Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Carotenoids from Sea Buckthorn Berries
2.2.1. Total Carotenoid Content of Sea Buckthorn Extract
2.2.2. In Vitro Antioxidant Activity of Sea Buckthorn Extract
2.3. Microencapsulation of Total Carotenoids from Sea Buckthorn Berries Extract
2.3.1. Powders Characterization and Encapsulation Efficiencies
2.3.2. Confocal Laser Scanning Microscopy of Powders
2.3.3. CIELAB Color Characterization of Powders
2.3.4. Carotenoid’s In Vitro Simulated Digestion
2.3.5. Storage and Color Stability of Powders
2.4. Mayonnaiese Formulation
2.4.1. Mayonnaise Characterization
2.4.2. Texture Analysis of Value-Added Mayonnaise
2.4.3. Sensorial Analysis of Value-Added Mayonnaise
2.5. Statistical Analysis of Data
3. Results and Discussion
3.1. Sea Buckthorn Fruits Extract and Microcapsules Characterization
3.2. Morphological Structure Analysis of Microcapsules by CLSM
3.3. Powders’ Color Analysis
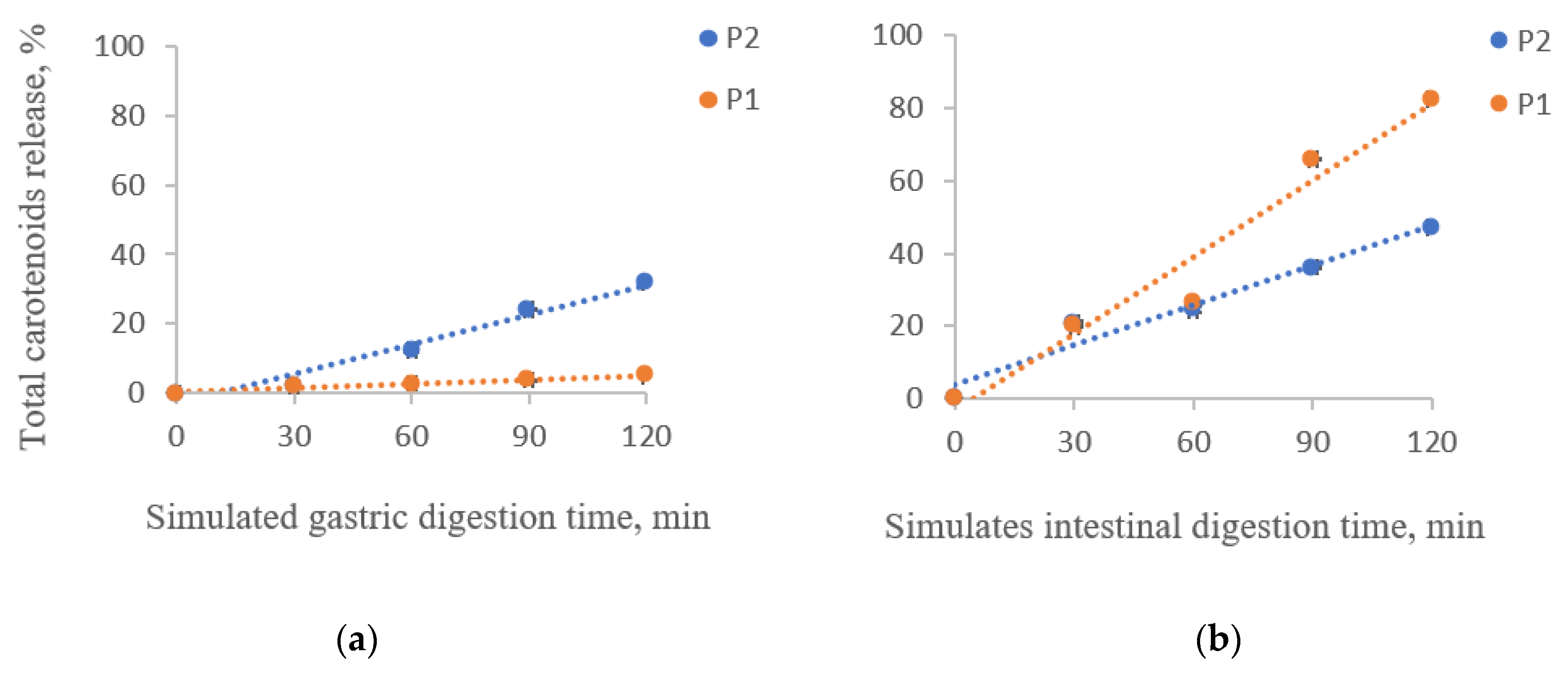
3.4. In Vitro Simulated Digestibility of Total Carotenoids from the Powders
3.5. Phytochemical and Color Stability of Powders
3.6. Value-Added Mayonnaise Characterization
3.7. Value-Added Mayonnaise Color Analysis
3.8. Value-Added Mayonnaise Texture Analysis
3.9. Value-Added Mayonnaise Sensorial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilarj, C.N.; et al. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2021, 153, 112527. [Google Scholar] [CrossRef]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ionita, E.; Cotarlet, M.; Dumitrascu, L.; Botez, E.; Rapeanu, G.; Stanciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, S.; Kuang, Y.; Jiang, Z.; Lin, Y.; Xie, Z.; Liu, E. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. Ethnopharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, K.Z.; Wani, W.; Dar, Z.A.; Mansoor, S.; Anam-ul-Haq, S.; Farooq, I.; Hussain, K.; Wani, S.A.; Nehvi, F.A.; Ahmed, N. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. J. Funct. Foods 2020, 73, 104102. [Google Scholar] [CrossRef]
- Xu, Y.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhall, N.S. Health benefits of sea buckthorn for the prevention of cardiovascular diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Cai, W.; Li, D. Effect of sea buckthorn (Hippophae rhamnoides L.) on blood lipid profiles: A systematic review and meta-analysis from 11 independent randomized controlled trials. Trends Food Sci. Technol. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of Apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Zu, Y.; Li, C.; Fu, Y.; Zhao, C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. 2006, 41, 714–719. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturica, M.; Barbu, V.; Ionita, E.; Patrascu, L.; Cotarlet, M.; Dumitrascu, L.; Aprodu, I.; Rapeanu, G.; Stanciuc, N. Transglutaminase mediated microencapsulation of sea buckthorn supercritical CO2 extract in whey protein isolate and valorization in highly value added food products. Food Chem. 2018, 262, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Condurache, N.N.; Aprodu, I.; Cracinescu, O.; Tatia, R.; Horincar, G.; Barbu, V.; Enachi, E.; Rapeanu, G.; Bahrim, G.E.; Oancea, A.; et al. Probing the functionality of bioactives from eggplant peel extracts through extraction and microencapsulation in different polymers and whey protein hydrolysates. Food Bioproc. Tech. 2019, 12, 1316–1329. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from hibiscus sabdariffa. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.E.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.R.S.T.E.N.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W.; Latimer, G. (Eds.) Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists Inc.: Washington, DC, USA, 2000; ISBN 093558467-6. [Google Scholar]
- Horincar, G.; Enachi, E.; Barbu, V.; Andronoiu, D.G.; Rapeanu, G.; Stanciuc, N.; Aprodu, I. Value-added pastry cream enriched with microencapsulated bioactive compounds from eggplant (Solanum melongena L.) peel. Antioxidants 2020, 9, 351. [Google Scholar] [CrossRef]
- Ursache, F.M.; Ghinea, I.O.; Turturica, M.; Aprodu, I.; Râpeanu, G.; Stanciuc, N. Phytochemicals content and antioxidant properties of sea buckthorn (Hippophae rhamnoides L.) as affected by heat treatment—Quantitative spectroscopic and kinetic approaches. Food Chem. 2017, 233, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, L.; Turturică, M.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleț, M.; Stănciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO2 supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. Technol. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Drozińska, E.; Kanclerz, A.; Kurek, M.A. Microencapsulation of sea buckthorn oil with β-glucan from barley as coating material. Int. J. Biol. Macromol. 2019, 131, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Neagu, C.; Mihalcea, L.; Enachi, E.; Barbu, V.; Borda, D.; Bahrim, G.E.; Stănciuc, N. Cross-linked microencapsulation of CO2 supercritical extracted oleoresins from sea buckthorn: Evidence of targeted functionality and stability. Molecules 2020, 25, 2442. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Córdoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef]
- Roman, D.; Condurache (Lazăr), N.N.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Stănciuc, N.; Râpeanu, G. Insights of sea buckthorn extract’s encapsulation by coacervation technique. Inventions 2021, 6, 59. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Moraes, I.C.F.; Favaro-Trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsifiers. Food Res. Int. 2020, 30, 108901. [Google Scholar] [CrossRef]



| Sample | Total Carotenoids (mg/g dw) | Antioxidant Activity, (μM TE/g dw) | Encapsulation (μM TE/g dw) | Efficiency, (%) |
|---|---|---|---|---|
| E | 32 ± 1 | 422 ± 1 | - | |
| P1 | 2.20 ± 0.13 a | 384 ± 2 a | 45 ± 2 a | |
| P2 | 2.89 ± 0.02 b | 454 ± 4 b | 61.2 ± 0.9 b |
| Sample | Colorimetric Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| P1 | 73.58 ± 0.04 a | 8.99 ± 0.02 a | 52.4 ± 0.6 a |
| P2 | 92.09 ± 0.6 b | 4.28 ± 0.05 b | 62 ± 1 b |
| Storage Period, Days | P1 | P2 | ||
|---|---|---|---|---|
| TC, (mg/g DW) | Antioxidant Activity, (μM Trolox/g dw) | TC, (mg/g DW) | Antioxidant Activity (μM Trolox/g dw) | |
| 0 | 2.20 ± 0.13 aA | 384 ± 2 aC | 2.89 ± 0.02 aB | 453 ± 2 aD |
| 30 | 2.03 ± 0.10 abA | 371.9 ± 0.2 aC | 2.74 ± 0.01 abB | 451.2 ± 0.3 aD |
| 60 | 1.79 ± 0.01 bcA | 366 ± 8 aC | 2.69 ± 0.10 abB | 426 ± 7 bD |
| 90 | 1.54 ± 0.01 cA | 335 ± 8 bC | 2.52 ± 0.01 bB | 404 ± 4 cD |
| Storage Time, Days | P1 | P2 | ||||
|---|---|---|---|---|---|---|
| Colorimetric Parameters | ||||||
| L* | a* | b* | L* | a* | b* | |
| 0 | 73.58 ± 0.04 a | 8.99 ± 0.02 a | 52.4 ± 0.6 a | 92.1 ± 0.6 a | 4.28 ± 0.05 a | 62 ± 1 a |
| 30 | 78.02 ± 0.14 a | 9.4 ± 0.4 a | 50.4 ± 0.5 ab | 93 ± 1 b | 5.3 ± 0.3 a | 60.7 ± 0.4 ab |
| 60 | 90.53 ± 0.05 b | 9.4 ± 0.4 a | 48.6 ± 0.6 b | 92.4 ± 0.6 b | 6.43 ± 0.19 b | 59.4 ± 0.3 ab |
| 90 | 91.3 ± 0.5 c | 10.9 ± 0.9 a | 45.6 ± 0.5 c | 99 ± 1 b | 6.94 ± 0.04 c | 58.3 ± 0.5 b |
| Physico-Chemical Characteristics | Control | Mayo 1 (2.5%) | Mayo 2 (5%) |
|---|---|---|---|
| Proteins, g/100 g | 8.06 ± 0.07 a | 6.94 ± 0.02 b | 6.93 ± 0.04 b |
| Lipids, g/100 g | 72.0 ± 0.2 ab | 72.7 ± 0.3 a | 71.3 ± 0.2 b |
| Carbohydrates, g/100 g | 2.5 ± 0.2 a | 2.1 ± 0.2 a | 2.61 ± 0.05 a |
| Humidity, g/100 g | 15.58 ± 0.04 a | 16.28 ± 0.10 b | 16.98 ± 0.12 c |
| Ash, g/100 g | 1.84 ± 0.02 a | 1.96 ± 0.04 a | 2.2 ± 0.2 a |
| Energetic value, %kcal | 713.2 ± 0.7 a | 713 ± 2 a | 702± 3 b |
| kJ | 2984 ± 3 a | 2985 ± 10 a | 2938 ± 11 b |
| Phytochemical Characteristics | Control | Mayo 1 (2.5%) | Mayo 2 (5%) |
|---|---|---|---|
| TC, mg /100 g dw | 0.26 ± 0.07 a | 1.23 ± 0.15 b | 1.85 ± 0.04 c |
| Antioxidant activity, μM Trolox/g dw | 10.0 ± 0.6 a | 152.1 ± 0.4 b | 293 ± 3 c |
| Sample | L* | a* | b* |
|---|---|---|---|
| Control | 65.7 ± 0.6 a | −1.01 ± 0.13 a | 30 ± 1 a |
| Mayo 1 (2.5%) | 61 ± 1 b | −0.53 ± 0.02 b | 42.9 ± 0.8 b |
| Mayo 2 (5%) | 54 ± 1 c | −0.34 ± 0.02 b | 57 ± 2 c |
| Textural Parameters | Control | Mayo 1 (2.5%) | Mayo 2 (5%) |
|---|---|---|---|
| Firmness, N | 0.22 ± 0.01 a | 0.23 ± 0.03 a | 0.34 ± 0.03 b |
| Adhesion, mJ | 1.63 ± 0.15 a | 2.32 ± 0.12 b | 3.4 ± 0.4 c |
| Cohesion | 0.68 ± 0.02 a | 0.65 ± 0.03 a | 0.62 ± 0.05 a |
| Elasticity, mm | 11.1 ± 0.2 a | 10.7 ± 0.7 a | 11.0 ± 0.3 a |
| Sensory Attributes | Control | Mayo 1 (2.5%) | Mayo 2 (5%) |
|---|---|---|---|
| Color | 7 ± 1 a | 8.3 ± 0.7 b | 8.7 ± 0.5 b |
| Aroma | 6 ± 1 a | 7.6 ± 0.8 b | 8.0 ± 0.9 b |
| Taste | 6.8 ± 0.9 a | 7.5 ± 0.9 b | 8.0± 0.5 b |
| Consistency | 7.5 ± 0.9 a | 8.0 ± 0.7 ab | 8.4 ± 0.6 b |
| Texture | 8.4 ± 0.8 a | 8.7 ± 0.6 a | 8.8 ± 0.4 a |
| Odor | 6.9 ± 1.3 a | 7.40 ± 0.18 ab | 8.1 ± 0.6 b |
| Aftertaste | 7.4 ± 0.8 a | 7.50 ± 0.19 a | 8 ± 1 a |
| Spreadability | 8 ± 1 a | 8.8 ± 0.4 a | 8.8 ± 0.4 a |
| Acceptability | 8.2 ± 0.6 a | 8.6 ± 0.5 b | 8.9 ± 0.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, D.; Condurache, N.N.; Stănciuc, N.; Andronoiu, D.G.; Aprodu, I.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Stanciu, S.; Râpeanu, G. Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment. Polymers 2022, 14, 548. https://doi.org/10.3390/polym14030548
Roman D, Condurache NN, Stănciuc N, Andronoiu DG, Aprodu I, Enachi E, Barbu V, Bahrim GE, Stanciu S, Râpeanu G. Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment. Polymers. 2022; 14(3):548. https://doi.org/10.3390/polym14030548
Chicago/Turabian StyleRoman, Diana, Nina Nicoleta Condurache (Lazăr), Nicoleta Stănciuc, Doina Georgeta Andronoiu, Iuliana Aprodu, Elena Enachi, Vasilica Barbu, Gabriela Elena Bahrim, Silvius Stanciu, and Gabriela Râpeanu. 2022. "Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment" Polymers 14, no. 3: 548. https://doi.org/10.3390/polym14030548
APA StyleRoman, D., Condurache, N. N., Stănciuc, N., Andronoiu, D. G., Aprodu, I., Enachi, E., Barbu, V., Bahrim, G. E., Stanciu, S., & Râpeanu, G. (2022). Advanced Composites Based on Sea Buckthorn Carotenoids for Mayonnaise Enrichment. Polymers, 14(3), 548. https://doi.org/10.3390/polym14030548










