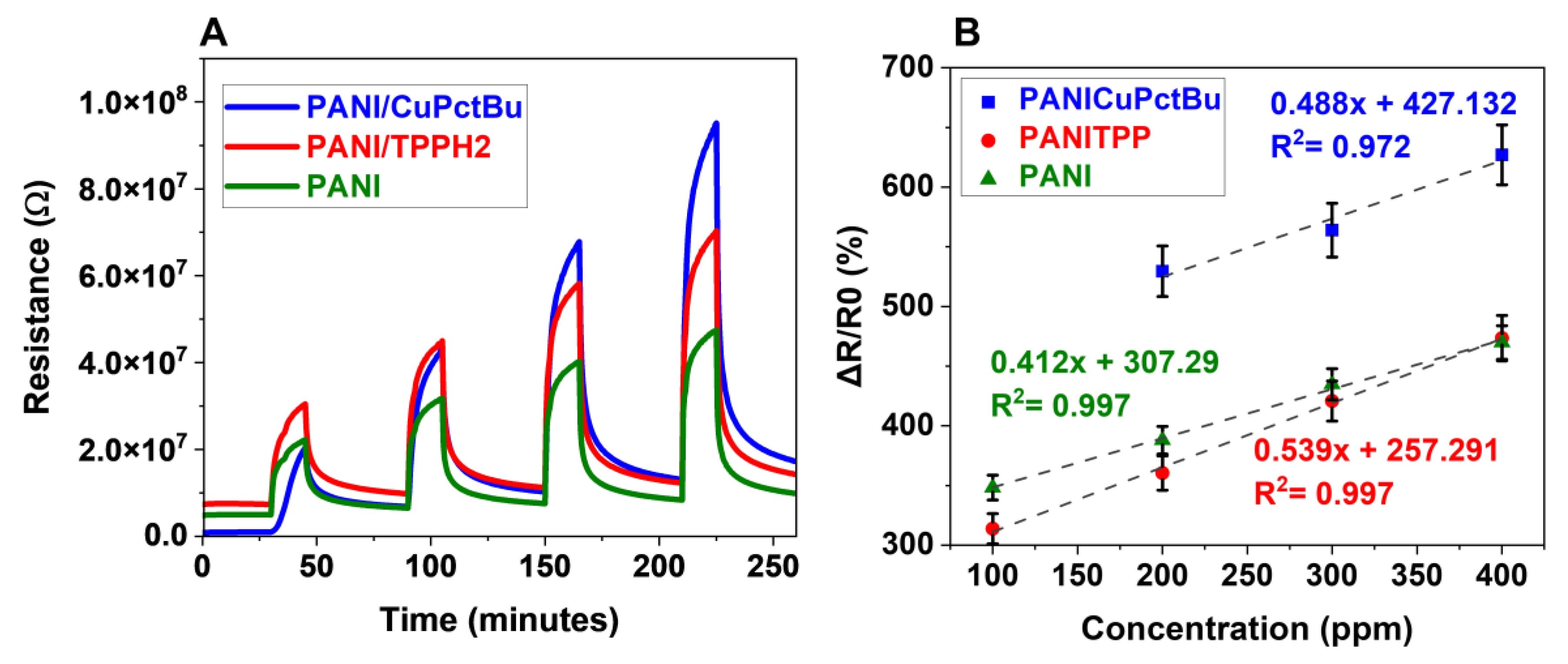
3.2.1. Typical Sensor Response toward Ammonia Exposure
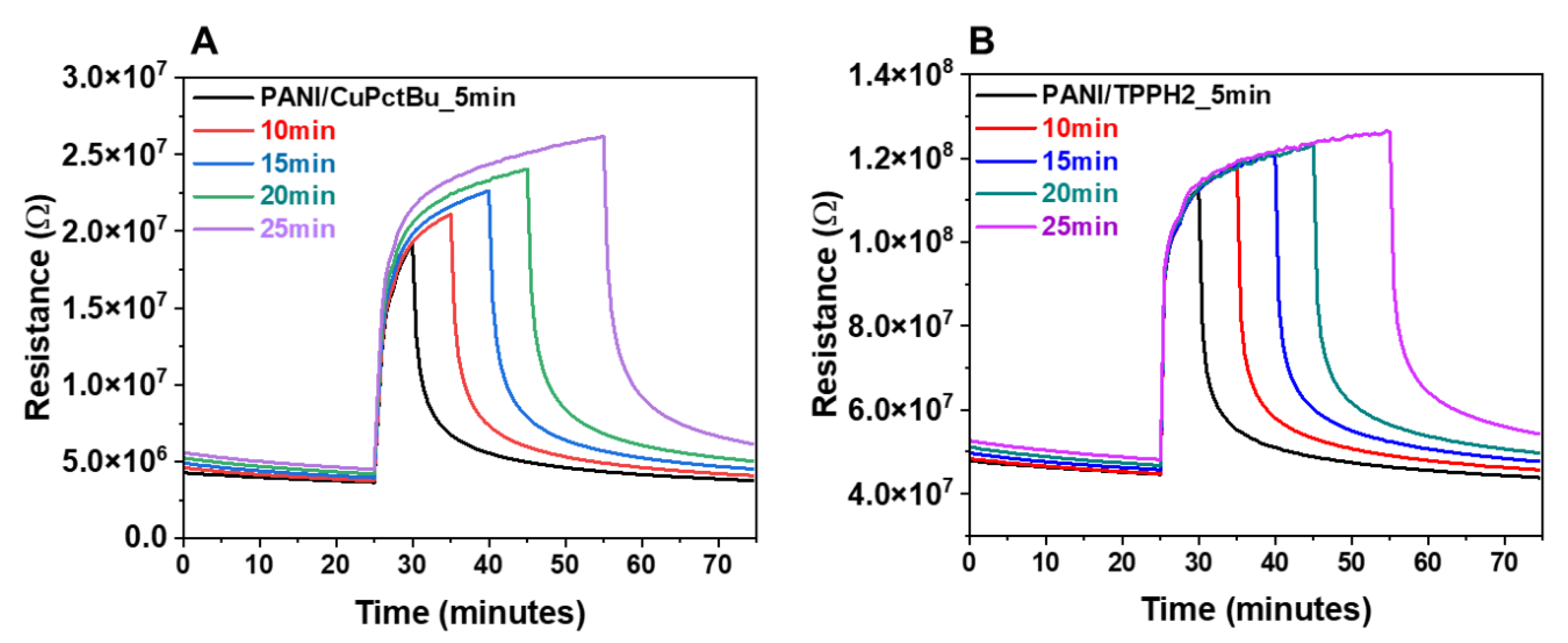
3.2.2. Sensing Performances toward Ammonia Exposure: Effect of the Exposure Time
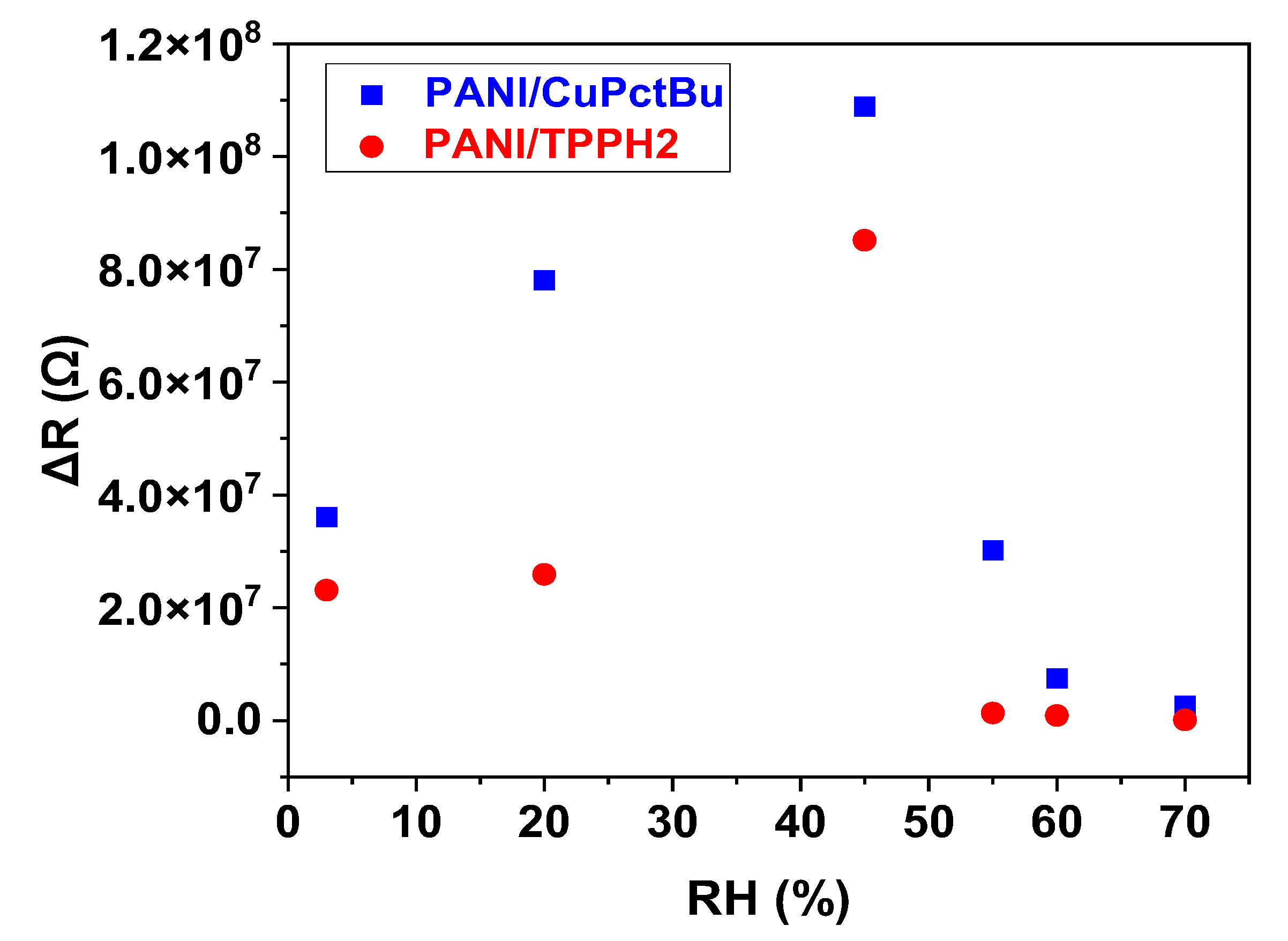
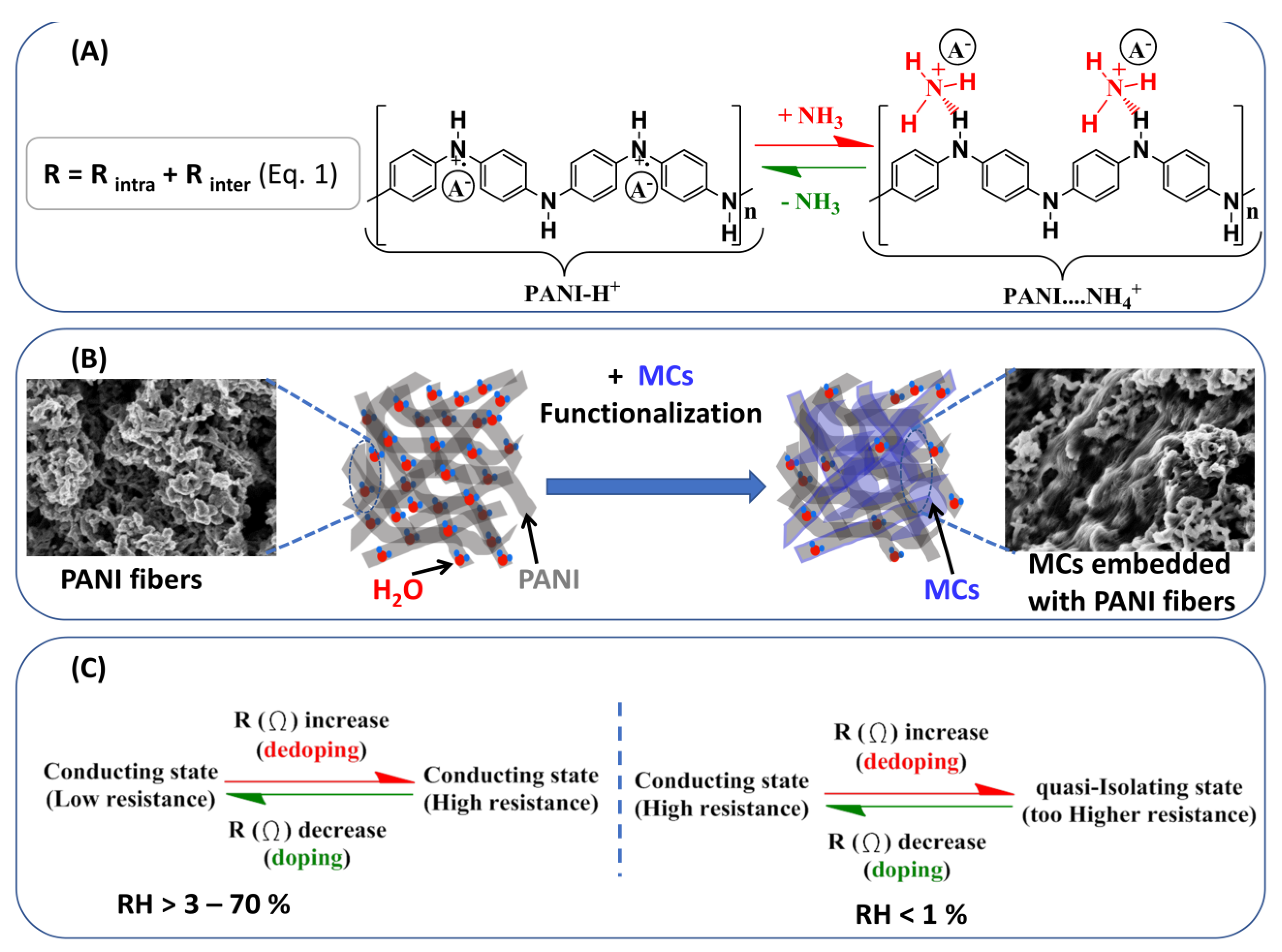
3.2.3. Sensing Performances toward Ammonia Exposure: Effect of Humidity
3.2.4. Sensor Behavior at Lower Humidity Level: On/Off State
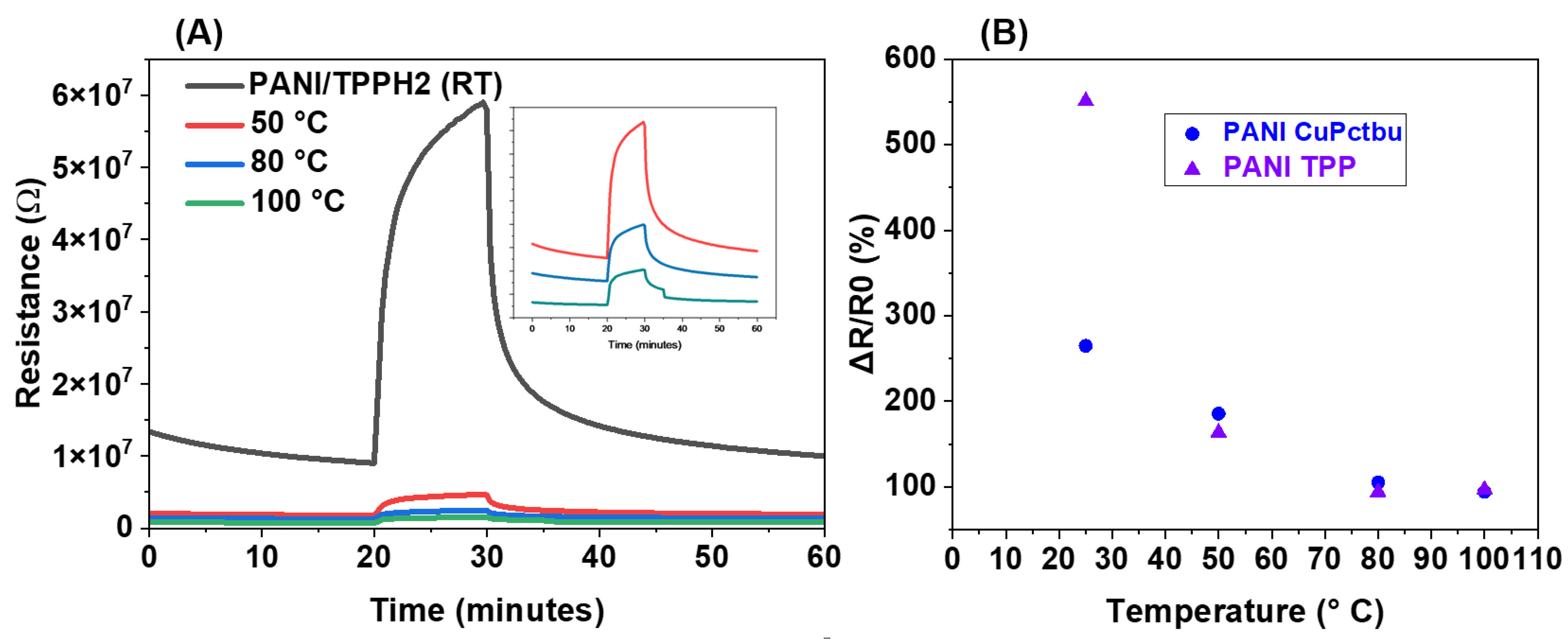
3.2.5. Sensor Behavior at Different Operating Temperatures
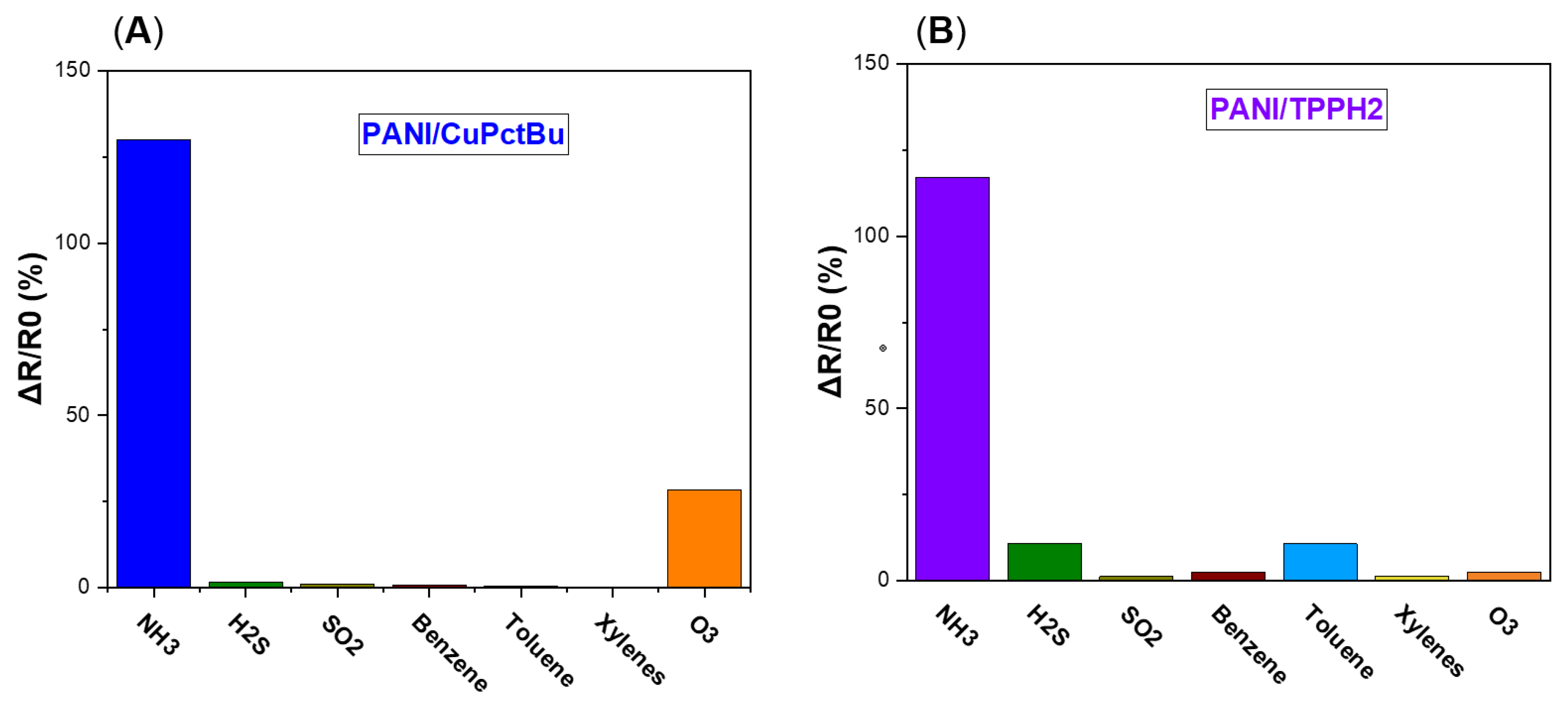
3.2.6. Cross-Sensitivity Experiment: Effect of the Interfering Gases
3.2.7. Sensing Performances toward Ammonia: Repeatability on Exposure Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trung, D.D.; Cuong, N.D.; Trung, K.Q.; Nguyen, T.-D.; Van Toan, N.; Hung, C.M.; Hieu, N.V. Controlled synthesis of manganese tungstate nanorods for highly selective nh3 gas sensor. J. Alloys Compd. 2018, 735, 787–794. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive room-temperature h2s gas sensors employing sno2 quantum wire/reduced graphene oxide nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/polyaniline nanocomposite for hydrogen sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Patni, N.; Jain, N.; Pillai, S.G. Polyaniline-based sensors for monitoring and detection of ammonia and carbon monoxide gases. In Trends and Applications in Advanced Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 145–162. [Google Scholar]
- Bengasi, G.; Meunier-Prest, R.; Baba, K.; Kumar, A.; Pellegrino, A.L.; Boscher, N.D.; Bouvet, M. Molecular engineering of porphyrin-tapes/phthalocyanine heterojunctions for a highly sensitive ammonia sensor. Adv. Electron. Mater. 2020, 6, 2000812. [Google Scholar] [CrossRef]
- Lobotka, P.; Kunzo, P.; Kovacova, E.; Vavra, I.; Krizanova, Z.; Smatko, V.; Stejskal, J.; Konyushenko, E.N.; Omastova, M.; Spitalsky, Z.; et al. Thin polyaniline and polyaniline/carbon nanocomposite films for gas sensing. Thin Solid Films 2011, 519, 4123–4127. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile fabrication of polyaniline/multi-walled carbon nanotubes/molybdenum disulfide ternary nanocomposite and its high-performance ammonia-sensing at room temperature. Sens. Actuators B Chem. 2018, 258, 895–905. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Das, S. Investigation on energy storage and conversion properties of multifunctional PANI-MWCNT composite. Int. J. Hydrogen Energy 2018, 43, 7128–7139. [Google Scholar] [CrossRef]
- Nagaraju, S.C.; Roy, A.S.; Kumar, J.B.P.; Anilkumar, K.R.; Ramagopal, G. Humidity sensing properties of surface modified polyaniline metal oxide composites. J. Eng. 2014, 2014, 925020. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Sun, P.; Liang, X.; Liu, F.; Yan, X.; et al. The room temperature gas sensor based on polyaniline@flower-like wo3 nanocomposites and flexible pet substrate for nh3 detection. Sens. Actuators B Chem. 2018, 259, 505–513. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, X.; Dong, X.; Xu, Y.M. Enhanced sub-ppm nh(3) gas sensing performance of pani/tio(2) nanocomposites at room temperature. Front. Chem. 2018, 6, 493. [Google Scholar] [CrossRef]
- Bai, S.; Tian, Y.; Cui, M.; Sun, J.; Tian, Y.; Luo, R.; Chen, A.; Li, D. Polyaniline@SnO2 heterojunction loading on flexible pet thin film for detection of nh3 at room temperature. Sens. Actuators B Chem. 2016, 226, 540–547. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Agbor, N.E.; Petty, M.C.; Monkman, A.P. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]
- Ouyang, M.; Hu, X.; Shao, X.; Chen, L.; Li, W.; Bai, R.; Zhang, L.; Lv, X.; Tameev, A.; Zhang, C. In situ preparation and determination of electrochemical and electrochromic properties of copper phthalocyanine-polyaniline nanocomposite films. RSC Adv. 2019, 9, 34382–34388. [Google Scholar] [CrossRef]
- Yunfen, S.; Yang, Z.; Ying, X. The synthesis and characterization of pani—Cupc organic semiconductor hybrid material. Energy Procedia 2012, 17, 1550–1554. [Google Scholar] [CrossRef][Green Version]
- Pakapongpan, S.; Mensing, J.P.; Lomas, T.; Tuantranont, A. Electrochemical sensor for ascorbic acid based on graphene/cupc/pani nanocomposites. In Proceedings of the 2012 IEEE International Conference on Electron Devices and Solid State Circuit (EDSSC), Bangkok, Thailand, 3–5 December 2012; pp. 1–3. [Google Scholar]
- Radhakrishnan, S.; Deshpande, S.D. Conducting polymers functionalized with phthalocyanine as nitrogen dioxide sensors. Sensors 2002, 2, 185–194. [Google Scholar] [CrossRef]
- Gorduk, O.; Gencten, M.; Gorduk, S.; Sahin, M.; Sahin, Y. Electrochemical fabrication and supercapacitor performances of metallo phthalocyanine/functionalized-multiwalled carbon nanotube/polyaniline modified hybrid electrode materials. J. Energy Storage 2021, 33, 102049. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Kotresh, H.M.N.; Devendrachari, M.C.; Khan, F. Synthesis and electrochemical investigation of tetra amino cobalt (ii) phthalocyanine functionalized polyaniline nanofiber for the selective detection of dopamine. Electroanalysis 2020, 32, 1807–1817. [Google Scholar] [CrossRef]
- Khalid, M.; Acuña, J.J.S.; Tumelero, M.A.; Fischer, J.A.; Zoldan, V.C.; Pasa, A.A. Sulfonated porphyrin doped polyaniline nanotubes and nanofibers: Synthesis and characterization. J. Mater. Chem. 2012, 22, 11340–11346. [Google Scholar] [CrossRef]
- Sinha, M.; Verma, P.; Panda, S. Metal-phthalocyanine modified doped polyaniline for voc sensing applications. Flex. Print. Electron. 2020, 5, 014014. [Google Scholar] [CrossRef]
- Ndiaye, A.; Bonnet, P.; Pauly, A.; Dubois, M.; Brunet, J.; Varenne, C.; Guerin, K.; Lauron, B. Noncovalent functionalization of single-wall carbon nanotubes for the elaboration of gas sensor dedicated to btx type gases: The case of toluene. J. Phys. Chem. C 2013, 117, 20217–20228. [Google Scholar] [CrossRef]
- Saad Ali, S.; Pauly, A.; Brunet, J.; Varenne, C.; Ndiaye, A.L. Mwcnts/pmma/ps composites functionalized pani: Electrical characterization and sensing performance for ammonia detection in a humid environment. Sens. Actuators B Chem. 2020, 320, 128364. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, S.Y.; Hwang, J.Y.; Choi, H.J. Synthesis and electrical properties of polymer composites with polyaniline nanoparticles. Mater. Sci. Eng. C 2004, 24, 15–18. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Šeděnková, I.; Stejskal, J. Spectroscopy of thin polyaniline films deposited during chemical oxidation of aniline. Chem. Pap. 2012, 66, 415–445. [Google Scholar] [CrossRef]
- Souto, J.; de Saja, J.A.; Gobernado-Mitre, M.I.; Rodriguez, M.L.; Aroca, R. Nox gas detection with langmuir-blodgett monolayers of tetra-tert-butyl phthalocyanine complexes. Sens. Actuators B Chem. 1993, 16, 306–311. [Google Scholar] [CrossRef]
- Basova, T.V.; Kiselev, V.G.; Schuster, B.-E.; Peisert, H.; Chassé, T. Experimental and theoretical investigation of vibrational spectra of copper phthalocyanine: Polarized single-crystal raman spectra, isotope effect and dft calculations. J. Raman Spectrosc. 2009, 40, 2080–2087. [Google Scholar] [CrossRef]
- Xu, L.-C.; Li, Z.-Y.; Tan, W.; He, T.-J.; Liu, F.-C.; Chen, D.-M. Density functional theory studies on the raman and ir spectra of meso-tetraphenylporphyrin diacid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 850–862. [Google Scholar] [CrossRef]
- Aydin, M. Dft and raman spectroscopy of porphyrin derivatives: Tetraphenylporphine (tpp). Vib. Spectrosc. 2013, 68, 141–152. [Google Scholar] [CrossRef]
- Kemp, N.T.; Fianagan, G.U.; Kaiser, A.B.; Trodahl, H.J.; Chapman, B.; Partridge, A.C.; Buckley, R.G. Temperature-dependent conductivity of conducting polymers exposed to gases. Synth. Met. 1999, 101, 434–435. [Google Scholar] [CrossRef]
- Long, Y.; Chen, Z.; Wang, N.; Li, J.; Wan, M. Electronic transport in pani-csa/pani-dbsa polyblends. Phys. B Condens. Matter 2004, 344, 82–87. [Google Scholar] [CrossRef]
- Kaiser, A.B. Systematic conductivity behavior in conducting polymers: Effects of heterogeneous disorder. Adv. Mater. 2001, 13, 927–941. [Google Scholar] [CrossRef]
- Zhihua, L.; Xucheng, Z.; Jiyong, S.; Xiaobo, Z.; Xiaowei, H.; Tahir, H.E.; Holmes, M. Fast response ammonia sensor based on porous thin film of polyaniline/sulfonated nickel phthalocyanine composites. Sens. Actuators B Chem. 2016, 226, 553–562. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, Q.; Jiang, B.; Huang, Y. Design of the novel polyaniline/polysiloxane flexible nanocomposite film and its application in gas sensor. Compos. Part B Eng. 2020, 196, 108131. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast nh3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Pang, Z.; Yildirim, E.; Pasquinelli, M.A.; Wei, Q. Ammonia sensing performance of polyaniline-coated polyamide 6 nanofibers. ACS Omega 2021, 6, 8950–8957. [Google Scholar] [CrossRef]
- Cai, S.; Chen, L.; Zhang, J.; Ke, Y.; Fu, X.; Yang, H.; Li, H.; Long, Y.; Liu, X. Facile fabrication of pani/zn-tpps4 flexible nh3 sensor based on the “bridge” of Zn-tpps4. Sens. Actuators B Chem. 2020, 321, 128476. [Google Scholar] [CrossRef]
- Hadano, F.S.; Gavim, A.E.X.; Stefanelo, J.C.; Gusso, S.L.; Macedo, A.G.; Rodrigues, P.C.; Mohd Yusoff, A.R.b.; Schneider, F.K.; Deus, J.F.d.; José da Silva, W. Nh3 sensor based on rgo-pani composite with improved sensitivity. Sensors 2021, 21, 4947. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-performance pani-based ammonia gas sensor promoted by surface nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007. [Google Scholar] [CrossRef]
- Rannou, P.; Nechtschein, M. Aging studies on polyaniline: Conductivity and thermal stability. Synth. Met. 1997, 84, 755–756. [Google Scholar] [CrossRef]
- Alix, A.; Lemoine, V.; Nechtschein, M.; Travers, J.P.; Menardo, C. Water absorption study in polyaniline. Synth. Met. 1989, 29, 457–462. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Okamoto, A.; Sakai, Y. Effect of humidity on nh3 gas sensitivity of polyaniline blend films. Sens. Actuators B Chem. 2003, 94, 46–52. [Google Scholar] [CrossRef]
- Zeng, F.-W.; Liu, X.-X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Wu, T.-F.; Hong, J.-D. Humidity sensing properties of transferable polyaniline thin films formed at the air–water interface. RSC Adv. 2016, 6, 96935–96941. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chang, S. Effect of humidity on electrical conductivity of transparent polyaniline thin films. In Proceedings of the 2014 9th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 22–24 October 2014; pp. 491–493. [Google Scholar]
- Joulazadeh, M.; Navarchian, A.H.; Niroomand, M. A comparative study on humidity sensing performances of polyaniline and polypyrrole nanostructures. Adv. Polym. Technol. 2014, 33, 21461. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, R.; Ghosh, R.; Pradhan, M.; Roy, S.; Singha, P.; Ghosh, D.; Banerjee, A.; Banerjee, D.; Pal Chowdhury, M. Relative humidity sensing properties of doped polyaniline-encased multiwall carbon nanotubes: Wearable and flexible human respiration monitoring application. J. Mater. Sci. 2020, 55, 3884–3901. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.Y.; Kim, D.H.; Lee, H.-K.; Shin, B.J.; Tae, H.-S. Humidity-independent conducting polyaniline films synthesized using advanced atmospheric pressure plasma polymerization with in-situ iodine doping. Appl. Phys. Lett. 2017, 110, 033502. [Google Scholar] [CrossRef]
- Nohria, R.; Khillan, R.K.; Su, Y.; Dikshit, R.; Lvov, Y.; Varahramyan, K. Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens. Actuators B Chem. 2006, 114, 218–222. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, S.J.; Kim, B.; Lim, G. A stretchable humidity sensor based on a wrinkled polyaniline nanostructure. RSC Adv. 2014, 4, 39767–39770. [Google Scholar] [CrossRef]
- Jain, S.; Chakane, S.; Samui, A.B.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sens. Actuators B Chem. 2003, 96, 124–129. [Google Scholar] [CrossRef]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Hino, T.; Kuramoto, N. Effect of temperature and moisture on electrical conductivity in polyaniline/polyurethane (pani/pu) blends. Synth. Met. 2006, 156, 1187–1193. [Google Scholar] [CrossRef]
- Kukla, A.L.; Shirshov, Y.M.; Piletsky, S.A. Ammonia sensors based on sensitive polyaniline films. Sens. Actuators B Chem. 1996, 37, 135–140. [Google Scholar] [CrossRef]
- Zheng, W.; Angelopoulos, M.; Epstein, A.J.; MacDiarmid, A.G. Experimental evidence for hydrogen bonding in polyaniline: Mechanism of aggregate formation and dependency on oxidation state. Macromolecules 1997, 30, 2953–2955. [Google Scholar] [CrossRef]
- Yin, C.; Gao, L.; Zhou, F.; Duan, G. Facile synthesis of polyaniline nanotubes using self-assembly method based on the hydrogen bonding: Mechanism and application in gas sensing. Polymers 2017, 9, 544. [Google Scholar] [CrossRef]
- Zhang, L.; Long, Y.; Chen, Z.; Wan, M. The effect of hydrogen bonding on self-assembled polyaniline nanostructures. Adv. Funct. Mater. 2004, 14, 693–698. [Google Scholar] [CrossRef]
- Azim-Araghi, M.E.; Jafari, M.J. Electrical and gas sensing properties of polyaniline-chloroaluminium phthalocyanine composite thin films. Eur. Phys. J. Appl. Phys. 2010, 52, 10402. [Google Scholar] [CrossRef]
- Zampetti, E.; Muzyczuk, A.; Macagnano, A.; Pantalei, S.; Scalese, S.; Spinella, C.; Bearzotti, A. Effects of temperature and humidity on electrospun conductive nanofibers based on polyaniline blends. J. Nanopart. Res. 2011, 13, 6193–6200. [Google Scholar] [CrossRef]


| Materials | Preparation | Range (ppm) | Response (at xx ppm) | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|---|---|
| PANI/NiTSPc | Electrodeposition | 5–2500 | 2.75 at 100 ppm | 10 s | 46 s | [35] |
| PANI/Poysiloxane | in situ polymerization | 5–100 | 2.5 * (at 50 ppm) | 208 s | 263 s | [36] |
| Pyrolle/CoPc/CNTs | Drop casting | 0.05–500 | 26.2% (at 50 ppm) | 11.7 s | 91.8 s | [37] |
| PA6/PANI | Paste | 50–250 | 125% (at 200 ppm) | 27 s | NA | [38] |
| PANI/ZnTPP | Electrodeposition | 50–500 | 205.18% (at 500 ppm) | 108 s | 450 s | [39] |
| PANI/RGO | Spin coating | 5–600 | 250% (at 100 ppm) | 97 s | 680 s | [40] |
| Nanostructured PANI | Spin coating | 3–990 | 219% (at 990 ppm) | NA | NA | [41] |
| PANI/CuPctbu | Drop casting | 5–500 | 529% (at 200 ppm) | 510 s | 990 s | This work |
| PANI/TPPH2 | Drop casting | 5–500 | 360% (at 200 ppm) | 480 s | 1080 s | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauly, A.; Saad Ali, S.; Varenne, C.; Brunet, J.; Llobet, E.; Ndiaye, A.L. Phthalocyanines and Porphyrins/Polyaniline Composites (PANI/CuPctBu and PANI/TPPH2) as Sensing Materials for Ammonia Detection. Polymers 2022, 14, 891. https://doi.org/10.3390/polym14050891
Pauly A, Saad Ali S, Varenne C, Brunet J, Llobet E, Ndiaye AL. Phthalocyanines and Porphyrins/Polyaniline Composites (PANI/CuPctBu and PANI/TPPH2) as Sensing Materials for Ammonia Detection. Polymers. 2022; 14(5):891. https://doi.org/10.3390/polym14050891
Chicago/Turabian StylePauly, Alain, Sahal Saad Ali, Christelle Varenne, Jérôme Brunet, Eduard Llobet, and Amadou L. Ndiaye. 2022. "Phthalocyanines and Porphyrins/Polyaniline Composites (PANI/CuPctBu and PANI/TPPH2) as Sensing Materials for Ammonia Detection" Polymers 14, no. 5: 891. https://doi.org/10.3390/polym14050891
APA StylePauly, A., Saad Ali, S., Varenne, C., Brunet, J., Llobet, E., & Ndiaye, A. L. (2022). Phthalocyanines and Porphyrins/Polyaniline Composites (PANI/CuPctBu and PANI/TPPH2) as Sensing Materials for Ammonia Detection. Polymers, 14(5), 891. https://doi.org/10.3390/polym14050891







