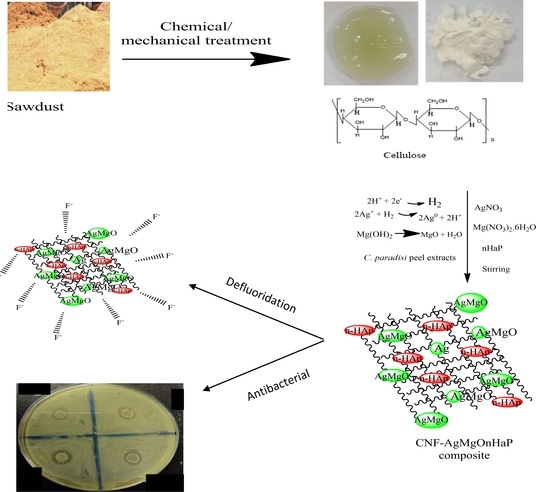
Sorption of Fluoride and Bacterial Disinfection Property of Biosynthesized Nanofibrous Cellulose Decorated Ag–MgO–Nanohydroxyapatite Composite for Household Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Adsorbent
2.2.1. Preparation of Citrus Paradisi Peel Extract
2.2.2. Preparation of Cellulose Nanofibers from Sawdust Biomass (CNF)
2.2.3. Preparation of Nanohydroxyapatite (nHaP)
2.2.4. Synthesis Conditions of Cellulose–Nanofiber–AgMgOnanohydoxyapatite
2.3. Instruments for Material Characterization
2.4. Batch Fluoride Adsorption Procedure
2.5. Point of Zero Charge (pHpzc)
2.6. Regeneration of CNF-AgMgOnHaP Composite
2.7. Antibacterial Evaluation of the Composite
2.8. Statistical Tools
3. Results and Discussions
3.1. Effects of Cellulose Nanofiber Loading (wt.%) in CNF-AgMgOnHaP Adsorbent on Adsorption
3.2. Structural Morphological Analysis
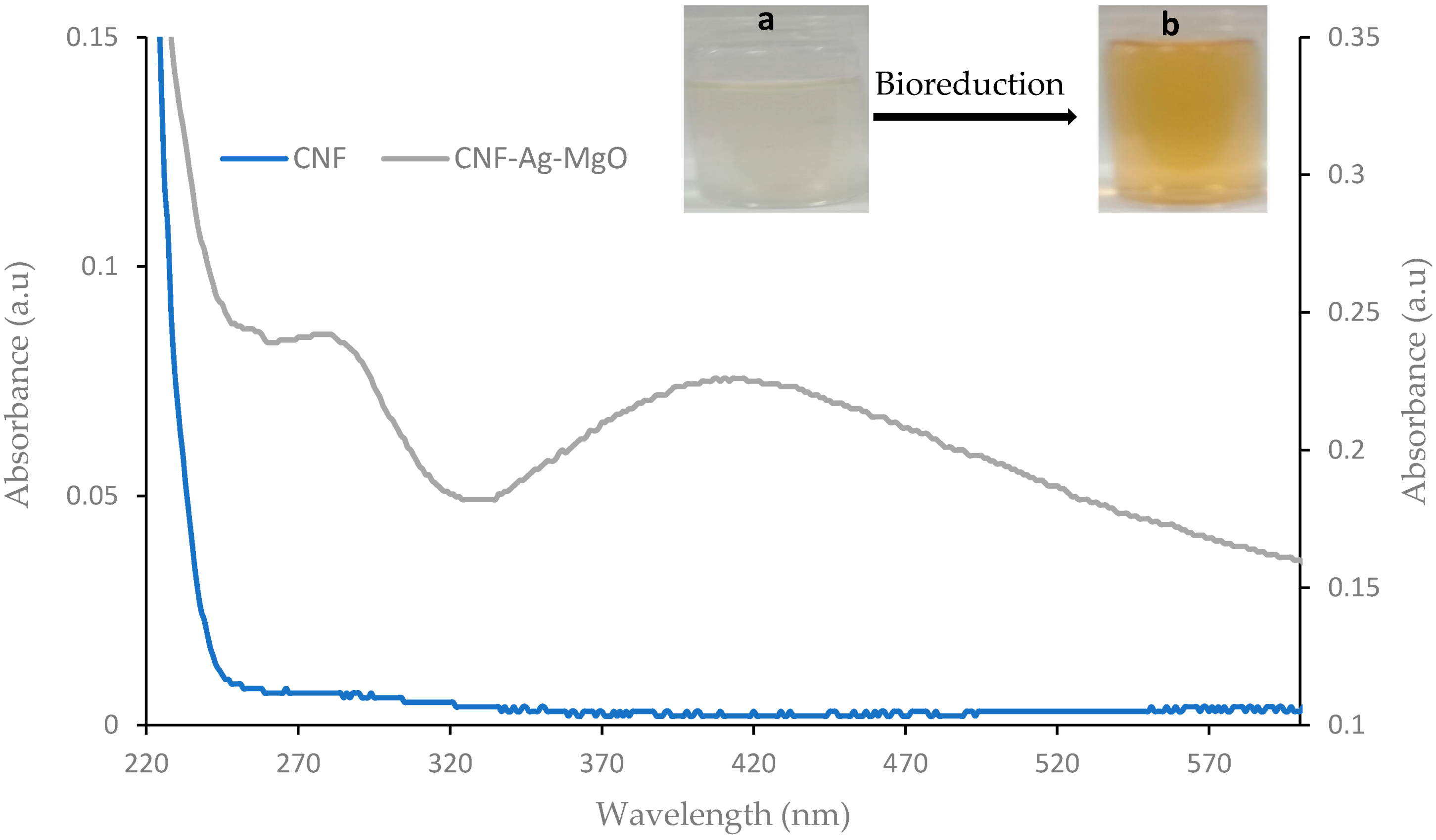
3.2.1. UV-Visible Study
3.2.2. Electron Microscopic Analysis
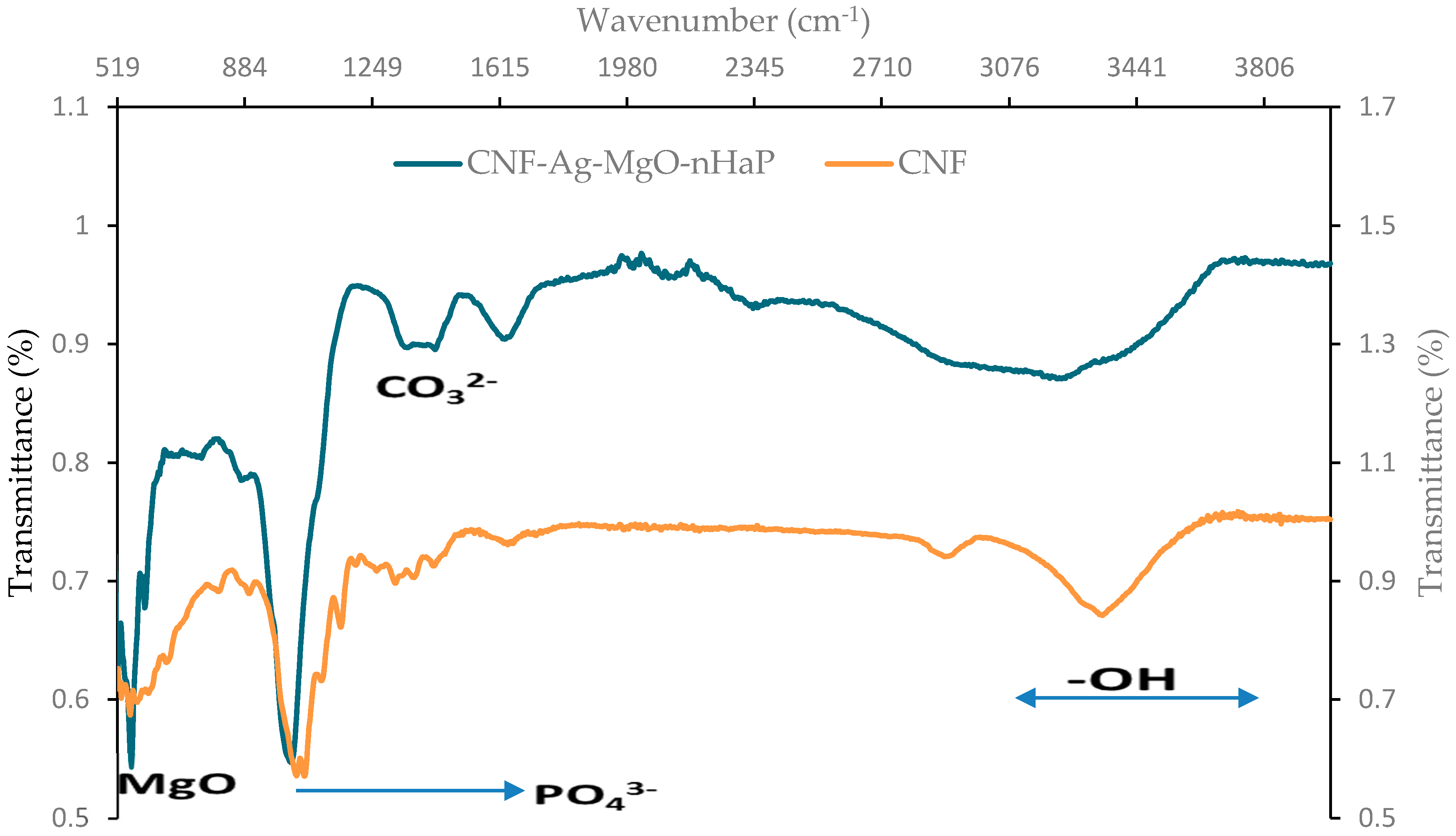
3.2.3. FTIR Spectroscopy
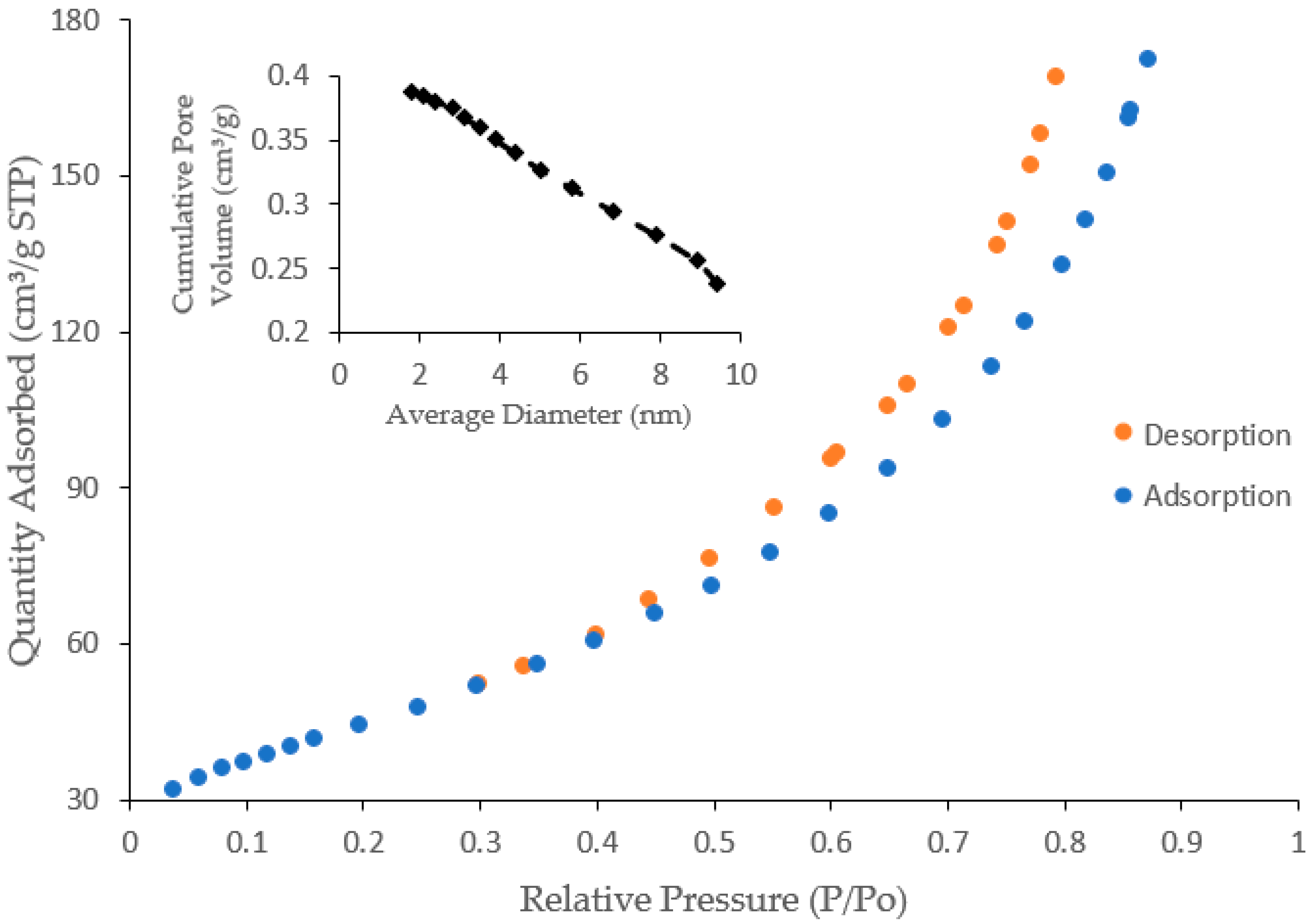
3.2.4. Specific Surface Area by Brunauer–Emmett–Teller (BET)
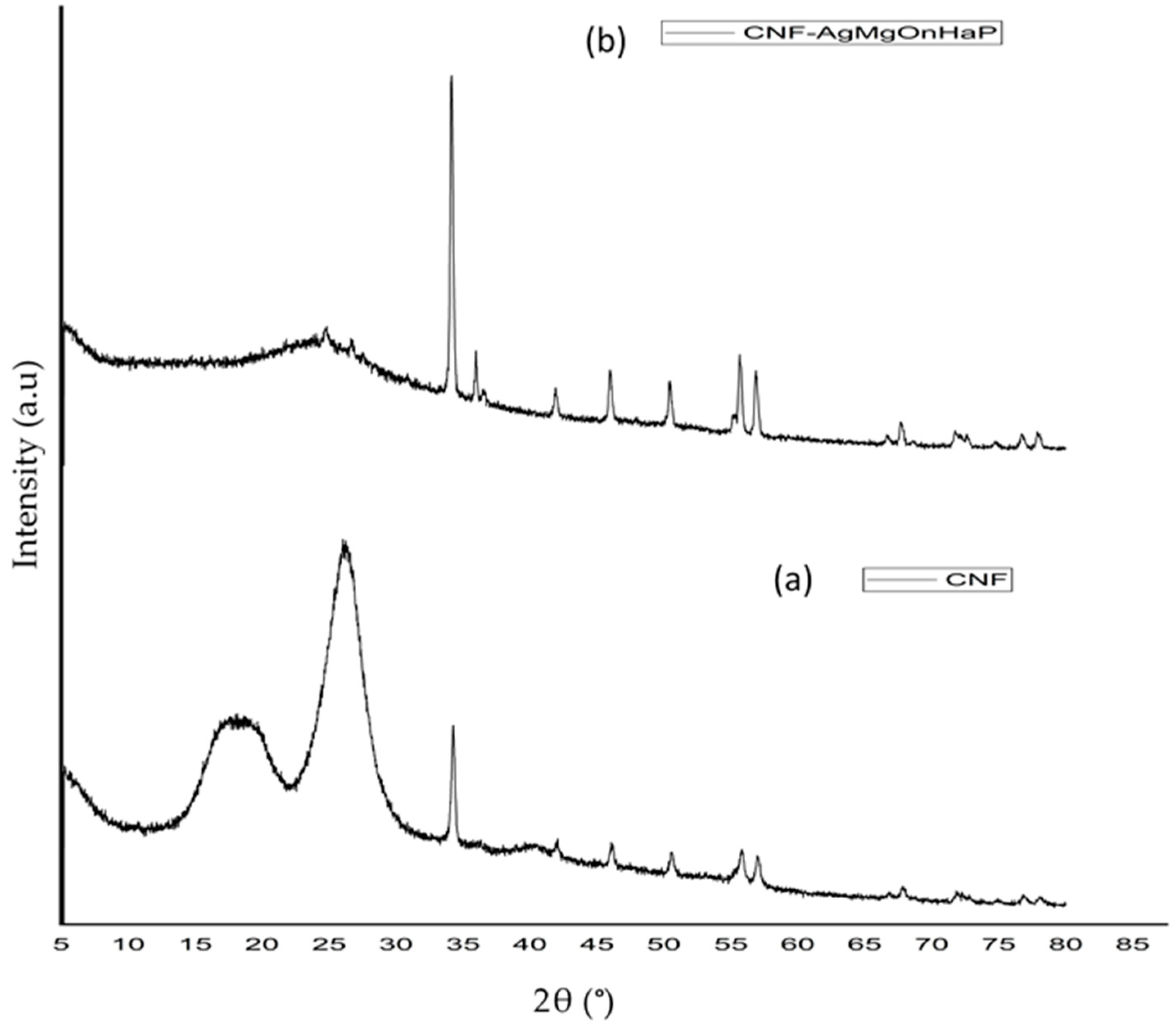
3.2.5. XRD Analysis
4. Batch Fluoride Adsorption Experimental Results
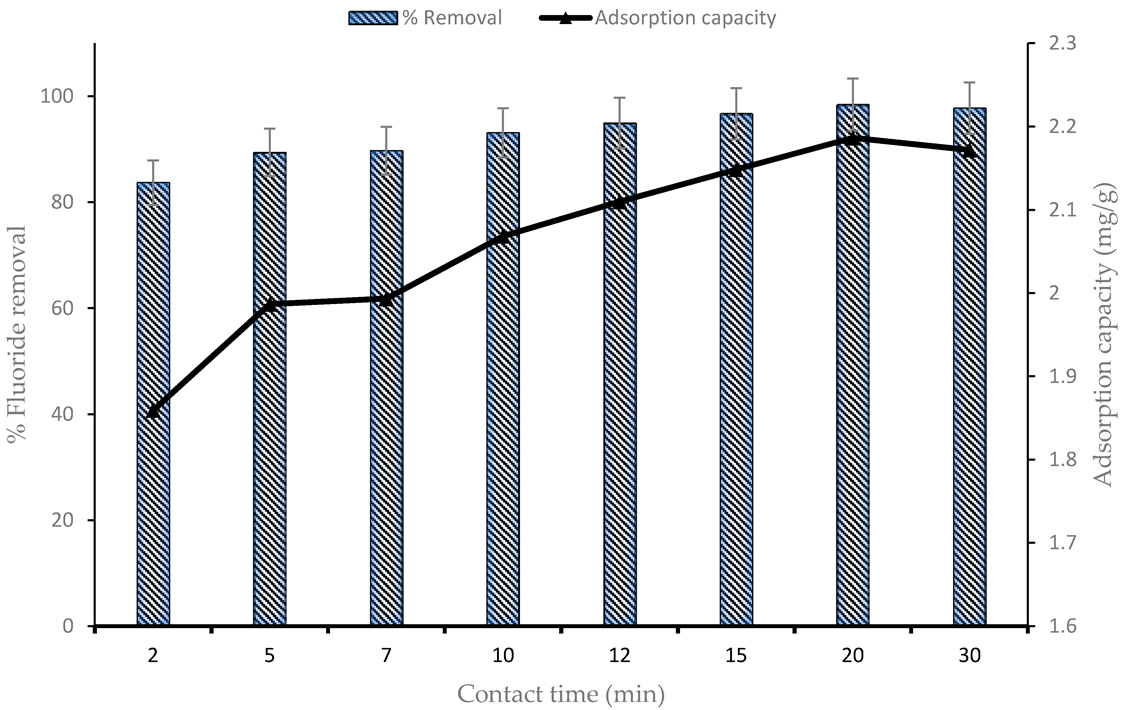
4.1. Effect of Contact Time
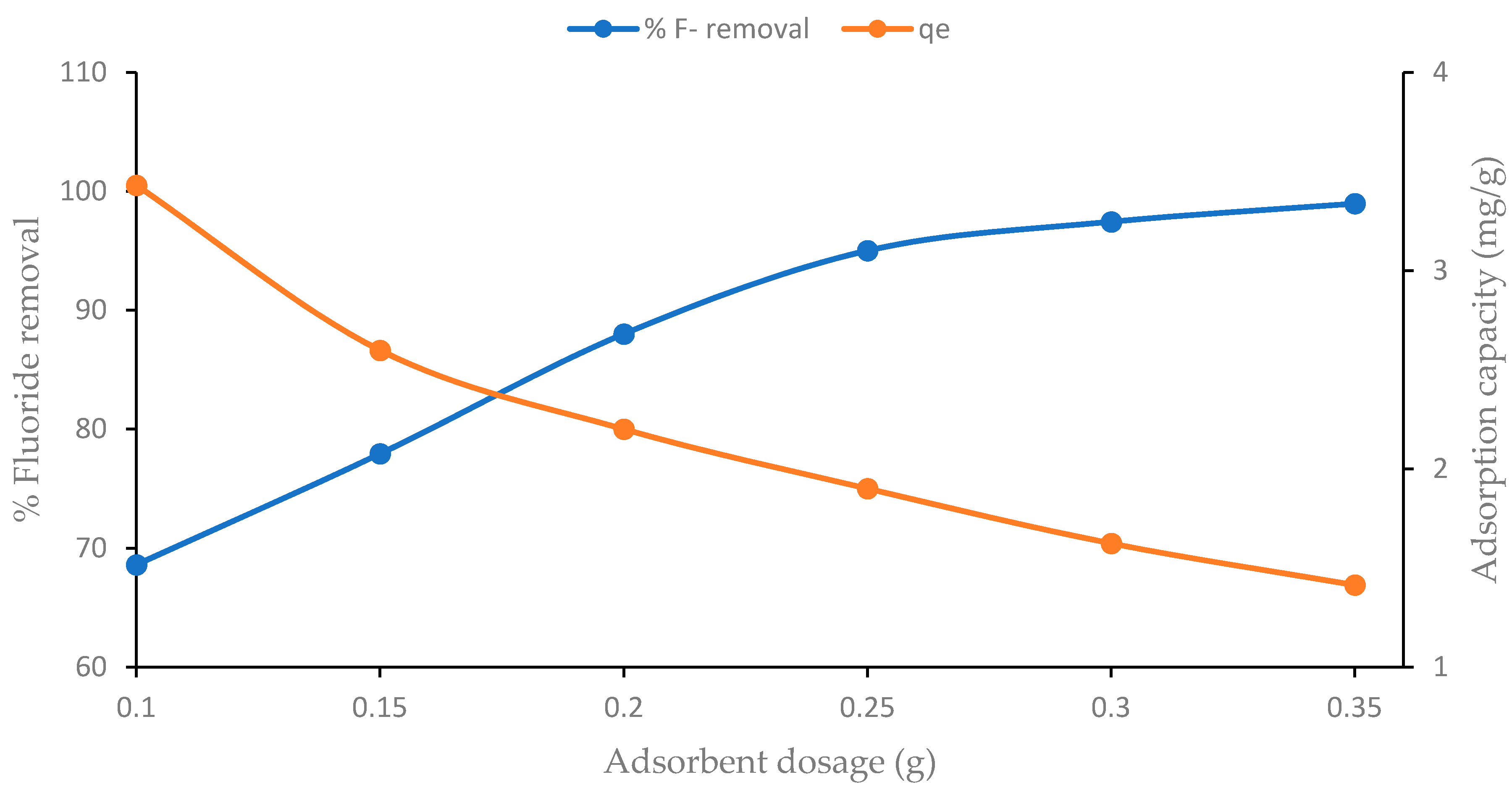
4.2. Effect of Sorbent Dosage
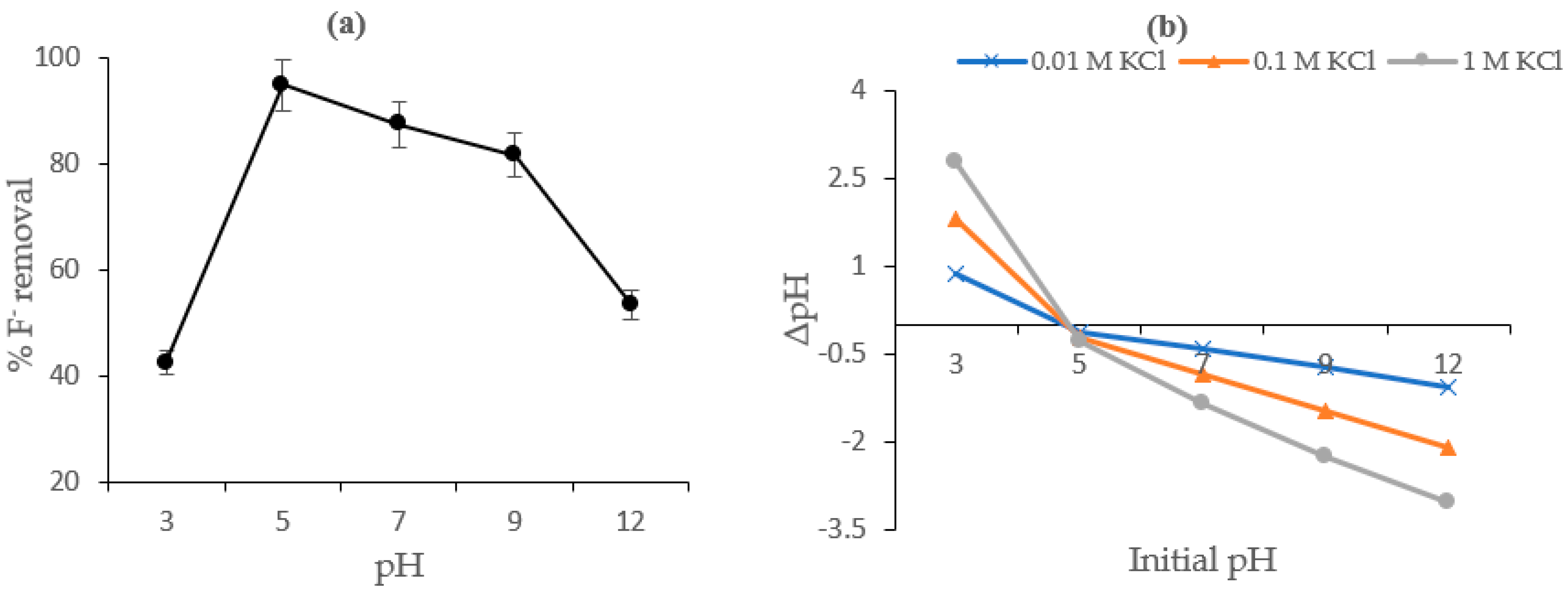
4.3. Effect of pH and Surface Charges on Fluoride Sorption
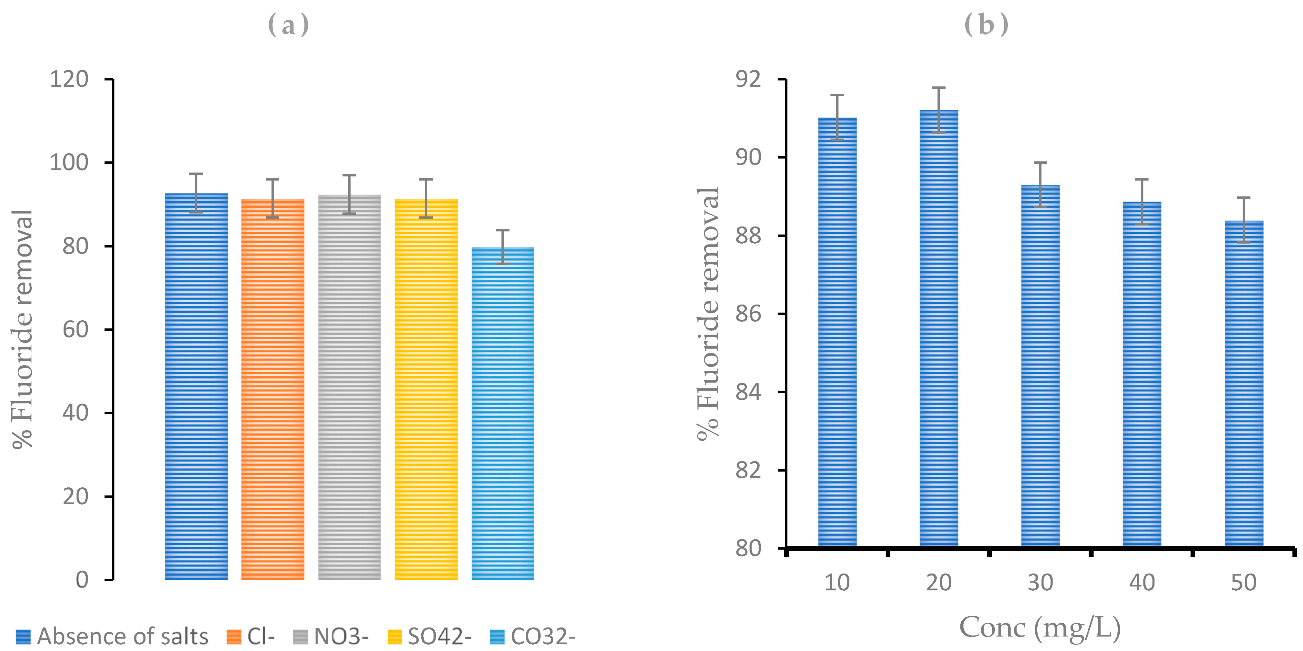
4.4. Effect of Coexisting Ions
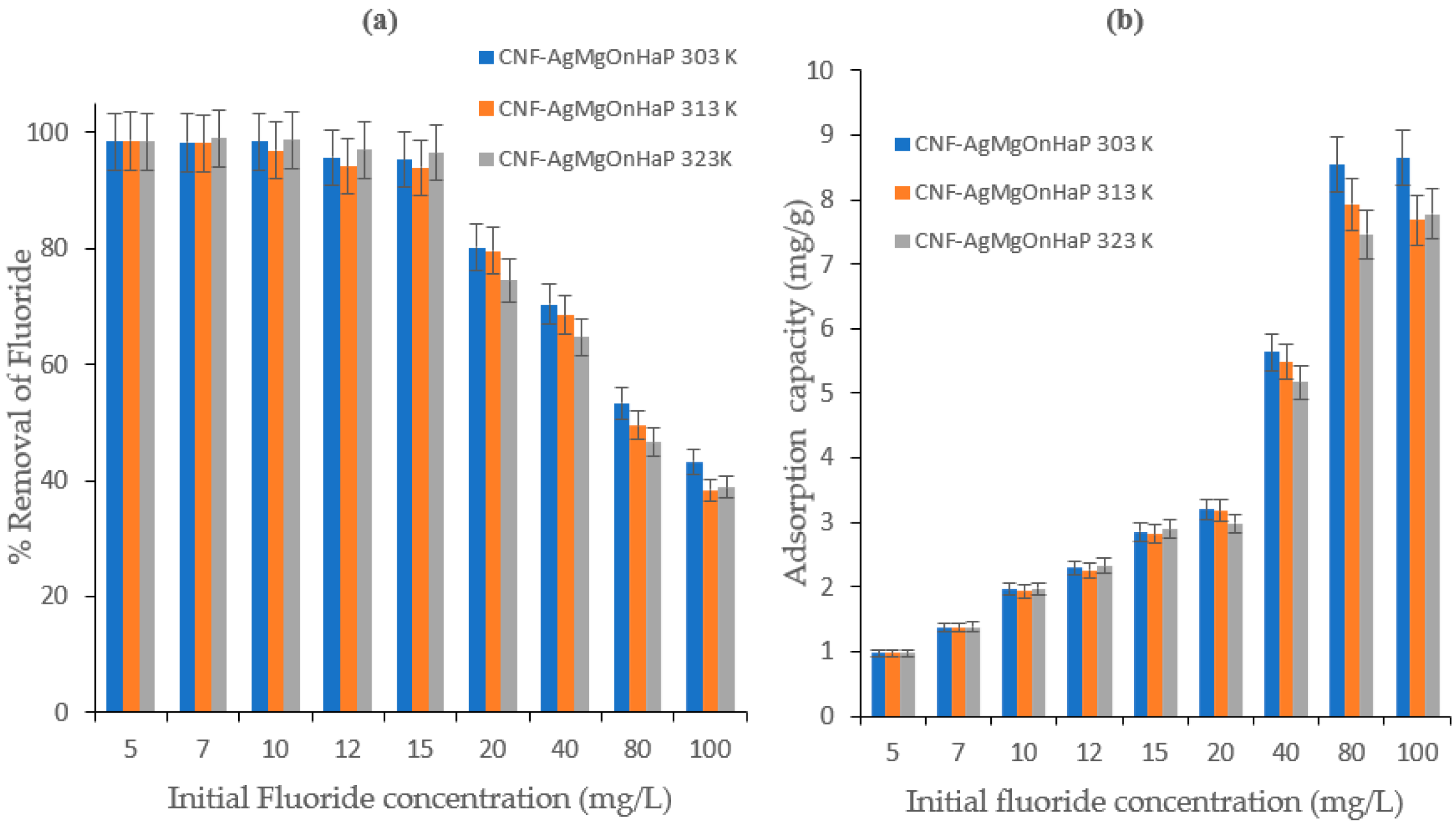
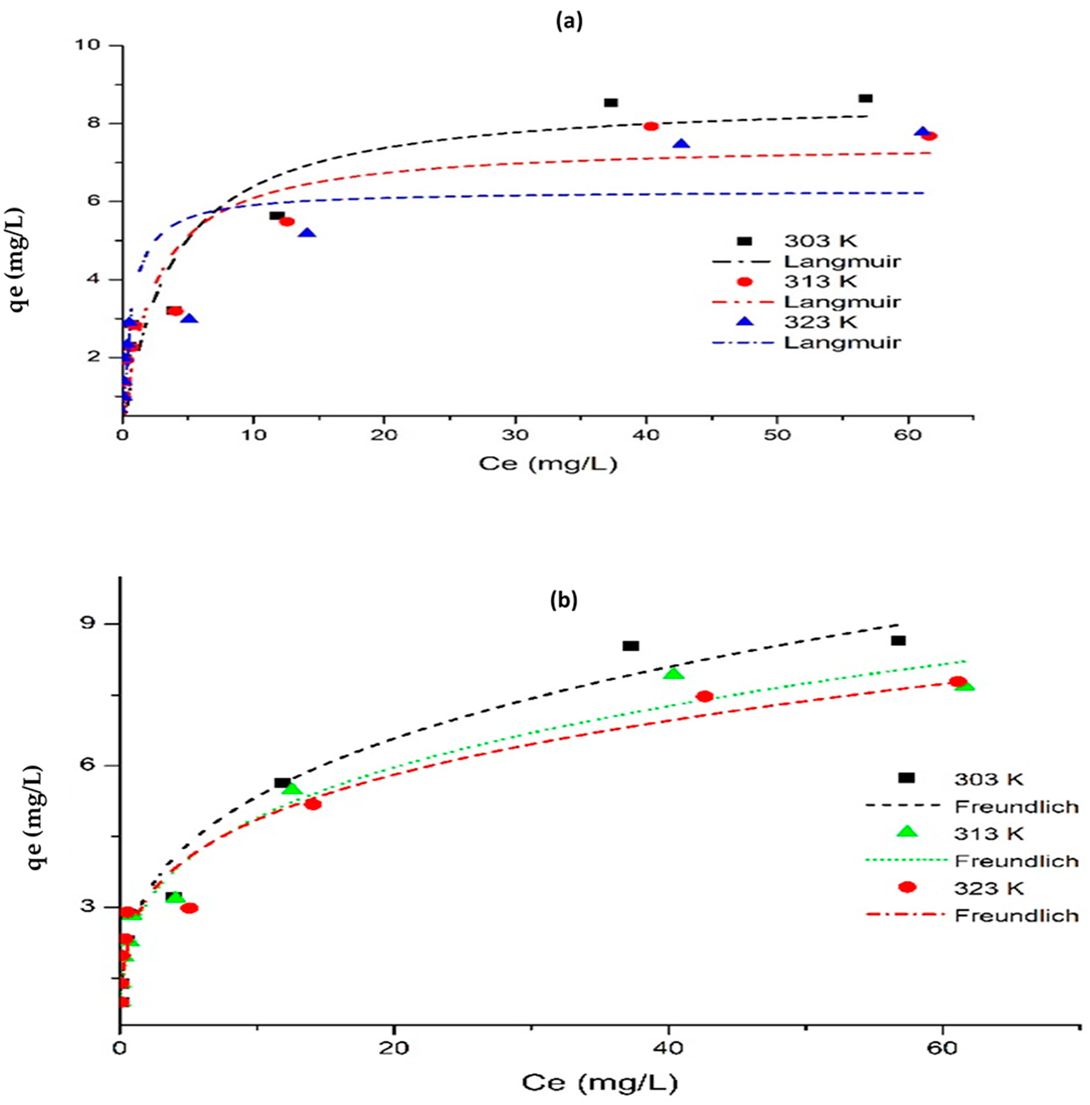
4.5. Effect of Initial Concentration and Adsorption Isotherm
4.6. Adsorption Kinetic
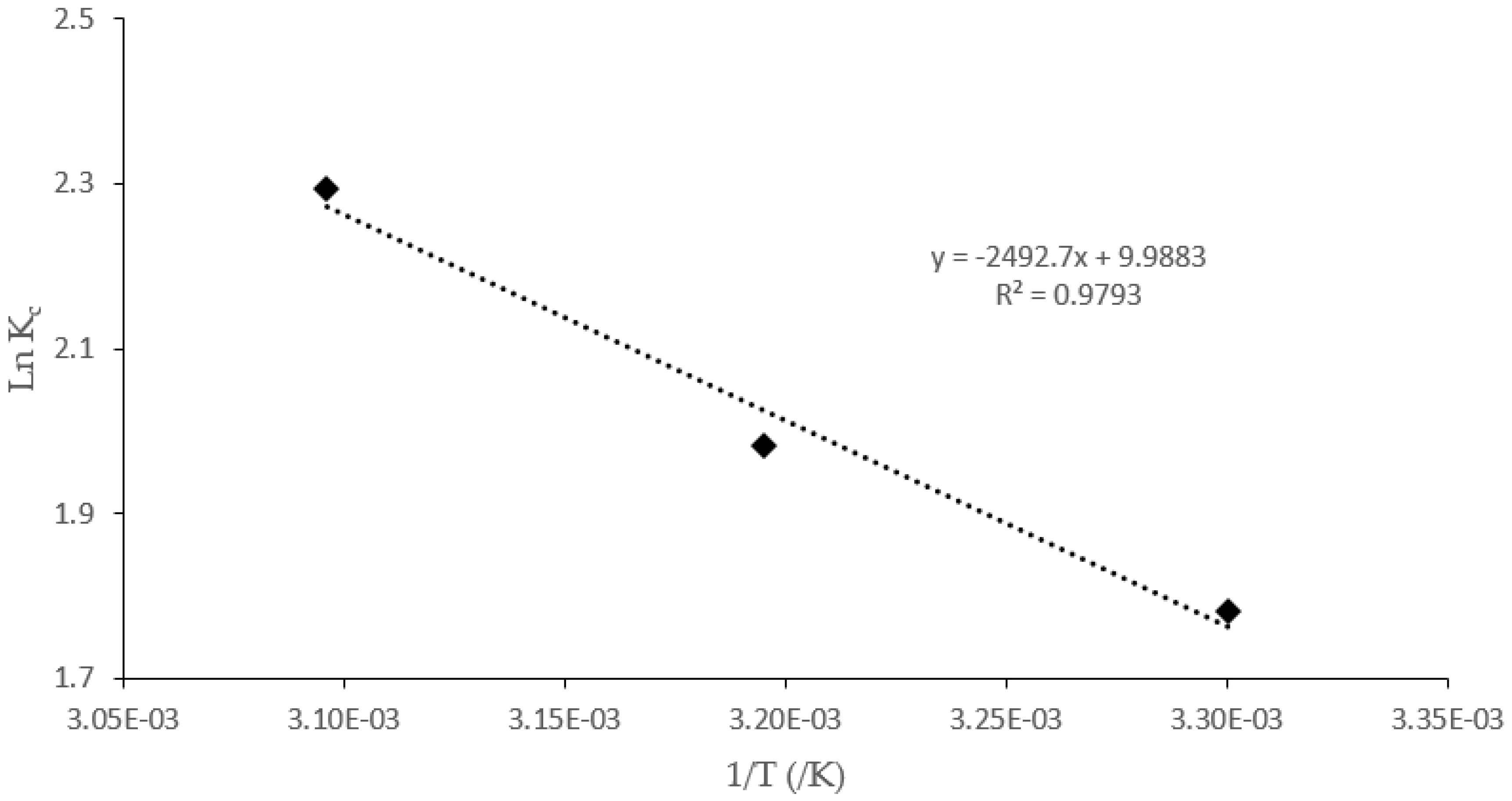
4.7. Thermodynamics
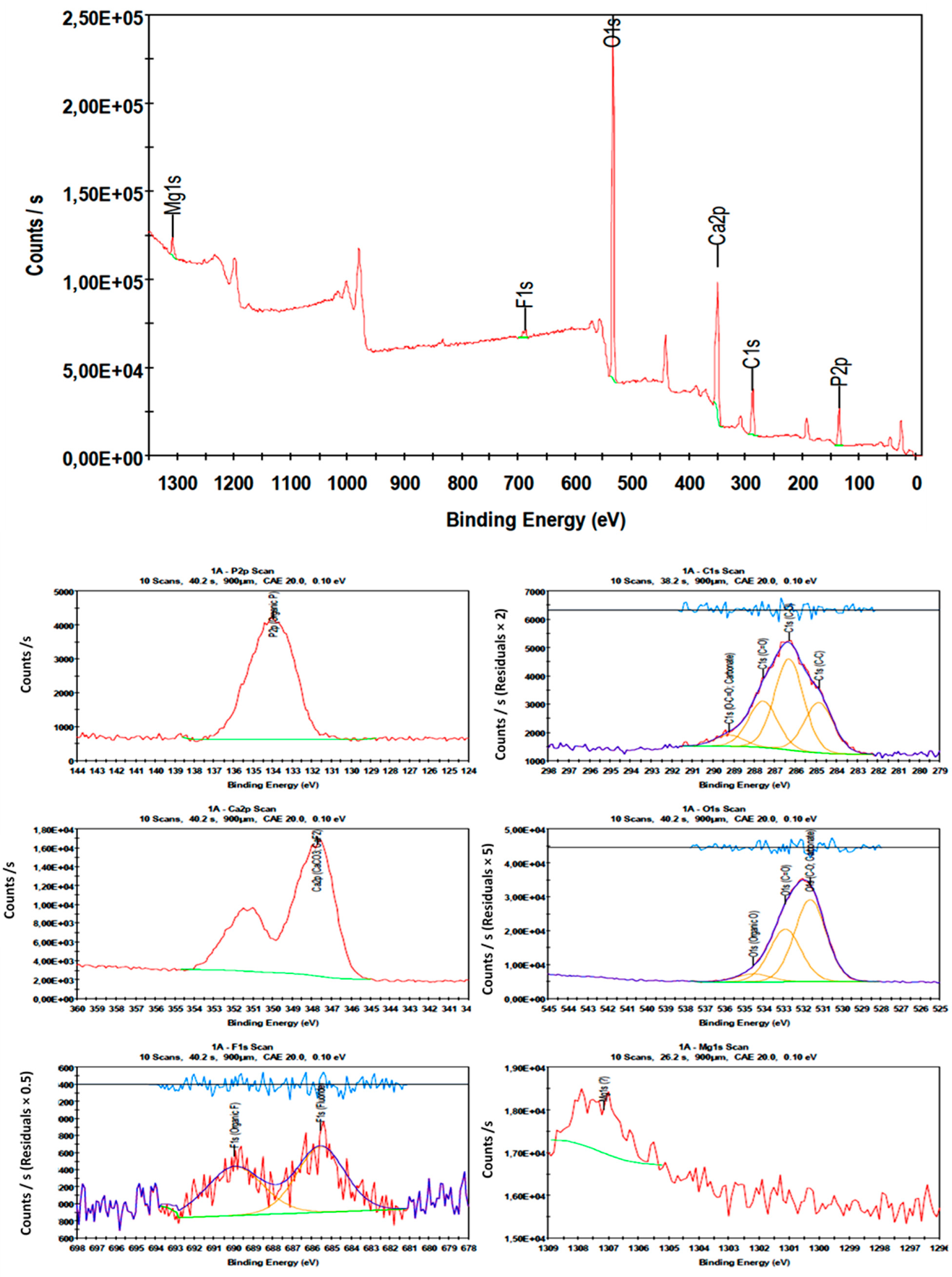
4.8. Surface Chemistry
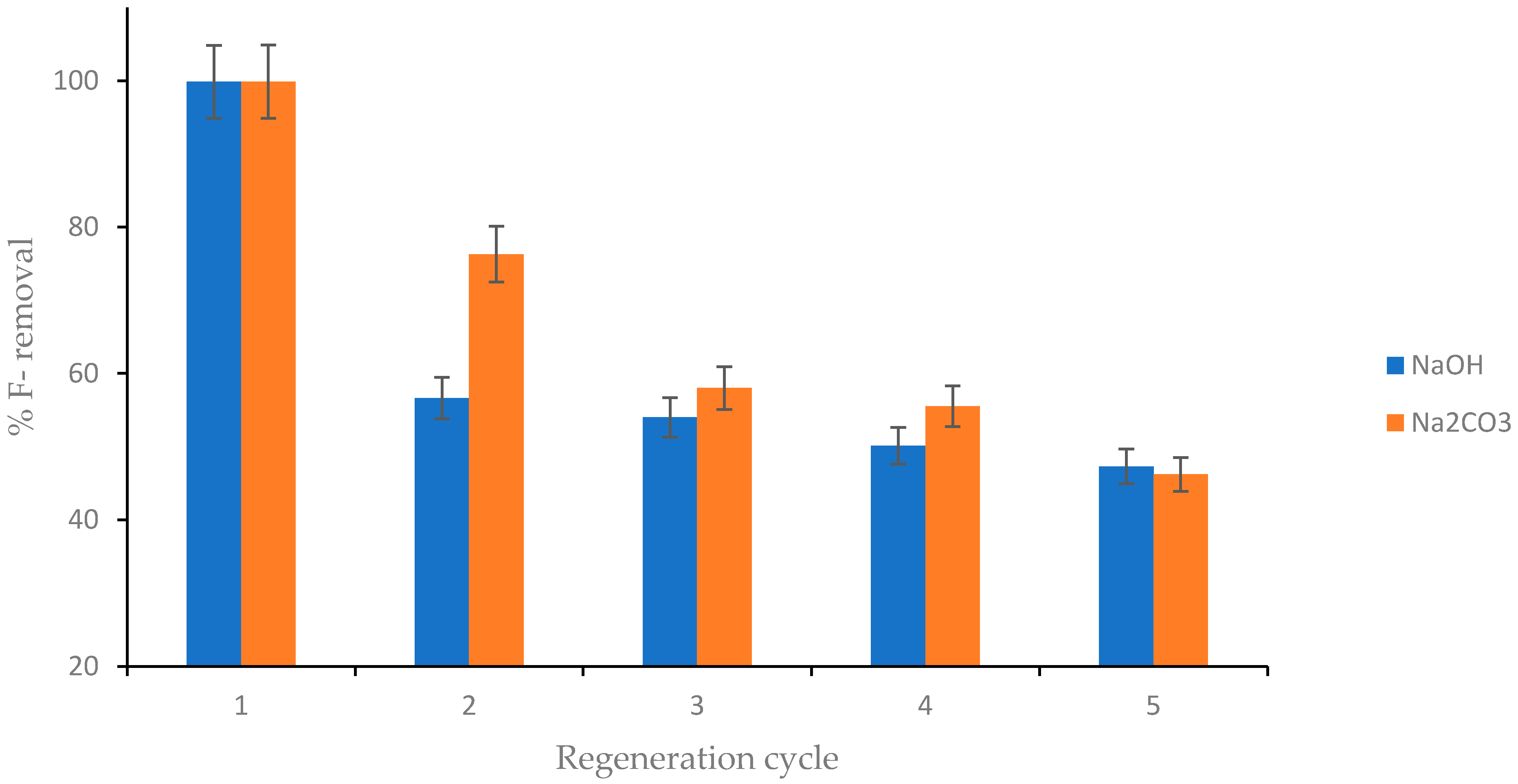
4.9. Regeneration and Reusability of CNF-AgMgOnHaP Adsorbent
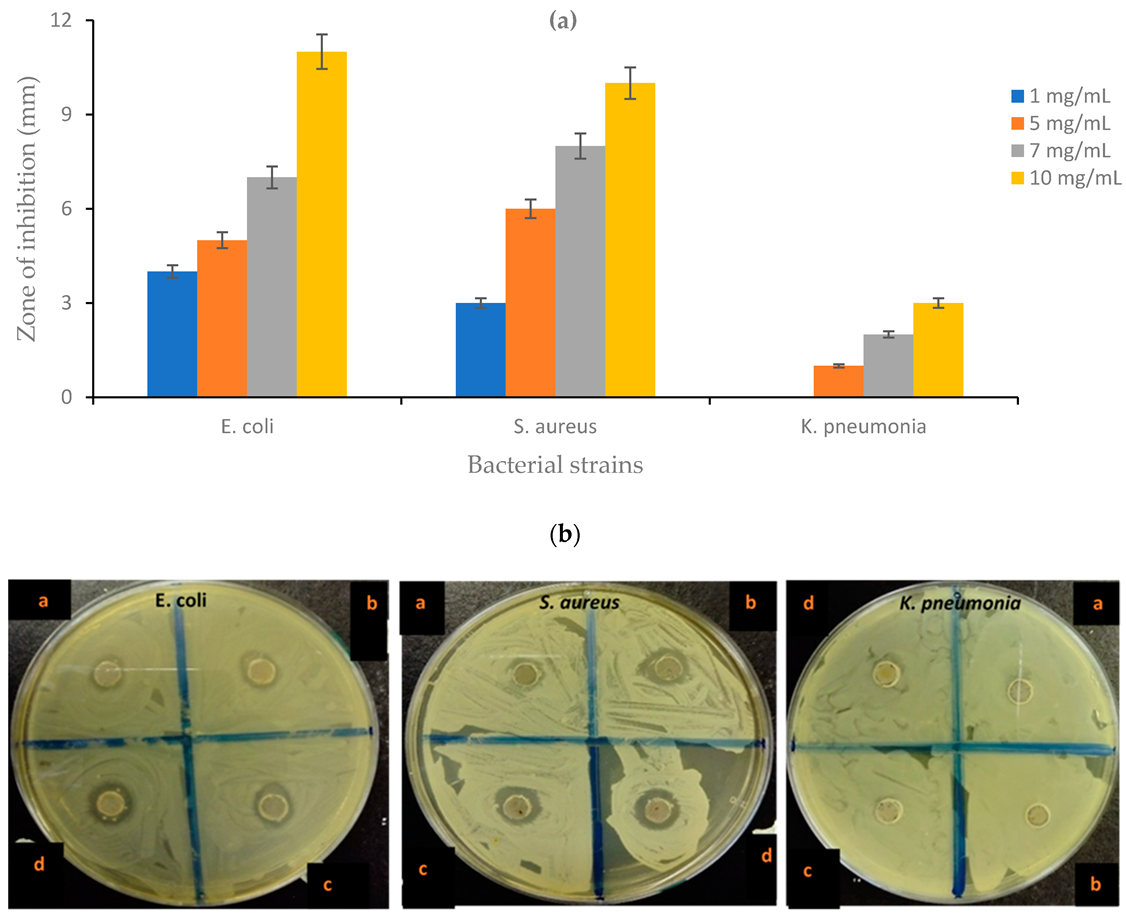
4.10. Antibacterial Activity of the CNF-AgMgOnHaP Adsorbent
5. Comparative Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WWAP (United Nations World Water Assessment Programme); UNESCO: Paris, France, 2017.
- World Health Organization. W.H.O Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Beard, V.A.; Mitlin, D. Water access in global South cities: The challenges of intermittency and affordability. World Dev. 2021, 147, 105625. [Google Scholar] [CrossRef]
- Mumtaz, N.; Pandey, G.; Labhasetwar, P.K. Global fluoride occurrence, available technologies for fluoride removal and electrolytic defluoridation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2357–2389. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Singh, J.; Singh, P.; Singh, A. Fluoride ions vs removal technologies: A study. Arab. J. Chem. 2016, 9, 815–824. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Fluoride removal from water by chitosan derivatives and composites: A review. J. Fluor. Chem. 2011, 132, 231–240. [Google Scholar] [CrossRef]
- Edition, F. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Defluoridation of drinking water using adsorption processes. J. Hazard. Mater. 2013, 248, 1–19. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef]
- Ramesh, M.; Malathi, N.; Ramesh, K.; Aruna, R.M.; Kuruvilla, S. Comparative evaluation of dental and skeletal fluorosis in an endemic fluorosed district, Salem, Tamil Nadu. J. Pharm. Bioallied Sci. 2017, 9, S88. [Google Scholar] [CrossRef]
- Hijnen, W.; Beerendonk, E.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G. Microbiology of Drinking Water Production and Distribution; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Chlorine Chemistry Council; American Chemistry Council. Drinking Water Chlorination: A Review of Disinfection Practices and Issues. Water Cond. Purif. Int. 2006, 68. [Google Scholar]
- Momba, M.N.B. Selection and Use of Home Water-treatment Systems and Devices: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2013.
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 22, 104516. [Google Scholar] [CrossRef]
- Nasker, P.; Mukherjee, M.; Kant, S.; Tripathy, S.; Sinha, A.; Das, M. Fluorine substituted nano hydroxyapatite: Synthesis, bio-activity and antibacterial response study. Ceram. Int. 2018, 4, 22008–22013. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Wang, D.; Lin, X.; Liu, W.; Ma, L.; Li, Y.; Huang, Y. Modified native cellulose fibers—A novel efficient adsorbent for both fluoride and arsenic. J. Hazard. Mater. 2011, 185, 93–100. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef]
- Ferroudj, N.; Nzimoto, J.; Davidson, A.; Talbot, D.; Briot, E.; Dupuis, V.; Bée, A.; Medjram, M.S.; Abramson, S. Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Appl. Catal. B Environ. 2013, 136, 9–18. [Google Scholar] [CrossRef]
- Gitari, W.M.; Izuagie, A.A.; Gumbo, J.R. Synthesis, characterization and batch assessment of groundwater fluoride removal capacity of trimetal Mg/Ce/Mn oxide-modified diatomaceous earth. Arab. J. Chem. 2017, 13, 1–16. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio-and nanocomposites: A review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Manna, S.; Pasquini, D.; Thomas, S.; Grohens, Y. Nanocellulose: Extraction and application as a sustainable material for wastewater purification. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 469–486. [Google Scholar]
- Fu, L.-H.; Xie, Y.-M.; Bian, J.; Ma, M.-G.; Tian, C.-H.; Jin, X.-J. Microwave-assisted rapid synthesis of lignocellulose/hydroxyapatite nanocomposites. Mater. Lett. 2015, 159, 51–53. [Google Scholar] [CrossRef]
- Ayinde, W.B.; Gitari, M.W.; Muchindu, M.; Samie, A. Biosynthesis of ultrasonically modified Ag-MgO nanocomposite and its potential for antimicrobial activity. J. Nanotechnol. 2018, 2018, 9537454. [Google Scholar] [CrossRef] [Green Version]
- Ayinde, W.; Gitari, W.; Samie, A. Optimization of microwave-assisted synthesis of silver nanoparticle by Citrus paradisi peel and its application against pathogenic water strain. Green Chem. Lett. Rev. 2019, 12, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Ayinde, W.; Gitari, W.; Munkombwe, M.; Samie, A.; Smith, J.A. Green synthesis of AgMgOnHaP nanoparticles supported on chitosan matrix: Defluoridation and antibacterial effects in groundwater. J. Environ. Chem. Eng. 2020, 8, 104026. [Google Scholar] [CrossRef]
- Kamphunthong, W.; Hornsby, P.; Sirisinha, K. Isolation of cellulose nanofibers from para rubberwood and their reinforcing effect in poly (vinyl alcohol) composites. J. Appl. Polym. Sci. 2012, 125, 1642–1651. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ghosh, M.K.; Ng, Y.-J.; Issa, T.B.; Anand, S.; Singh, P. Defluoridation behavior of nanostructured hydroxyapatite synthesized through an ultrasonic and microwave combined technique. J. Hazard. Mater. 2011, 185, 29–37. [Google Scholar] [CrossRef]
- Athar, T. Synthesis of MgO Nanoparticles via Sol–Gel Method. Mater. Focus 2013, 2, 493–496. [Google Scholar] [CrossRef]
- Karthiga, P. Preparation of silver nanoparticles by Garcinia mangostana stem extract and investigation of the antimicrobial properties. Biotechnol. Res. Innov. 2018, 2, 30–36. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Sterrer, M.; Berger, T.; Diwald, O.; Knözinger, E. Energy transfer on the MgO surface, monitored by UV−induced H2 chemisorption. J. Am. Chem. Soc. 2003, 125, 195–199. [Google Scholar] [CrossRef]
- Stankic, S.; Sterrer, M.; Hofmann, P.; Bernardi, J.; Diwald, O.; Knözinger, E. Novel optical surface properties of Ca2+-doped MgO nanocrystals. Nano Lett. 2005, 5, 1889–1893. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; Regiani, T.; Marques, R.F.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J. Antimicrobial bacterial cellulose-silver nanoparticles composite membranes. J. Nanomater. 2011, 2011, 721631. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Zhang, W.; Zhou, Z.; Zhang, X. Facile synthesis of tunable silver nanostructures for antibacterial application using cellulose nanocrystals. Carbohydr. Polym. 2013, 95, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, K.; Samec, D.; Kvitek, O.; Reznickova, A.; Rimpelova, S.; Svorcik, V. Preparation and characterization of silver nanoparticles in methyl cellulose matrix and their antibacterial activity. Jpn. J. Appl. Phys. 2017, 56, 06GG09. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, M.; Sarfraz, S.; Amin, S. On the formation of hydroxyapatite nano crystals prepared using cationic surfactant. Mater. Res. 2015, 18, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Maryan, A.S.; Montazer, M.; Harifi, T. Synthesis of nano silver on cellulosic denim fabric producing yellow colored garment with antibacterial properties. Carbohydr. Polym. 2015, 115, 568–574. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier transform infrared spectroscopy for natural fibres. Fourier Transform.-Mater. Anal. 2012, 3, 45–68. [Google Scholar]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ashna, L.; Parthiban, P. Synthesis of nanocrystalline MgO particles by combustion followed by annealing method using hexamine as a fuel. J. Nanotechnol. 2014, 2014, 841803. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.M.; Elanchezhiyan, S.S.; Lee, G.; Khan, A.; Meenakshi, S. Assembly of nano-sized hydroxyapatite onto graphene oxide sheets via in-situ fabrication method and its prospective application for defluoridation studies. Chem. Eng. J. 2016, 300, 334–342. [Google Scholar] [CrossRef]
- Hwang, N.; Barron, A.R. BET surface area analysis of nanoparticles. Connex. Proj. 2011, 39, 445–446. [Google Scholar]
- Lu, L.; Lu, D.; Chen, L.; Luo, F. Removal of Cd (II) by modified lawny grass cellulose adsorbent. Desalination 2010, 259, 120–130. [Google Scholar] [CrossRef]
- Banavath, H.N.; Bhardwaj, N.K.; Ray, A.K. A comparative study of the effect of refining on charge of various pulps. Bioresour. Technol. 2011, 102, 4544–4551. [Google Scholar] [CrossRef]
- Akram, M.; Alshemary, A.Z.; Goh, Y.F.; Ibrahim, W.A.; Lintang, H.O.; Hussain, R. Continuous microwave flow synthesis of mesoporous hydroxyapatite. Mater. Sci. Eng. 2015, 56, 356–362. [Google Scholar] [CrossRef]
- Mohammad, N.F.; Othman, R.; Yeoh, F.Y. Controlling the pore characteristics of mesoporous apatite materials: Hydroxyapatite and carbonate apatite. Ceram. Int. 2015, 41, 10624–10633. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Antony, K.; Pradeep, T. High yield combustion synthesis of nanomagnesia and its application for fluoride removal. Sci. Total Environ. 2010, 408, 2273–2282. [Google Scholar] [CrossRef]
- Hou, S.; Li, J.; Huang, X.; Wang, X.; Ma, L.; Shen, W.; Kang, F.; Huang, Z.-H. Silver nanoparticles-loaded exfoliated graphite and its anti-bacterial performance. Appl. Sci. 2017, 7, 852. [Google Scholar] [CrossRef] [Green Version]
- Wada, M.; Heux, L.; Sugiyama, J. Polymorphism of cellulose I family: Reinvestigation of cellulose IVI. Biomacromolecules 2004, 5, 1385–1391. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X.; King, P.L. Accommodation of the carbonate ion in apatite: An FTIR and X-ray structure study of crystals synthesized at 2–4 GPa. Am. Miner. 2004, 89, 1422–1432. [Google Scholar] [CrossRef]
- Averbuch, P.; MT, A.P. Structure Cristalline D’un Tripolyphosphate Acide Mixte Zinc-Argent Nonahydrate: Zn2Ag0.62H0.38P3O10. 9H2O. Acta Crystallogr. 1976, B32, 2270–2274. [Google Scholar] [CrossRef]
- Calvo, C. The crystal structure of α-Mg2P2O7. Acta Crystallogr. 1967, 23, 289–295. [Google Scholar] [CrossRef]
- Samadi, N.; Hasanzadeh, R.; Rasad, M. Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. J. Appl. Polym. Sci. 2015, 132, 41642. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Wang, X.; Chen, J.; Xu, C.; Liu, Y.; Yu, Z.; Wang, X. Fabrication of magnetic Fe/Zn layered double oxide@ carbon nanotube composites and their application for U (VI) and 241Am (III) removal. ACS Appl. Nano Mater. 2018, 1, 2386–2396. [Google Scholar] [CrossRef]
- Tran, H.N.; Wang, Y.F.; You, S.; Chao, H.P. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Swain, S.; Mishra, S.; Patnaik, T.; Patel, R.; Jha, U.; Dey, R. Fluoride removal performance of a new hybrid sorbent of Zr (IV)–ethylenediamine. Chem. Eng. J. 2012, 184, 72–81. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Nayak, B.; Samant, A.; Patel, R.; Misra, P.K. Comprehensive understanding of the kinetics and mechanism of fluoride removal over a potent nanocrystalline hydroxyapatite surface. ACS Omega 2017, 2, 8118–8128. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I Solids J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Dubinin, M. The equation of the characteristic curve of activated charcoal. Doklady Akademii Nauk SSSR 1947, 55, 327–329. [Google Scholar]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; Ng, J.; McKay, G. Kinetics of pollutant sorption by biosorbents. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Equilibria and capacities for adsorption on carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Machado, F.M.; Bergmann, C.P.; Lima, E.C.; Royer, B.; de Souza, F.E.; Jauris, I.M.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Blue 4 dye from water solutions by carbon nanotubes: Experiment and theory. Phys. Chem. Chem. Phys. 2012, 14, 11139–11153. [Google Scholar] [CrossRef]
- Viswanathan, N.; Pandi, K.; Meenakshi, S. Synthesis of metal ion entrapped silica gel/chitosan biocomposite for defluoridation studies. Int. J. Biol. Macromol. 2014, 70, 347–353. [Google Scholar] [CrossRef]
- Biggar, J.; Cheung, M. Adsorption of picloram (4-amino-3, 5, 6-trichloropicolinic acid) on panoche, ephrata, and palouse soils: A thermodynamic approach to the adsorption mechanism. Soil Sci. Soc. Am. J. 1973, 37, 863–868. [Google Scholar] [CrossRef]
- Summaira, S.; Zhenggang, X. Facile preparation of metal-organic frameworks-8 (ZIF-8) and its simultaneous adsorption of tetracycline (TC) and minocycline (MC) from aqueous solutions . Mater. Res. Bull. 2021, 141, 111372. [Google Scholar]
- Bomben, K.; Moulder, J.F.; Sobol, P.E.; Stickle, W.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Newberg, J.T.; Starr, D.E.; Yamamoto, S.; Kaya, S.; Kendelewicz, T.; Mysak, E.R.; Porsgaard, S.; Salmeron, M.B.; Brown, G.E., Jr.; Nilsson, A. Formation of hydroxyl and water layers on MgO films studied with ambient pressure XPS. Surf. Sci. 2011, 605, 89–94. [Google Scholar] [CrossRef]
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Regeneration and reuse of polymeric nanocomposites in wastewater remediation: The future of economic water management. Polym. Bull. 2019, 76, 647–681. [Google Scholar] [CrossRef]
- Vivekanandhan, S.; Christensen, L.; Misra, M.; Mohanty, A.K. Green process for impregnation of silver nanoparticles into microcrystalline cellulose and their antimicrobial bionanocomposite films. J. Biomater. Nanobiotechnol. 2012, 3, 20556. [Google Scholar] [CrossRef]
- Kim, M.K.; Sundaram, K.S.; Iyengar, G.A.; Lee, K.-P. A novel chitosan functional gel included with multiwall carbon nanotube and substituted polyaniline as adsorbent for efficient removal of chromium ion. Chem. Eng. J. 2015, 267, 51–64. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, D.; Zhu, X.; Wang, W.; Tan, F.; Chen, J.; Qiao, X.; Qiu, X. Sol-gel preparation of Ag-doped MgO nanoparticles with high efficiency for bacterial inactivation. Ceram. Int. 2017, 43, 1066–1072. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Hagihara, Y. Association of the physical and chemical properties and the cytotoxicity of metal oxidenanoparticles: Metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350–360. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sun, R.; Wei, Z.G.; Zhao, H.Y.; Li, H.X.; Hu, F. Size-dependent defluoridation properties of synthetic hydroxyapatite. J. Fluor. Chem. 2009, 130, 550–556. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.; Gupta, A.; Dadashzadeh, M. Removal of fluoride from aqueous solution and groundwater by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol. Eng. 2013, 52, 211–218. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J. Removal of fluoride from drinking water by cellulose@ hydroxyapatite nanocomposites. Carbohydr. Polym. 2013, 92, 269–275. [Google Scholar] [CrossRef]

| CNF (w/w %) | Average Equilibrium Fluoride Concentration (Ce) (mg/L) | % Fluoride Removal |
|---|---|---|
| 10 | 3.307 | 66.93 |
| 30 | 1.364 | 86.36 |
| 50 | 0.476 | 95.24 |
| 70 | 0.343 | 96.57 |
| 100 | 0.096 | 99.04 |
| BET Parameter | Methods | Values |
|---|---|---|
| Surface area | BET surface area | 160.17 m2/g |
| Langmuir surface area | 220.45 m2/g | |
| t-Plot external surface area | 154.71 m2/g | |
| Pore Area | ||
| Micropore area | t-Plot micropore area | 5.46 m2/g |
| Mesopore area | BJH adsorption | 196.12 m2/g |
| BJH desorption | 223.89 m2/g | |
| Pore volume | ||
| Micropore volume | Single point adsorption | 0.38 cm3/g |
| Single point desorption | 0.38 cm3/g | |
| Mesopore volume | BJH adsorption | 0.39 cm3/g |
| BJH desorption | 0.40 cm3/g | |
| Pore size | ||
| Adsorption average pore width | 4 V/A by BET | 9.55 nm |
| Desorption average pore width | 4 V/A by BET | 9.53 nm |
| Mesopore size | BJH adsorption | 7.91 nm |
| BJH desorption | 6.91 nm |
| Temperature (K) | 303 | 313 | 323 | |
|---|---|---|---|---|
| Non-Linear | Langmuir isotherm | |||
| Qm (mg/g) | 8.715 | 7.52 | 6.286 | |
| KL (L/mg) | 0.275 | 0.426 | 1.566 | |
| RL | 0.27 | 0.19 | 0.06 | |
| Adj. R2 | 0.813 | 0.845 | 0.709 | |
| Red. χ2 | 1.632 | 1.085 | 1.879 | |
| RSS | 11.42 | 7.59 | 13.16 | |
| Freundlich isotherm | ||||
| KF [(mg/g)/(mg/L)n] | 2.686 | 2.547 | 2.671 | |
| n | 3.345 | 3.519 | 3.853 | |
| Adj. R2 | 0.973 | 0.973 | 0.952 | |
| Red. χ2 | 0.308 | 0.188 | 0.308 | |
| RSS | 2.158 | 1.318 | 2.158 | |
| Linear | Dubinin–Radushkevvich | |||
| βDR (mol2/kJ2) | 4.00 × 10−8 | 3.00 × 10−8 | 3.00 × 10−8 | |
| qmax (mg/g) | 5.046 | 4.448 | 4.804 | |
| E (kJ/mol) | 3.535 | 4.083 | 4.083 | |
| R2 | 0.749 | 0.698 | 0.759 |
| Model | Values |
|---|---|
| Pseudo-first order | |
| qcal (mg/g) | 0.84 |
| k1 (min−1) | 0.23 |
| R2 | 0.89 |
| RMSE | 0.24 |
| S. D. (%) | 63.53 |
| Pseudo-second order | |
| qcal (mg/g) | 2.24 |
| k2 (g/min mg) | 0.67 |
| R2 | 0.99 |
| RMSE | 0.082 |
| S. D. (%) | 18.21 |
| Intraparticle diffusion | |
| C1 (mg/g) | 1.64 |
| C2 (mg/g) | 1.62 |
| C3 (mg/g) | 1.9 |
| Ki1 (mg/g.min0.5) | 0.16 |
| Ki2 (mg/g.min0.5) | 0.14 |
| Ki3 (mg/g.min0.5) | 0.064 |
| R21 | 1 |
| R22 | 0.99 |
| R23 | 1 |
| Temperature (K) | ΔH° (KJ/mol) | ΔS° (J/mol K) | ΔG° (kJ/mol) |
|---|---|---|---|
| 20.73 | 83.05 | ||
| 303 | −4.44 | ||
| 313 | −5.27 | ||
| 323 | −6.10 |
| Sorbent Materials | F− Sorption Capacity (mg/g) | Optimized Condition | Microbial Removal Potential | Reference |
|---|---|---|---|---|
| HaP nanorods | 1.49 | 3 h. pH 7; 7 g/L | Nil | [88] |
| Sawdust raw | 1.73 | pH 6, 0.5 g/25 mL | Nil | [89] |
| Cellulose–hydroxyapatite | 4.2 | pH 6.5. | Nil | [90] |
| Ag/MgOnHaP | 2.15 | 60 min; pH 6; 0.3 g | Yes | [26] |
| AgMgOnHaP@CSn | 6.86 | 40 min; pH 7; 0.25 g | Yes | [28] |
| CNF-AgMgOnHaP | 8.71 | 0.25 g; 10 min; pH 5 | Yes | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayinde, W.B.; Gitari, M.W.; Smith, J.A.; Samie, A. Sorption of Fluoride and Bacterial Disinfection Property of Biosynthesized Nanofibrous Cellulose Decorated Ag–MgO–Nanohydroxyapatite Composite for Household Water Treatment. Polymers 2022, 14, 890. https://doi.org/10.3390/polym14050890
Ayinde WB, Gitari MW, Smith JA, Samie A. Sorption of Fluoride and Bacterial Disinfection Property of Biosynthesized Nanofibrous Cellulose Decorated Ag–MgO–Nanohydroxyapatite Composite for Household Water Treatment. Polymers. 2022; 14(5):890. https://doi.org/10.3390/polym14050890
Chicago/Turabian StyleAyinde, Wasiu B., Mugera W. Gitari, James A. Smith, and Amidou Samie. 2022. "Sorption of Fluoride and Bacterial Disinfection Property of Biosynthesized Nanofibrous Cellulose Decorated Ag–MgO–Nanohydroxyapatite Composite for Household Water Treatment" Polymers 14, no. 5: 890. https://doi.org/10.3390/polym14050890
APA StyleAyinde, W. B., Gitari, M. W., Smith, J. A., & Samie, A. (2022). Sorption of Fluoride and Bacterial Disinfection Property of Biosynthesized Nanofibrous Cellulose Decorated Ag–MgO–Nanohydroxyapatite Composite for Household Water Treatment. Polymers, 14(5), 890. https://doi.org/10.3390/polym14050890