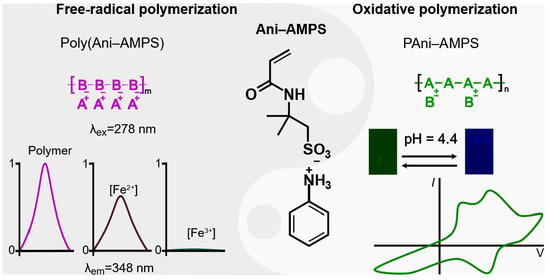
Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
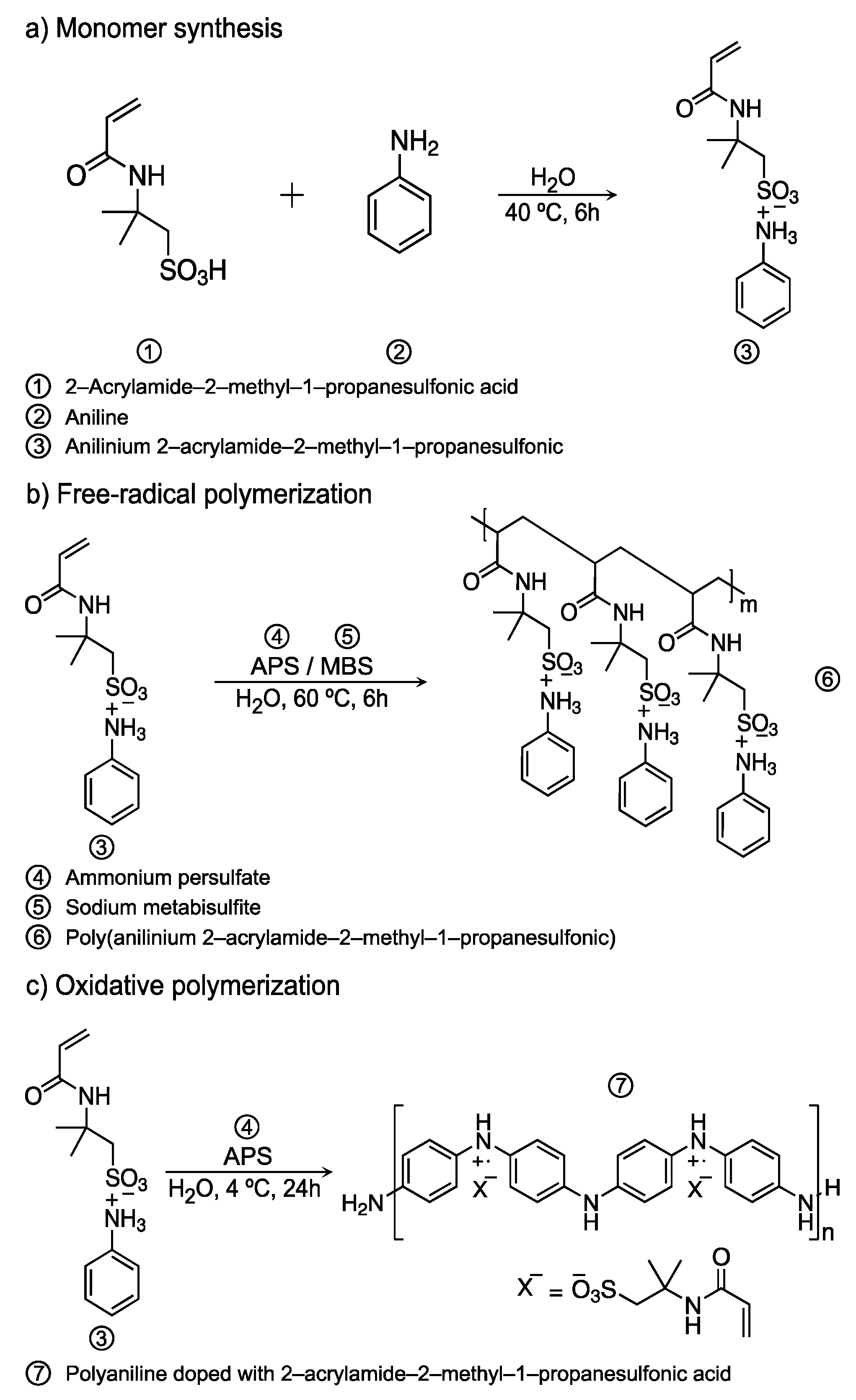
2.2. Synthesis of Anilinium Salt
2.3. Free-Radical Polymerization of Ani-AMPS
2.4. Oxidative Polymerization
2.5. Characterization
3. Results and Discussion
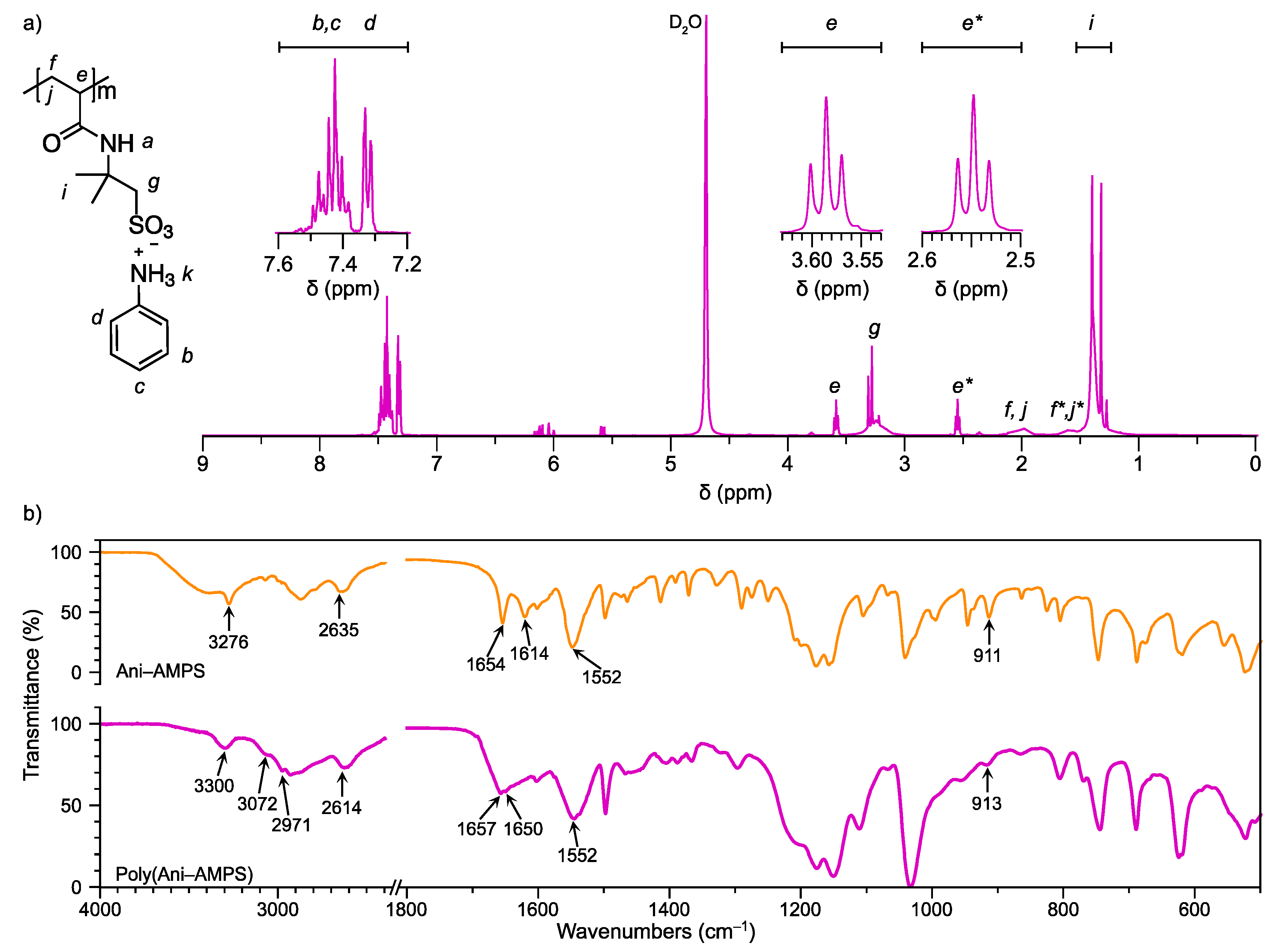
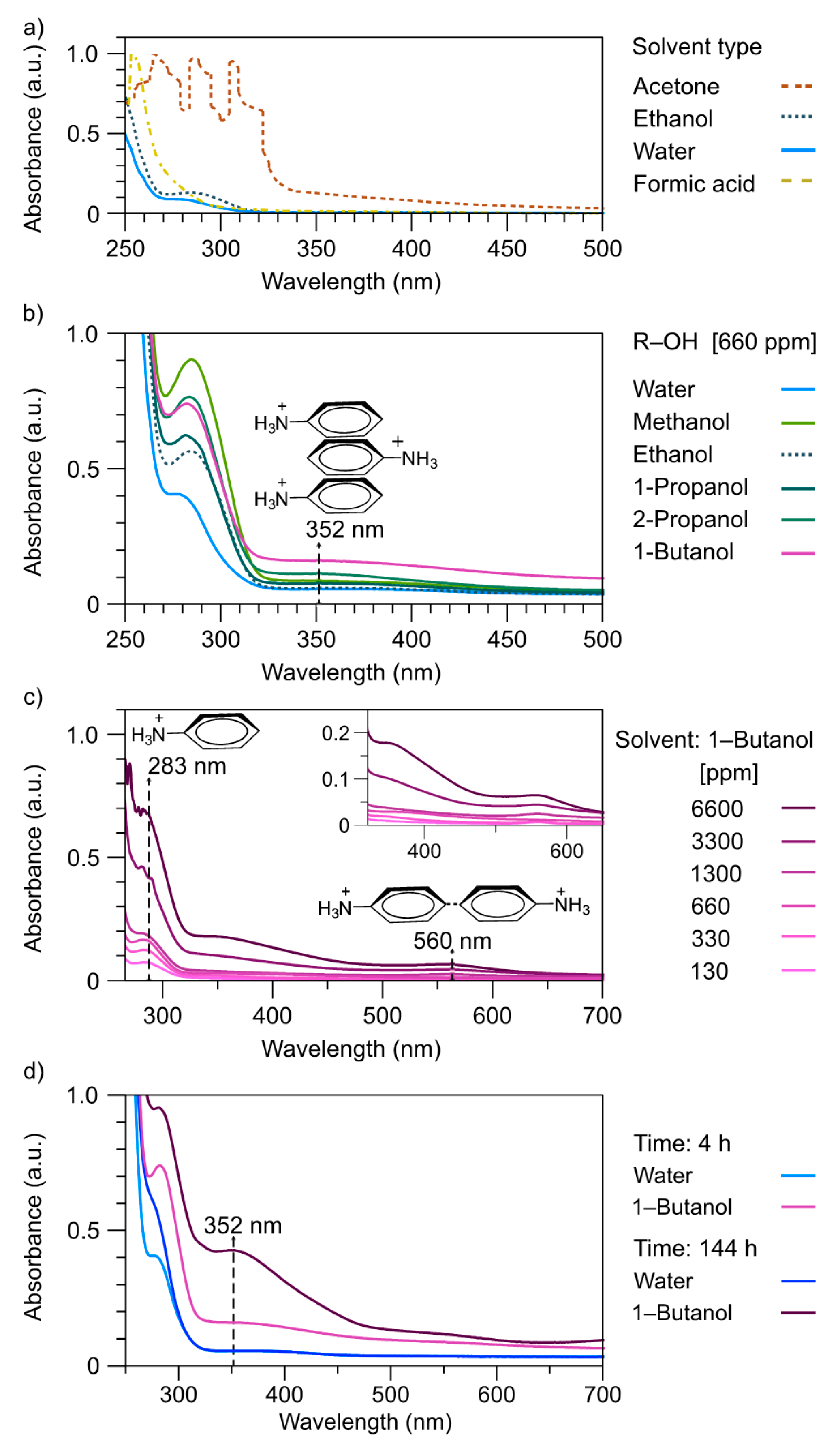
3.1. Monomer Characterization
3.2. Characterization of the Product of Free-Radical Polymerization
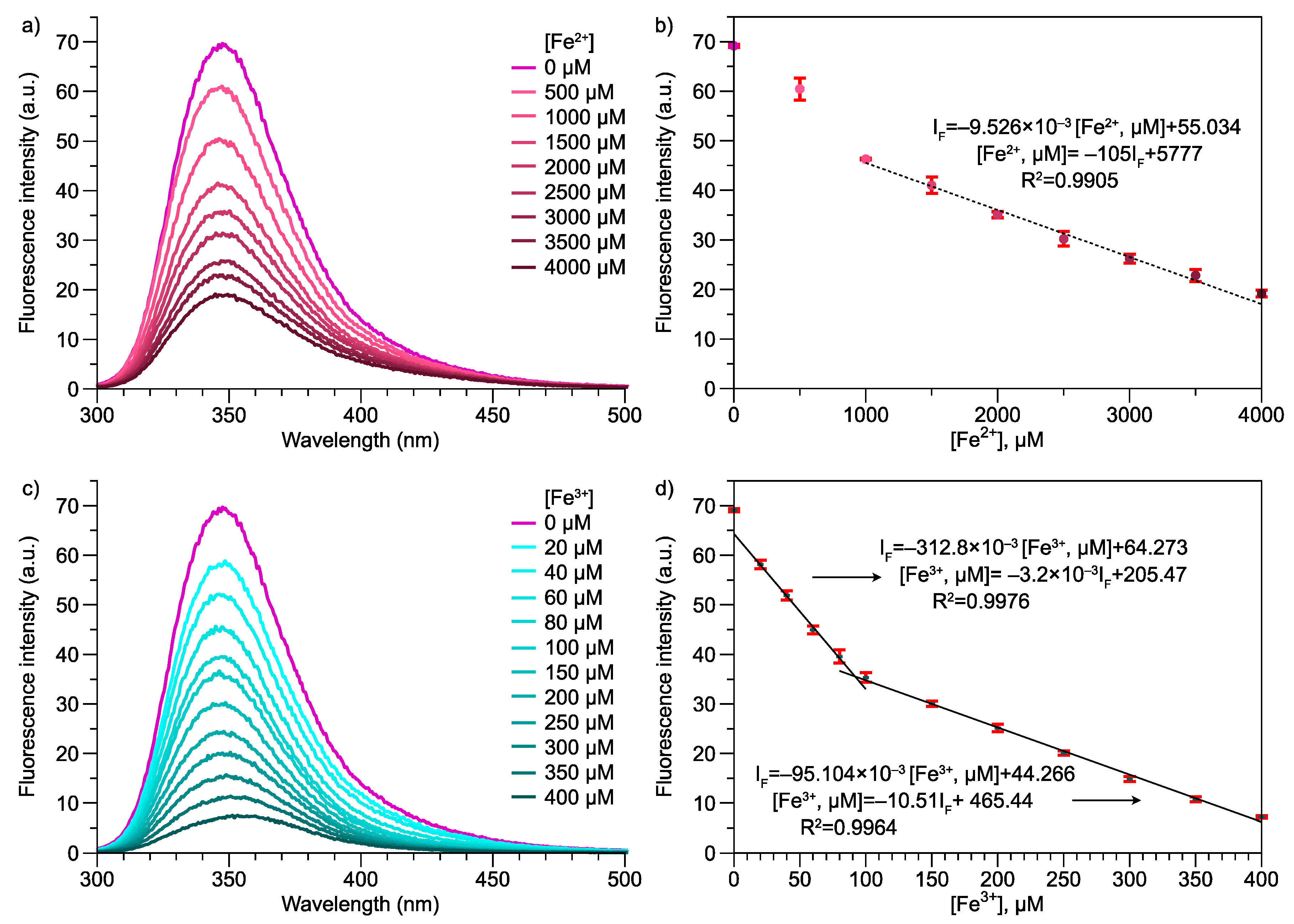
3.3. Photoluminescence Properties of Poly(Ani-AMPS)
3.4. Oxidative Polymerization
3.5. Comparison of Poly(Ani-AMPS) and PAni-AMPS
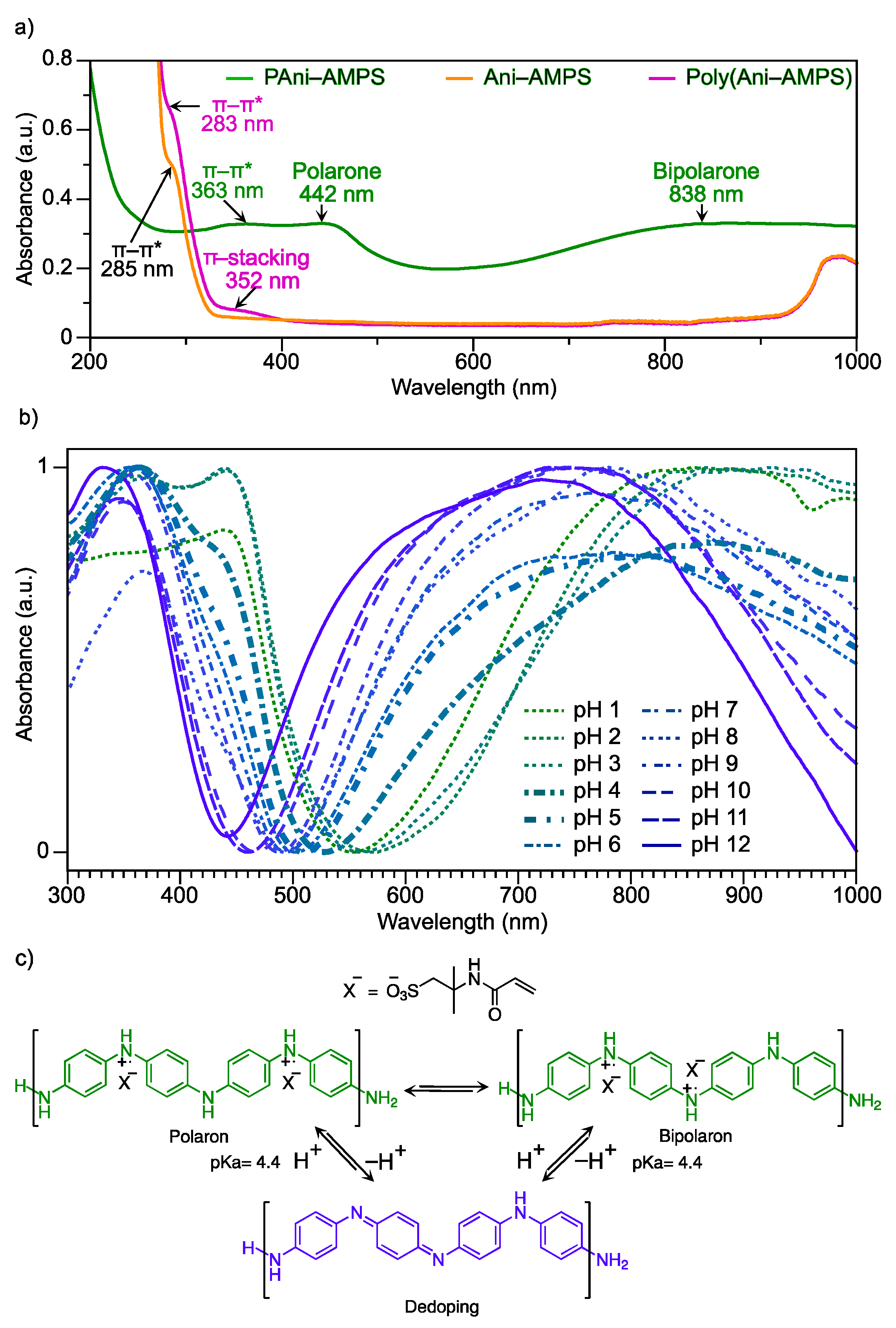
3.5.1. Optical Properties
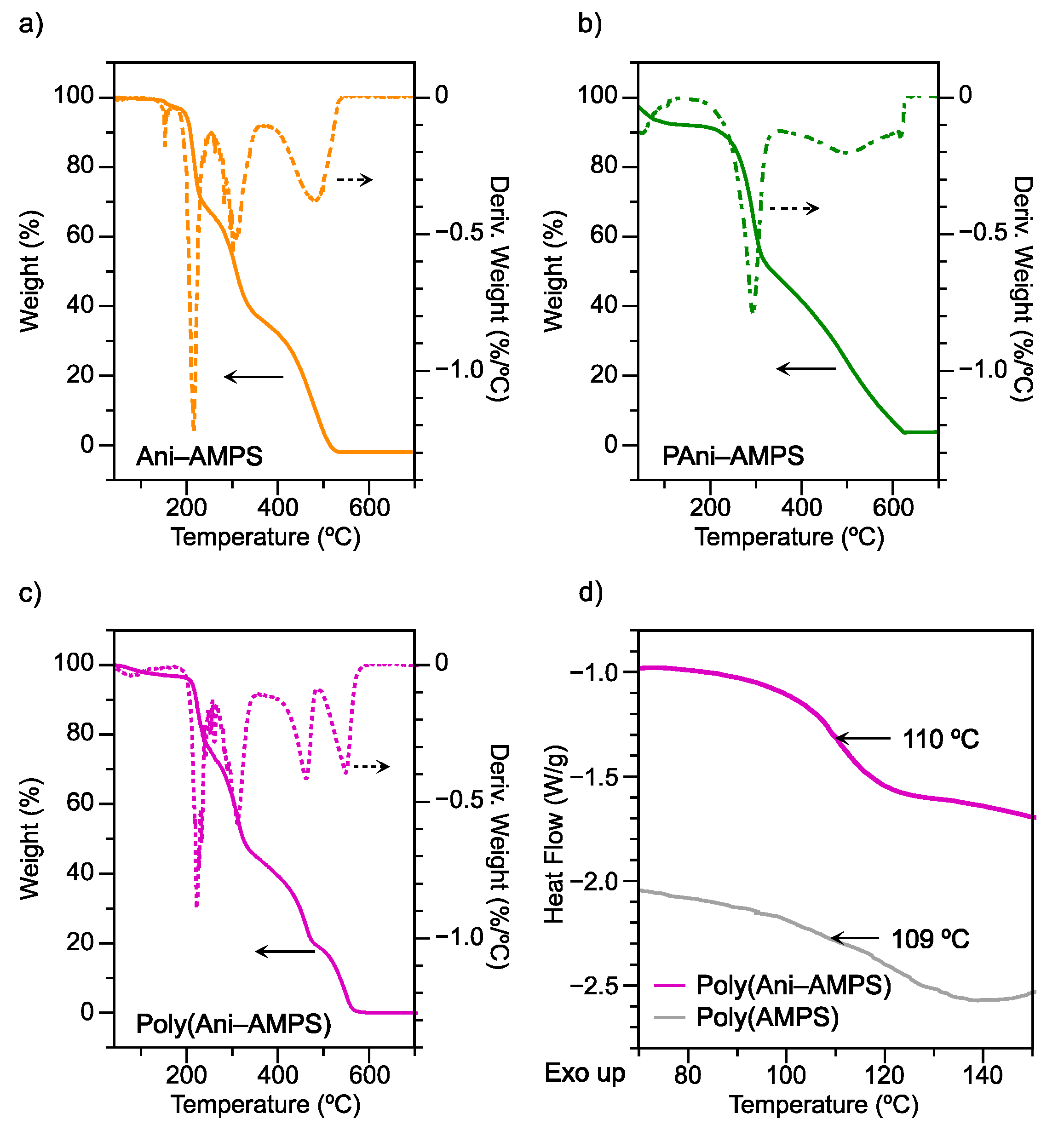
3.5.2. Thermal Analysis
3.5.3. Morphology
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerrits, L.; Hammink, R.; Kouwer, P.H.J. Semiflexible polymer scaffolds: An overview of conjugation strategies. Polym. Chem. 2021, 12, 1362–1392. [Google Scholar] [CrossRef]
- Plummer, C.M.; Li, L.; Chen, Y. The post-modification of polyolefins with emerging synthetic methods. Polym. Chem. 2020, 11, 6862–6872. [Google Scholar] [CrossRef]
- Brännström, S.; Johansson, M.; Malmström, E. Enzymatically Synthesized Vinyl Ether-Disulfide Monomer Enabling an Orthogonal Combination of Free Radical and Cationic Chemistry toward Sustainable Functional Networks. Biomacromolecules 2019, 20, 1308–1316. [Google Scholar] [CrossRef]
- Liu, H.; Dong, X.; Liu, F.; Zheng, J.; Sun, Y. Iminodiacetic acid-conjugated nanoparticles as a bifunctional modulator against Zn2+-mediated amyloid β-protein aggregation and cytotoxicity. J. Colloid Interface Sci. 2017, 505, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, L.; Zhang, H.; Lin, W.; Wang, Y. Investigation on multifunctional monomer modified polypropylene and its foamability. J. Appl. Polym. Sci. 2013, 130, 1675–1681. [Google Scholar] [CrossRef]
- Sivanesan, D.; Seo, B.K.; Lim, C.S.; Kim, S.; Kim, H.G. Trifunctional cycloaliphatic epoxy-based thermoset polymers: Synthesis, polymerization, and characterization. Polymer 2021, 220. [Google Scholar] [CrossRef]
- Chan, E.W.; Baek, P.; Barker, D.; Travas-Sejdic, J. Highly functionalisable polythiophene phenylenes. Polym. Chem. 2015, 6, 7618. [Google Scholar] [CrossRef]
- Klein, R.; Übel, F.; Frey, H. Maleimide Glycidyl Ether: A Bifunctional Monomer for Orthogonal Cationic and Radical Polymerizations. Macromol. Rapid Commun. 2015, 36, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Conejo-Dávila, A.S.; Hernández-Escobar, C.A.; Vega-Rios, A.; Rodríguez-Sánchez, I.; Estrada-Monje, A.; de León-Gómez, R.E.; Zaragoza-Contreras, E.A. Selective polymerization of a new bifunctional monomer via free radical polymerization and oxidative route. Synth. Met. 2020, 259, 116258. [Google Scholar] [CrossRef]
- Usha, R.; Hema, N.; Ambika, V.R.; Shalini, D.; Jayalakshmi, D. Synthesis, growth and characterization of nonlinear optical organic crystal: 4-Methoxy Anilinium 5-Sulfo Salicylate. Mater. Res. Innov. 2019, 23, 107–112. [Google Scholar] [CrossRef]
- Cruz-Medina, R.; Vega-Rios, A.; Hernández-Escobar, C.A.; Estrada-Monje, A.; Rodríguez-Sánchez, I.; Zaragoza-Contreras, E.A. Polystyrene-polyaniline core-shell composite particles using a bifunctional selectively polymerizable monomer as the interfacial linkage. Synth. Met. 2020, 265, 116402. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Marchewka, M.K.; Pietraszko, A. Structural and vibrational studies of anilinium nitrate. J. Phys. Chem. Solids. 2005, 66, 1039–1048. [Google Scholar] [CrossRef]
- Vivek, P.; Jauhar, R.M.; Suvitha, A.; Era, P.; Ananth, S.; Murugakoothan, P. Habitual growth and its influence on the properties of anilinium perchlorate (AP) single crystal for nonlinear optical device applications. J. Mater. Sci. Mater. Electron. 2018, 29, 5718–5725. [Google Scholar] [CrossRef]
- Sudharsana, N.; Subramanian, G.; Krishnakumar, V.; Nagalakshmi, R. Growth and characterization of anilinium hydrogen sulfate (AHS) single crystals: An organic nonlinear optical material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-Contreras, E.A.; Stockton-Leal, M.; Hernández-Escobar, C.A.; Hoshina, Y.; Guzmán-Lozano, J.F.; Kobayashi, T. Synthesis of core-shell composites using an inverse surfmer. J. Colloid Interface Sci. 2012, 377, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Yu, X.; Zhang, J.; Cheng, N.; Huang, X. Electropolymerization in a novel proton functionalized room temperature ionic liquid anilinium acetate. Synth. Met. 2015, 204, 76–83. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sumi, S.; Terauchi, T.; Hashizume, D. Ionic semiconductor: DC and AC conductivity of anilinium tetrathiafulvalene-2-carboxylate. Dalt. Trans. 2013, 42, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Hoshino, N.; Takeda, T.; Moritomo, H.; Kawamata, J.; Nakamura, T.; Akutagawa, T. The Formation of Organogels and Helical Nanofibers from Simple Organic Salts. Chem. A Eur. J. 2014, 20, 16279–16285. [Google Scholar] [CrossRef]
- Du, X.-S.; Zhou, C.-F.; Wang, G.-T.; Mai, Y.-W. Novel Solid-State and Template-Free Synthesis of Branched Polyaniline Nanofibers. Chem. Mater. 2008, 20, 3806–3808. [Google Scholar] [CrossRef]
- Raghava, K.; Pill, K.; Iyengar, A. Self-assembly approach for the synthesis of electro-magnetic functionalized Fe3O4/polyaniline nanocomposites: Effect of dopant on the properties. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 320, 49–56. [Google Scholar] [CrossRef]
- Tutgun, M.S.; Sinirlioglu, D.; Celik, S.U.; Bozkurt, A. Preparation and characterization of hexagonal boron nitride and PAMPS-NMPA-based thin composite films and investigation of their membrane properties. Ionics 2015, 21, 2871–2878. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Wang, C.; Xiang, K.; Deng, M.; Yin, H. PAMPS/MMT composite hydrogel electrolyte for solid-state supercapacitors. J. Alloys Compd. 2017, 709, 596–601. [Google Scholar] [CrossRef]
- Pehlivan, L.; Métay, E.; Laval, S.; Dayoub, W.; Demonchaux, P.; Mignani, G.; Lemaire, M. Alternative method for the reduction of aromatic nitro to amine using TMDS-iron catalyst system. Tetrahedron Lett. 2011, 67, 1971–1976. [Google Scholar] [CrossRef]
- Aguilar, R.; Gallardo, A.; Ferna, M. In Situ Quantitative 1 H NMR Monitoring of Monomer Consumption: A Simple and Fast Way of Estimating Reactivity Ratios. Macromolecules 2002, 35, 2036–2041. [Google Scholar] [CrossRef]
- Nadim, E.; Bouhendi, H.; Ziaee, F.; Nouri, A. Kinetic Study of the Aqueous Free-Radical Polymerization of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid via an Online Proton Nuclear Magnetic Resonance Technique. J. Appl. Polym. Sci. 2012, 126, 156–161. [Google Scholar] [CrossRef]
- Evans, J.C. The vibrational assignments and configuration of aniline, aniline-NHD and aniline-ND2. Spectrochim. Acta. 1960, 16, 428–442. [Google Scholar] [CrossRef]
- Das, M.; Devi, N.; Sarma, J.; Devi, N. Preparation, characterization, and water sorption study of 2-acrylamido-2- methylpropane sulfonic acid ( AMPS ) based hydrogel. J. Chem. Pharm. Res. 2014, 6, 800–806. [Google Scholar]
- Kim, S.K.; Lim, J.M.; Pradhan, T.; Jung, H.S.; Lynch, V.M.; Kim, J.S.; Kim, D.; Sessler, J.L. Self-Association and Nitroaromatic-Induced Deaggregation of Pyrene Substituted Pyridine Amides. J. Am. Chem. Soc. 2014, 136, 495–505. [Google Scholar] [CrossRef]
- Morisaki, Y.; Ishida, T.; Chujo, Y. Synthesis and Optical Properties of Novel Through-Space π-Conjugated Polymers Having a Dithia[3.3]metacyclophane Skeleton in the Main Chain. Polym. J. 2003, 35, 501–506. [Google Scholar] [CrossRef][Green Version]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Shen, Y.; Xi, J.; Qiu, X.; Zhu, W. A new proton conducting membrane based on copolymer of methyl methacrylate and 2-acrylamido-2-methyl-1-propanesulfonic acid for direct methanol fuel cells. Electrochim. Acta. 2007, 52, 6956–6961. [Google Scholar] [CrossRef]
- Dai, J.; Coh, S.H.; Lee, S.Y.; Slow, K.S. Complexation between poly(2-hydroxypropyl methacrylate) and three tertiary amide polymers. J. Appl. Polym. Sci. 1994, 54, 1585. [Google Scholar] [CrossRef]
- Sun, J.; Shang, K.; Wu, Y.; Zhang, Q.; Yao, X.; Yang, Y.; Hu, D.; Liu, J. Three new Coordination Polymers based on a 1-(3,5-dicarboxy-benzyl)-1H-pyrazole-3,5-dicarboxylic acid ligand: Synthesis, crystal structures, magnetic properties and selectively sensing properties. Polyhedron 2018, 141, 223–229. [Google Scholar] [CrossRef]
- Joshi, J.C.; Pant, D.D. Characteristic luminescence of the anilinium ion and the pKa values of excited singlet and triplet states. Chem. Phys. Lett. 1978, 59, 529–532. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Cai, X.; Caiyun, L.; Jia, P.; Li, Z.; Zhu, H.; Yu, Y.; Wang, K.; Li, X.; et al. A highly sensitive rapid-response fluorescent probe for specifically tracking endogenous labile Fe2+ in living cells and zebrafish. Dye. Pigment. 2020, 174, 108065. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Y.; Hu, Y.; Yuan, M.; Zheng, X.; Huang, X. Facile synthesis of amino-functionalized polyphosphazene microspheres and their application for highly sensitive fluorescence detection of Fe3+. J. Appl. Polym. Sci. 2020, 137, 48937. [Google Scholar] [CrossRef]
- Selvaraj, M.; Rajalakshmi, K.; Nam, Y.-S.; Lee, Y.; Song, J.-W.; Lee, H.-J.; Lee, K.-B. On-off-on relay fluorescence recognition of ferric and fluoride ions based on indicator displacement in living cells. Anal. Chim. Acta 2019, 1066, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, B.; Jiang, X.; Zhu, X.; Zheng, X. Fluorescent linear polyurea based on toluene diisocyanate: Easy preparation, broad emission and potential applications. Chem. Eng. J. 2020, 399, 125867. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, I.K. Optical and electrochemical properties and sensing application for iron (II) and mercury (II) ions of polyfluorenes with imidazole in the main chain. Polym. Int. 2019, 68, 1033–1041. [Google Scholar] [CrossRef]
- Fu, S.; Cai, Z.; Liu, L.; Yang, L.; Jin, R.; Lu, Z.; Ai, H. Controlled aggregation of amphiphilic aggregation-induced emission polycation and superparamagnetic iron oxide nanoparticles as fluorescence/magnetic resonance imaging probes. J. Appl. Polym. Sci. 2020, 137, 48760. [Google Scholar] [CrossRef]
- Tang, T.; Guo, W.; Xu, Y.; Xu, D. Synthesis and characterization of novel hyperbranched fluorescent polymers that can be precisely used for metal ion detection and quantification. J. Appl. Polym. Sci. 2020, 137, 48933. [Google Scholar] [CrossRef]
- Yang, C.B.; Jiang, C.B.; Zhang, M.Y.; Chen, X.; Zou, P.; Yang, R.W.; Rao, H.B.; Wang, G.T. A multifunctional Eu-based coordination polymer luminescent sensor for highly sensitive and selective detection of Fe3+ and acetone. Polyhedron 2020, 175, 1–7. [Google Scholar] [CrossRef]
- Sendenkova, I.; Trchová, M.; Blinova, N.V.; Stejskal, J. In-situ polymerized polyaniline films. Preparation in solutions of hydrochloric, sulfuric, or phosphoric acid. Thin Solid Films 2006, 515, 1640–1646. [Google Scholar] [CrossRef]
- Belaabed, B.; Lamouri, S.; Naar, N.; Bourson, P.; Hamady, S.O.S. Polyaniline-doped benzene sulfonic acid/epoxy resin composites: Structural, morphological, thermal and dielectric behaviors. Polym. J. 2010, 42, 546–554. [Google Scholar] [CrossRef]
- Arasi, A.Y.; Jeyakumari, J.J.L.; Sundaresan, B.; Dhanalakshmi, V.; Anbarasan, R. The structural properties of Poly(aniline)-Analysis via FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Chou, C.T.; Chen, Y.W.; Kuo, K.T.; Yang, S.M. Electrochemical behaviors of polyaniline-poly(styrene-sulfonic acid) complexes and related films. J. Appl. Polym. Sci. 2006, 100, 4023–4044. [Google Scholar] [CrossRef]
- Hechavarría, L.; Hu, H.; Rincón, M.E. Polyaniline-poly(2-acrylamido-2-methyl-1-propanosulfonic acid) composite thin films: Structure and properties. Thin Solid Films 2003, 441, 56–62. [Google Scholar] [CrossRef]
- Tawde, S.; Mukesh, D.; Yakhmi, J.V. Redox behavior of polyaniline as in¯uenced by aromatic sulphonate anions: Cyclic voltammetry and molecular modeling. Synth. Met. 2002, 125, 401–413. [Google Scholar] [CrossRef]
- Vivier, V.; Cachet-vivier, C.; Michel, D.; Nedelec, J.; Yu, L.T. Voltamperommetric study of chemically made polyaniline powder with cavity microelectrode technique. Synth. Met. 2002, 126, 253–262. [Google Scholar] [CrossRef]
- Shao, L.; Qiu, J.; Liu, M.; Feng, H.; Lei, L.; Zhang, G.; Zhao, Y.; Gao, C.; Qin, L. Synthesis and characterization of water-soluble polyaniline films. Synth. Met. 2011, 161, 806–811. [Google Scholar] [CrossRef]
- Pruneanu, S.; Veress, E.; Marian, I.; Oniciu, L. Characterization of polyaniline by cyclic voltammetry and UV-Vis absorption spectroscopy. J. Mater. Sci. 1999, 4, 2733–2739. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Kuswandi, B.; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control. 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding Graphene Paper Supported Three-Dimensional Porous Graphene−Polyaniline Nanocomposite Synthesized by Inkjet Printing and in Flexible All-Solid-State Supercapacitor. Appl. Mater. Interfaces. 2014, 6, 16312–16319. [Google Scholar] [CrossRef]
- Thompson, J.O.F.; Saalbach, L.; Crane, S.W.; Paterson, M.J.; Townsend, D. Ultraviolet relaxation dynamics of aniline, N, N -dimethylaniline and 3,5-dimethylaniline at 250 nm. J. Chem. Phys. 2015, 142. [Google Scholar] [CrossRef]
- Gospodinova, N.; Mokreva, P.; Terlemezyan, L. Chemical oxidative polymerization of aniline in aqueous medium without added acids. Polymer 1993, 34, 2438–2439. [Google Scholar] [CrossRef]
- Kumar, D.; Iwamoto, M. Investigation of the chiroptical behavior of optically active polyaniline synthesized from naturally occurring amino acids. Polym. J. 2013, 45, 160–165. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Devi, J.S.A.; Aparna, R.S.; Anjana, R.R.; Nebu, J.; Anju, S.M.; George, S. Solvent Effects: A Signature of J- and H-Aggregate of Carbon Nanodots in Polar Solvents. J. Phys. Chem. A. 2019, 123, 7420–7429. [Google Scholar] [CrossRef] [PubMed]
- So, Y.; Zaleski, J.M.; Murlick, C.; Ellaboudy, A. Synthesis and Photophysical Properties of Some Benzoxazole and Benzothiazole Compounds. Macromolecules 1996, 29, 2783–2795. [Google Scholar] [CrossRef]
- Shibahara, M.; Watanabe, M.; Iwanaga, T.; Matsumoto, T.; Ideta, K.; Shinmyozu, T. Synthesis, structure, and transannular π-π interaction of three- and four-layered [3.3]paracyclophanes. J. Org. Chem. 2008, 73, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Wojtyk, J.; McKerrow, A.; Kazmaier, P.; Buncel, E. Quantitative investigations of the aggregation behaviour of hydrophobic anilino squaraine dyes through UV/vis spectroscopy and dynamic light scattering. Can. J. Chem. 1999, 77, 903–912. [Google Scholar] [CrossRef]
- Hu, R.; Zhu, Q.; Chen, W.; Liu, H.; Yao, B.; Zhan, J.; Hao, J.; Han, C.C. Ordered dichloro-biphenylene-bridged silsesquioxanes fabricated by interfacial polymerization. Polymer 2012, 53, 267–271. [Google Scholar] [CrossRef]
- Griffiths, T.R.; Pugh, D.C. Correlations among solvent polarity scales, dielectric constant and dipole moment, and a means to reliable predictions of polarity scale values from current data. Coord. Chem. Rev. 1979, 29, 129–211. [Google Scholar] [CrossRef]
- Petr, A.; Wei, D.; Kvarnström, C.; Ivaska, A.; Dunsch, L. π-Dimer of an Aniline Dimer: An ESR−UV−Vis Spectroelectrochemical Study. J. Phys. Chem. B 2007, 111, 12395–12398. [Google Scholar] [CrossRef]
- Celebioglu, A.; Durgun, E.; Uyar, T. Selective and Efficient Removal of Volatile Organic Compounds by Channel-type Gamma-Cyclodextrin Assembly through Inclusion Complexation. Ind. Eng. Chem. Res. 2017, 56, 7345–7354. [Google Scholar] [CrossRef]
- Gong, C.; Pinatti, L.; Lavigne, G.; Shaw, M.T.; Scola, D.A. Thermal stability of end-capped and linear sulfonated polyimides, sulfonated polystyrene, and Nafion 117. J. Appl. Polym. Sci. 2018, 135, 45694. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, R.; Guha, S.; Chatterjee, U.; Jewrajka, S.K. Homogeneous phase crosslinked poly(acrylonitrile-co-2-acrylamido-2-methyl-1-propanesulfonic acid) conetwork cation exchange membranes showing high electrochemical properties and electrodialysis performance. Polymer 2019, 180, 121680. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Teo, M.Y.B.; Khor, E.; Lim, C.N. Thermal analysis of conducting polymers part I. J. Therm. Anal. 1989, 35, 765–774. [Google Scholar] [CrossRef]
- Amano, K.; Ishikawa, H.; Kobayashi, A.; Satoh, M.; Hasegawa, E. Thermal stability of chemically synthesized polyaniline. Synth. Met. 1994, 62, 229–232. [Google Scholar] [CrossRef]
- Gong, X.; Li, G.; Wu, Y.; Zhang, J.; Feng, S.; Liu, Y.; Li, C.; Ma, W.; Bo, Z. Enhancing the Performance of Polymer Solar Cells by Using Donor Polymers Carrying Discretely Distributed Side Chains. ACS Appl. Mater. Interfaces. 2017, 9, 24020–24026. [Google Scholar] [CrossRef]
- Fang, F.F.; Dong, Y.-Z.; Choi, H.J. Effect of oxidants on morphology of interfacial polymerized polyaniline nanofibers and their electrorheological response. Polymer 2018, 158, 176–182. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef]
- Shishov, M.A.; Moshnikov, V.A.; Sapurina, I.Y. Nanostructures of oligoaniline and polyaniline and their properties. Glas. Phys. Chem. 2011, 37, 106–110. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conejo-Dávila, A.S.; Moya-Quevedo, M.A.; Chávez-Flores, D.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate. Polymers 2021, 13, 2349. https://doi.org/10.3390/polym13142349
Conejo-Dávila AS, Moya-Quevedo MA, Chávez-Flores D, Vega-Rios A, Zaragoza-Contreras EA. Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate. Polymers. 2021; 13(14):2349. https://doi.org/10.3390/polym13142349
Chicago/Turabian StyleConejo-Dávila, Alain Salvador, Marco Armando Moya-Quevedo, David Chávez-Flores, Alejandro Vega-Rios, and Erasto Armando Zaragoza-Contreras. 2021. "Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate" Polymers 13, no. 14: 2349. https://doi.org/10.3390/polym13142349
APA StyleConejo-Dávila, A. S., Moya-Quevedo, M. A., Chávez-Flores, D., Vega-Rios, A., & Zaragoza-Contreras, E. A. (2021). Role of the Anilinium Ion on the Selective Polymerization of Anilinium 2-Acrylamide-2-methyl-1-propanesulfonate. Polymers, 13(14), 2349. https://doi.org/10.3390/polym13142349