Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
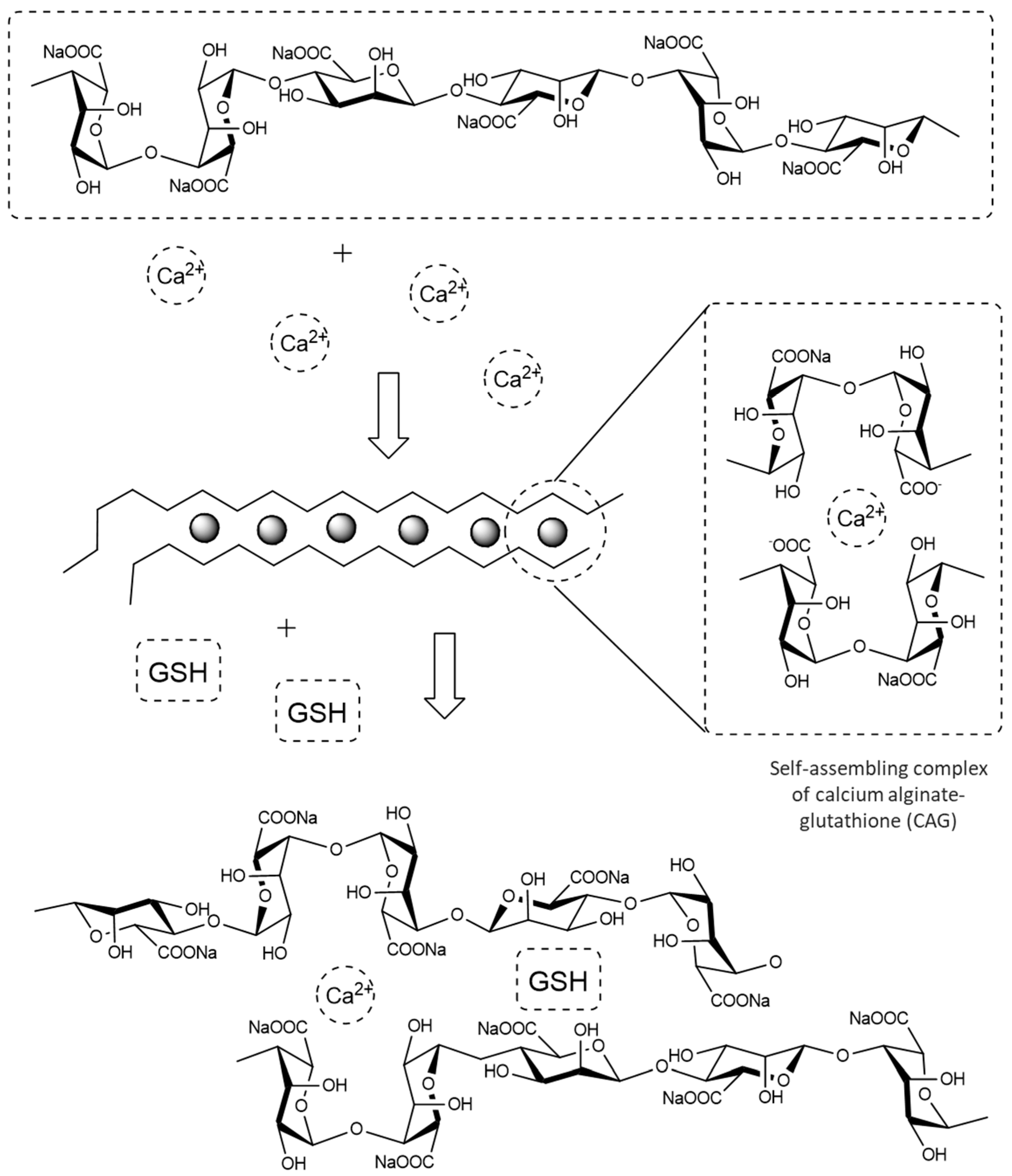
2.2. Experimental Design and Preparation of the Microcapsules
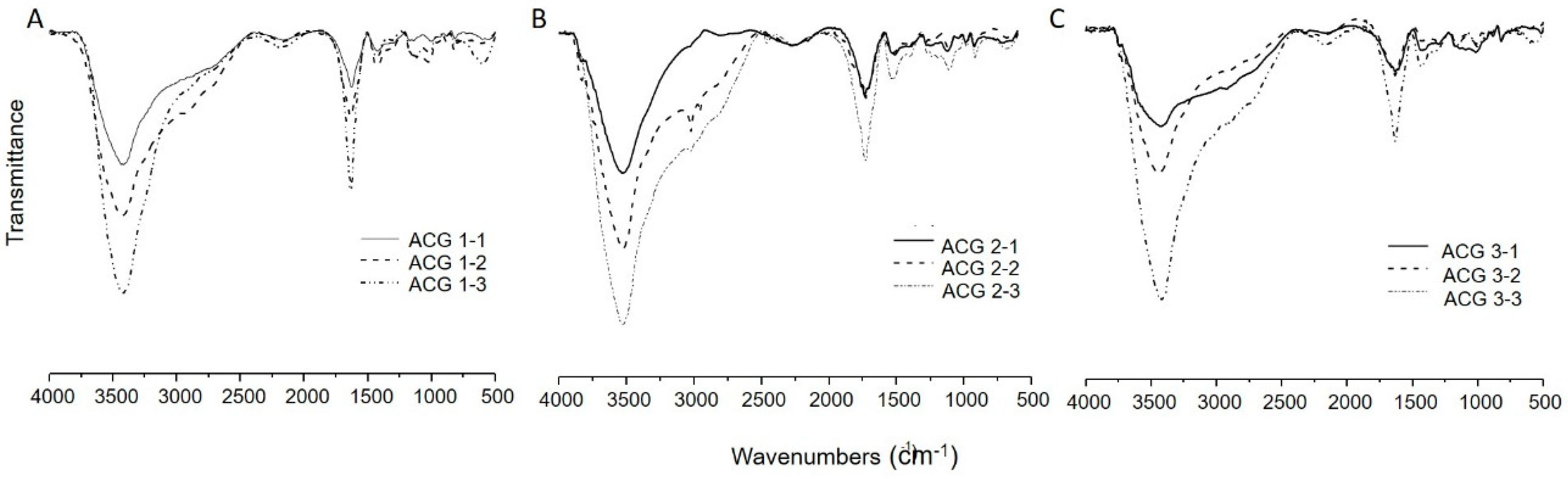
2.3. FTIR Characterization of the Alginate Complexes
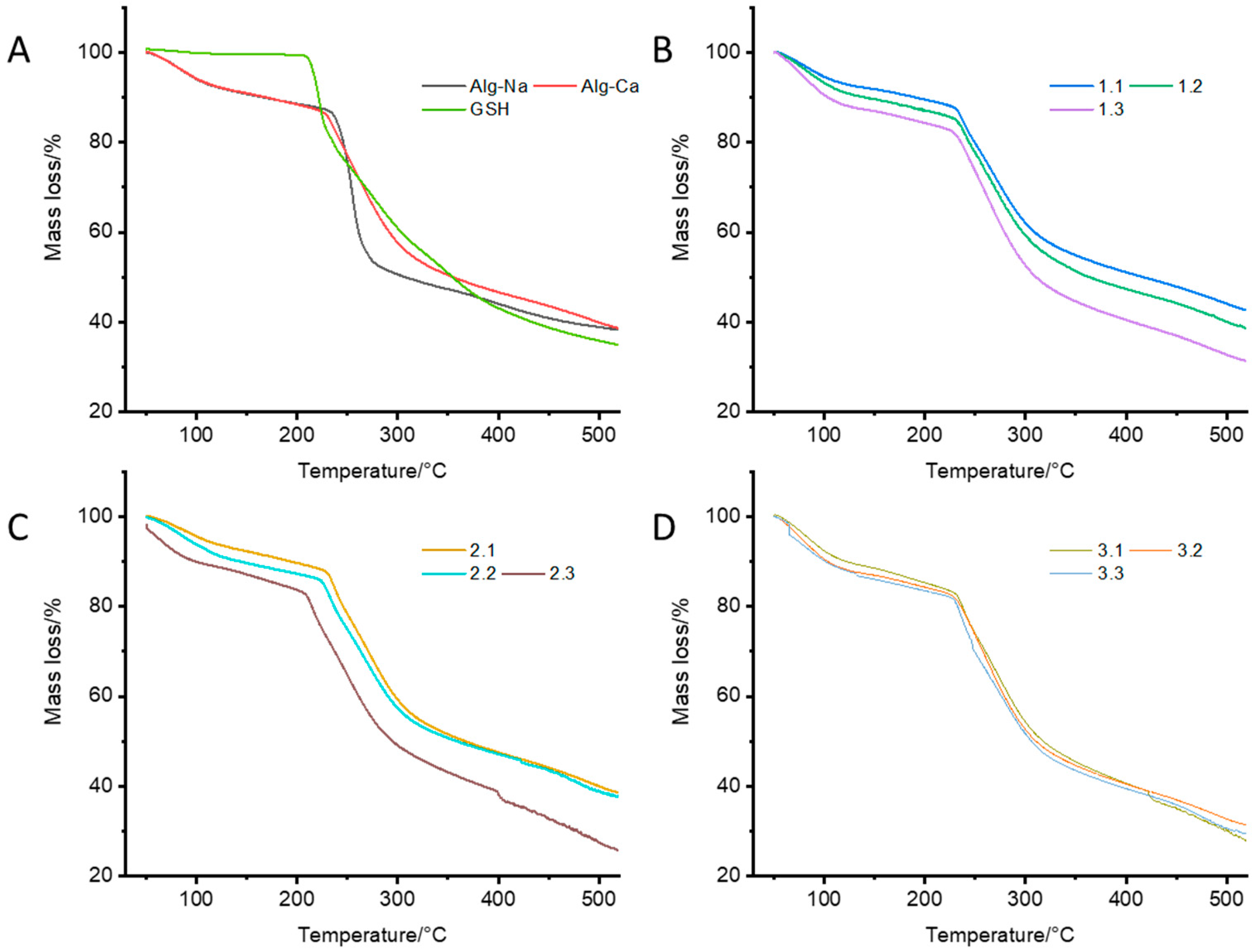
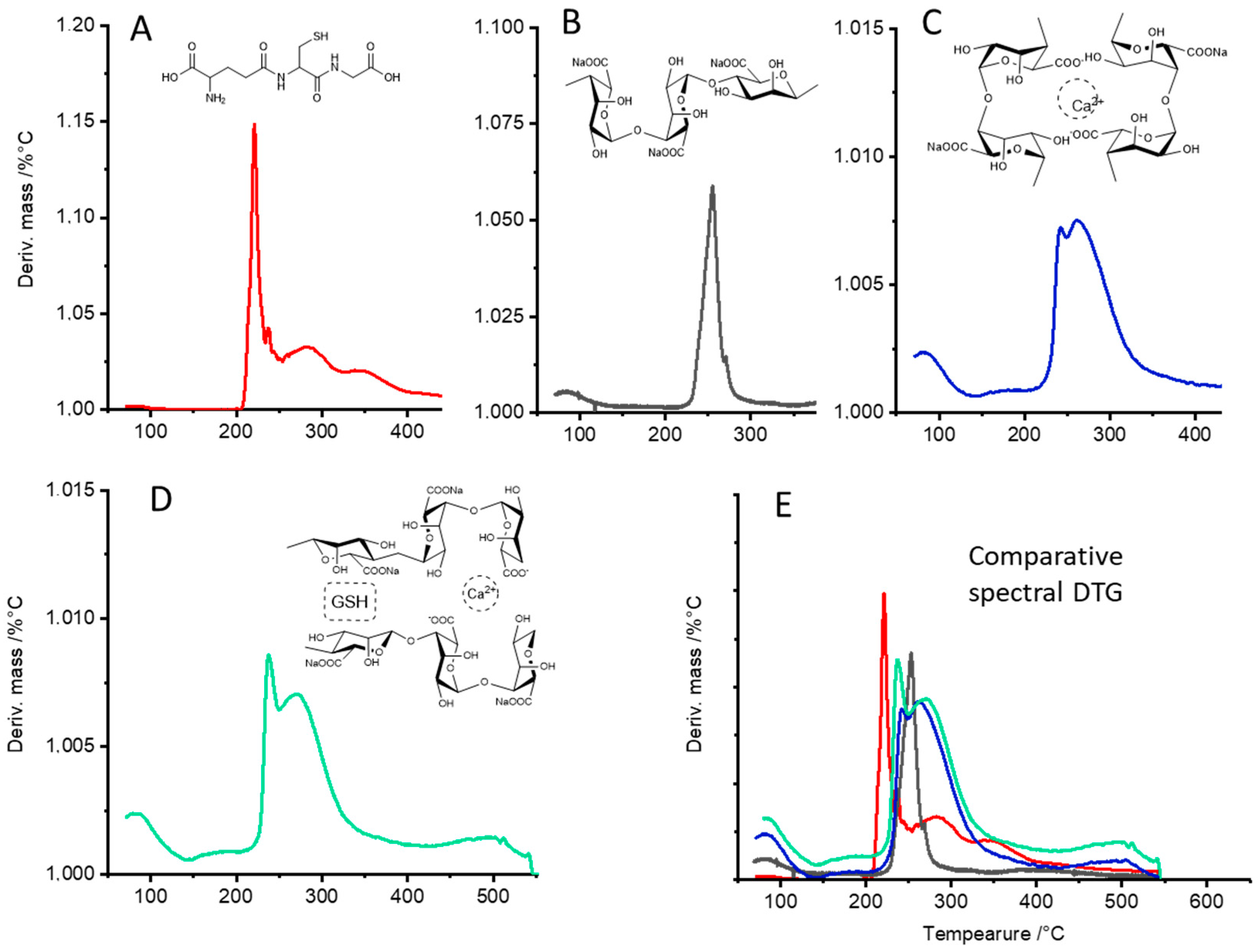
2.4. Thermogravimetric (TG) Characterization and Differential Thermogravimetric (DTG) Analyses of CAG Complexes
2.5. Computational Building of the Molecular Structures and Polymeric Systems
2.6. Determination of Reduced Glutathione
2.7. Statistical Analysis and Optimization Studies for the Encapsulation Process
3. Results and Discussion
3.1. FTIR Characterization of the Alginate Complexes
3.2. Thermogravimetric (TG) Characterization and Differential Thermogravimetric (DTG) Analyses of CAG Complex
3.3. Computational Building of the Molecular Structures and Polymeric Systems
3.4. Determination of Reduced Glutathione
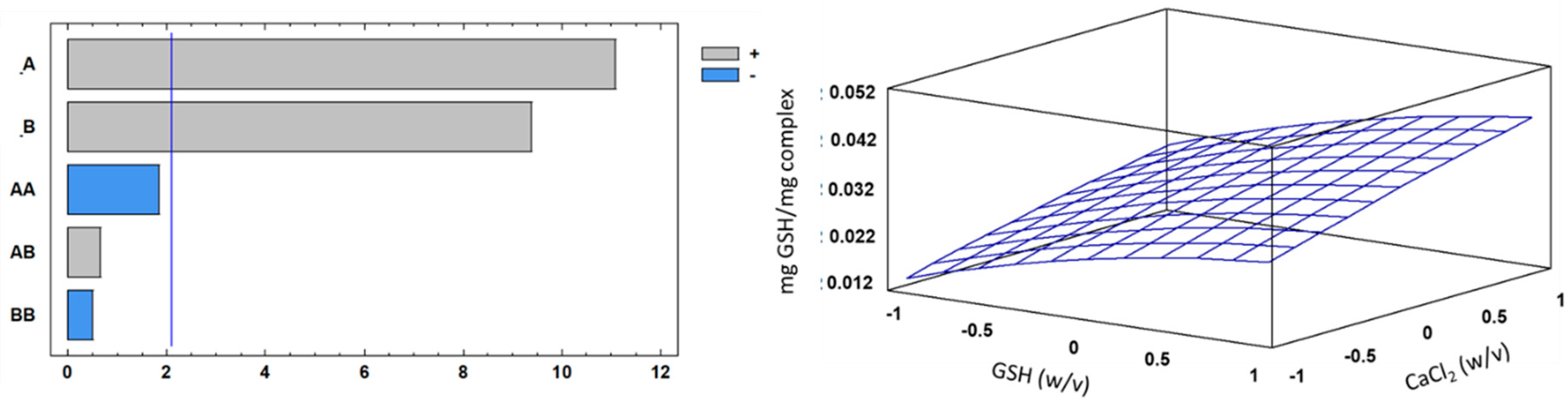
3.5. Optimization Studies for the Encapsulation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications: Understanding Mechanisms of Oxidation and Antioxidant Activity; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 0857090445. [Google Scholar]
- Díaz, I.; Castro, R.I.; Ubeda, C.; Loyola, R.; Laurie, V.F. Combined effects of sulfur dioxide, glutathione and light exposure on the conservation of bottled Sauvignon blanc. Food Chem. 2021, 356, 129689. [Google Scholar] [CrossRef]
- Stebbins, N.B.; Howard, L.R.; Prior, R.L.; Brownmiller, C.; Mauromoustakos, A. Stabilization of anthocyanins in blackberry juice by glutathione fortification. Food Funct. 2017, 8, 3459–3468. [Google Scholar] [CrossRef]
- Yano, H. Comparison of Oxidized and Reduced Glutathione in the Bread-Making Qualities of Rice Batter. J. Food Sci. 2012, 77, 182. [Google Scholar] [CrossRef]
- Wu, J.; Jin, X.; Zhu, C.; Yan, Y.; Ding, C.-F.; Tang, K. Gold nanoparticle-glutathione functionalized MOFs as hydrophilic materials for the selective enrichment of glycopeptides. Talanta 2021, 228, 122263. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Z.; Cui, X.; Liang, Y.; Wang, T.; Kong, J. Peptide transporter-related protein 2 plays an important role in glutathione transport of Streptococcus thermophilus. J. Dairy Sci. 2021, 104, 3990–4001. [Google Scholar] [CrossRef] [PubMed]
- Asantewaa, G.; Harris, I.S. Glutathione and its precursors in cancer. Curr. Opin. Biotechnol. 2021, 68, 292–299. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burford, N.; Eelman, M.D.; Groom, K. Identification of complexes containing glutathione with As (III), Sb (III), Cd (II), Hg (II), Tl (I), Pb (II) or Bi (III) by electrospray ionization mass spectrometry. J. Inorg. Biochem. 2005, 99, 1992–1997. [Google Scholar] [CrossRef] [PubMed]
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2012, 61, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, S.; Li, Y.; Di, B.; Zhang, J.; Liu, Y. Effect of high temperature and excessive light on glutathione content in apple peel. Front. Agric. China 2008, 2, 97–102. [Google Scholar] [CrossRef]
- Grøndahl, L.; Lawrie, G.; Anitha, A.; Shejwalkar, A. Applications of alginate biopolymer in drug delivery. In Biointegration of Medical Implant Materials; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 375–403. [Google Scholar]
- Zhang, Z.; Zhang, R.; Chen, L.; Tong, Q.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanoemulsions as edible coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Ghahfarrokhi, M.G.; Es, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 014104. [Google Scholar] [CrossRef]
- Aditya, N.; Espinosa, Y.G.; Norton, I.T. Encapsulation systems for the delivery of hydrophilic nutraceuticals: Food application. Biotechnol. Adv. 2017, 35, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.C.G.; Feltre, G.; Hubinger, M.D.; Sato, A.C.K. Protection and targeted delivery of β-carotene by starch-alginate-gelatin emulsion-filled hydrogels. J. Food Eng. 2021, 290, 110205. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.; El-Fiqi, A. Controlled delivery of therapeutic ions and antibiotic drug of novel alginate-agarose matrix incorporating selenium-modified borosilicate glass designed for chronic wound healing. J. Non-Cryst. Solids 2020, 534, 119889. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymer 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- ANS; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U. Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400–E 404) as food additives. EFSA J. 2017, 15, e05049. [Google Scholar] [PubMed] [Green Version]
- Yu, C.-J.; Ri, B.-H.; Kim, C.-H.; Hwang, U.-S.; Ri, K.-C.; Song, C.-J.; Kim, U.-C. Formation and characterization of ceramic coating from alumino silicate mineral powders in the matrix of cement composite on the concrete wall. Mater. Chem. Phys. 2019, 227, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Ghaffarian, R.; Pérez-Herrero, E.; Oh, H.; Raghavan, S.R.; Muro, S. Chitosan–Alginate Microcapsules Provide Gastric Protection and Intestinal Release of ICAM-1-Targeting Nanocarriers, Enabling GI Targeting In Vivo. Adv. Funct. Mater. 2016, 26, 3382–3393. [Google Scholar] [CrossRef] [Green Version]
- Agulhon, P.; Robitzer, M.; Habas, J.-P.; Quignard, F. Influence of both cation and alginate nature on the rheological behavior of transition metal alginate gels. Carbohydr. Polym. 2014, 112, 525–531. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and functional assessment of alginate fibers prepared by metal-calcium ion complex coagulation bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Tankhiwale, R. Investigation of water uptake behavior and stability of calcium alginate/chitosan bi-polymeric beads: Part-1. React. Funct. Polym. 2006, 66, 645–658. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Valdés, O.; Marican, A.; Avila-Salas, F.; Castro, R.; Mirabal, Y.; Amalraj, J.; Abril, D.; Durán-Lara, E.; Santos, L. Simple approach for cleaning up 2,4,6-trichloroanisole from alcoholic-beverage-reconstituted solutions using polymeric materials. Aust. J. Grape Wine Res. 2019, 25, 327–337. [Google Scholar] [CrossRef]
- Case, D.A.; Cerutti, D.S.; Cheatham III, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Greene, D.; Homeyer, N. AMBER Reference Manual. Univ. Calif. 2018, pp. 1–927. Available online: http://ambermd.org/#AmberTools (accessed on 10 March 2021).
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, X.; Chen, Y.; Guo, J.; Chen, Q.; Jiang, X. pH-induced self-assembly and capsules of sodium alginate. Biomacromolecules 2005, 6, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Perez, T.A.; Pedreiro, L.N.; Prezotti, F.G.; Boni, F.I.; Cardoso, V.M.d.O.; Venâncio, T.; Gremião, M.P.D. A novel pH-responsive hydrogel-based on calcium alginate engineered by the previous formation of polyelectrolyte complexes (PECs) intended to vaginal administration. Drug Dev. Ind. Pharm. 2017, 43, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Release, S. 4: LigPrep, Version 3.6; Schrödinger, LLC.: New York, NY, USA, 2015. [Google Scholar]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput. Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.I.; Valenzuela-Riffo, F.; Morales-Quintana, L. In Silico and In Vitro Analysis of the 4,4’,4’’-((1,3,5-Triazine-2,4,6-triyl)tris(azanediyl))triphenol), an Antioxidant Agent with a Possible Anti-Inflammatory Function. BioMed Res. Int. 2019, 2019. Available online: https://www.hindawi.com/journals/bmri/2019/9165648/ (accessed on 10 March 2021). [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Sahoo, S.; Awasthi, J.P.; Sunkar, R.; Panda, S.K. Determining Glutathione Levels in Plants. In Advanced Structural Safety Studies; Springer Science and Business Media LLC.: Berlin, Germany, 2017; Volume 1631, pp. 273–277. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, A practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly (vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Voo, W.-P.; Lee, B.-B.; Idris, A.; Islam, A.; Tey, B.-T.; Chan, E.-S. Production of ultra-high concentration calcium alginate beads with prolonged dissolution profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Donoso, W.; Castro, R.; Guzman, L.; López-Cabaña, Z.; Nachtigall, F.M.; Santos, L.S. Fast detection of Listeria monocytogenes through a nanohybrid quantum dot complex. Anal. Bioanal. Chem. 2017, 409, 5359–5371. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, Y.A.R.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S.; et al. Preparation of Hydrogel/Silver Nanohybrids Mediated by Tunable-Size Silver Nanoparticles for Potential Antibacterial Applications. Polymer 2019, 11, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plazinski, W. Molecular basis of calcium binding by polyguluronate chains. Revising the egg-box model. J. Comput. Chem. 2011, 32, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajry, H.A.; Al-Maskry, S.A.; Al-Kharousi, L.M.; El-Mardi, O.; Shayya, W.H.; Goosen, M.F.A. Electrostatic Encapsulation and Growth of Plant Cell Cultures in Alginate. Biotechnol. Prog. 1999, 15, 768–774. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surfaces B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Bilalis, P.; Katsigiannopoulos, D.; Avgeropoulos, A.; Sakellariou, G. Non-covalent functionalization of carbon nanotubes with polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.; Kadirvelu, K. Investigation of Cr(VI) adsorption onto chemically treated Helianthus annuus: Optimization using Response Surface Methodology. Bioresour. Technol. 2011, 102, 600–605. [Google Scholar] [CrossRef]



| Value | Coded | ||||
|---|---|---|---|---|---|
| Samples | GSH (w/v) | CaCl2 (w/v) | GSH (w/v) | CaCl2 (w/v) | |
| 1 | CAG-1.1 | 5 | 0.75 | −1 | −1 |
| 2 | CAG-1.2 | 10 | 0.75 | 0 | −1 |
| 3 | CAG-1.3 | 15 | 0.75 | 1 | −1 |
| 4 | CAG-2.1 | 5 | 1.00 | −1 | 0 |
| 5 | CAG-2.2 | 10 | 1.00 | 0 | 0 |
| 6 | CAG-2.3 | 15 | 1.00 | 1 | 0 |
| 7 | CAG-3.1 | 5 | 1.25 | −1 | 1 |
| 8 | CAG-3.2 | 10 | 1.25 | 0 | 1 |
| 9 | CAG-3.3 | 15 | 1.25 | 1 | 1 |
| Value | Result | ||
|---|---|---|---|
| Samples | GSH (w/v) | CaCl2 (w/v) | GSH Content (mg GSH per mg Complex) |
| CAG-1.1 | 5 | 0.75 | 0.014 ± 0.002 |
| CAG-1.2 | 10 | 0.75 | 0.022 ± 0.005 |
| CAG-1.3 | 15 | 0.75 | 0.027 ± 0.005 |
| CAG-2.1 | 5 | 1.00 | 0.018 ± 0.002 |
| CAG-2.2 | 10 | 1.00 | 0.030 ± 0.002 |
| CAG-2.3 | 15 | 1.00 | 0.038 ± 0.003 |
| CAG-3.1 | 5 | 1.25 | 0.026 ± 0.003 |
| CAG-3.2 | 10 | 1.25 | 0.037 ± 0.001 |
| CAG-3.3 | 15 | 1.25 | 0.041 ± 0.002 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| A:Factor_A | 0.00113606 | 1 | 0.00113606 | 122.80 | 0.0000 |
| B:Factor_B | 0.000813389 | 1 | 0.000813389 | 87.92 | 0.0000 |
| AA | 0.0000311296 | 1 | 0.0000311296 | 3.37 | 0.0823 |
| AB | 0.00000408333 | 1 | 0.00000408333 | 0.44 | 0.5144 |
| BB | 0.00000224074 | 1 | 0.00000224074 | 0.24 | 0.6282 |
| Error | 0.000175769 | 19 | 0.00000925097 | ||
| Total ss | 0.00216719 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, R.I.; Morales-Quintana, L.; Alvarado, N.; Guzmán, L.; Forero-Doria, O.; Valenzuela-Riffo, F.; Laurie, V.F. Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology. Polymers 2021, 13, 2080. https://doi.org/10.3390/polym13132080
Castro RI, Morales-Quintana L, Alvarado N, Guzmán L, Forero-Doria O, Valenzuela-Riffo F, Laurie VF. Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology. Polymers. 2021; 13(13):2080. https://doi.org/10.3390/polym13132080
Chicago/Turabian StyleCastro, Ricardo I., Luis Morales-Quintana, Nancy Alvarado, Luis Guzmán, Oscar Forero-Doria, Felipe Valenzuela-Riffo, and V. Felipe Laurie. 2021. "Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology" Polymers 13, no. 13: 2080. https://doi.org/10.3390/polym13132080
APA StyleCastro, R. I., Morales-Quintana, L., Alvarado, N., Guzmán, L., Forero-Doria, O., Valenzuela-Riffo, F., & Laurie, V. F. (2021). Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology. Polymers, 13(13), 2080. https://doi.org/10.3390/polym13132080








