Abstract
Xylo-oligosaccharides are sugar oligomers with 2~7 xylose units considered non-digestible fibers that can be produced from biodegradable and low-cost biomass like wheat straw. An integrated approach consisting of hydrothermal pretreatment, alkaline treatment, enzymatic treatment and the combinations thereof was applied to overcome the recalcitrance structure of the wheat straw and allow selective fractioning into fermentable sugars and xylo-oligosaccharides. The hydrolysates and processed solids were chemically characterized by High-performance liquid chromatography and Ion chromatography, and the results were expressed as function of the severity factor and statistically interpreted. The concentration of fermentable sugars (glucose, xylose, arabinose) was the highest after the combination of alkaline and enzymatic treatment with xylanase (18 g/L sugars), while xylo-oligosaccharides (xylotriose and xylotetraose) were released in lower amounts (1.33 g/L) after the same treatment. Refining experiments were carried out to obtain a purified fraction by using anion and cation exchange chromatography. The polymer adsorber resin MN-502 showed efficient removal of salts, phenols and furan derivatives. However, the xylo-oligosaccharides yields were also slightly reduced. Although still requiring further optimization of the treatments to obtain higher purified oligomer yields, the results provide information on the production of xylo-oligosaccharides and fermentable sugars from wheat straw for potential use in food applications.
1. Introduction
Oligosaccharides have attained a growing commercial interest in the last decades due to their application as food ingredients and putative prebiotic compounds, which might exert beneficial effects on the host health [1,2,3,4,5,6,7,8,9]. Some of these polymers, specifically xylo-oligosaccharides (XOS), are considered non-digestible fibers and can be produced from biodegradable and low-cost agricultural biomass, especially lignocellulosic material such as wheat straw, by chemical, auto-hydrolytic, enzymatic processes or a combination thereof [2,4,10,11,12,13,14,15,16,17,18,19,20,21,22].
Xylo-oligosaccharide products are sugar oligomers with 2~7 xylose units, and their monomer (xylose) is connected by β-(1–4)-linkages, which can contain different side groups (e.g., α-d-glucopyranosyl uronic acid or its 4-O-methyl derivative, acetyl groups, or arabinofuranosyl residues), forming branched structures [23]. Associations in the scientific literature were made between consumption of XOS and potential prebiotic effects since these compounds pass through the upper gastrointestinal tract without being digested. They are metabolized by lactic acid bacteria (bifido bacteria and lactobacilli) in the lower intestine, which facilitates their conversion into short-chain fatty acids (SCFAs) like acetate, propionate and butyrate. Lately, research has highlighted the implications of the gut microbiota on human health, with SCFAs being correlated with reducing luminal pH and having protective action against acid-sensitive enteropathogens [24,25,26,27,28]. In recent years, XOS have been incorporated in various food applications (cookies, dairy products, beverages, fruit juices and chewing gums) [29,30,31,32], animal feed [33] and nutraceuticals [16,34], showing better physicochemical properties such as thermal stability (up to 100 °C) and acidity (pH 2.5 to 8) compared to the well-known inulin and fructooligosaccharides [5,12,35]. In Japan, XOS are approved as food ingredients by the Japanese Ministry of Health, Labour and Welfare, as Foods for Specified Health Uses (FOSHU), while in China they have been on the market since 2000 as food supplements [36]. In the European Union (EU), XOS obtained from corncobs have been authorized to be used as a novel food ingredient in various food categories (bread, breakfast cereals, biscuits, yoghurt, soy drink, fruit spreads and chocolate confectionary) at maximum levels varying from 3.5 g/kg to 30 g/kg, by the European Commission, as proved by the Commission Implementing Regulation (EU) 2018/1648 of 29 October 2018, based on the scientific opinion on the safety of XOS by the European Food Safety Authority [37,38]. In the EU, foodstuffs produced with new technologies, derived from new sources, and made from new substances, as well as traditional foods consumed in non-EU countries that were not consumed to a significant degree within the EU before 15 May 1997, are considered “novel foods” in Regulation (EU) 2015/2283; therefore, they require premarket authorization by the European Comission after their safety is assessed by the European Food Safety Authority [39,40].
Over the last years, various processes (enzymatic, chemical and autohydrolysis) have been exploited to produce XOS and fermentable sugars from lignocellulosic biomasses such as wheat by-products (straw, chaff and bran) [2,3,10,13,14,15,17,18,19,20,41,42,43], corn cobs [44], almond shells [20], olive stones [20], rice husks [44], barley straw [44], sugarcane straw [45] and poplar wood chips [46]. The state of the art in the manufacture and application of XOS up to 2019 was reviewed recently [10]. The use of agricultural biomass has a positive impact on the environment, by contributing to the valorization of the residues generated annually; however, there are some challenges arising for XOS production, since they present a spoilage risk during storage, are seasonal and have variable chemical composition [47,48]. Wheat straw (WS) is the third most produced cereal in the world, after maize and rice, being a promising feedstock for high value-added products [42]. It was shown to have good xylan/lignin ratios, be biodegradable and have low-cost, but owing an inherent complex structure of the biopolymers cellulose, hemicellulose and lignin in different proportions, a strong native recalcitrance is formed, which blocks its enzymatic hydrolysis [10,21]. Therefore, an integrated approach that would allow selective fractionation of the wheat straw seems a crucial step. Generally, XOS can be produced from xylan, which is the main hemicellulose in wheat straw, consisting of a linear backbone of β-1,4-linked xylopyranose (Xyl) residues. A first pretreatment step of the wheat straw, to break down the complex polymeric structure, and enhancing the accessibility of enzymes to the substrate during a enzymatic hydrolysis step were shown to be effective for generating fermentable sugars and XOS [6,10]. Previous studies showed that autohydrolysis, or hydrothermal pretreatment (HTP), might be an attractive method, since it was low-cost and used only water as a reaction media, effectively depolymerizing hemicelluloses by the hydrolytic action of hydronium ions (generated from water autoionization and from in situ generated organic acids) into soluble sugars and XOS and enhancing the accessibility of enzymes to the solid fraction (cellulose and lignin) [10,15,34]. The extent of depolymerization depends on the treatment severity (temperature and time), with studies showing a varied range of temperatures as effective (130–230 °C), but also on other factors such as particle size, pH and liquid-solid ratio [18]. To remove the lignin from the pretreated residues, post-treatment technologies are used, such as alkaline, acidic and enzymatic processes or a combination thereof. Alkali treatment was shown to improve enzymatic digestibility, by degrading the lignin structure and swelling the cellulose fibers [10,49]. However, the chemical and autohydrolysis methods showed shortcomings, such as degradation of pentoses to contaminants like furfural and hexoses to hydroxymethylfurfural (HMF), low control over the degree of polymerization (DP) and high downstream costs [50]. A second step consisting of applying an enzymatic treatment for xylan hydrolysis, using endo-1,4-β-xylanases (EC 3.2.1.8) and endo-1,3-β-xylanases (EC3.2.1.32), affected the yield of XOS, depending on various factors such as enzyme activity and incubation conditions (pH, hydrolysis time and temperature) [4,12]. The enzymatic process was reported to be more environmentally friendly, since no use of chemicals is needed, and it can be conducted at milder temperatures; however, the cost of the various enzymes needed might be quite high.
Against this background, the main objectives of this paper were to characterize the liquid fractions of wheat straw obtained during the autohydrolysis, alkali and enzymatic treatment and the combinations thereof in terms of XOS production (xylobiose, xylotriose and xylotetraose) and fermentable sugars. A downstream process method consisting of filtration, decolorization, and an- and cation exchange resins was tested in order to obtain a purified fraction of oligosaccharides and fermentable sugars for potential use in food applications (Figure 1).
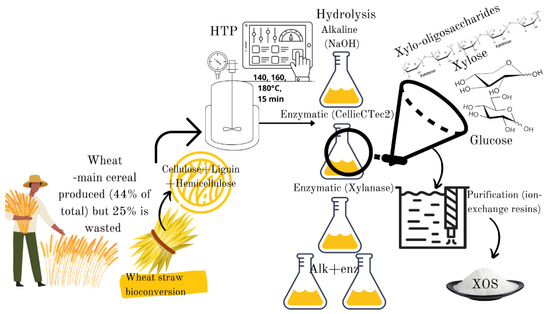
Figure 1.
Graphical abstract: Chemical and enzymatic synthesis of biobased xylo-oligosaccharides and fermentable sugars from wheat straw.
2. Materials and Methods
2.1. Material
Wheat straw was kindly provided from a farm (SC ALBATROS SRL) in Romania. The feedstock material was ground with a knife mill to particles of 0.5 mm, homogenized in a defined lot and stored in plastic containers at room temperature.
2.2. Hydrothermal Pretreatment (HTP)
The hydrothermal pretreatments (autohydrolysis) were performed according to Budde (2014), in a stainless-steel reactor (Parr Instruments Company, Moline, IL, USA) with a total volume of 600 mL [51]. The reactor was fitted with two four-blade turbine impellers, heated by an external fabric mantle and cooled down with ice water manually. Temperature was controlled through a Parr PID controller, model 4843.
The wheat straw was mixed with water in the reactor in order to obtain a L/S ratio of 10 (g water/g dry feedstock). The agitation speed was set at 350 rpm, and the reactor was heated to reach final temperatures ranging between 140 °C and 180 °C for 15 min (isothermal conditions). Three repetitions were made for each batch. Typically, the average heating rate (from 100 °C) was 3.8 °C/min. When the desired temperature was attained, the reactor was maintained for a reaction time of 15 min, and then it was rapidly cooled down to 70 °C. The liquid and solid phases were recovered by filtration (Whatman filter paper No. 1), and the whole slurry was filtered for solid and liquid recovery. After the filtration, the liquid phase was collected in a plastic vial and stored in a refrigerator at 4 °C for pH measurement as well as sugar and byproduct analyses. The solid residues were washed under tap water and centrifuged and stored in a refrigerator at 4 °C for enzymatic hydrolysis.
The severity of autohydrolysis pretreatment is often expressed in the literature as the “severity factor”, which refers to the combination of temperature and residence time in the autohydrolysis pretreatment process. It was calculated using the following equation [52]:
where t1 and T1 are the pretreatment time (min) and temperature (°C), respectively. The value of 14.75 is an empirical parameter related to temperature and activation energy. Severity factor was calculated in the range of 2.35–3.53, depending on pretreatment temperature and time.
2.3. Chemicals
Glucose (G), xylose (X), arabinose (A) and standards were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA) to determine the chemical composition of wheat straw and monomeric sugars in the liquid fraction. The xylo-oligosaccharides standards (X2) xylobiose, (X3) xylotriose and (X4) xylotetraose were acquired from Megazyme International (Ireland). All of the reagents were of analytical grade.
2.4. Analytical Methods
2.4.1. Chemical Characterization of Feedstock and Processed Solids
The materials were ground in a knife mill to a particle size of 0.5 mm, and the moisture content was determined by oven-drying at 105 °C to a constant weight. The ash content and organic dry matter was determined by igniting the contents at 550 °C for 5 h in a muffle furnace. The main components of the wheat straw were determined using the laboratory analytical protocol (LAP) developed by the National Renewable Energy Laboratory (NREL).
2.4.2. Chemical Characterization of Hydrolysates
Glucose, xylose, arabinose, acetic acid, 5-hydroxymethylfurfural (HMF) and furfural were analyzed by HPLC (Waters, Milford, DE, USA) using an Aminex HPX-87P column (Bio-Rad, Hercules, CA, USA) and a Shodex sugar SP0810 column. All samples were filtered through 0.2 μm membranes before analysis. A sample of the liquors was directly analyzed by HPLC. The yields of cellulose in the liquid phase were calculated based on Equation (2), with 0.9 being the conversion factors for glucose.
2.4.3. Enzyme Assays
Cellulase Assay
The cellulase activity was assayed by measuring the amount of reducing sugars released from the wheat straw according to Ghose (1987) and recommended by IUPAC, using filter paper Whatman No. 1 as a substrate [53]. A sodium citrate buffer solution of 0.05 M and pH 4.8 was prepared, of which 1 mL was added in a glass tube, covering the paper strip together with 0.5 mL of enzyme. The tube was vortexed and incubated in a water bath at 50 °C for 60 min. Then, 3 mL of DNS reagent was added at the end of the incubation, and the tubes were incubated in a water bath for 10 min at 95 °C and then placed in a cold-water bath for 5 min. Dilutions were made, and the absorbance was measured at 540 nm against a reagent blank using an LLG–uniSPEC 2 spectrophotometer. The amount of reducing sugar liberated was determined by the Dinitrosalicylic (DNS) acid method, using glucose as the standard. One FPU/g represents the enzyme unit per gram of the initial dry solid substrate.
Xylanase Assay
The crude endo-β-1-4-xylanase used in this study was produced from Trichoderma viride and provided by Sigma-Aldrich (USA). The reaction mixture containing equal volumes of 1% (w/v) beechwood xylan and the suitably diluted enzyme solution with 50 mM phosphate buffer (pH 7) at 37 °C for 30 min, allowing depolymerization of xylan. The reaction was stopped by adding 1500 μL DNS reagent, and then the mixture was heated at 100 °C for 10 min, allowing the color-forming reaction. The intensity of red-brown color was measured at 515 nm to estimate the concentration of reducing sugar in the reaction system. One xylanase unit was defined as the amount of enzyme needed for the liberation of one µM of reducing sugar (xylose) in one minute [18].
2.4.4. Alkaline and Enzymatic Hydrolysis of the Solid Residues
The residues obtained from the HTP straw were further treated with 1% NaOH 20% at 50 °C or 30 °C for 48 h. Currently, most xylo-oligosaccharides are produced at the industrial level from enzymatic hydrolysis of alkaline-extracted xylan with xylanase.
Enzymatic hydrolysis of the solid residues was performed with 10% of residue (w/v) in 300 mL distilled water in Erlenmeyer flasks. The flasks were put in a a shaking incubator (Ecotron, Infors HT, Berlin, Germany) at 50 °C or 30 °C, for 48 h at 150 rpm. Commercial cellulase (Cellic® CTec2, 100 FPU/mL) and Xylanase from Trichoderma viride (endo-1,4-β-Xylanase, 100–300 units/mg protein) were provided by Novozymes (Beijing, China) and Sigma-Aldrich (USA) and tested for saccharification experiments. The hydrolysates were monitored at specific time intervals and analyzed by HPLC using an UltiMate 3000 HPLC (Thermo Fisher DIONEX, Bavaria, Germany) with the column Eurokat H (KNAUER, Berlin, Germany). The dosages used for the saccharification experiments were as follows: Cellic® CTec2: 4 FPU/g residue; 2 mL endo-b-1-4-xylanase from T. viride per 100 g residue. All the experiments were performed in duplicate, and the results were averaged.
2.4.5. Filtration and Decolorization
The liquors were filtered manually using a 150 μm filter bag (Schwegmann Filtrations-Technik GmbH, Grafschaft, Germany) to remove the residues made of the lignocellulosic material. They were further centrifuged (4800 rpm 15 min—Sigma 4-16KS; Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) and decolorized using PUROLITE MN-502 (Purolite, Ratingen, Germany) or active charcoal. The flow was set to 6 bed volumes h−1. Decolorization was finished after rinsing the column with purified water until conductivity was below 1 mS cm−1.
2.4.6. An- and Cation Exchange Chromatography
An- and cation exchange chromatography was performed in order to separate the sugars from the salt-ions. First, the weak anion exchange resin A 103 S (NH3 form, styrene-DVB) and second the strong cation exchange resin C 150 S (Na+ form, polystyrene- DVB) (Resindion S. R. L., Binasco, Italy) were applied. Before use, the resins were regenerated with the specific acid or alkaline solution, then carefully washed with distilled water. Column volumes were 1 L, and the loading was carried out from below at 6 bed volumes h−1. Adsorption experiments were conducted in flasks shaking at 150 rpm for 2 h at 50 °C, and the pH was adjusted with NaOH 20% and H2SO4. The pH and conductivity of the samples were measured. Phenol, kresol, catechol and guaiacol were chosen as representatives for phenols with inhibiting properties, obtained from Sigma-Aldrich (St. Louis, MO, USA).
The concentration of inorganic anions and cations were measured by ion chromatography using an ICS-1000 system (Thermo Scientific Dionex, Germering, Germany). For quantification of anions, 25 μL of sample volume was added on a IonPac AS9-HC column (4 × 250 mm) (Thermo Fisher DIONEX, Germany) and eluted isocratically with 1.2 ml/min−1 of 9 mM Na2CO3 at room temperature. For quantification of cations, 25 μL of sample volume was added on an IonPac CS 16 column (250 mm × 5) (Thermo Fisher DIONEX) and eluted isocratically with 1.0 mL min−1 of 30 mM CH3SO3H at 40 °C. Detection of cat- and anions was carried out by a conductivity cell. Each analysis was carried in duplicate, and peak areas were compared to analyses of known concentrations of salt-solutions consisting of the cat- and anions of interest. The mean concentration of the two analyses is presented.
The purified fraction was concentrated in a spray-dryer (Mobil minor; GEA, Düsseldorf, Germany) at a temperature between 120–135 °C, a pressure of 35 bar and a flow rate of approximately 10 mL/min.
2.5. Statistical Analysis
Statistical analysis was performed with IBM SPSS Statistics 19. All tests/experiments were conducted in triplicate, and the results were expressed as the means ± standard deviation (SD). Data normality was studied using the Shapiro-Wilk test [54], and the homogeneity of variances (homoelasticity) was studied using Levene test. A one-way ANOVA test was applied for normally distributed and homogeneous data. For p < 0.05 corresponding to statistical F, calculations with the Tukey post hoc test were used to study whether the treatments had significant differences. For non-homogeneous data, the Welch test was used, followed by the Kruskal–Wallis test, in which, if p < 0.05 was obtained, the calculations were continued with the Mann–Whitney test to verify which treatments had significant differences. At the parameters where the data were not homogeneous and we used Mann–Whitney, the Bonferroni correction was applied: 0.05/6 = 0.008 and 0.01/6 = 0.001; for 3 treatments, 0.05/3 = 0.016 and 0.01/3 = 0.003.
3. Results and Discussion
3.1. Chemical Characterization of Feedstock and Processed Solids
The tight structure of wheat straw cell wall is hard to hydrolyze without pretreatment processes that could overcome the recalcitrance of lignocellulose for the enzyme action. In this work, hydrothermal pretreatment (HTP), alkaline treatment and enzymatic treatment, or the combinations thereof, were used to improve the accessibility of the enzymes to the WS. Hydrothermal treatment was chosen due to the advantages highlighted in the literature, such as low cost, reduced chemical consumption due to the increase in acetic acid and water ionization that catalyze the process and facilitate depolymerization of the wheat straw structure, production of oligosaccharides and monosaccharides with decreased formation of degradation products, and the formation of residues that could further be fractioned [21].
Milling was applied to reduce the particle size and improve the hydrolysis by increasing the surface area [21]. The chemical composition of wheat straw is shown in Table 1. The holocellulose of wheat straw accounts for 68.67 ± 0.4% of the total weight and consists of 27.67 ± 0.1% hemicelluloses and 36% ± 0.3 celluloses. Lignin was quantified as acid detergent lignin 12.91 ± 0.8%, ash 5 ± 0.1% and extractives 1.2 ± 0.1%. During the HTP, the hemicelluloses decreased with the severity factor, from 24.7 ± 0.2% at 2.35 when treated at 140 °C for 15 min, to 11.3 ± 0.1% at a severity factor of 2.94 when HTP reached 180 °C. The dissolution of hemicellulose was caused by the ionization of water and formation of acetic acid [55]. Consequently, cellulose increased with the severity factor, from 45.12 ± 0.1% to 51 ± 0.5%. As for lignin, the content increased with the severity factor, with the highest content of 9.9% at 180 °C (Table 1). The pH profile after HTP shows the treatment severity and indicates the solubility of hemicelluloses in the liquid phase. The pH of the filtrate at the severity factor of 2.35 decreased from 5.2 to 4.5 at the severity of 2.94 (160 °C, 15 min) to 3.9 at the severity of 3.53 (180 °C, 15 min). Similar results were obtained by Chen et al. in 2018, who applied hydrothermal pretreatment combined with ethanol extraction to obtain oligosaccharides from wheat straws [10]. They observed that hemicellulose decreased with the HTP increasing temperature (from 27.06% w/w at 120 °C to 25.09% w/w at 140 °C and 3.09% w/w at 180 °C), while cellulose content increased with the increase of temperature from 120 °C to 180 °C.

Table 1.
Chemical composition (% DM) of the raw material (WS) and pre-treated residues after the hydrothermal pretreatment.
3.2. Chemical Characterization of Hydrolysates after HTP, Alkaline and Enzymatic Treatments of the Solid Residues
Hydrothermal pretreatment was shown to degrade the hemicellulose present in the WS, improving the accessibility of cellulose to enzymes, with the formation of soluble sugars, xylose oligomers (xylo-oligosaccharides “XOS”), weak acids, furan derivatives and phenolic compounds. The results concerning the production of soluble sugars (glucose, xylose and arabinose) and xylo-oligosaccharides are presented in Table 2 and Table 3, respectively. In Supplementary File S1 the results obtained by other researchers in terms of wheat straw fractioning, sugars, xylo-oligosaccharides and inhibitor release are described.

Table 2.
Sugar yields in the hydrolysates after HTP, alkaline and enzymatic treatments of the solid residues.

Table 3.
Xylo-oligosaccharides yields in the hydrolysates after HTP, alkaline and enzymatic treatments of the solid residues.
3.2.1. Sugar Release
The release of soluble sugars during HTP increased with the severity factor, from 0.17 ± 0.1 g/L at a severity factor of 2.35 to 0.89 ± 0.6 g/L at log R0 = 3.53. The depolymerization of hemicellulose occurred due to the hydrolytic action of the hydronium ions, which cleaved the acetyl groups to generate acetic acid [42]. The acetic acid content increased with the severity factor, from 0.15 ± 0.02 g/L at a severity factor of 2.35 (140 °C, 15 min) to 1.32 ± 0.01 g/L at a severity factor of 3.53 (180 °C, 15 min), as observed in Figure 2a. Huang et al. [18] observed the same trend after liquid hot water pretreatment of waste wheat straw for fermentable sugars and acetic acid, which increased with the rising temperature. Additionally, the authors obtained higher yields of sugars, with 15.8 g/L fermentable sugars at 180 °C for 40 min compared to our work, due to the different processing conditions. Chen et al. applied the same hydrothermal treatment as in our work but for longer residence time (0.5 h) and further applied an acid treatment with sulfuric acid to the hydrolysates to determine the oligosaccharide concentrations; they obtained higher yields of oligosaccharides than monosaccharides, which increased with the severity of the treatment from 2.49 g/kg at 120 °C to 61.69 g/kg at 180 °C [10]. Similarly, Ilanidis et al. reported comparable concentrations of monosaccharides to our work after HTP treatment of wheat straw at temperatures between 160 and 205 °C for 15 min (0.1 g/L xylose in the hydrolysate treated at 160 °C; 4.4 g/L xylose at the highest temperature of 205 °C) [56].
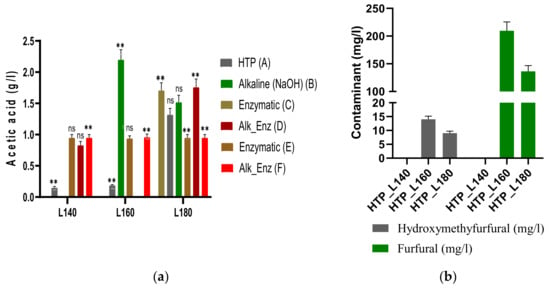
Figure 2.
(a) Yield of acetic acid (g/L) detected in the hydrolysates after HTP, alkaline and enzymatic treatments of the solid residues; ns: not statistically significant; ** Correlation is significant at the 0.05 level (2-tailed); (b) Yield of furfural and HMF (mg/L) detected in the hydrolysates after HTP treatment.
The solid residues after HTP were used to carry out the alkaline and enzymatic treatments. The alkaline treatment was shown to improve the removal of lignin and hemicellulose and swelling of the cellulose fibers [57]. Glucose release was highest at 160 °C (3.73 ± 0.28 g/L); however, the differences between the pretreatment temperatures were not statistically significant. Xylose and arabinose had the highest release at the highest severity factor (3.53) (3.80 ± 0.2 g/L and 0.59 ± 0.04 g/L, respectively) after the alkaline treatment method. Faryar et al. observed that xylose and arabinose were the main sugars extracted after alkaline treatment of wheat straw (0.047 g–0.056 g xylose/g WS) [58]. Akpinar et al. [59] applied an alkaline treatment (4% KOH, 1% w/v NaBH4) to extract xylan from different agricultural wastes (wheat straw, tobacco stalk, cotton stalk and sunflower stalk) and observed that WS had the highest amount of arabinose and a more arabinoxylan structure compared to the other biomasses.
Enzymatic hydrolysis was performed with two different enzymes for xylan and cellulose hydrolysis using Xylanase from T. viride and commercial cellulases (Cellic® CTec2) in different concentrations. Alkaline enzymatic treatment was also applied to the residues to improve the accessibility of the enzymes, since lignin is soluble in highly alkaline media [57].
With regards to the enzymatic hydrolysis with xylanase, xylose release was highest in the alkaline hydrolysates of the residue treated at 180 °C, showing a quantity of 4.76 ± 0.35 g/L after 48 h of enzymatic hydrolysis at a pH of 4.9. When combining enzymatic with alkaline treatment, the xylose release almost doubled, showing a release of 9.47 ± 0.71 g/L in the hydrolysate of the residue treated at 180 °C. Glucose release was highest when the residues were treated with the enzymatic mix Cellic®CTec2, which contains aggressive cellulases, β-glucosidases and hemicellulases. Statistically significant differences were observed regarding conversion of cellulose to glucose after the different enzymatic treatments; the maximum glucose yield after 48 h of enzymatic hydrolysis with Cellic®CTec2 was found to be 10.72 ± 0.23 g/L at the autohydrolysis condition of 140 °C, which means about 19% of cellulose in the solid residue. Huang et al. used the same enzymatic cocktail to pretreat waste wheat straw (180 °C, 40 min) to recover soluble sugars (xylose, glucose) and obtained a recovery of maximum 33.4 g glucose and 6.1 g xylose after applying a washing treatment to remove the ash [18]. The authors also suggested that the ash in the residues conducted to reduced enzymatic hydrolysis efficiency; thus, the prewashing step was necessary. Han et al. reported that alkaline treatment (1%) improved the efficiency of enzymatic hydrolysis of wheat straw using a cellulase produced by P. waksmanii and obtained a maximal concentration of reducing sugar (343.95 mg/g substrate) [60]. Moreover, Silva-Fernandes et al. [61] applied an enzymatic hydrolysis to pretreated residues of WS by HTP (at 210 and 230 °C) and observed that the conditions applied for the enzymatic hydrolysis allowed a higher recovery of glucose (30.6 g/L glucose representing around 90% yield) from the residues pretreated at 230 °C compared to those at 210 °C (78.8%). They concluded that enzymatic hydrolysis allowed a high digestibility of cellulose fibers due to the physical degradation of lignocellulose, while diminishing the hemicellulose content in the residues and their inhibition towards cellulolytic enzymes [61]. When xylanase was used in the hydrolysis, the highest yield of glucose was detected in the hydrolysate at 180 °C, 6.67 ± 0.50 g/L, after enzymatic and alkaline treatment of the residues.
3.2.2. Xylo-Oligosaccharide Production and Byproduct Release
In addition to monosaccharides, xylo-oligosaccharides with a low degree of polymerization (DP < 6) (xylotriose, X3, and xylotetraose, X4) were also detected in the hydrolysates after enzymatic and alkaline treatment of the residues. We observed that during the hydrothermal pretreatment, xylo-oligosaccharides were not detected, except a very low amount of xylobiose (0.01 g/L) in the hydrolysate at 180 °C, which might be caused by the autohydrolysis conditions, for instance, the long residence time (30–40 min) and pressure conditions (12 bar) in the reactor until it reached the desired temperatures. Guo et al. applied an acidolysis treatment with acetic acid to wheat straw and reported that the yield of released XOS was also dependent on the structural composition of the raw material, specifically the wax content and inert structure, which inhibit the production of XOS [42]. However, after the enzymatic and alkaline treatment of the residues, statistically significant differences were noticed. During the enzymatic treatment, xylanase was the most efficient in the hydrolysates obtained at 160 °C, with an amount of 1.23 ± 0.12 g/L XOS released, compared to the enzymatic mix, which showed an increasing trend of liberated XOS from 0.59 ± 0.03 g/L in the hydrolysates at 140 °C and at 160 °C to a maximum amount of 0.78 ± 0.03 g/L in the liquids at 180 °C. When combining enzymatic with alkaline treatment, which was shown to improve enzymatic digestibility, higher yields of XOS were obtained, showing statistically significant differences when compared to the enzymatic treatment. Xylotriose and xylotetraose were detected in the hydrolysates at 140 °C, with a content of 1.48 ± 0.2 g/L, and 1.33 ± 0.13 g/L in the filtrate at 180 °C, after 48 h of enzymatic hydrolysis with xylanase at pH 4.6. Faryar et al. reported concentrations of 1.94 ± 0.045–2.07 ± 0.068 mg XOS/mL (X2–X5) at pH 7 and 8, respectively, after enzymatic hydrolysis at 60 °C of wheat straw using an endoxylanase from B. halodurans [58]. Production of XOS from wheat straw was also studied by Akpinar et al., who reported 0.079 g XOS/g xylan after enzymatic hydrolysis with endoxylanase from Aspergillus niger [59].
Slightly lower yields were obtained when using the enzymatic mix Cellic CTec2, with an increasing trend of XOS being observed in the hydrolysates, from 1.11 ± 0.05 g/L in the hydrolysate at log R0 = 2.35 to a maximum of 1.26 ± 0.06 g/L in the hydrolysate at log R0 = 3.53. Huang et al. obtained higher yields of xylotryose and xylotetraose after enzymatic hydrolysis of pretreated wheat straw at 180 °C for 20 min (X3 + X4: xylotriose: 3.1 ± 0.7 g/L). The literature shows that XOS produced via alkaline enzymatic treatment have low aqueous solubility, since the acetyl and uronic groups are completely degraded [2,21].
During autohydrolysis, polysaccharides and monosaccharides could further decompose in degradation products like acetic acid, furfural and hydroxymethylfurfural (HMF). Figure 2a,b show the results of these degradation products. It was noticed that the yield of acetic acid increased with the severity of the hydrothermal treatment, from 0.15 ± 0.02 g/L to 1.32 ± 0.10 g/L in the hydrolysate at 180 °C. The highest content of acetic acid was detected after enzymatic treatment with xylanase and the combined alkali enzymatic with the same enzyme in the hydrolysates at 180 °C, with yields of 1.71 ± 0.12 g/L and 1.76 ± 0.13 g/L, showing statistically significant differences when compared to the other enzymatic treatment applied. Acetic acid originated from the hydrolysis of acetyl groups on the hemicellulose backbone, with the increase in the concentrations of byproducts leading to a decrease in the pH of the filtrate (from 5.2 to 3.9 after HTP). The results indicate a relationship between acid generation and hemicellulose solubilization, similar to other papers [43].
The other byproducts detected, furfural and HMF, resulted from the degradation of pentose (xylose and arabinose) and hexose (glucose); they were shown to hinder the enzymatic hydrolysis by inhibiting the activity of the enzyme and could inhibit the upgrade of both the liquid and solid fractions [10]. As observed in Figure 2a, the amounts of these metabolites released during the hydrothermal pretreatment increased with the severity factor from 2.35 to 3.53. For instance, furfural had a content of 209 ± 15.8 mg/L and HMF 14.81 ± 1.1 mg/L in the hydrolysate at log R0 = 2.94, while in the hydrolysate at log R0 = 3.53, furfural was detected in an amount of 136 ± 10 mg/L and HMF 9.54 ± 0.7 mg/L, due to the conversion of xylose and glucose. Chen et al. showed that furfural and HMF were detected only in the hydrolysate after pretreatment of wheat straw at 180 °C and 200 °C, with higher yields compared to our work (furfural: 8.4–17.6 g/kg and HMF: 0.3–4.5 g/kg) [10].
3.3. Material Balance
Material balances of hydrothermal pretreatment and subsequent alkaline, enzymatic and alkali enzymatic treatments are shown in Table 4. The material balance shows that the higher solid recovery of 88.9% was at the lowest severity of the hydrothermal pretreatment, and it decreased to 76.8% at the highest severity. The total amount of sugars released during HTP and alkaline treatment increased with the severity, from 2.35 (1.62 g/L) to 3.53 (8.76 g/L). It was noticed that the treatments applied showed high efficiency in releasing the sugars from the lignocellulosic materials, with the highest sugar recovery of 94% at the severity of 3.53 (180 °C, 15 min), obtained by considering the total amount of sugars released (glucose, xylose and arabinose) after combining all treatments applied (hydrothermal, alkaline, enzymatic hydrolysis and the alkali enzymatic). Discriminating between treatments, it was noticed that the hydrothermal pretreatment at the highest severity combined with enzymatic hydrolysis with xylanase had the highest sugar recovery of 64.86%, followed by the combined alkaline and enzymatic treatment with the enzymatic mix (Cellic®CTec2), which showed a recovery of 54.41%. In contrast, the lowest sugar recovery (18.79%) was shown at the lowest severity (140 °C), after enzymatic hydrolysis with xylanase.

Table 4.
Material balances from hydrothermal pretreatment followed by alkali, enzymatic and combined alkali enzymatic treatments.
3.4. Downstream Processing
In order to obtain a purified fraction of XOS for potential food applications, the hydrolysates obtained during hydrothermal pretreatment and enzymatic treatments must be refined. A variety of compounds like monosaccharides, acetic acid, furfural and HMF from pentose and hexose dehydration, soluble inorganic compounds or protein-derived products could appear in the hydrolysates [34]. Commercial xylo-oligosaccharides have a purity in the range of 75–95% [12]. The literature shows that purification and separation of XOS requires several processing steps, consisting of physicochemical treatments [62].
Adsorption was employed in combination with other treatments for the refining of XOS, intended to remove undesired compounds and separate oligo from monosaccharides. Chromatographic separation was carried out for XOS purification at an analytical level, yielding high purity fraction, while ion exchange was used for purification of XOS alone or in multi-step processing [12,34,62,63].
In this work, refining experiments were carried out in two steps: a first step of adsorption using the surface-active material activated charcoal or the polymer adsorber resin PUROLITE MN-502 to remove phenols and reduce the concentration of salt ions. The hydrolysate resulting after alkaline and enzymatic hydrolysis of the residues treated at the severity factor of 3.53 was used for the downstream processing experiments. As observed in Table 5, statistically significant differences were obtained when comparing removal of sulphate, phosphate and cations (K+, Mg2+, Ca2+, NH4+-N) by activated charcoal or the MN-502 resin, the latter showing higher efficiency. For instance, phosphate concentrations reduced from the initial 59 mg/L in the hydrolysate to 16 mg/L or 23 mg/L after 2 h of decolorization with the resin or the activated charcoal, respectively. Sulphate concentrations dropped almost to half, from 80 mg/L to 40 mg/L after treatment with the resin, showing statically significant differences when compared to the filtrate resulting after decolorization with charcoal (54 mg/L). Good results were obtained also in the case of HMF and furfural removal, which showed a decrease from 9.7 mg/L to 0.5 mg/L (MN 502) and <0.004 mg/L (charcoal) in the case of HMF. Furfural decreased from 139 mg/L to 2.3 mg/L after treatment with MN502 and <0.01 mg/L after charcoal treatment. In contrast, phenol concentrations did not decrease significantly. Monosaccharide concentrations were also reduced, with xylose being lowered from 9.4 mg/L to 1.3 mg/L after treatment with the resin and 0.09 mg/L after charcoal treatment. XOS registered a slight decrease, from 1.33 mg/L in the initial hydrolysate to 1.31 mg/L in the hydrolysate treated with the resin and to 1.26 mg/L in the hydrolysate treated with charcoal. Xu et al. used activate carbon treatment at different dosages (0.2%, 0.4%, 0.5%, 0.8%, 1% and 1.2%) to purify wood chip hydrolysates and observed that at higher dosage (1.2%), a loss of 20% xylosugars was noticed (1.5% xylose, 21% XOS loss), even if lignin and furfural were removed at a high percentage 70% [64]. It was reported that the adsorption behavior of the materials was affected by the molecular structure and weight [65].

Table 5.
Overview of the down-stream processing experiments of 1 L of hydrolysate after alkaline and enzymatic treatment of the residues treated at the highest severity factor (3.53).
An- and cation exchange chromatography was performed to further reduce the concentration of salts and minerals. It was previously shown that this method was successful in removing salt ions and other undesirable compounds and obtaining purified fractions of specific compounds such as lactic acid [66,67,68,69,70]. The anion exchange resin was more efficient in reducing the concentrations of sulphate, phosphates and nitrate, showing statistically significant differences (1.36 ± 0.01 mg/L PO43—P vs. the initial content of 58.58 ± 0.50 mg/L PO43—P; 24.3 ± 0.04 mg/L SO42− vs. the initial content of 80.82 ± 0.50 mg/L SO42−). Dupoiron et al. showed that a weak anion exchange resin (Amberlyst A2) was successful in removing carbohydrate fractions, chlorides and other anions such as sulfates and phosphates from ferulic acid contained in wheat bran, due to the resin strong affinity for OH- [71].
Cation concentrations were lowered after the cation exchange, showing statistically significant differences; however, sodium concentrations increased significantly (507.22 ± 38 mg/L). Levels of HMF, furfural and phenols were also reduced during the downstream process (Table 6). Monosaccharide (xylose and arabinose) removal was the most efficient after the activated charcoal adsorption (92%). It was noticed that XOS concentrations registered a slight decrease during the down-streaming process; however, no statistically significant differences were noted.

Table 6.
Removal of furfural, HMF and phenols after the down-streaming process.
Finally, it should be noted that the optimization of the downstream processing was not aim of the present work but is certainly needed to create feasible processes.
4. Conclusions
Our work showed that wheat straw could represent a low-cost alternative for production of fermentable sugars and xylo-oligosaccharides with low DP. Among the treatments applied, alkaline and enzymatic treatment with xylanase showed better results in terms of sugar release (glucose, xylose and arabinose) (18 g/L sugars), while XOS were generally released in lower amounts (highest concentration of 1.33 g/L). Refining experiments were carried out to obtain a purified fraction for potential food applications by using anion and cation exchange chromatography. The polymer adsorber resin MN-502 showed efficient removal of salts, cations, phenols and furan derivatives. However, further optimization treatments targeting a more efficient fractioning of the wheat straw are needed to obtain higher XOS yields in a purified form. Therefore, the integrated strategies in sugar-based biorefineries should target maximal sugar recoveries and fractioning processes that facilitate further conversion processes [72].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14071336/s1, Supplementary File S1. State of the art regarding wheat straw fractioning after various treatments in terms of monosaccharides, xylo-oligosaccharides and inhibitors yields.
Author Contributions
Conceptualization, G.P.; methodology, G.P., J.V.; M.H., R.S. and I.D.P.; software, I.D.P.; validation, G.P. and D.C.V.; formal analysis, G.P.; investigation, G.P. and D.C.V.; resources, J.V. and D.C.V.; data curation, G.P.; writing—original draft preparation, G.P.; writing—review and editing G.P., J.V., M.H. and R.S.; visualization, G.P.; supervision, D.C.V.; project administration, G.P. and D.C.V.; funding acquisition, D.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCI-UEFISCDI, Grant Number TE 184, Project PN–III–P1-1.1-TE-2019-1748, and by funds from the National Research Development Projects to finance excellence (PFE)-14/2022-2024 granted by the Romanian Ministry of Research and Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article. Other data that support the findings of this study are available upon request from the corresponding authors.
Acknowledgments
This work was carried out in the Leibniz Institute for Agricultural Engineering & Bioeconomy (ATB), Germany. The authors would like to thank Anne-Katrin Thoma, Sigrid Quilitz, Giovanna Rehde, Mandy Jäkel, Miriam Felgentreu, Linda Schroedter, Andreas Toursel, Leontina Lipan and Bernadette Emőke Teleky for their helpful collaboration. Financial support from a grant from the Deutsche Bundesstiftung Umwelt is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| XOS | Xylo-oligosaccharides |
| X3 | Xylotriose |
| X4 | Xylotetraose |
| SCFA | Short-chain fatty acids |
| WS | Wheat straw |
| HTP | Hydrothermal pretreatment |
| HMF | Hydroxymethylfurfural |
| DP | Degree of polymerization |
| DNS | Dinitrosalicylic method |
| CCTec2 | Cellic CTec 2 enzyme |
| ADL | acid detergent lignin |
| NS | Not statistically significant |
| ND | Not determined |
| AC | Active charcoal |
References
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic Profiling of Xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef]
- Isci, A.; Thieme, N.; Lamp, A.; Zverlov, V.; Kaltschmitt, M. Production of xylo-oligosaccharides from wheat straw using microwave assisted deep eutectic solvent pretreatment. Ind. Crop. Prod. 2021, 164, 113393. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Pintado, M.M.; Gomes, A.M.; Alonso, J.L.; Parajó, J.C. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J. Funct. Foods 2014, 6, 438–449. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Song, L.; Xie, W.; Zhao, Y.; Lv, X.; Yang, H.; Zeng, Q.; Zheng, Z.; Yang, X. Synthesis, Antimicrobial, Moisture Absorption and Retention Activities of Kojic Acid-Grafted Konjac Glucomannan Oligosaccharides. Polymers 2019, 11, 1979. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, T.S.; Retter, A.; Hobbs, P.J.; Budde, J.; Heiermann, M.; Plöchl, M.; Ravella, S.R. Production of xylooligosaccharides from renewable agricultural lignocellulose biomass. Biofuels 2015, 6, 147–155. [Google Scholar] [CrossRef]
- Martău, G.A.; Emoke, T.B.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple pomace as a sustainable substrate in sourdough fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Co-production of oligosaccharides and fermentable sugar from wheat straw by hydrothermal pretreatment combined with alkaline ethanol extraction. Ind. Crop. Prod. 2018, 111, 78–85. [Google Scholar] [CrossRef]
- Samanta, A.K.; Senani, S.; Kolte, A.P.; Sridhar, M.; Sampath, K.T.; Jayapal, N.; Devi, A. Production and in vitro evaluation of xylooligosaccharides generated from corn cobs. Food Bioprod. Process. 2012, 90, 466–474. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Ren, J.; Zhang, Y.; Liu, X.; Ouyang, J. Simultaneously separation of xylo-oligosaccharide and lignosulfonate from wheat straw magnesium bisulfite pretreatment spent liquor using ion exchange resin. Bioresour. Technol. 2018, 249, 189–195. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, C. Extracting xylooligosaccharides in wheat bran by screening and cellulase assisted enzymatic hydrolysis. Int. J. Biol. Macromol. 2016, 92, 748–752. [Google Scholar] [CrossRef]
- Ertas, M.; Han, Q.; Jameel, H.; Chang, H.-M. Enzymatic hydrolysis of autohydrolyzed wheat straw followed by refining to produce fermentable sugars. Bioresour. Technol. 2014, 152, 259–266. [Google Scholar] [CrossRef]
- Jagtap, S.; Deshmukh, R.A.; Menon, S.; Das, S. Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour. Technol. 2017, 245, 283–288. [Google Scholar] [CrossRef]
- Antov, M.G.; Đorđević, T.R. Environmental-friendly technologies for the production of antioxidant xylooligosaccharides from wheat chaff. Food Chem. 2017, 235, 175–180. [Google Scholar] [CrossRef]
- Huang, C.; Lai, C.; Wu, X.; Huang, Y.; He, J.; Huang, C.; Li, X.; Yong, Q. An integrated process to produce bio-ethanol and xylooligosaccharides rich in xylobiose and xylotriose from high ash content waste wheat straw. Bioresour. Technol. 2017, 241, 228–235. [Google Scholar] [CrossRef]
- Duarte, L.C.; Silva-Fernandes, T.; Carvalheiro, F.; Gírio, F.M. Dilute Acid Hydrolysis of Wheat Straw Oligosaccharides. Appl. Biochem. Biotechnol. 2008, 153, 116. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Ebringerová, A.; Montané, D. Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohydr. Polym. 2007, 69, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.E.; García-Martin, A.; Comendador Morales, P.; Wojtusik, M.; Santos, V.E.; Kovensky, J.; Ladero, M. Production of Oligosaccharides from Agrofood Wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Christophersen, C.T.; Petersen, A.; Licht, T.R.; Conlon, M.A. Xylo-Oligosaccharides and Inulin Affect Genotoxicity and Bacterial Populations Differently in a Human Colonic Simulator Challenged with Soy Protein. Nutrients 2013, 5, 3740–3756. [Google Scholar] [CrossRef] [Green Version]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Mitrea, L.; Vodnar, D.C. Klebsiella pneumoniae—A useful pathogenic strain for biotechnological purposes: Diols biosynthesis under controlled and uncontrolled pH levels. Pathogens 2019, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Juhász, R.; Penksza, P.; Sipos, L. Effect of xylo-oligosaccharides (XOS) addition on technological and sensory attributes of cookies. Food Sci. Nutr. 2020, 8, 5452–5460. [Google Scholar] [CrossRef]
- Dai, H.; Leung, C.E.; Corradini, M.G.; Xiao, H.; Kinchla, A.J. Increasing the nutritional value of strawberry puree by adding xylo-oligosaccharides. Heliyon 2020, 6, e03769. [Google Scholar] [CrossRef]
- Ferrão, L.L.; Ferreira, M.V.S.; Cavalcanti, R.N.; Carvalho, A.F.A.; Pimentel, T.C.; Silva, H.L.A.; Silva, R.; Esmerino, E.A.; Neto, R.P.C.; Tavares, M.I.B.; et al. The xylooligosaccharide addition and sodium reduction in requeijão cremoso processed cheese. Food Res. Int. 2018, 107, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Călinoiu, L.F.; Mitrea, L.; Precup, G.; Bindea, M.; Păcurar, A.M.; Szabo, K.; Ştefănescu, B.E. A New Generation of Probiotic Functional Beverages Using Bioactive Compounds from Agro-Industrial Waste. Funct. Med. Beverages 2019, 11, 483–528. [Google Scholar]
- Ribeiro, T.; Cardoso, V.; Ferreira, L.M.A.; Lordelo, M.M.S.; Coelho, E.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A.; Bedford, M.R.; Fontes, C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult. Sci. 2018, 97, 4330–4341. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Aragon, C.C.; Santos, A.F.; Ruiz-Matute, A.I.; Corzo, N.; Guisan, J.M.; Monti, R.; Mateo, C. Continuous production of xylooligosaccharides in a packed bed reactor with immobilized–stabilized biocatalysts of xylanase from Aspergillus versicolor. J. Mol. Catal. B Enzym. 2013, 98, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Mäkeläinen, H.; Juntunen, M.; Hasselwander, O. Prebiotic Potential of Xylo-Oligosaccharides. In Prebiotics and Probiotics Science and Technology; Charalampopoulos, D., Rastall, R.A., Eds.; Springer: New York, NY, USA, 2009; pp. 245–258. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of xylo-oligosaccharides (XOS) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, e05361. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Implementing Regulation (EU) 2018/1648 of 29 October 2018 Authorising the Placing on the Market of Xylo-Oligosaccharides as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2018, L 275/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R1648 (accessed on 20 January 2022).
- Ververis, E.; Ackerl, R.; Azzollini, D.; Colombo, P.A.; de Sesmaisons, A.; Dumas, C.; Fernandez-Dumont, A.; da Costa, L.F.; Germini, A.; Goumperis, T. Novel foods in the European Union: Scientific requirements and challenges of the risk assessment process by the European Food Safety Authority. Food Res. Int. 2020, 137, 109515. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; et al. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar]
- Kont, R.; Kurašin, M.; Teugjas, H.; Väljamäe, P. Strong cellulase inhibitors from the hydrothermal pretreatment of wheat straw. Biotechnol. Biofuels 2013, 6, 135. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Huang, K.; Zhang, S.; Xu, Y. Optimization of selective acidolysis pretreatment for the valorization of wheat straw by a combined chemical and enzymatic process. J. Chem. Technol. Biotechnol. 2020, 95, 694–701. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat Straw Autohydrolysis: Process Optimization and Products Characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.C.; Garrote, G.; Cruz, J.M.; Dominguez, H. Production of xylooligosaccharides by autohydrolysis of lignocellulosic materials. Trends Food Sci. Technol. 2004, 15, 115–120. [Google Scholar] [CrossRef]
- Jayapal, N.; Samanta, A.K.; Kolte, A.P.; Senani, S.; Sridhar, M.; Suresh, K.P.; Sampath, K.T. Value addition to sugarcane bagasse: Xylan extraction and its process optimization for xylooligosaccharides production. Ind. Crop. Prod. 2013, 42, 14–24. [Google Scholar] [CrossRef]
- Yang, G.; Xu, F.; Ji, X.; Dong, J.; Ni, Y.; Fatehi, P.; Zhang, K.; Chen, J. Enhanced biochemical process for purifying xylo-oligosaccharides from pre-hydrolysis liquor. Res. Sq. 2020, 1–26. [Google Scholar] [CrossRef]
- Kumar, V.; Satyanarayana, T. Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermo-alkali-stable endoxylanase of the polyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour. Technol. 2015, 179, 382–389. [Google Scholar] [CrossRef]
- Batidzirai, B.; Valk, M.; Wicke, B.; Junginger, M.; Daioglou, V.; Euler, W.; Faaij, A.P.C. Current and future technical, economic and environmental feasibility of maize and wheat residues supply for biomass energy application: Illustrated for South Africa. Biomass Bioenergy 2016, 92, 106–129. [Google Scholar] [CrossRef] [Green Version]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The Effect of Alkaline Pretreatment Methods on Cellulose Structure and Accessibility. ChemSusChem 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Bian, J.; Peng, P.; Peng, F.; Xiao, X.; Xu, F.; Sun, R.-C. Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food Chem. 2014, 156, 7–13. [Google Scholar] [CrossRef]
- Budde, J.; Heiermann, M.; Quiñones, T.S.; Plöchl, M. Effects of thermobarical pretreatment of cattle waste as feedstock for anaerobic digestion. Waste Manag. 2014, 34, 522–529. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. London Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Ghose, T. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257. [Google Scholar] [CrossRef]
- Royston, P. Approximating the Shapiro-Wilk W-test for non-normality. Stat. Comput. 1992, 2, 117–119. [Google Scholar] [CrossRef]
- Ibbett, R.; Gaddipati, S.; Greetham, D.; Hill, S.; Tucker, G. The kinetics of inhibitor production resulting from hydrothermal deconstruction of wheat straw studied using a pressurised microwave reactor. Biotechnol. Biofuels 2014, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Hydrothermal pretreatment of wheat straw: Effects of temperature and acidity on byproduct formation and inhibition of enzymatic hydrolysis and ethanolic fermentation. Agronomy 2021, 11, 487. [Google Scholar] [CrossRef]
- Li, J.; Feng, P.; Xiu, H.; Zhang, M.; Li, J.; Du, M.; Zhang, X.; Kozliak, E.; Ji, Y. Wheat straw components fractionation, with efficient delignification, by hydrothermal treatment followed by facilitated ethanol extraction. Bioresour. Technol. 2020, 316, 123882. [Google Scholar] [CrossRef]
- Faryar, R.; Linares-Pastén, J.A.; Immerzeel, P.; Mamo, G.; Andersson, M.; Stålbrand, H.; Mattiasson, B.; Karlsson, E.N. Production of prebiotic xylooligosaccharides from alkaline extracted wheat straw using the K80R-variant of a thermostable alkali-tolerant xylanase. Food Bioprod. Process. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Akpinar, O.; Erdogan, K.; Bostanci, S. Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food Bioprod. Process. 2009, 87, 145–151. [Google Scholar] [CrossRef]
- Han, L.; Feng, J.; Zhang, S.; Ma, Z.; Wang, Y.; Zhang, X. Alkali pretreated of wheat straw and its enzymatic hydrolysis. Braz. J. Microbiol. 2012, 43, 53–61. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Marques, S.; Loureiro-Dias, M.C.; Fonseca, C.; Gírio, F. Biorefining strategy for maximal monosaccharide recovery from three different feedstocks: Eucalyptus residues, wheat straw and olive tree pruning. Bioresour. Technol. 2015, 183, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, M.; Garrote, G.; Alonso, J.; Domínguez, H.; Parajó, J. Refining of autohydrolysis liquors for manufacturing xylooligosaccharides: Evaluation of operational strategies. Bioresour. Technol. 2005, 96, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Vegas, R.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Processing of rice husk autohydrolysis liquors for obtaining food ingredients. J. Agric. Food Chem. 2004, 52, 7311–7317. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chen, J.; Yang, G.; Ji, X.; Wang, Q.; Liu, S.; Ni, Y. Combined Treatments Consisting of Calcium Hydroxide and Activate Carbon for Purification of Xylo-Oligosaccharides of Pre-Hydrolysis Liquor. Polymers 2019, 11, 1558. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, P.; Ryan, J.; Ni, Y. Adsorption of lignocelluloses of model pre-hydrolysis liquor on activated carbon. Bioresour. Technol. 2013, 131, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Neu, A.-K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Mandl, M.; Venus, J. Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice. Fermentation 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Pleissner, D.; Schneider, R.; Venus, J.; Koch, T. Separation of lactic acid and recovery of salt-ions from fermentation broth. J. Chem. Technol. Biotechnol. 2017, 92, 504–511. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane Technologies for Lactic Acid Separation from Fermentation Broths Derived from Renewable Resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Dupoiron, S.; Lameloise, M.-L.; Pommet, M.; Bennaceur, O.; Lewandowski, R.; Allais, F.; Teixeira, A.R.; Rémond, C.; Rakotoarivonina, H. A novel and integrative process: From enzymatic fractionation of wheat bran with a hemicellulasic cocktail to the recovery of ferulic acid by weak anion exchange resin. Ind. Crop. Prod. 2017, 105, 148–155. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.-F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).