Automatic Detoxification Medicine Delivery by Thermo-Sensitive Poly(ethylene glycol)-Based Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polystyrene (PS) Core Microspheres
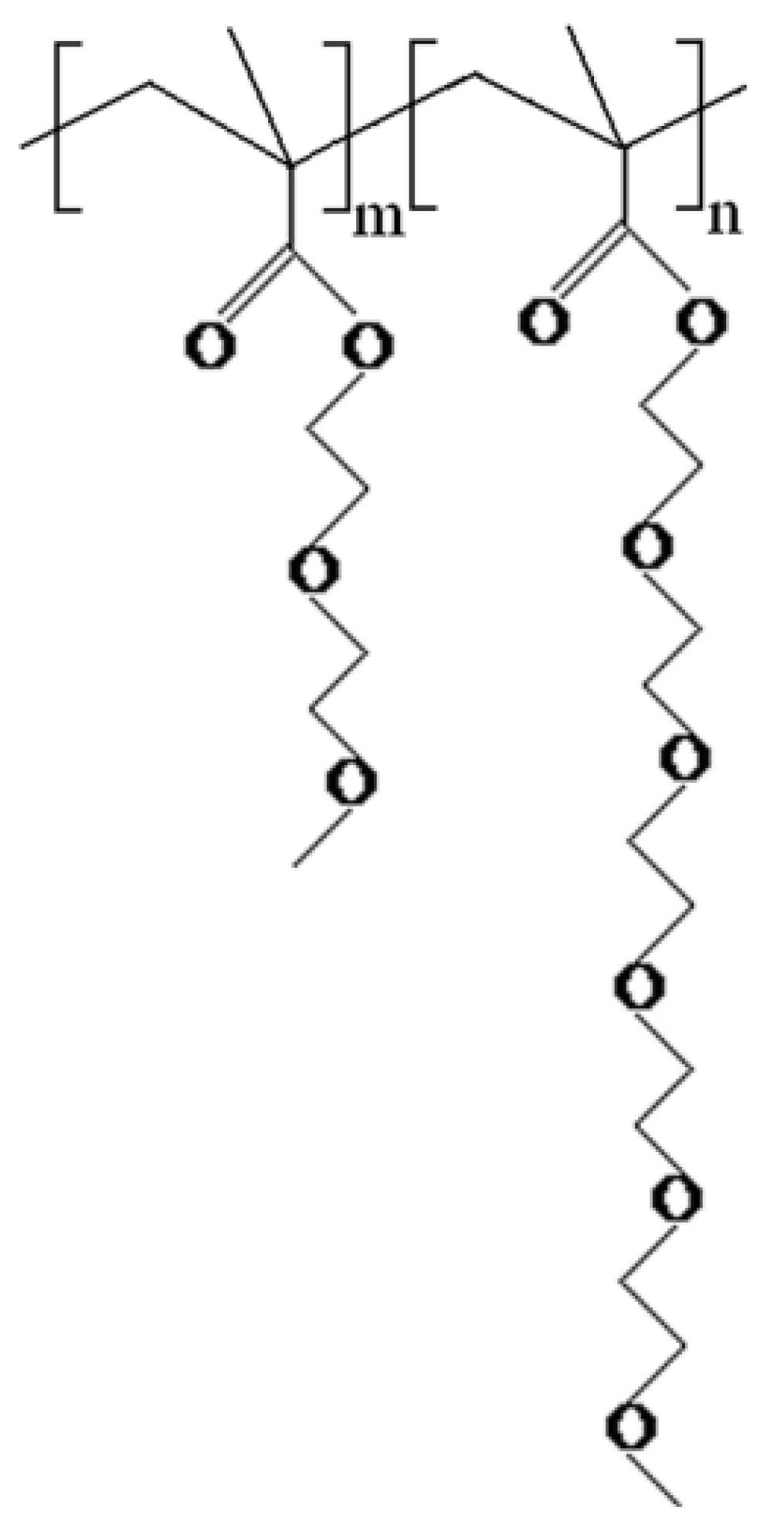
2.3. Preparation of PS@P(MEO2MA-co-MEO5MA) Nanogels
2.4. Cell Viability Evaluation of Nanogels
2.5. Drug Loading and Release
2.6. Characterization
3. Results and Discussion
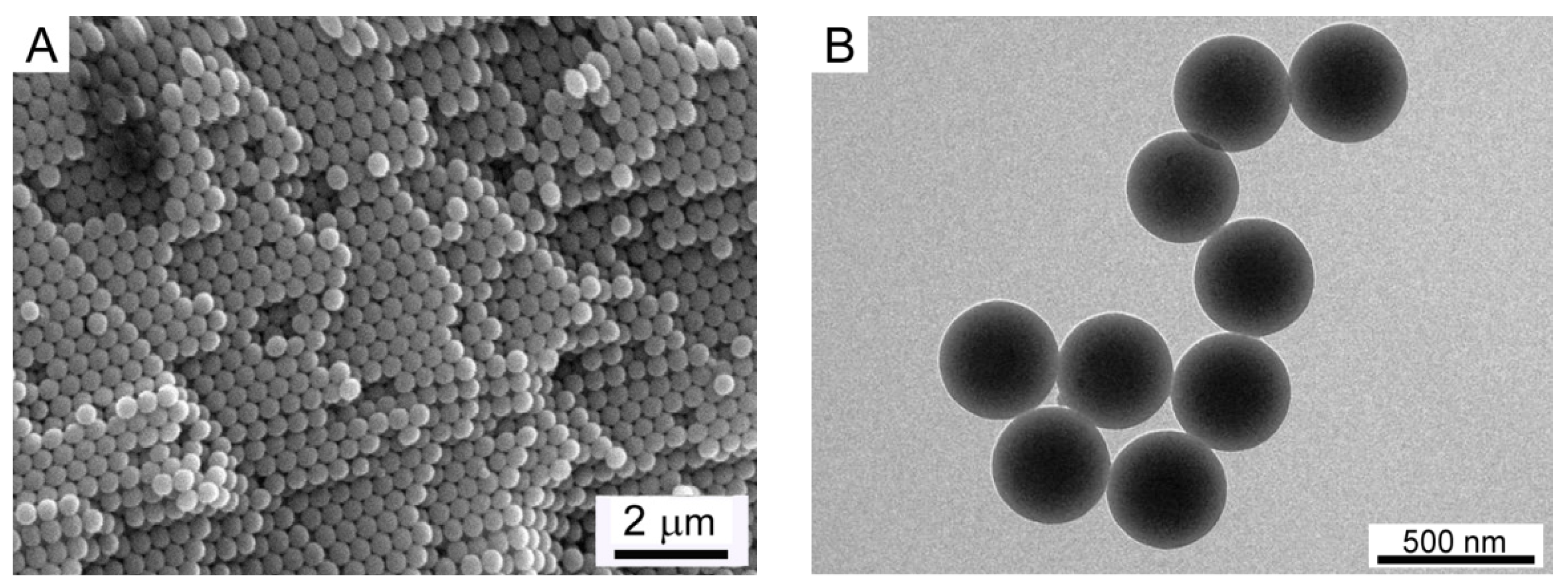
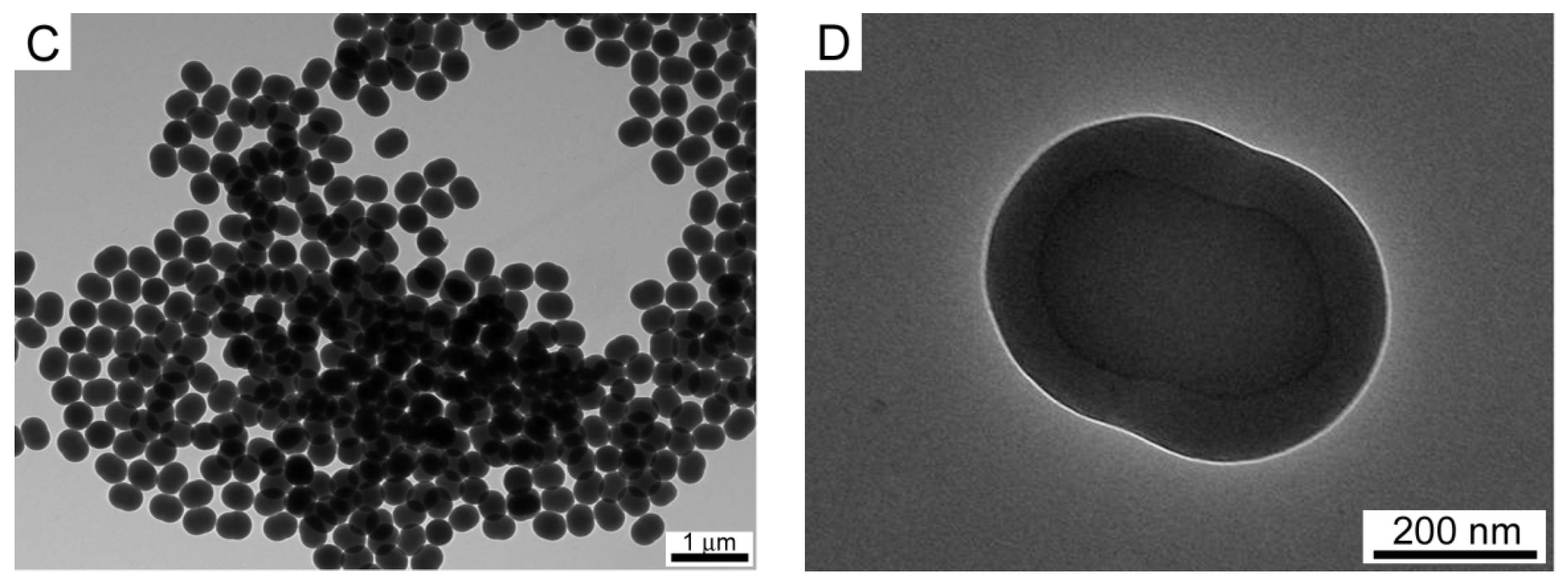
3.1. Morphology of PS Core and PS@(MEO2MA-co-MEO5MA) Nanogels
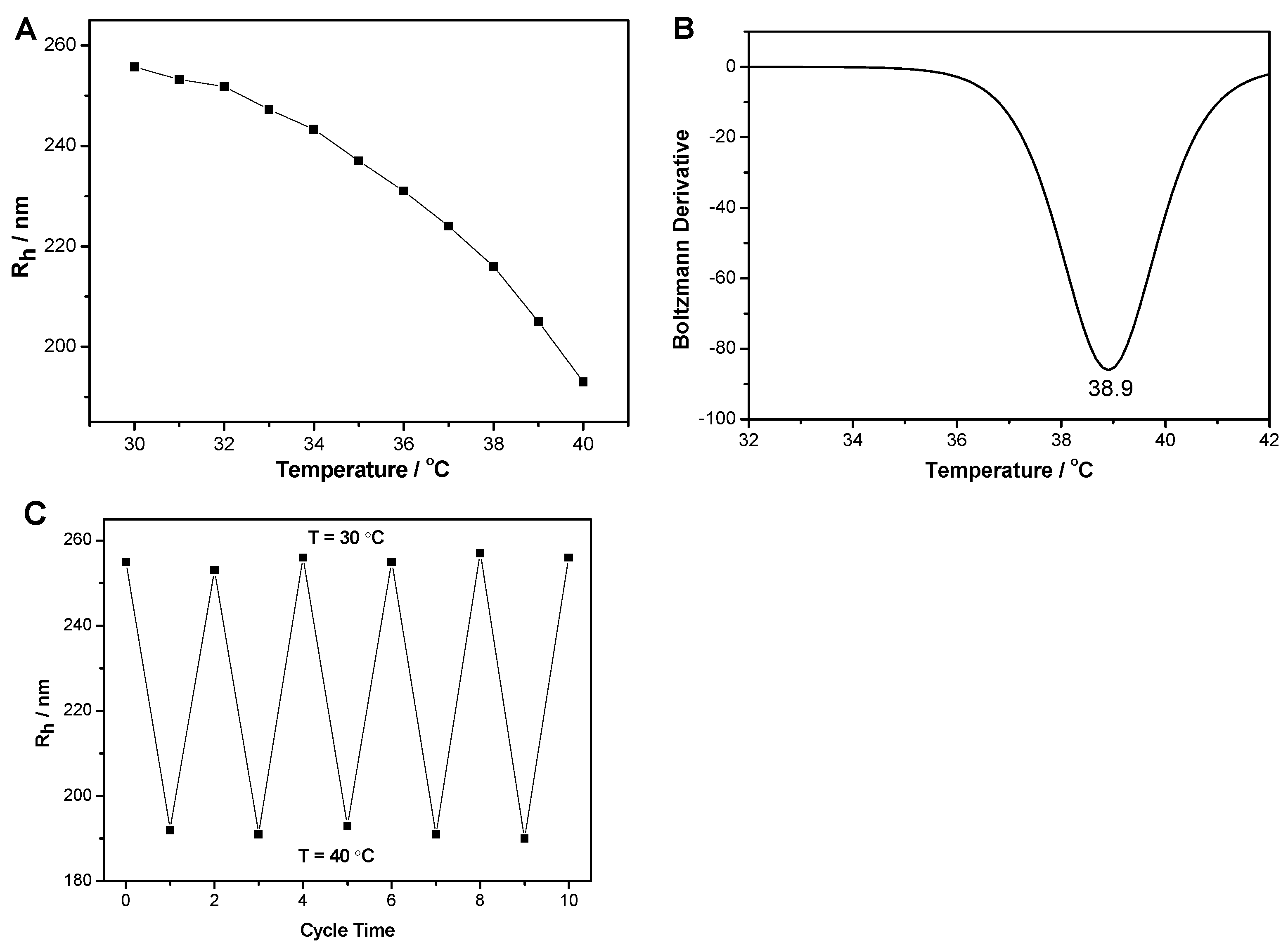
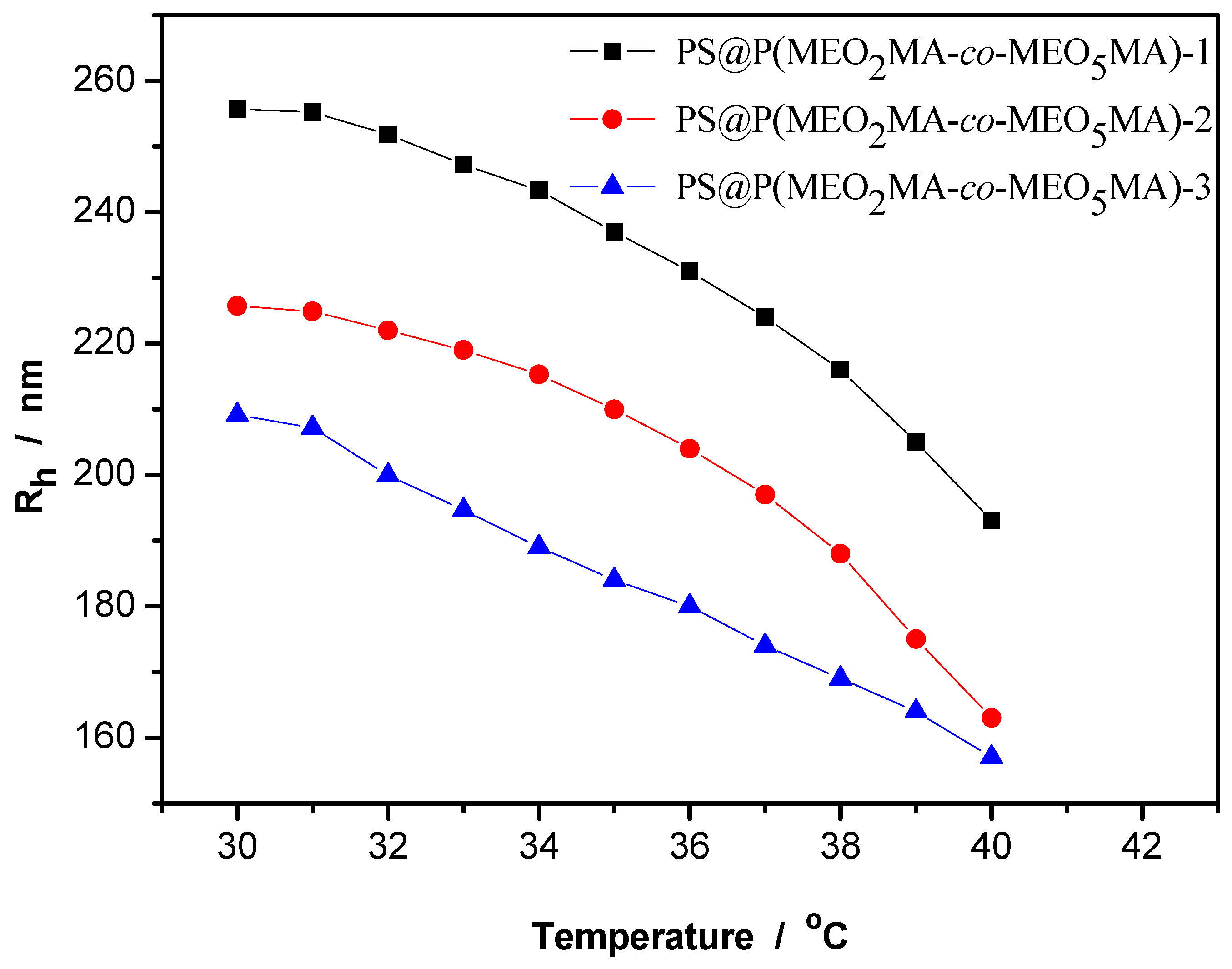
3.2. Thermo-Sensitive Behavior of PS@(MEO2MA-co-MEO5MA) Nanogels
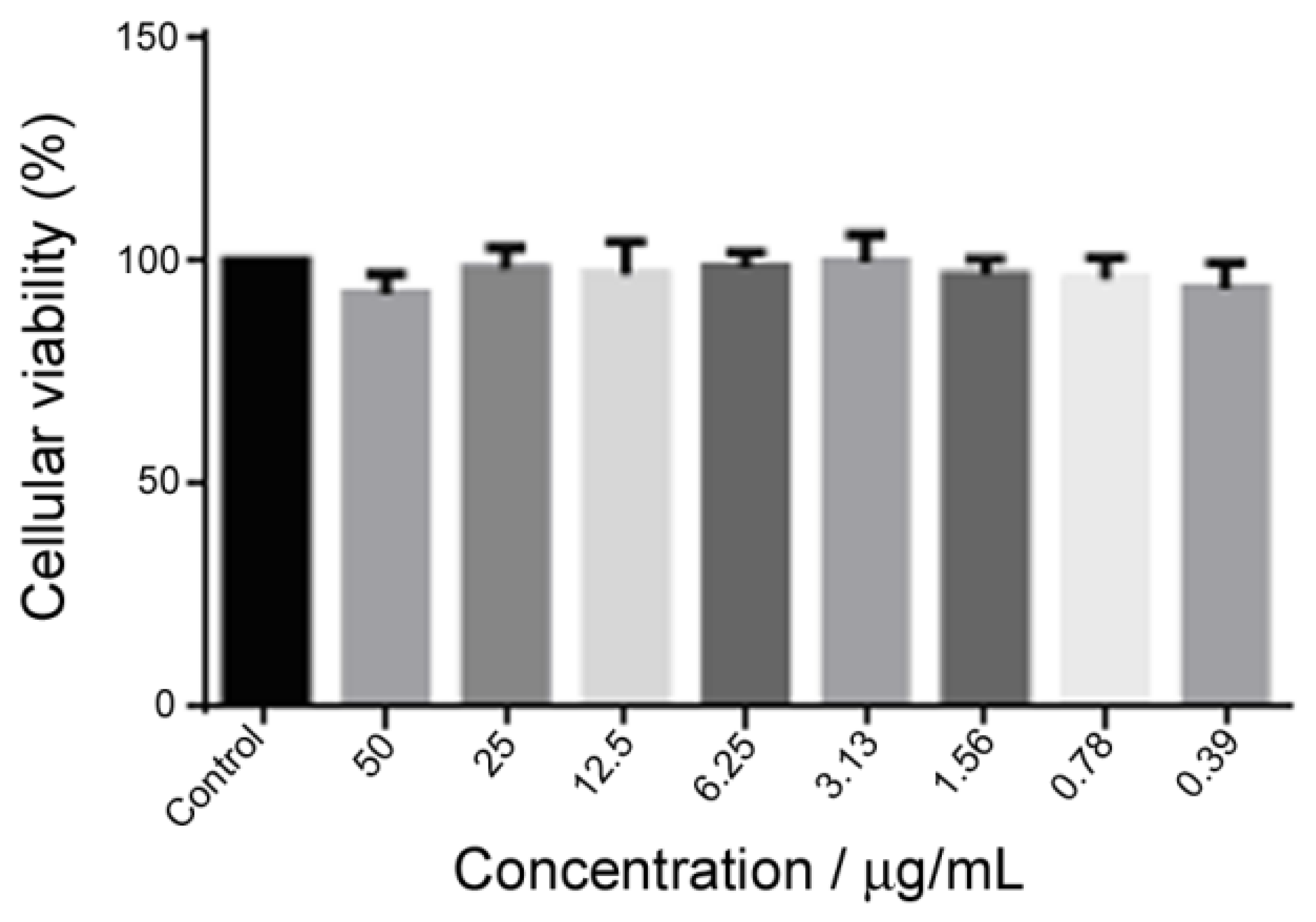
3.3. Biocompatibility Assay
3.4. Drug Loading and In Vitro Thermo-Sensitive Regulated Drug Release
3.4.1. Drug Loading Efficiency
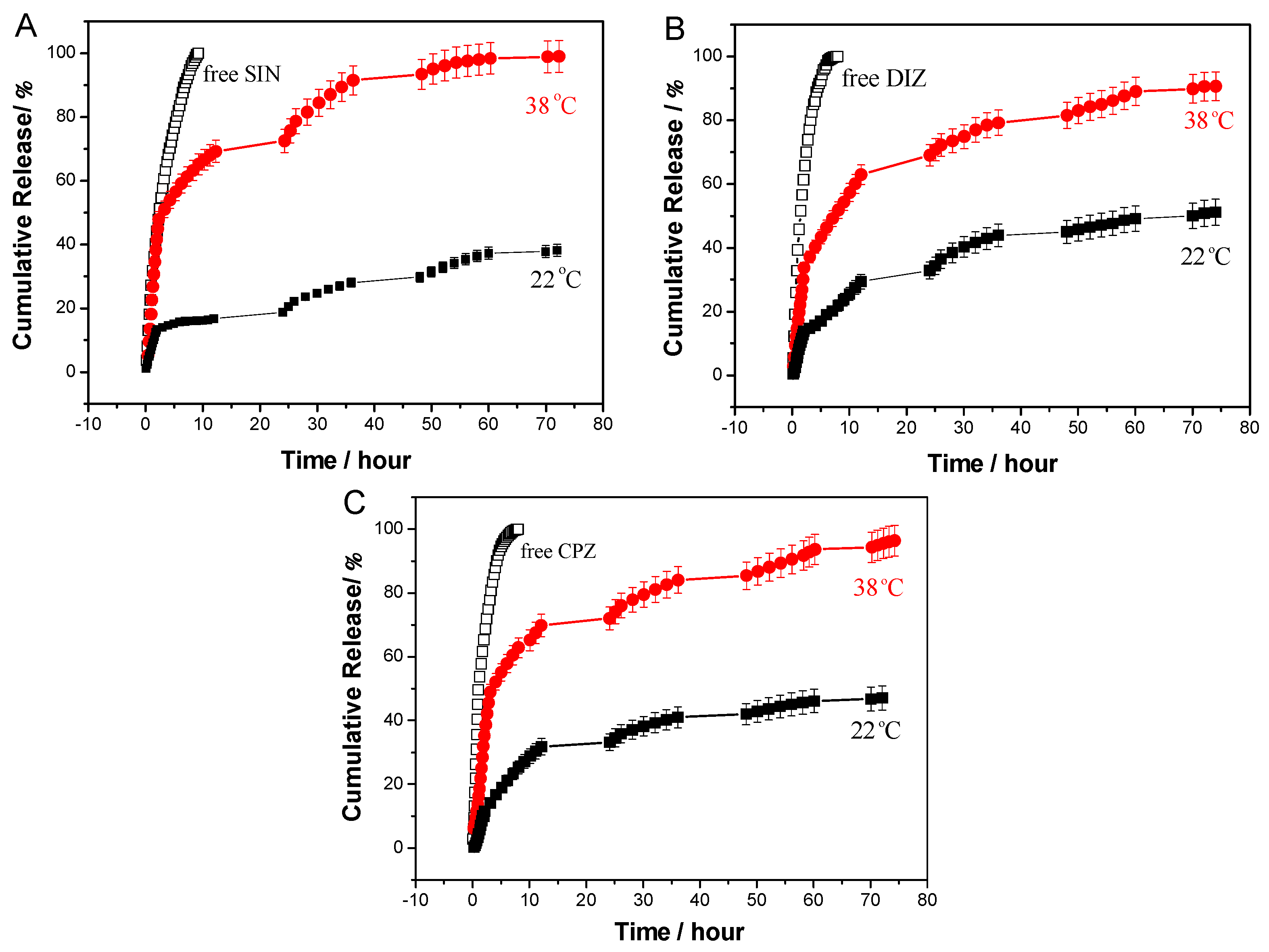
3.4.2. In Vitro Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shulman, M.; Choo, T.H.; Scodes, J.; Pavlicova, M.; Wai, J.; Haenlein, P.; Tofighi, B.; Campbell, A.N.C.; Lee, J.D.; Rotrosen, J.; et al. Association between methadone or buprenorphine use during medically supervised opioid withdrawal and extended-release injectable naltrexone induction failure. J. Subst. Abuse Treat. 2021, 124, 108292. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Tang, Y.; Axon, R.; Casarella, J.; Whitfield, N.; Rauch, S. Effectiveness of a substance use treatment program for veterans with chronic pain and opioid use disorder. J. Subst. Abuse Treat. 2022, 132, 108635. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Wu, Z.; Cao, X.; Liu, E.; Detels, R. Continued drug use during methadone treatment in China: A retrospective analysis of 19,026 service users. J. Subst. Abuse Treat. 2014, 47, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, K.; Johnson, M.; Wassermann, M.; Evans-Wall, J. Drugs of Abuse—Opioids, Sedatives, Hypnotics. Crit. Care Clin. 2021, 37, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Ko, M. Potential therapeutic targets for the treatment of opioid abuse and pain. Adv. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Lu, J.; Yan, C.; Liu, C. Observation on the changes of body temperature in amphetamine abusers. Chin. J. Drug Abuse Prevent. Treat. 2006, 12, 46–47. (In Chinese) [Google Scholar]
- Shi, J.; Votru Ba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Lavrador, P.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive nanocarriers for delivery of bone therapeutics—Barriers and progresses. J. Control. Release 2018, 273, 51–67. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Dong, S.; Cui, J.; Hao, J. Microgels in biomaterials and nanomedicines. Adv. Colloid Interf. Sci. 2019, 266, 1–20. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Maitland, D.; Oszustowicz, T.; Hoare, T. Tuning drug release from smart microgel–hydrogel composites via cross-linking. J. Colloid Interf. Sci. 2013, 392, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, A.; Yuan, X.; Li, H.; Ma, D.; Xue, W. Glucose-sensitive and blood-compatible nanogels for insulin controlled release. J. Appl. Polym. Sci. 2016, 133, 43504. [Google Scholar] [CrossRef]
- Shen, J.; Ye, T.; Chang, A.; Wu, W.; Zhou, S. A colloidal supra-structure of responsive microgels as a potential cell scaffold. Soft Matter 2012, 8, 12034–12042. [Google Scholar] [CrossRef]
- Samah, N.; Heard, C.M. Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc). Int. J. Pharm. 2013, 453, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ghugare, S.V.; Mozetic, P.; Paradossi, G. Temperature-Sensitive Poly(vinyl alcohol)/Poly(methacrylate-co-N-isopropyl acrylamide) Microgels for Doxorubicin Delivery. Biomacromolecules 2009, 10, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Badi, N. Non-linear PEG-based thermoresponsive polymer systems. Prog. Polym. Sci. 2017, 66, 54–79. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, O.; Hoth, A. Point by Point Comparison of Two Thermosensitive Polymers Exhibiting a Similar LCST: Is the Age of Poly(NIPAM) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Lutz, J.F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Han, S.; Hagiwara, M.; Ishizone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Ishizone, T.; Seki, A.; Hagiwara, M.; Han, S.; Yokoyama, H.; Oyane, A.; Deffieux, A.; Carlotti, S. Anionic Polymerizations of Oligo(ethylene glycol) Alkyl Ether Methacrylates: Effect of Side Chain Length and ω-Alkyl Group of Side Chain on Cloud Point in Water. Macromolecules 2008, 41, 2963–2967. [Google Scholar] [CrossRef]
- Aguirre, G.; Deniau, E.; Brûlet, A.; Chougrani, K.; Alard, V.; Billon, L. Versatile oligo(ethylene glycol)-based biocompatible microgels for loading/release of active bio(macro)molecules. Colloids Surf. B Biointerfaces 2019, 175, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Coreshell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef]
- Meng, Y.; Shen, J.; Fu, T.; Feng, X.; Wang, S.; Wang, T.; Zhang, X. Thermosensitive PMMA core/oligo(ethylene glycol)-based shell microgels as drug carriers in detoxification treatment. J. Appl. Polym. Sci. 2021, 138, e51454. [Google Scholar] [CrossRef]
- Xie, P.; Liu, P. pH-responsive surface charge reversal carboxymethyl chitosan-based drug delivery system for pH and reduction dual-responsive triggered DOX release. Carbohydr. Polym. 2020, 236, 116093. [Google Scholar] [CrossRef]
- Shen, J.; He, W.; Wang, T. Multifunctional amphiphilic ionic liquid pathway to create water-based magnetic fluids and magnetically-driven mesoporous silica. RSC Adv. 2019, 9, 3504–3513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Z.; Wang, Z.; Zhang, W.; Ming, N. Preparation of monodisperse polystyrene spheres in aqueous alcohol system. Mater. Lett. 2003, 57, 4466–4470. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; He, M.; Chen, Z.; Wang, Y.; Ye, Q.; Zhang, H.; Zhang, L. Construction of cellulose–phosphor hybrid hydrogels and their application for bioimaging. J. Mater. Chem. B 2014, 2, 7559–7566. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Chen, K. Lanthanide-doped chitosan nanospheres as cell nuclei illuminator and fluorescent nonviral vector for plasmid DNA delivery. Dalton Trans. 2012, 41, 490–497. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, W.; Wang, Y.; Zhao, Y.; Chen, H.; Xu, H.; Yang, X. Dual temperature/pH-sensitive drug delivery of poly(N-isopropylacrylamide-co-acrylic acid) nanogels conjugated with doxorubicin for potential application in tumor hyperthermia therapy. Colloids Surf. B Biointerfaces 2011, 84, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhou, J.; Wang, C. Experimental study on physical and psychic dependence of sinomenine. Chin. J. Drug Abuse Prevent. Treat. 2004, 10, 190–193. (In Chinese) [Google Scholar]
- Zheng, J.; Liu, Z.; Zhu, Y.; Liu, J.; Hu, B. Effects of sinomenine on the mRNA expression of morphine dependence in mouse bone marrow and HO2, sGCα1, sGCα2 gene of cerebellum. Chin. Pharmacol. Bull. 2008, 24, 1044–1048. (In Chinese) [Google Scholar]
- Chen, H.; Zhang, W.; Yang, N.; Chen, C.; Zhang, M. Chitosan-Based Surface Molecularly Imprinted Polymer Microspheres for Sustained Release of Sinomenine Hydrochloride in Aqueous Media. Appl. Biochem. Biotechnol. 2018, 185, 370–384. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y. New progress in drug treatment. Prog. Mod. Biomed. 2009, 9, 3163–3175. (In Chinese) [Google Scholar]
- Gao, J.; Zhao, H.; Wang, S. Effects of calcium antagonist diltiazem hydrochloride on morphine withdrawal and serum monoamine transmitters in mice. Chin. J. Drug Depend. 2001, 10, 101–103. (In Chinese) [Google Scholar]
- Xu, P.; Mark, J.E.; Wang, S.B.; Nathan, P.; Korol, B. Controlled release of diltiazem hydrochloride. Polym. Int. 1995, 38, 311–317. [Google Scholar] [CrossRef]
- Jiang, T.; Li, W.; Tan, L.; Deng, C.; Li, M.; Yu, M. Preparation and stability of chlorpromazine hydrochloride microcapsules on yeast cell wall. Chin. J. Biol. 2012, 25, 1550–1553. (In Chinese) [Google Scholar]
- Mohammed, H.; Urszula, D. PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release. Int. J. Mol. Sci. 2014, 15, 23909–23923. [Google Scholar]

| Sample | MEO2MA (mmoL) | MEO5MA (mmoL) | AAPH (mmoL) | PEGDMA (mmoL) |
|---|---|---|---|---|
| PS@P(MEO2MA-co-MEO5MA)-1 | 0.25 | 0.75 | 0.02 | 0.75 × 10−2 |
| PS@P(MEO2MA-co-MEO5MA)-2 | 0.50 | 1.50 | 0.02 | 1.51 × 10−2 |
| PS@P(MEO2MA-co-MEO5MA)-3 | 0.75 | 2.25 | 0.02 | 2.06 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, T.; Shen, J.; Meng, Y.; Wang, J.; Wang, S.; Zhang, Y.; Wang, T.; Zhang, X. Automatic Detoxification Medicine Delivery by Thermo-Sensitive Poly(ethylene glycol)-Based Nanogels. Polymers 2022, 14, 892. https://doi.org/10.3390/polym14050892
Fu T, Shen J, Meng Y, Wang J, Wang S, Zhang Y, Wang T, Zhang X. Automatic Detoxification Medicine Delivery by Thermo-Sensitive Poly(ethylene glycol)-Based Nanogels. Polymers. 2022; 14(5):892. https://doi.org/10.3390/polym14050892
Chicago/Turabian StyleFu, Ting, Jing Shen, Yuting Meng, Jun Wang, Siping Wang, Yuhui Zhang, Tongwen Wang, and Xufeng Zhang. 2022. "Automatic Detoxification Medicine Delivery by Thermo-Sensitive Poly(ethylene glycol)-Based Nanogels" Polymers 14, no. 5: 892. https://doi.org/10.3390/polym14050892
APA StyleFu, T., Shen, J., Meng, Y., Wang, J., Wang, S., Zhang, Y., Wang, T., & Zhang, X. (2022). Automatic Detoxification Medicine Delivery by Thermo-Sensitive Poly(ethylene glycol)-Based Nanogels. Polymers, 14(5), 892. https://doi.org/10.3390/polym14050892





